Abstract
Background
Early endoscopic lesions following resection for Crohn’s disease (CD) are often observed. Currently, the relationship between this endoscopic observation and subsequent occurrence of CD lesions or recurrence is not understood well, but should be valuable in the context of predicting CD course. This prospective study was to investigate the impact of early endoscopic lesions on future clinical recurrence rates following ileocolonic resection for CD.
Methods
Forty patients who had maintained clinical remission, CD activity index (CDAI) <150 with mesalazine during 6 months after ileocolonic resection for CD were included. At 6 months after surgery, ileocolonoscopy was performed, and the endoscopic activity score at the proximal site of the anastomosis was determined according to Rutgeerts. All patients were regularly monitored for 5 years, and clinical recurrence was defined as CDAI ≥ 150. Corticosteroids, immunosuppressants, or biological agents were not given unless there was clinical recurrence.
Results
At 6 months after surgery, the endoscopic scores were i0 or i1 in 27 patients, i2 in seven patients, i3 in four patients, and i4 in two patients. During the following 5 years, the clinical recurrence occurred in three (11%) patients with endoscopic score of i0 or i1, four (57%) patients with i2 score, three (75%) patients with i3 score, and two (100%) patients with i4 score, showing a significant positive correlation (p = 0.001) between the endoscopic severity of the proximal site of the anastomosis at 6 months after surgery and the clinical recurrence rate during the following 5 years.
Conclusions
The assessment of endoscopic lesions at the proximal site of the anastomosis appeared to be valuable for predicting subsequent clinical recurrence after ileocolonic resection for CD. Further studies in larger cohorts of patients are warranted to strengthen our findings.
Keywords: Crohn’s disease, endoscopic lesions, postoperative recurrence, prophylactic medications, resection
Introduction
In the management of Crohn’s disease (CD), postoperative recurrence is common, and many patients require repeat operations.1 Within 1 year after ileocolonic resection for CD, endoscopic lesions were observed at the proximal site of the anastomosis in approximately 70% of the patients, although only 20% of the patients developed recurrent clinical symptoms (clinical recurrence).2 Thus, early endoscopic lesions are frequently observed in patients without clinical recurrence after resection for CD.
Rutgeerts and colleagues2 reported that patients with severe endoscopic lesions within 1 year after resection developed early clinical recurrence. In contrast, patients with no or mild endoscopic lesions had a low frequency of subsequent clinical recurrence. The severity of the endoscopic inflammation at the proximal site of the anastomosis during the first year after resection was reported to be a reliable predictive risk factor for future clinical recurrence.
To the best of our knowledge, since the report by Rutgeerts and colleagues in 1990,2 there have been no prospective studies examining the impact of endoscopic lesions at the proximal site of the anastomosis on subsequent clinical recurrence rates after resection for CD. Currently, the relationship between this endoscopic observation and subsequent recurrence is not understood well, but it should be valuable in the context of predicting CD course. This prospective study was undertaken to investigate the impact of early endoscopic lesions on the clinical course of patients following ileocolonic resection for CD. The relationship between endoscopic findings at the proximal site of the anastomosis and subsequent clinical recurrence rates were analysed. Furthermore, factors affecting endoscopic findings and clinical recurrence rates were investigated.
Methods
Patients
This was a prospective cohort study conducted at a single centre. The study was carried out in accordance with the principle of good clinical practice, the Declaration of Helsinki and the study protocol was reviewed and approved by our Institutional Review Board. Inclusion criteria were: (1) patients who were between 15 and 70 years of age; (2) those who underwent ileocolonic (including previous anastomosis) resection with primary ileocolonic anastomosis for active CD; (3) those who remained in clinical remission (CD activity index, CDAI, score3 < 150) with mesalazine treatment (Pentasa 3 g/day) during 6 months after resection; (4) those who agreed to undergo endoscopic examination at 6 months after surgery; and (5) those who agreed to continue the medical treatment with mesalazine until clinical recurrence (CDAI score ≥ 150) was confirmed. Exclusion criteria were: (1) patient who had gastroduodenal, jejunal, or proximal ileal disease at the time of operation; (2) those with active colonic or anorectal disease in endoscopic examination at 6 months after resection; and (3) those who had received corticosteroids, immunosuppressants, or biological agents after surgery. Forty patients who met our inclusion criteria were entered into this study at 6 months after surgery. The baseline characteristics of the patients are presented in Table 1.
Table 1.
Baseline characteristics
| Characteristic | Patient population (n = 40) |
|---|---|
| Age at entry (years) | 32 ± 4 |
| Male: female | 26 : 14 |
| Duration of CD before entry (months) | 38 ± 8 |
| Smoker | 4 |
| Previous surgery | 8 |
| Preoperative medication | |
| Mesalazine | 31 |
| Corticosteroids | 37 |
| Immunosuppressive drugs or biological | 0 |
| Indication for surgery | |
| Bowel obstruction | 40 |
| Concomitant abscess/fistula | 4 |
Values are mean ± standard error or n.
CD, Crohn’s disease; Previous surgery, patients who had undergone bowel surgery before the current operation; Concomitant abscess/fistula, abscess or fistula detected at the time of laparotomy.
Endoscopic examination
At 6 months after surgery, ileocolonoscopy was performed in all patients, and the endoscopic activity score at the proximal site of the anastomosis was determined according to Rutgeerts.2 Rutgeerts score is a well-established endoscopic scoring system based on examination of the ileal segment proximal to ileocolonic anastomosis: i0, no lesions; i1, up to five aphthous lesions; i2, more than five aphthous lesions with normal mucosa between the lesions, or skip areas of larger lesions or lesions confined to the ileocolonic anastomosis; i3, diffuse aphthous ileitis with diffusely inflamed mucosa; and i4, diffuse inflammation with larger ulcers, nodules, and/or narrowing. The patients were blinded to endoscopic findings because if not blinded then the endoscopic findings could impact on subjective symptoms and lead to lower or higher CDAI scores.
Follow up
All patients were reviewed regularly in our clinic for the following 5 years after the endoscopic examination. All patients were instructed to record their symptoms in a diary every day. At clinic visits, height, bodyweight, general wellbeing, fever, stool frequency and consistency, and presence or absence of abdominal pain and tenderness, tenesmus, and rectal bleeding were examined. Peripheral blood samples were collected for measurements of white cell count, haemoglobin, platelet count, C-reactive protein, and albumin. The clinical disease activity was assessed according to the CDAI score, and clinical recurrence was defined as a CDAI score of ≥150. Corticosteroids, immunosuppressants, or biological agents were not given, and all patients were treated with continuing mesalazine (Pentasa 3 g/day) unless there was clinical recurrence.
Statistical analysis
Comparisons of frequencies were done using the chi-squared test with Yates’ correction. Means between the groups were compared using the unpaired Student’s t-test. p < 0.05 was considered statistically significant.
Results
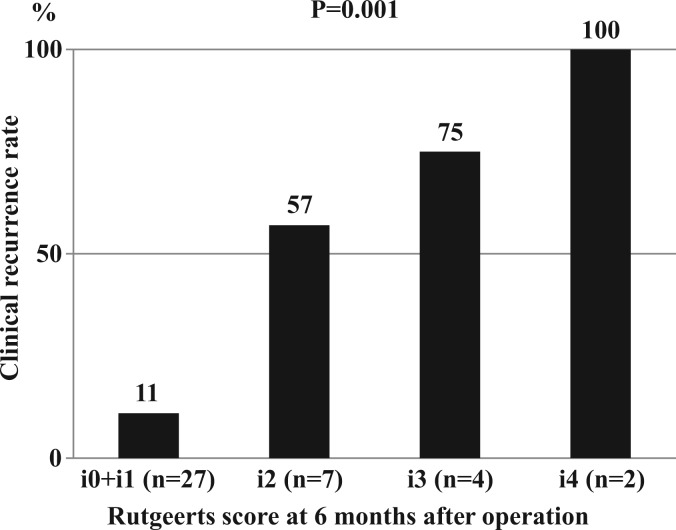
At 6 months after surgery, the endoscopic scores were i0 or i1 in 27 patients, i2 in seven patients, i3 in four patients, and i4 in two patients. During the following 5 years, 12 (30%) of the 40 patients had clinical recurrence. Clinical recurrence (CDAI score ≥ 150) occurred in three (11%, 95% confidence interval –1 to 23%) patients with endoscopic score of i0 or i1, four (57%, 95% CI 20 to 94%) patients with i2 score, three (75%, 95% CI 33 to 117%) patients with i3 score, and two (100%) patients with i4 score, showing a significant positive correlation (p = 0.001) between the endoscopic severity of the proximal site of the anastomosis at 6 months after surgery and the clinical recurrence rate during the following 5 years (Figure 1).
Figure 1.
The relationship between the endoscopic severity of the proximal site of the anastomosis at 6 months after ileocolonic resection and the clinical recurrence rate during the following 5 years.
To search for parameters which might have impacted the endoscopic severity at 6 months after surgery, the relationship between endoscopic severity and clinical and laboratory parameters was investigated. Patients were classified into two groups according to endoscopic severity: low-grade inflammation (n = 27), an endoscopic score of i0 or i1; and high-grade inflammation (n = 13), a score of i2, i3, or i4. The relationship between the baseline clinical parameters and endoscopic severity at 6 months after surgery is presented in Table 2. None of these parameters affected the endoscopic severity. The mean ± SE CDAI score at 6 months after surgery was 99 ± 4 in patients with low-grade inflammation vs. 104 ± 5 in those with high-grade inflammation (p = 0.39).
Table 2.
Baseline clinical parameters
| Parameter | Endoscopic severity |
|
|---|---|---|
| Low-grade inflammation (n = 27) | High-grade inflammation (n = 13) | |
| Age at entry (years) | ||
| ≤32 (n = 20) | 11 | 9 |
| >32 (n = 20) | 16 | 4 |
| Gender | ||
| Male (n = 26) | 17 | 9 |
| Female (n = 14) | 10 | 4 |
| Duration of CD before entry (months) | ||
| ≤38 (n = 21) | 13 | 8 |
| >38 (n = 19) | 14 | 5 |
| Smoking habit at entry | ||
| Smoker (n = 4) | 2 | 2 |
| Non-smoker (n = 36) | 25 | 11 |
| Previous surgery | ||
| Yes (n = 8) | 5 | 3 |
| No (n = 32) | 22 | 10 |
| Concomitant abscess/fistula at surgery | ||
| Yes (n = 4) | 3 | 1 |
| No (n = 36) | 24 | 12 |
Values are n. There were no statistically significant differences (chi-squared test).
Low-grade inflammation, endoscopic score i0 or i1; high-grade inflammation, endoscopic score i2, i3, or i4.
The relationship between endoscopic severity and laboratory parameters at 6 months after surgery is presented in Table 3. The mean serum C-reactive protein level was higher in patients with high-grade inflammation than in those with low-grade inflammation. However, the difference did not statistically significant. The mean serum albumin level was lower in patients with high-grade inflammation than in those with low-grade inflammation, but the difference was not statistically significant.
Table 3.
Laboratory parameters
| Parameter | Endoscopic severity |
|
|---|---|---|
| Low-grade inflammation (n = 27) | High-grade inflammation (n = 13) | |
| White cell count (/mm3) | 6200 ± 460 | 7800 ± 1050 |
| Haemoglobin (g/dl) | 12.2 ± 0.3 | 11.9 ± 0.5 |
| Platelet count (/mm3) | 310,000 ± 17,000 | 340,000 ± 34,000 |
| C-reactive protein (mg/dl) | 0.5 ± 0.2 | 1.3 ± 0.4 |
| Albumin (g/dl) | 3.8 ± 0.1 | 3.6 ± 0.1 |
Values are mean ± standard error. There were no statistically significant differences (unpaired Student’s t-test).
Low-grade inflammation, endoscopic score i0 or i1; high-grade inflammation, endoscopic score i2, i3, or i4.
Factors affecting the occurrence of clinical recurrence (CDAI score ≥ 150) were also investigated (Table 4). Age, gender, duration of CD before entry, smoking habit, previous surgery, and concomitant abscess or fistula presented at laparotomy did not significantly affect the incidence of clinical recurrence. Only endoscopic severity of the proximal site of anastomosis at 6 months after surgery significantly affected the clinical recurrence rates.
Table 4.
Clinical recurrence rates after surgery
| Factor | Recurrence (n = 12) | No recurrence (n = 28) | p-value |
|---|---|---|---|
| Age at entry (years) | |||
| ≤32 (n = 20) | 7 | 13 | 0.49 |
| >32 (n = 20) | 5 | 15 | |
| Gender | |||
| Male (n = 26) | 8 | 18 | 0.88 |
| Female (n = 14) | 4 | 10 | |
| Duration of CD before entry (months) | |||
| ≤38 (n = 21) | 7 | 14 | 0.62 |
| >38 (n = 19) | 5 | 14 | |
| Smoking habit at entry | |||
| Smoker (n = 4) | 2 | 2 | 0.36 |
| Non-smoker (n = 36) | 10 | 26 | |
| Previous surgery | |||
| Yes (n = 8) | 4 | 4 | 0.17 |
| No (n = 32) | 8 | 24 | |
| Concomitant abscess/fistula at surgery | |||
| Yes (n = 4) | 2 | 2 | 0.36 |
| No (n = 36) | 10 | 26 | |
| Endoscopic severity at 6 months after surgery | |||
| i0, i1 (n = 27) | 3 | 24 | 0.0002 |
| i2, i3, i4 (n = 13) | 9 | 4 |
Values are n. p-values calculated using chi-squared test.
Discussion
In this study, we selected a well-defined and homogeneous group of patients according to the rigorous inclusion and exclusion criteria. We included patients who underwent curative ileocolonic resection with primary anastomosis for CD. The indication for surgery was bowel obstruction in all patients. Only four patients had concomitant perforating disease (abscess or fistula) at laparotomy. In our clinical practice, when patients have severe abscess or fistula at laparotomy, we avoid anastomosis or create diverting stoma following bowel resection. In this study, we excluded patients who did not have primary anastomosis or who required diverting stoma to protect anastomosis. During this study, all patients were treated with continuing mesalazine (Pentasa 3 g/day) unless there was clinical recurrence.
We have decided to perform endoscopic examination at 6 months after surgery because severe endoscopic recurrence confirmed at 1 year or later postoperatively does not respond well to any medications in our experience. This timing is earlier than that in the study by Rutgeerts and colleagues.2 More evidence is necessary to determine the most appropriate timing for postoperative endoscopy.
In this rigorous study, we confirmed a very significant positive correlation between the endoscopic severity of the proximal site of the anastomosis at 6 months after ileocolonic resection and the clinical recurrence rate during the following 5 years. The assessment of endoscopic lesions at the proximal site of the anastomosis was valuable for predicting subsequent clinical recurrence after ileocolonic resection for CD.
We failed to find any significant correlation between the clinical parameters at baseline and the endoscopic severity of the proximal site of the anastomosis at 6 months after surgery. Similarly, we found no significant correlation between the laboratory parameters at 6 months after surgery and the endoscopic severity. Further, the CDAI score at 6 months after surgery did not significantly correlate with the endoscopic severity. These results suggest that it is not possible to predict endoscopic severity using the clinical findings at the time of surgery or laboratory markers after surgery.
Several studies have reported that faecal calprotectin showed a close correlation with endoscopic inflammation in patients with CD and ulcerative colitis.4–7 Faecal calprotectin appears to be useful for the assessment of endoscopic disease activity. After surgery for CD, faecal calprotectin measurement may be valuable for detecting early endoscopic lesions. We are now conducting a prospective study to investigate the relationship between faecal calprotectin and endoscopic findings after ileocolonic resection for CD.
The identification of risk factors for postoperative recurrence can help to determine optimal strategies for medical therapy after surgery. Smoking and perforating disease are reported to be significant risk factors for postoperative recurrence.8–10 We also searched for factors which might have affected the clinical recurrence rates. None of the following factors appeared to affect the incidence of the clinical recurrence: age, gender, duration of CD, smoking habit, previous surgery, and concomitant abscess or fistula at laparotomy. Only endoscopic severity of the proximal site of anastomosis at 6 months after surgery significantly affected the clinical recurrence rates. Therefore, endoscopic examination is the optimal method to precisely predict subsequent recurrence after surgery for CD.
The main limitation of our study is the relatively small sample size. We had a well-defined and homogeneous group of patients, and this should partly compensate for the sample size not being large enough. The findings of this investigation should be strengthened by a future study involving a large cohort of patients to fully evaluate the impact of early endoscopic lesions on the clinical course of patients following ileocolonic resection.
Based on the results in our study, patients who postoperatively develop early endoscopic lesions (i2, i3, or i3) despite optimal mesalazine therapy may not benefit from continuing mesalazine. For such patients, more powerful medications should be considered. In contrast, the majority of patients with no (i0) or mild (i1) endoscopic lesions have a low frequency of subsequent clinical recurrence and prolonged periods of remission with mesalazine treatment.
In conclusion, the results in the present study are valuable not only for identifying patients with a high risk of recurrence after surgery for CD but also for creating an integrated management strategy for prevention of postoperative recurrence. The early endoscopic inflammation at the proximal site of the anastomosis after ileocolonic resection is a suitable model to investigate the pathogenesis of CD and also to evaluate new therapeutic modalities for prevention of progressive recurrence.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Lock MR, Farmer RG, Fazio VW, et al. Recurrence and reoperation for Crohn’s disease: the role of disease location in prognosis. N Engl J Med 1981; 304: 1586–1588 [DOI] [PubMed] [Google Scholar]
- 2.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990; 99: 956–963 [DOI] [PubMed] [Google Scholar]
- 3.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index: National Cooperative Crohn’s Disease Study. Gastroenterology 1976; 70: 439–444 [PubMed] [Google Scholar]
- 4.Sipponen T, Kärkkäinen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther 2008; 28: 1221–1229 [DOI] [PubMed] [Google Scholar]
- 5.Jones J, Loftus EV, Jr, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2008; 6: 1218–1224 [DOI] [PubMed] [Google Scholar]
- 6.Sipponen T, Björkesten CG, Färkkilä M, et al. Faecal calprotectin and lactoferrin are reliable surrogate markers of endoscopic response during Crohn’s disease treatment. Scand J Gastroenterol 2010; 45: 325–331 [DOI] [PubMed] [Google Scholar]
- 7.D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012; 18: 2218–2224 [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T. Factors affecting recurrence after surgery for Crohn’s disease. World J Gastroenterol 2005; 11: 3971–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simillis C, Yamamoto T, Reese GE, et al. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus non-perforating Crohn’s disease. Am J Gastroenterol 2008; 103: 196–205 [DOI] [PubMed] [Google Scholar]
- 10.Reese GE, Nanidis T, Borysiewicz C, et al. The effect of smoking after surgery for Crohn’s disease: a meta-analysis of observational studies. Int J Colorectal Dis 2008; 23: 1213–1221 [DOI] [PubMed] [Google Scholar]