Abstract
Background
The factors associated with inadequate bowel cleansing leading to colonoscopy failure are not well known.
Objective
We conducted a multicentre, prospective, observational case–control study in order to identify these factors.
Methods
Investigators included one patient with colonoscopy failure due to inadequate bowel cleansing (failure group) and the following patient with complete colonoscopy (success group). Data related to demographics, medical history, and preparation were collected and compared. Factors associated with bowel cleansing failure were identified by multivariate logistical regression analysis.
Results
A total of 101 gastroenterologists included 202 patients from 1 November 2009 to 15 January 2010. There was no difference between both groups with regards to baseline characteristics. In univariate analysis, factors significantly associated with bowel cleansing failure were vomiting during preparation (p = 0.0007), incomplete intake of the preparation (p = 0.002), and constipation (p = 0.02). Type and timing of preparation were not significantly different between groups. In multivariate analysis, incomplete intake of the preparation (OR 4.5, 95% CI 1.2–17.3), constipation (OR 4.2, 95% CI 1.2–14.9), and treatment with neuroleptics or antidepressants (OR 9.9 (95% CI 1.4–71.0) were independent predictors of colonoscopy failure.
Conclusions
Factors associated with bowel cleansing failure were incomplete intake of the preparation, constipation, and treatment with psychotropic drugs. Interventions to reduce poor colonoscopy preparations should be targeted at these at-risk patients.
Keywords: Bowel preparation, inadequate bowel cleansing, risk factors
Introduction
Colonoscopy is the gold standard for the investigation of the colon. Colonoscopies also enable biopsies and treatment of superficial tumours (polypectomy and mucosal resection). According to a French epidemiological survey, out of 1.12 million colonoscopies performed in 2008, 5% had to be redone because they were incomplete. Inadequate bowel cleansing was responsible for failure in 40% of cases.1 Poor bowel preparation is associated with increased technical difficulties, enhanced risks of perforation, longer examination durations, and reduced adenoma detection rates.2–4 The costs related to repeated colonoscopies and prolonged hospital stays are significant.4 Finally, undetected carcinomas may also lead to lawsuits.
The factors associated with bowel preparation failure remain largely unknown. Interventional studies are not entirely appropriate to answer this type of question because, first, they focus on a single parameter while reasons for failure are often multiple and intertwined, and second, they do not reflect real life. In this respect, case–control studies are probably more informative because they can alert to trivial issues that occur in daily practice. The main objective of the PACOME study (Préparation A la Coloscopie: Observatoire des Motifs d’Echec) was to identify the factors related to insufficient bowel preparation prior to colonoscopy and describe groups of at-risk patients. In addition, we investigated how medical instructions are really implemented.
Materials and methods
This prospective observational study was performed with private and hospital-based gastroenterologists. They were invited to participate in the study by the Société Française d’Endoscopie Digestive (SFED).
All adult patients who underwent a routine colonoscopy, whatever its indication were eligible to the study. Each investigator had to include two subjects: the first patient with colonoscopy failure due to poor bowel preparation (failure group) and the second one, the consecutive patient, with successful examination of the colon by colonoscopy (success group, controls). Colonoscopy failure was defined as unperformed colonoscopy, incomplete colonoscopy (no cecal intubation), or complete colonoscopy but requiring a further control colonoscopy, due to poor bowel preparation quality according to the personal opinion of the endoscopist. The endoscopists were not trained in bowel preparation objective assessment using specific scales.
Exclusion criteria were the following: patients below 18 years of age, history of colon surgery and/or inflammatory bowel disease, colonoscopy failure due to any other reason than insufficient preparation, absence of bowel cleansing, and any condition potentially hampering compliance with the study protocol.
Investigators were free to choose bowel preparation type, dose, and regimen.
Data collection
Data were collected from self-administered questionnaires. Each investigator filled in a general questionnaire concerning its activities, main habits for colonoscopy (location, timing), and usual modalities of patients’ information (timing and duration of the precolonoscopy consultation, documents provided to the patient, type of information). In addition, the investigators recorded the following items in a questionnaire for each included patient: patients’ characteristics and timing of colonoscopy, indication for colonoscopy, existence of digestive symptoms (symptomatic) or not (asymptomatic), comorbidities, modalities of preparation and information, and colonoscopy results. Lastly, each patient also filled in a feedback form, documenting demographics and comorbidities, description of the preparation (what was prescribed and what was really done, tolerance, and acceptability), and information provided before the examination. Information given was not standardized; each investigator proceeded according to his usual practice.
The following data was analysed: patient characteristics (age, gender, comorbidities, previous colonoscopy history, and current treatments), indication of the colonoscopy, acceptability of the preparation and compliance, type of bowel cleansing and administration protocol, tolerability and side effects of the preparation, and instructions given to the patient. The latter being asked to the patients and physicians, consistency between their responses could be measured. The patient questionnaire is indicated in Appendix A (available online).
Statistical analysis
The primary outcome was to identify the factors related to insufficient bowel preparation prior to colonoscopy. Each gastroenterologist had to include a pair of patients, one patient with colonoscopy failure and the subsequent patient with a successful colonoscopy. Baseline demographic and clinical factors of the patient groups were described and compared using chi-squared test for qualitative data and Student’s t-test for quantitative data in order to assess properly paired groups and identify the potential variables predictors of success or failure of colonoscopy.
As the study design was conducted according to a ‘match box 1–1 control design’, conditional logistic regression analyses were used to evaluate the association between baseline demographic and clinical factors and successful colonoscopy. The analyses were performed using PROC LOGISTIC in SAS with STRATA option. All variables have been retained in the analyses with the exception of subjective variables, variables with a low relative frequency (<5%) – e.g. chronic heart problems (3%), thyroid disorders (1%) – ‘trivial’ variables which have a direct causal relationship with another one: for example, ‘vomiting while taking preparation’ generates almost always ‘incomplete taking the bowel preparation’.
The potential predictors of success or failure of colonoscopy are the difference between the two patients in each pair. The variables were first dichotomized (0 or 1), then they took the following values: 0 if the two patients were equivalent, –1 or 1 if the patients’ success/failure statuses were different (–1 or 1 depending on whether the failure or success patient had the characteristic, respectively).
The logistic regression model had 37 variables introduced. The type 1 error was α = 0.05.
Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC, USA). All p-values are two-sided. The adjusted odds ratio (OR) with their 95% confidence interval (CI) and p-value were calculated for each variable retained in the multivariate conditional logistic regression analysis.
Results
One thousand French gastroenterologists registered in the SFED database received an invitation to take part in the study, and 102 accepted to participate. One gastroenterologist who only included one instead of two patients was excluded. Among investigators, 43 and 27% had an exclusive private or public practice, respectively; the remaining 30% were working in both types of institutions. The mean number of colonoscopies performed monthly per physician was about 50, and 38% of participants declared more than 60/month.
Patient characteristics
This study included 202 patients from 1 November 2009 to 15 January 2010: 101 patients in each group. There was no difference between the failure and success groups regarding gender, body mass index, colonoscopy indications including existence of digestive symptoms, previous colonoscopy, and medical history, including abdominal or pelvic surgery, radiotherapy, concomitant chronic diseases (e.g. arterial hypertension, diabetes, cancer; Table 1). Long-term administration of antidepressants and/or neuroleptics (17 vs. 9% p < 0.09) and constipation (42 vs. 26% p < 0.02) occurred more frequently in the failure group than in the success group (Table 1).
Table 1.
Characteristics of the patients
| Failure (n = 101) | Success (n = 101) | p-value | |
|---|---|---|---|
| Male | 49 | 43 | NS |
| Age (years) | |||
| <40 | 13 | 12 | NS |
| 40–59 | 45 | 44 | |
| 60–74 | 30 | 40 | |
| >75 | 12 | 4 | |
| Body mass index (kg/m2) | |||
| <30 | 82 | 79 | NS |
| ≥75 | 18 | 21 | |
| History | |||
| Digestive surgery | 23 | 16 | NS |
| Minor pelvic surgery | 13 | 16 | |
| Pelvic irradiation | 3 | 3 | |
| Chronic disease | 40 | 33 | NS |
| Constipation | 42 | 26 | <0.02 |
| Long-term treatment | |||
| Any treatment | 35 | 28 | NS |
| Antihypertensive drug | 20 | 24 | NS |
| Antidiabetic drug | 7 | 9 | NS |
| Antidepressant/antipsychotic | 17 | 9 | <0.09 |
| Previous colonoscopies | |||
| Mean n | 1.8 | 1.9 | NS |
| Previous failures | 22 | 8 | NS |
Values are % unless otherwise stated.
NS, non significant.
Colonoscopy and colonoscopy preparation characteristics
Colonoscopy failures were due to inadequate bowel preparation. When asked about result of colonoscopy for their patients in the failure group, physicians reported: examination was not performed, was incomplete, or required another colonoscopy (2, 27, and 71% of cases, respectively).
Examinations were performed in the morning (before 13.00 hours) in 69% of the failure group and in 77% of the success group (p = 0.20), most between 10.00 and 13.00 hours (51 and 54% for failure and success, respectively). Split-dose preparation was less frequently used in the failure group than the success group (38 vs. 46%), but the difference did not reach statistical significance (p = 0.20). Time between end of ingestion and colonoscopy was similar in both groups whatever the bowel preparation regimen (mean ± SD, 13.5 ± 3.4 vs. 12.7 ± 4.4 h for nonsplit-dose regimens and 5.2 ± 2.8 vs. 5.5 ± 2.4 h for split-dose regimens in the failure and success groups, respectively. Almost all patients had a fibre-free diet before colonoscopy (95 and 94% in failure and success groups, respectively).
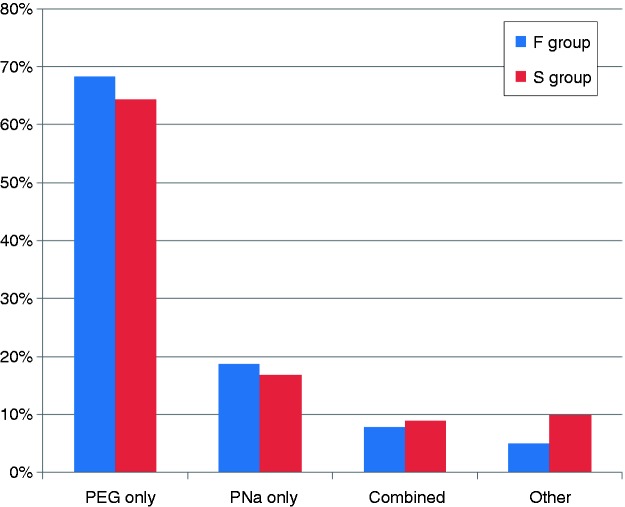
The type of preparation was similarly distributed in both groups, with about two-thirds of patients receiving polyethylene glycol (PEG) and 18% receiving sodium-phosphate-based (PNa) preparation only (Figure 1). The proportion of patients who did not take the bowel-cleansing solution completely was higher in the failure group than in the success group (30 vs. 12%, p = 0.0002; Table 2). In the failure group, the difference between prescription and real intake was 0.43 l in patients who received PEG only (4.06 ± 0.87) vs. 3.63 ± 1.13 l) and 0.56 l in those who were prescribed PNa only (1.66 ± 2.08 vs. 1.10 ± 0.93 l).
Figure 1.
Types of colonic cleansing preparation in the failure and success groups.
There were no significant differences. F, failure; PEG, polyethylene glycol; PNa, sodium phosphate; S, success.
Table 2.
Parameters associated with colonoscopy failure in uni- and multivariate analyses
| Failure (n = 101) | Success (n = 101) | Univariate p-value | Multivariate p-value | OR (95% CI) | |
|---|---|---|---|---|---|
| Vomiting during preparation | 26 | 8 | 0.0007 | – | – |
| Incomplete preparation intake | 30 | 12 | 0.0002 | 0.03 | 4.5 (1.2–17.3) |
| Constipation | 42 | 26 | 0.02 | 0.03 | 4.2 (1.2–14.9) |
| Psychotropic drugs | 17 | 9 | 0.09 | 0.02 | 9.9 (1.4–71.0) |
Values are % unless otherwise stated.
Vomiting was not included in the multivariate analysis because it was one of the main reasons for incomplete preparation intake.
CI, confidence interval; OR, odds ratio.
In the failure group, the main reason for incomplete intake of the preparation was vomiting (48% of cases). Vomiting was significantly more frequent in the failure group than in the success group (26 vs. 8%, respectively, p = 0.0007). The overall tolerance of the preparation was higher in the success group, although not significantly, than in the failure group (40 vs. 28%). There were 27 and 24% of patients in the failure and success groups, respectively, who experienced abdominal pain.
Patient information
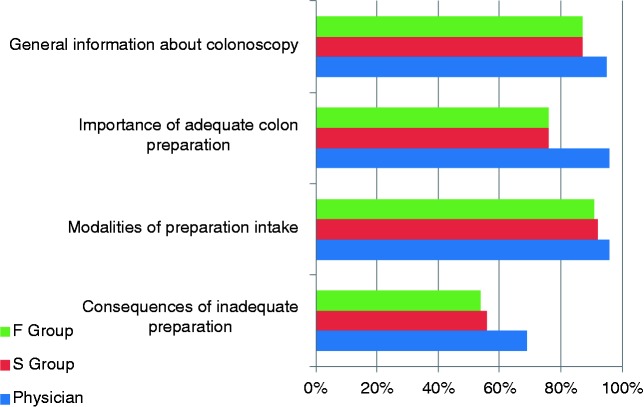
The mean duration of the precolonoscopy visit were similar in the failure and success groups (20 and 21 min, respectively: <11 min, 4 and 14%; 11–15 min, 32 and 31%; 16–20 min, 36 and 25%; >20 min, 28 and 30%). Patients of both groups received information about colonoscopy similarly (Figure 2). However, the perceptions of the physicians were different from that of the patients, in particular information about the quality of adequate colon preparation, the modalities of intake, and the consequences of poor bowel cleansing were less often received by the patients than the physicians thought it was (Figure 2). Especially, only 55% of the patients were aware of the risk of colonoscopy failure in case of inadequate bowel cleansing, while 96% of physicians thought they had delivered appropriate information.
Figure 2.
Proportions of physicians that indicated that the four types of information had been correctly given and patients in the failure and success groups that self-reported having received the information.
F, failure; S, success.
The mean time from the precolonoscopy visit to the procedure was 37 and 35 days in failure and success groups, respectively. The percentage of colonoscopy failure was higher when the waiting time was >20 days, although not significantly (55% for ≥20 days vs. 44% for <20 days).
Factors associated with colonoscopy failure
In univariate analysis, factors associated with colonoscopy failure were vomiting during the preparation (26 vs. 8%, p = 0.0007), incomplete preparation intake (30 vs. 12%, p = 0.002), and constipation (42 vs. 26%, p = 0.02; Table 2). Long-term administration of psychotropic drugs (17 vs. 9%) did not reach significance (p = 0.09).
We included in the multivariate analysis all parameters but those with <5% occurrence, subjective parameters, and those that were clinically linked with major parameters. Especially, we did not include vomiting because it was the main reason of incomplete preparation intake. In multivariate analysis, factors associated with failure because of inadequate preparation were incomplete intake of the bowel preparation (OR 4.5, 95% CI 1.2–17.3, p = 0.028), previous constipation (OR 4.2, 95% CI 1.2 – 14.9, p = 0.026), and long-term administration of psychotropics (OR 9.9, 95% CI 1.4–71.0, p = 0.023; Table 2). Previous constipation and long-term administration of psychotropics were partially linked. When the psychotropics variable was excluded from the analysis, the significance of constipation increased from p = 0.026 to p = 0.012. Split-dose regimen did not reach significance (OR 4.7, 95% CI 0.8–28.4, p = 0.096)
The main self-reported reasons for noncompliance by the patients were vomiting (50%), nausea (36%), palatability (11%), saturation (4%), and flatulence (4%) in the failure group (n = 28) and saturation (27%), nausea (18%), flatulence (18%), and palatability (9%), but not vomiting (0%), in the success group (n = 11).
Discussion
In this prospective case–control study, the factors associated with poor bowel cleansing resulting in colonoscopy failure in the univariate analysis were constipation, vomiting during preparation intake, and incomplete drinking of the preparation. The three factors selected by the multivariate analysis were related either to patients’ characteristics (constipation and psychotropics) or to preparation intake (incomplete drinking). Our results differ from those of some other studies that found an association with male sex in several studies,5–10 obesity and increased body mass index,8–11 or other factors such as colorectal surgery,10–12 diabetes,10,12 cirrhosis,7,10 or Parkinson disease.10 These differences might be due to different methodology, as will be discussed.
In observational studies, about 20% of patients with colonoscopy failure were not compliant with instructions, and this parameter has been shown to be an independent risk factor for inadequate bowel cleansing.7 In this cohort, as we did, the authors also identified treatment with tricyclic antidepressants, late colonoscopy starting time (preparation was ingested the day prior to colonoscopy), and constipation as independent predictors of failure. The importance of compliance with instructions as a key success factor seems to be perfectly obvious. In our study, the average compliance rate was 79% in the studied population, and 70 and 88% in the failure and success groups, respectively. These percentages are higher in randomized controlled trials, ranging from 91 to 99%,5,13–15 but the definition of compliance is rarely given and the specific setting of randomized trials does not reflect real life.
Why do patients not comply with instructions? In our study, patients described nausea and vomiting as the main reasons for noncompliance. Vomiting was associated with colonoscopy failure in one-quarter of patients in the failure group (main reason for incomplete preparation drinking). Thus, this adverse event can be considered as a main cause of poor bowel cleansing and colonoscopy failure. Preparations that require a low volume of drinking and have a low emetic effect would likely reduce vomiting, thus increasing compliance with instructions and improving bowel cleansing quality.
Poor understanding of instructions and a low awareness of the consequences of inadequate bowel preparation might be another cause of noncompliance to instructions. We showed that only 55% of patients were conscious of these consequences, while 96% of physicians were convinced to have given appropriate and sufficient information. Other studies have showed that misunderstanding instructions is an important factor of poor preparation quality.16 Education programmes have been proved to be efficient in improving bowel cleansing preparation.17–19 Such interventions are easy to implement and inexpensive. The utilization of written documents, demonstrative pictures, electronic presentations, and/or videos might be useful.
The precolonoscopy consultation can also detect constipation that should be treated several days before starting the preparation; special attention must be paid to patients taking medicines like antidepressants or neuroleptics which cause or aggravate constipation. The date of consultation is also critical. It has been shown that the quality of bowel cleansing is inversely proportional to the waiting time between the information provided and the colonoscopy.19
Randomized trials comparing the various colon preparations gave conflicting results, some demonstrating the superiority of PNa over PEG,20 with others showing opposite13,21 or similar efficacy,15 or heterogeneous results, depending on the time of colonoscopy6 or the criteria used.22 Two recent meta-analyses of randomized published studies also showed conflicting results, one showing that PNa tablets resulted in better preparation and completion rates than PEG23 and the other showing similar results, except in some subgroups in favour of PEG.24 Afternoon colonoscopies might have lower success rates with more frequent inadequate bowel preparations.25,26 This was not shown in our study. Similarly, we were not able to statistically demonstrate better cleansing in split regimens as demonstrated by numerous other studies,15 probably because our study was underpowered.
Our study adds to the literature relating to bowel cleansing failure. In most publications, randomized clinical trials investigated the quality of bowel cleansing using various preparations, but few studies were designed to identify factors associated with failure. Among recently published papers, we found observational studies7–10,12,27,28 but no case–control studies, which makes our work original and relevant for clinical application. The gastroenterologists enrolled were representative of French gastroenterologists for the type of activity and sex ratio: 73% had a private practice while 27% had a public hospital-based practice, and these data are similar with those of the whole French gastroenterologist population according to a professional French database (CEGEDIM) including 3720 gastroenterologists. The process used to identify the study population and select subjects was achieved to reduce selection bias in a realistic manner. Indeed, we have selected cases and controls whose characteristics (e.g. gender, body mass index, history, chronic concomitant diseases, colonoscopy indications) were similar. Endoscopists were not specifically trained in bowel preparation objective assessment by using specific scales, but they were members of our national society (SFED), which organizes annual training courses in endoscopy for their members. It would have been impossible, considering the design of the study with a potential recruitment of 1000 gastroenterologists registered in the SFED database, to organize a specific course for the study. Secondly, we wanted to be as close as possible to real life to allow the conclusions to be applicable to general practice. A major limitation is that the study was underpowered, as suggested by the fact that split versus nonsplit regimens, a well-known factor of the quality of the colon cleansing,15 did not reach statistical significance in univariate and multivariate analyses. However, the three independent factors selected by the multivariate analysis had a major clinical relevance for daily practice. Extending the recruitment probably would have not changed the key message. A limitation is related to self-reported studied data with no data control. Finally, we did not collect colonoscopy findings and adverse events occurring during the examination, but it was not the aim of the study.
In conclusion, in this observational case–control study, we found that constipation, long-term use of psychotropics, and incomplete ingestion of the preparation, mainly due to vomiting, were associated with colonoscopy failure due to poor bowel cleansing. These findings have implications for practice. Colonoscopy failure can be overcome by a careful evaluation of comorbidities (constipation and treatment inducing constipation, especially psychotropics) and a clear explanation of the consequences of inadequate preparation. The tolerability of bowel cleansing procedures, especially avoiding vomiting, could be improved by the choice of preparations requiring lower volume intake and better taste. Although we could not show a statistically significant difference, a split course is likely to be preferable, at least in afternoon colonoscopies.
Acknowledgements
We warmly thank the gastroenterologists that participated in the study and Mrs Anne Visbecq for translation.
Funding
This work was supported by Mayoly Spindler, France.
Conflict of interest
JL, SC, TP, BRM, PC, RL, and GC have served as advisory board members for Mayoly Spindler France. FH is an employee of Mayoly Spindler, France. GH declares no conflict of interest.
References
- 1. Canard JM. Colonoscopy in France in 2008: results of the two-day survey of endoscopy in France. Acta Endosc 2010; 40: 58–65 [Google Scholar]
- 2. Froehlich F, Wietlisbach V, Gonvers JJ, et al. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc 2005; 61: 378–384 [DOI] [PubMed] [Google Scholar]
- 3. Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 2003; 58: 76–79 [DOI] [PubMed] [Google Scholar]
- 4. Rex DK, Imperiale TF, Latinovich DR, et al. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol 2002; 97: 1696–1700 [DOI] [PubMed] [Google Scholar]
- 5. Marmo R, Rotondano G, Riccio G, et al. Effective bowel cleansing before colonoscopy: a randomized study of split-dosage versus non-split dosage regimens of high-volume versus low-volume polyethylene glycol solutions. Gastrointest Endosc 2010; 72: 313–320 [DOI] [PubMed] [Google Scholar]
- 6. Lawrance IC, Willert RP, Murray K. Bowel cleansing for colonoscopy: prospective randomized assessment of efficacy and of induced mucosal abnormality with three preparation agents. Endoscopy 2011; 43: 412–418 [DOI] [PubMed] [Google Scholar]
- 7. Ness RM, Manam R, Hoen H, et al. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol 2001; 96: 1797–1802 [DOI] [PubMed] [Google Scholar]
- 8. Borg BB, Gupta NK, Zuckerman GR, et al. Impact of obesity on bowel preparation for colonoscopy. Clin Gastroenterol Hepatol 2009; 7: 670–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu KL, Rayner CK, Chuah SK, et al. Impact of low-residue diet on bowel preparation for colonoscopy. Dis Colon Rectum 2011; 54: 107–112 [DOI] [PubMed] [Google Scholar]
- 10. Hassan C, Fuccio L, Bruno M, et al. A predictive model identifies patients most likely to have inadequate bowel preparation for colonoscopy. Clin Gastroenterol Hepatol 2012; 10: 501–506 [DOI] [PubMed] [Google Scholar]
- 11. Lim SW, Seo YW, Sinn DH, et al. Impact of previous gastric or colonic resection on polyethylene glycol bowel preparation for colonoscopy. Surg Endosc 2012; 26: 1554–1559 [DOI] [PubMed] [Google Scholar]
- 12. Chung YW, Han DS, Park KH, et al. Patient factors predictive of inadequate bowel preparation using polyethylene glycol: a prospective study in Korea. J Clin Gastroenterol 2009; 43: 448–452 [DOI] [PubMed] [Google Scholar]
- 13. Worthington J, Thyssen M, Chapman G, et al. A randomised controlled trial of a new 2 litre polyethylene glycol solution versus sodium picosulphate + magnesium citrate solution for bowel cleansing prior to colonoscopy. Curr Med Res Opin 2008; 24: 481–488 [DOI] [PubMed] [Google Scholar]
- 14. Park SS, Sinn DH, Kim YH, et al. Efficacy and tolerability of split-dose magnesium citrate: low-volume (2 liters) polyethylene glycol vs. single- or split-dose polyethylene glycol bowel preparation for morning colonoscopy. Am J Gastroenterol 2010; 105: 1319–1326 [DOI] [PubMed] [Google Scholar]
- 15. Seo EH, Kim TO, Kim TG, et al. Efficacy and tolerability of split-dose PEG compared with split-dose aqueous sodium phosphate for outpatient colonoscopy: a randomized, controlled trial. Dig Dis Sci 2011; 56: 2963–2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nguyen DL, Wieland M. Risk factors predictive of poor quality preparation during average risk colonoscopy screening: the importance of health literacy. J Gastrointestin Liver Dis 2010; 19: 369–372 [PubMed] [Google Scholar]
- 17. Abuksis G, Mor M, Segal N, et al. A patient education program is cost-effective for preventing failure of endoscopic procedures in a gastroenterology department. Am J Gastroenterol 2001; 96: 1786–1790 [DOI] [PubMed] [Google Scholar]
- 18. Rosenfeld G, Krygier D, Enns RA, et al. The impact of patient education on the quality of inpatient bowel preparation for colonoscopy. Can J Gastroenterol 2010; 24: 543–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodger J, Steele RJ. Telephone assessment increases uptake of colonoscopy in a FOBT colorectal cancer-screening programme. J Med Screen 2008; 15: 105–107 [DOI] [PubMed] [Google Scholar]
- 20. Ben Chaabane N, Ben Mansour W, Hellara O, et al. Bowel preparation before colonoscopy. Presse Med 2012; 41: 37–42 [DOI] [PubMed] [Google Scholar]
- 21. Jansen SV, Goedhard JG, Winkens B, et al. Preparation before colonoscopy: a randomized controlled trial comparing different regimes. Eur J Gastroenterol Hepatol 2011; 23: 897–902 [DOI] [PubMed] [Google Scholar]
- 22. Kambe H, Yamaji Y, Sugimoto T, et al. A randomized controlled trial of sodium phosphate tablets and polyethylene glycol solution for polyp detection. J Dig Dis 2012; 13: 374–380 [DOI] [PubMed] [Google Scholar]
- 23. Juluri R, Eckert G, Imperial TF. Polyethylene glycol vs. sodium phosphate for bowel preparation: a treatment arm meta-analysis of randomized controlled trials. BMC Gastroenterol 2011; 11: 38–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Belsey J, Crosta C, Epstein O, et al. Meta-analysis: the relative efficacy of oral bowel preparations for colonoscopy 1985–2010. Aliment Pharmacol Ther 2012; 35: 222–237 [DOI] [PubMed] [Google Scholar]
- 25. Sanaka MR, Shah N, Mullen KD, et al. Afternoon colonoscopies have higher failure rates than morning colonoscopies. Am J Gastroenterol 2006; 101: 2726–2730 [DOI] [PubMed] [Google Scholar]
- 26. Wells CD, Heigh RI, Sharma VK, et al. Comparison of morning versus afternoon cecal intubation rates. BMC Gastroenterol 2007; 7: 19–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Athreya PJ, Owen GN, Wong SW, et al. Achieving quality in colonoscopy: bowel preparation timing and colon cleanliness. ANZ J Surg 2011; 81: 261–265 [DOI] [PubMed] [Google Scholar]
- 28. Gurudu SR, Ratuapli S, Heigh R, et al. Quality of bowel cleansing for afternoon colonoscopy is influenced by time of administration. Am J Gastroenterol 2010; 105: 2318–2322 [DOI] [PubMed] [Google Scholar]