Abstract
Background
Gastrointestinal symptoms and malabsorption following fructose ingestion (fructose intolerance) are common in functional gastrointestinal disorders (FGID). The underlying mechanism is unclear, but is hypothesized to be related an abnormality of intestinal fructose transporter proteins.
Objective
To assess the expression of the main intestinal fructose transporter proteins, glucose transport protein 5 (GLUT5) and 2 (GLUT2), in FGID.
Methods
The expression of GLUT5 and GLUT2 protein and mRNA in small intestinal biopsy tissue was investigated using real-time reverse-transcription PCR and Western immunoblotting in 11 adults with FGID and fructose intolerance ascertained by breath testing and in 15 controls.
Results
Median expression levels of GLUT5 mRNA normalized to beta-actin were 0.18 (interquartile range, IQR, 0.13–0.21) in patients and 0.17 (IQR 0.12–0.19) in controls (p > 0.05). Respective levels of GLUT2 mRNA were 0.26 (IQR 0.20–0.31) and 0.26 (IQR 0.19–0.31) (p > 0.05). Median expression levels of GLUT5 protein normalized to alpha-tubulin were 0.95 (IQR 0.52–1.68) in patients and 0.95 (IQR 0.59–1.15) in controls (p > 0.05). Respective protein expression levels for GLUT2 were 1.56 (IQR 1.06–2.14) and 1.35 (IQR 0.96–1.79) (p > 0.05).
Conclusions
Human fructose intolerance may not be associated with marked changes in GLUT5 and GLUT2 expression. Replication of these results in a larger subject group, including measures of transporter activation and membrane and subcellular localization, is warranted.
Keywords: FODMAP, fructose intolerance, fructose transporters, GLUT5, GLUT2, irritable bowel syndrome, malabsorption, visceral pain
Introduction
Gastrointestinal symptoms following ingestion of fermentable sugars are common in patients with functional gastrointestinal disorders (FGID) or with inflammatory bowel disorders, with prevalence rates of fructose intolerance approaching 60% in FGID.1,2 These intolerances are generally attributed to saccharide malabsorption, with osmotic loading and microbial fermentation in the lower intestine. A contributing factor to the prevalence of fructose intolerance may be the high current level of fructose consumption, which has increased from a daily average of 37 g in 1978 to 49 g in 2004 in the USA.3,4 Most of the increased intake is derived from fructose-based sweeteners, as the proportion of naturally occurring fructose has decreased from 35% to 16%.5 A mismatch between an individual’s saccharide ingestion and the absorptive or digestive capacity is assumed. In lactose maldigestion there is a deficiency in lactase activity, but the underlying cause for the even more common fructose malabsorption is unknown.2 An intuitive mechanistic hypothesis is a change in the expression or activation of the main fructose transporter proteins. Animal data has shown glucose transport protein 5 (GLUT5, Slc2a5) to be the main apical fructose transporter, while GLUT2 (Slc2a2) plays a facilitative and inducible role.6,7 Other GLUT can also transport fructose, but are less relevant (GLUT7, 8, 9, 11, and 12).6 GLUT5 is expressed in several tissues, including the small intestine, kidney, musculoskeletal tissues, testis, fat, and brain.8 While often invoked, current evidence for a role of GLUT5 in human fructose intolerance and malabsorption is inferential. In a rodent GLUT5 knockout model, fructose ingestion resulted in malabsorption, intestinal distension, and fluid retention, resembling some of the changes seen in patients with fructose intolerance.9 Furthermore, infants with reduced expression of GLUT5 also have higher rates of fructose malabsorption.10,11 Increased expression of GLUT5 and GLUT2 in the human intestine in non-insulin-dependent diabetes mellitus was shown to be associated with augmented absorption of fructose.12 The expression of mutant GLUT5 protein as a cause for isolated fructose malabsorption was excluded in eight young children.13 We are not aware of any published data examining the role of GLUT5 and GLUT2 in human adult fructose intolerance or malabsorption. It should be emphasized that, at present, it is unclear whether the symptoms of fructose intolerance are primarily caused by malabsorption or other underlying mechanisms.
Consequently, the aim of this study was to investigate the expression of GLUT5 and GLUT2 protein and mRNA in small intestinal tissue in adults with symptomatic fructose malabsorption, in the following referred to as fructose intolerance, in comparison with controls. We hypothesized that patients with fructose intolerance would show decreased expression of GLUT5 or GLUT2.
Methods
Subjects
Successive male or female patients aged between 18–60 years referred to our gastroenterology practice by general practitioners for evaluation of FGID were included in this prospective study. Patients with evidence of organic disease, assessed by medical history, physical examination, haematology and biochemistry blood testing, stool calprotectin and pancreas elastase quantification, and upper and lower endoscopies with biopsies, were excluded. Parasite and bacterial stool cultures were performed if clinically indicated. Further exclusion criteria were diabetes mellitus, major obesity (body mass index > 35 kg/m2), clinically relevant hypertension requiring treatment, systemic inflammatory disease, and the use of anti-inflammatory drugs within 7 days before endoscopy. One consultant gastroenterologist performed all examinations and endoscopies. All patients completed a standardized questionnaire, which included the specific questions for classification of gastrointestinal symptoms into FGID groups according to the Rome III criteria and additional questions regarding allergies, childhood and family history, central nervous, musculoskeletal and cardiac system symptoms, dietary history, and the use of polyol-containing sweets and chewing gum. A fructose breath test, as described below, was performed in all patients. Patients were classified into FGID subgroups according to the Rome III criteria.14 All patients gave their written informed consent and the study was approved by the Cantonal Ethics Committee. The study was performed in accordance with the Helsinki Declaration of 1975 as revised in 1983 and the study was registered at www.clinicaltrials.gov with the identifier NCT01705171.
Twenty-five male or female age-matched controls undergoing endoscopy for reasons other than FGID, without symptoms of food intolerance, conforming to the same exclusion criteria as the FGID patients above and with a negative fructose breath test as defined below were screened as controls.
Breath test protocol
The fructose breath test was performed in standardized fashion.2 Patients arrived for testing in the morning after fasting for at least 8 hours overnight and without having smoked, chewed gum, or performed vigorous exercise for at least 4 hours. No antibiotics, colonoscopy, or laxatives were permitted within 14 days and a specific low-saccharide diet was adhered to 1 day before the breath test. Chlorhexidine mouthwash was used and teeth were brushed before testing. Breath samples were collected in sealed glass tubes (Quintron Instruments, Milwaukee, USA) before and hourly for 5 hours after ingestion of fructose (∼640 mM, 35 g dissolved in 300 ml water). Hydrogen, methane, and CO2 concentrations were measured within 72 hours using a BreathTracker SC (Quintron Instruments, Milwaukee, USA). Malabsorption was defined as an increase of >20 ppm in hydrogen or >10 ppm in methane levels over baseline twice in succession. Intolerance was defined as an increase of more than 2 over baseline using a symptom score index, which was the sum of the intensities (0 = none, 1 = mild, 2 = intense) of abdominal distension or bloating, flatulence, fullness, nausea, diarrhoea, abdominal cramps, borborygmi, and gastro-oesophageal reflux symptoms, which were scored hourly concurrently with the collection of the breath samples.2 Fructose intolerance was defined as the co-existence of a positive symptom score and malabsorption.
Endoscopy
After an overnight fast and 2–4 hours after waking, subjects underwent upper gastrointestinal endoscopy with a EF450HR endoscope (Fujifilm, Tokyo, Japan) during sedation with propofol. A potential diurnal rhythm effect in GLUT expression was minimized by obtaining all samples between 08:00 and 10:00 hours. Four duodenal biopsies were removed from the duodenum pars 2–3 (Radial Jaw 3 biopsy forceps, standard capacity; Boston Scientific, Solothurn, Switzerland). Additionally, two biopsies from the gastric body and antrum were obtained for histological exclusion of gastritis and Helicobacter pylori infection, which were study exclusion criteria.
Handling of duodenal biopsies
Directly after removal, biopsies were placed into a code-labelled screw-capped cryotube with 1 ml of RNAlater solution (Qiagen, Magden, Switzerland) to stabilize and protect RNA and stored overnight at 2–8℃. The next morning the tissue was removed from the reagent and transferred to a cryotube for storage at −80℃ in liquid nitrogen. The biopsies for Western blot analysis were immediately flash frozen in liquid nitrogen after removal and stored at −80℃. Biopsies were shipped on dry ice via courier to the Molecular Diagnosis Centre, Department of Laboratory Medicine, National University Hospital, Singapore.
Multiplex reverse-transcription quantitative real-time PCR
Total RNA was isolated using TRIzol (Ambion, Life Technologies, Carlsbad, CA) according to standard procedure and manufacturer’s recommendations. GLUT2 and GLUT5 were co-amplified together with the reference gene, beta-actin (ACTB). All primers (AITbiotech, Singapore) and hydrolysis probes (TIB MOLBIOL, Berlin, Germany) were designed carefully to minimize formation of secondary structures during multiplex reverse-transcription quantitative real-time PCR (RT-qPCR) (Table 1). To ensure mRNA specificity and minimum non-specific amplification of genomic DNA, forward and reverse primers were spaced to include intron and exon sequences that were 900 bp (ACTB, NC_000007.13), 4488 bp (GLUT2, NG_008108.1), and 11,310 bp (GLUT5, NC_000001.10) apart. Multiplex RT-qPCR was performed using the SuperScript III Platinum One-Step qRT-PCR reagent kit (Invitrogen, Carlsbad, CA, USA) using a LightCycler 480 II System (Roche Molecular Systems, Pleasanton, CA, USA) according to the manufacturer’s recommendations. Fluorescence signals were captured at 60℃ for each cycle at 465–510 nm (FAM), 618–660 nm (Cy5), and 533–580 nm (Yellow Yakima), for ACTB, GLUT2, and GLUT5 respectively, and fluorescence signals were colour-compensated. RNA dilution standards and no-template controls were included in every run. GLUT5 and GLUT2 levels are expressed as the fraction of ACTB.
Table 1.
Primers used for RT-qPCR of ACTB, GLUT2, and GLUT5
| Primers/probe | Reference gene | Orientation | Nucleotide position | Sequence (5′–3′) |
|---|---|---|---|---|
| ACTB | ||||
| BActin_33F20 | NC_000007.13 | Forward | 33–22 | GAGCCTCGCCTTTGCCGATC |
| BActin_93R18 | NC_000007.13 | Reverse | 970–953 | ACGAGCGCGGCGATATCA |
| BActin_66U23 | NM_001101.3 | Forward | 66–88 | 6FAM–CACCCGCCGCCAGCTCACCATGG–BHQ1 |
| GLUT2 | ||||
| Glut2_591F24 | NC_008108.1 | Forward | 12,422–12,445 | CATGCTCTGGTCCCTGTCTGTATC |
| Glut2_717R24 | NC_008108.1 | Reverse | 16,975–16,934 | AACCCCATCAAGAGAGCTCCAACT |
| Glut2_664L29 | NM_000340.1 | Reverse | 692–664 | Cy5–ATGGCTTTGATTCTTCCAAGTGTGTCCCC–BBQ |
| GLUT5 | ||||
| Glut5_282F21 | NC_000001.10 | Forward | 282–302 | CCAGAGCAAGCATGGAGCAAC |
| Glut5_360R21 | NC_000001.10 | Reverse | 11,633–11,613 | AGGATGACCCAAAGGCAGCTA |
| Glut5_314U26 | NM_003039.2 | Forward | 314–339 | HEX–ATGAAGGAAGGGAGGCTGACGCTTGT–BHQ1 |
Specificity of primers
As the PCR amplicons of ACTB and GLUT5 are short (78–99 bp), they were cloned into pCR2.1-TOPO cloning vector (Invitrogen) before Sanger sequencing to confirm the primer’s specificity (Table 1). The longer GLUT2 PCR amplicons were Sanger-sequenced without cloning.
GLUT5 and GLUT2 protein quantification by Western immunoblotting
Western immunoblotting was performed according to standard procedures. The protein concentration was determined using the Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad, Berkeley, USA), and the colorimetric assay was read at 595 nm. Protein extract (20 µg) was separated by SDS-PAGE under reducing conditions, transferred to nitrocellulose membranes, and stained with 0.1% Ponceau S dye to visualize the transferred protein bands. Membranes were blocked with 5% milk in Tris-buffered saline with Tween (TBST) overnight at room temperature. Membranes were then incubated with 2% milk in TBST with (i) anti-Glut2 (1 : 1000) (sc-9117; Santa Cruz Biotechnology), (ii) anti-Glut5 (1 : 1000) (sc-271055; Santa Cruz Biotechnology), (iii) anti-GAPDH (1 : 2000) (sc-137179; Santa Cruz Biotechnology), or (iv) anti-alpha tubulin (1 : 1000) (2144; Cell Signaling Technology, Danvers, USA) overnight at 4℃. Membranes were next incubated with peroxidase-labelled goat anti-rabbit or anti-mouse IgG (1 : 1000) (sc-2030 and sc-358917, respectively; Santa Cruz Biotechnology) for 90 minutes. All membranes were visualized using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, West Palm Beach, USA) and exposure to ECL Hyperfilm (GE Healthcare Life Sciences, New York, USA). Intensities of bands were quantified using ImageJ (National Institutes of Health, Bethesda, USA). Of the two reference (housekeeping) proteins, levels of alpha-tubulin were more stable and consistent across all samples than GAPDH. Hence, GLUT5 and GLUT2 protein levels are expressed relative to both tubulin and total protein.
Statistics
Data are shown as medians and interquartile ranges. Group comparisons of protein and mRNA expression of GLUT5 and GLUT2 between the subject groups were performed using the Mann–Whitney U-test using the Statistica 9.0 software package (Statsoft, Tulsa, USA). The 2-sided test for sample size calculation was based on differences of 50% in mRNA and protein expression of GLUT5 and GLUT2 between-subject groups, as differences greater than 50% are seen with fructose feeding in rodents, and on an alpha value of 0.05 and a power of 0.8.15,16 Based on these assumptions – no similar data in humans exists – the required sample size estimates were between 12 and 18.
Results
Thirteen patients were screened for inclusion in the study and all had fructose intolerance with malabsorption, as defined in the Methods section. Two patients were excluded because of relevant pathology (mesenteric ischaemia and chromogranin B elevation in one patient each). Eleven patients (eight females) of median age 44 (IQR 32–56) years were therefore evaluated in the study. Eight of the 11 patients were classified as irritable bowel syndrome (IBS) of the diarrhoea subtype, two had IBS of the constipation subtype, and one had IBS of the mixed diarrhoea-constipation subtype. All patients had experienced symptoms for at least 3 years and all had significant bloating and abdominal pain.
Of the 25 controls screened and without fructose intolerance and malabsorption, 10 were excluded: four due to inflammatory changes in histology, one due to microscopic colitis, and a further five due to symptoms suggestive of FGID, despite not corresponding to the full Rome III criteria. Thus, 15 controls (nine females) of median age 49 (IQR 38–60) years were evaluable in the study. Twelve of these 15 controls underwent endoscopy for assessment of reflux as the cause for dental erosions. The final diagnoses were as follows: oesophagitis grade A in two, non-erosive gastro-oesophageal reflux disease in eight, and no abnormal finding in two controls. In the remaining three controls the reason for endoscopy was dysphagia and no specific pathology was found during endoscopy.
GLUT5 and GLUT2 mRNA expression
Quantification by real-time PCR demonstrated similar levels of both GLUT5 and GLUT2 mRNA in the 11 patients with fructose intolerance and the 15 controls (Figure 1). There were no significant differences in mRNA expression between all males and females for GLUT5 (0.154, IQR 0.123–0.232, and 0.165, IQR 0.122–0.208, respectively; p > 0.05) and GLUT2 (0.257, IQR 0.212–0.298, and 0.240, IQR 0.190–0.300, respectively; p > 0.05).
Figure 1.
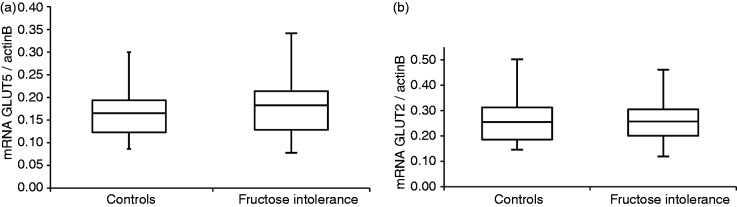
GLUT5 (a) and GLUT2 (b) mRNA expression in 11 patients with fructose intolerance compared to 15 controls, normalized to beta-actin B.
The box-Whisker plots show medians (central horizontal line), interquartile ranges (upper and lower ends of boxes), and absolute ranges of values (ends of whiskers). The expression of GLUT5 and GLUT2 mRNA was similar between patients and controls.
GLUT5 and GLUT2 protein expression
The Western blot analysis of all subjects is shown in Figure 2. Two protein bands are visible for GLUT2, as seen in several earlier reports and also in the sample Western blot of the manufacturer.17–19 We chose to report both bands, but the results were identical when only the top protein band was analysed (data not shown).
Figure 2.
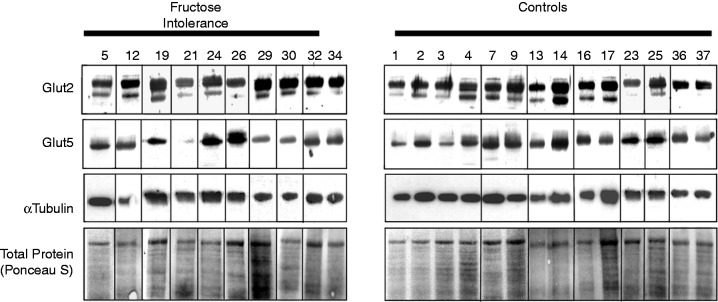
Western immunoblots of GLUT5 and GLUT2 showing the control protein, alpha-tubulin, and total protein (Ponceau S staining) in 10 patients with fructose intolerance compared to 14 controls.
GLUT5 and total GLUT2 protein expression relative to tubulin concentration is shown in Figure 3. There were no significant differences in protein expression between the 10 evaluable patients with fructose intolerance and the 14 evaluable controls. One patient and control each were excluded from Western blot analysis due to insufficient sample material. When normalized to total protein from the tissue homogenate, the results were similar between groups for GLUT5, where median expression levels were 0.166 (IQR 0.130–0.237) in patients and 0.198 (IQR 0.156–0.212) in controls (p > 0.05), and for GLUT2, where respective values were 0.344 (IQR 0.186–0.414) and 0.336 (IQR 0.275–0.414) (p > 0.05). There were no significant differences between all males and females in GLUT5 (0.155, IQR 0.092–0.224, and 0.180, IQR 0.128–0.224, respectively; p > 0.05) and in GLUT2 (0.359, IQR 0.284–0.418, and 0.323, IQR 0.251–0.450, respectively; p > 0.05) protein expression normalized to total protein in the tissue homogenate.
Figure 3.
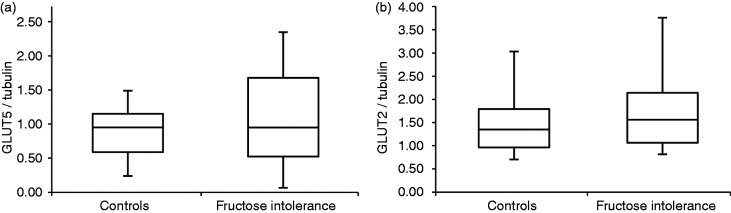
GLUT5 (a) and GLUT2 (b) protein expression in 11 patients with fructose intolerance compared to 15 controls, normalized to alpha-tubulin.
The box-whisker plots show medians (central horizontal line), interquartile ranges (upper and lower ends of boxes), and absolute ranges of values (ends of whiskers). The expression of GLUT5 and GLUT2 protein was similar between patients and controls.
Discussion
In this study, the expression of the main fructose transporters, GLUT5 and GLUT2, and their mRNA in duodenal mucosa did not differ significantly between patients with FGID patients and controls. Changes in these transporters have been suggested as the underlying cause of fructose intolerance or malabsorption and there is some circumstantial evidence supporting this hypothesis. GLUT5 knockout mice fed a high-fructose diet revealed signs reminiscent of malabsorption.9 Malabsorption of fructose decreases with age, while in rodent models GLUT5 expression increases with age and in human adults small intestinal tissue GLUT5 expression is greater than in fetal tissue.10,11,20 Inflammation, one of the postulated mechanisms in IBS, has been shown to decrease GLUT5 activity and mRNA expression in rodents.6 Current data indicates GLUT5 is responsible for the majority of luminal fructose uptake, with GLUT2 becoming relevant when high doses of fructose are ingested, at least in the mouse.21 GLUT2 expression, on the other hand, has been shown to be susceptible to stress and corticosteroids, another pathophysiological factor in IBS, with some studies in the rat demonstrating an upregulation and others a downregulation, depending on the stressor model.22,23 Very little data on fructose transporters exist in adult humans and we are not aware of any such data in patients with FGID. This first data clearly suggests fructose intolerance with malabsorption is not secondary to changes in the duodenal expression of the fructose transporters or their production. The power calculation indicates the chosen sample sizes were adequate to assess differences of at least 50% in transporter mRNA and protein expression, which is the minimal reversible change seen with feeding in rodents.15,16 No similar data has been reported in humans, but it does indicate the study power is in a reasonable range.
The current data obtained from mucosal homogenates do not completely exclude a role of the fructose transporters through differences in membrane abundance, cellular activation or subcellular localization. In contrast to the above-mentioned studies with changes in GLUT expression, age- and diet-associated changes in jejunal and ileal fructose uptake in rats were not related to changes in either GLUT5 or GLUT2 abundance in an earlier study.24 Although not investigated in the current study, the food-ingestion-related facultative localization of GLUT2 to the apical membrane, and not only to the basolateral membrane, may have an influence on absorption rates. As all our subjects were fasted for 8 hours due to the requirements of anaesthesia, our results reflect the fasting state and not the GLUT5 upregulation seen with fructose ingestion or the GLUT2 upregulation seen in rats consuming fructose and glucose.21,25 Differential expression of the transporters along the small intestine could be another explanation for our absence of group differences, as we investigated the distal duodenum accessible by endoscopy. However, available data suggest a uniform distribution of GLUT5 and GLUT2 along the small intestine in humans.20 A potential limitation in the interpretation of the GLUT2 results is the specificity of the primer used in the PCR assay for one of several identified human splice variants.20 The impact of this limitation is difficult to assess, as the importance of the further splice variants is currently unknown. However, the Western blot data clearly confirms the RNA expression data obtained with the chosen primer.
These data, if replicated in larger patient numbers and with immunohistochemistry, do raise the issue of whether fructose intolerance in FGID patients is primarily due to malabsorption. In a recent study, malabsorption was demonstrated by the fructose breath test in only 45% of 1372 FGID patients and the presence of malabsorption did not correlate with the clinical symptoms of FGID.2 Furthermore, fructose malabsorption appears to be similarly prevalent in patients with IBS and healthy controls, while the latter have more symptoms following sugar ingestion for breath testing.26–30 Indeed, the symptoms induced by the sugar ingestion during breath testing, rather than the malabsorption, correlate very significantly with the clinical symptoms of FGID.2 Several potential mechanisms for the high incidence of fructose intolerance in FGID besides malabsorption can be hypothesized, with supportive evidence emerging for several. These include an altered enteric microbiome and bacterial fermentation products, increased intestinal permeability, rapid small bowel transit, and aberrant nervous system and immune responsiveness.31–36 The interaction between several of these alternative pathogenic mechanisms and fructose transporters is in itself of interest, which will be the subject of further investigation.
We conclude that duodenal GLUT5 and GLUT2 protein and mRNA expression did not differ significantly between FGID patients with fructose intolerance and controls. Our results suggest that human fructose intolerance may not be associated with marked changes in GLUT5 and GLUT2 expression. However, replication of these results in a larger group of subjects and with measures of transporter activation, as well as membrane and subcellular localization is warranted.
Acknowledgements
We gratefully acknowledge the expert support of Lee Hong Kai and Christopher Ng Wai Siong, both of the Molecular Diagnosis Centre, Department of Laboratory Medicine at the National University Hospital, Singapore.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1. Barrett JS, Irving PM, Shepherd SJ, et al. Comparison of the prevalence of fructose and lactose malabsorption across chronic intestinal disorders. Aliment Pharmacol Ther 2009; 30: 165–174 [DOI] [PubMed] [Google Scholar]
- 2. Wilder-Smith CH, Materna A, Wermelinger C, et al. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther 2013; 37: 1074–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 2009; 139: 1228S–1235S [DOI] [PubMed] [Google Scholar]
- 4. Park YK, Yetley EA. Intakes and food sources of fructose in the United States. Am J Clin Nutr 1993; 58: 737S–747S [DOI] [PubMed] [Google Scholar]
- 5. Welsh JA, Sharma AJ, Grellinger L, et al. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr 2011; 94: 726–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Douard V, Ferraris RP. Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab 2008; 295: E227–E237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones HF, Butler RN, Brooks DA. Intestinal fructose transport and malabsorption in humans. Am J Physiol Gastrointest Liver Physiol 2011; 300: G202–G206 [DOI] [PubMed] [Google Scholar]
- 8. Schurmann A. Insight into the ‘odd’ hexose transporters GLUT3, GLUT5, and GLUT7. Am J Physiol Endocrinol Metab 2008; 295: E225–E226 [DOI] [PubMed] [Google Scholar]
- 9. Barone S, Fussell SL, Singh AK, et al. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J Biol Chem 2009; 284: 5056–5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Douard V, Ferraris RP. The role of fructose transporters in diseases linked to excessive fructose intake. J Physiol 2013; 591: 401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones HF, Butler RN, Moore DJ, et al. Developmental changes and fructose absorption in children: effect on malabsorption testing and dietary management. Nutr Rev 2013; 71: 300–309 [DOI] [PubMed] [Google Scholar]
- 12. Dyer J, Wood IS, Palejwala A, et al. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol 2002; 282: G241–G248 [DOI] [PubMed] [Google Scholar]
- 13. Wasserman D, Hoekstra JH, Tolia V, et al. Molecular analysis of the fructose transporter gene (GLUT5) in isolated fructose malabsorption. J Clin Invest 1996; 98: 2398–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rome Foundation. Rome III disorders and criteria. Rome: Rome Foundation. Available at: www.romecriteria.org/criteria (consulted May 2013)
- 15. Cui XL, Schlesier AM, Fisher EL, et al. Fructose-induced increases in neonatal rat intestinal fructose transport involve the PI3-kinase/Akt signaling pathway. Am J Physiol Gastrointest Liver Physiol 2005; 288: G1310–G1320 [DOI] [PubMed] [Google Scholar]
- 16. Douard V, Suzuki T, Sabbagh Y, et al. Dietary fructose inhibits lactation-induced adaptations in rat 1,25-(OH)2D3 synthesis and calcium transport. FASEB J 2012; 26: 707–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaudhry RM, Scow JS, Madhavan S, et al. Acute enterocyte adaptation to luminal glucose: a posttranslational mechanism for rapid apical recruitment of the transporter GLUT2. J Gastrointest Surg 2012; 16: 312–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei N, Liu R, Ou Y, et al. Effects of octreotide on glucose transporter type 2 expression in obese rat small intestine. World J Gastroenterol 2011; 17: 4434–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santa Cruz Biotechnology. ‘Glut2 antibody (H-67): sc-9117’. Dallas, TX: Santa Cruz Biotechnology. Available at: www.scbt.com/datasheet-9117-glut2-h-67-antibody.html (consulted April 2013)
- 20. Davidson NO, Hausman AM, Ifkovits CA, et al. Human intestinal glucose transporter expression and localization of GLUT5. Am J Physiol 1992; 262: C795–C800 [DOI] [PubMed] [Google Scholar]
- 21. Gouyon F, Caillaud L, Carriere V, et al. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice. J Physiol 2003; 552: 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boudry G, Cheeseman CI, Perdue MH. Psychological stress impairs Na+-dependent glucose absorption and increases GLUT2 expression in the rat jejunal brush-border membrane. Am J Physiol Regul Integr Comp Physiol 2007; 292: R862–R867 [DOI] [PubMed] [Google Scholar]
- 23. Shepherd EJ, Helliwell PA, Mace OJ, et al. Stress and glucocorticoid inhibit apical GLUT2-trafficking and intestinal glucose absorption in rat small intestine. J Physiol 2004; 560: 281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drozdowski LA, Woudstra TD, Wild GE, et al. Age-associated changes in intestinal fructose uptake are not explained by alterations in the abundance of GLUT5 or GLUT2. J Nutr Biochem 2004; 15: 630–637 [DOI] [PubMed] [Google Scholar]
- 25. Cui XL, Jiang L, Ferraris RP. Regulation of rat intestinal GLUT2 mRNA abundance by luminal and systemic factors. Biochim Biophys Acta 2003; 1612: 178–185 [DOI] [PubMed] [Google Scholar]
- 26. Kyaw MH, Mayberry JF. Fructose malabsorption: true condition or a variance from normality. J Clin Gastroenterol 2011; 45: 16–21 [DOI] [PubMed] [Google Scholar]
- 27. Law D, Conklin J, Pimentel M. Lactose intolerance and the role of the lactose breath test. Am J Gastroenterol 2010; 105: 1726–1728 [DOI] [PubMed] [Google Scholar]
- 28. Rao SS, Attaluri A, Anderson L, et al. Ability of the normal human small intestine to absorb fructose: evaluation by breath testing. Clin Gastroenterol Hepatol 2007; 5: 959–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rumessen JJ, Gudmand-Hoyer E. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut 1986; 27: 1161–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao J, Fox M, Cong Y, et al. Lactose intolerance in patients with chronic functional diarrhoea: the role of small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2010; 31: 892–900 [DOI] [PubMed] [Google Scholar]
- 31. He T, Venema K, Priebe MG, et al. The role of colonic metabolism in lactose intolerance. Eur J Clin Invest 2008; 38: 541–547 [DOI] [PubMed] [Google Scholar]
- 32. Kennedy PJ, Clarke G, Quigley EM, et al. Gut memories: towards a cognitive neurobiology of irritable bowel syndrome. Neurosci Biobehav Rev 2012; 36: 310–340 [DOI] [PubMed] [Google Scholar]
- 33. Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol 2010; 7: 163–173 [DOI] [PubMed] [Google Scholar]
- 34. Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 2013; 62: 159–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spiller R, Garsed K. Infection, inflammation, and the irritable bowel syndrome. Dig Liver Dis 2009; 41: 844–849 [DOI] [PubMed] [Google Scholar]
- 36. Spiller R, Lam C. The shifting interface between IBS and IBD. Curr Opin Pharmacol 2011; 11: 586–592 [DOI] [PubMed] [Google Scholar]


