Abstract
Background
In gastrointestinal bleeding, a physician often has to make a decision between two possible choices. Endoscopic management of the bleeding could be initiated immediately, or it could be delayed until the patient has become haemodynamically stable or the conditions for a successful endoscopy have otherwise improved.
Objective
The present article serves to present such situations and highlights their characteristic features.
Methods
The choice between immediate and delayed endoscopy is analysed in terms of a decision tree, comparing the expected results of the two management alternatives. The decision tree is applied to three different clinical scenarios associated with gastrointestinal bleeding, where performing endoscopy later rather than sooner represents the preferred management option.
Results
The work up of chronic iron-deficient anaemia in patients with serious cardiac problems should be deferred until resolution of their reduced cardiovascular status. It is also recommended that, even in acute bleeding, endoscopy is deferred until the patient has become haemodynamically stable. Lastly, for nonemergency treatment of oesophageal varices bleeding, a long rather than short interval between consecutive banding sessions appears more beneficial.
Conclusions
The results illustrate how to use threshold analysis as a simple bedside tool to solve seemingly complex decisions associated with management of gastrointestinal bleeding.
Keywords: Decision analysis, gastrointestinal bleeding, gastrointestinal endoscopy, threshold analysis
Introduction
Bleeding into the gastrointestinal tract constitutes a medical emergency that requires medical intervention to localize the bleeding source and stop the bleeding.1,2 During the past decades, a large variety of methods of interventional endoscopy and radiology have been developed to efficiently diagnose and treat gastrointestinal bleeding. It is common for gastroenterology practices to provide a 24/7 on-call service to deal with gastrointestinal emergencies, the majority of which relate to acute gastrointestinal bleeding. The success in managing most of such emergencies has also increased the expectations towards gastroenterologists of being able to intervene whenever gastrointestinal bleeding occurs. However, sometimes situations arise when such heightened expectations need to be disappointed and when it is not in the patient’s best interest to rush into action but rather delay the onset of endoscopic work up. The present article serves to present such situations and highlight their characteristic features.
Methods
In patients with signs and symptoms of gastrointestinal bleeding, a physician often has to make a decision between two possible choices. Endoscopic management of the bleeding could be initiated immediately, or it could be delayed until the patient has become haemodynamically stable or the conditions for a successful endoscopy have otherwise improved. In the present analysis, the choice between immediate and delayed endoscopy is mapped out as a decision tree, comparing the expected results of the two management alternatives. Costs are entered into the tree as estimates of lost life incurred from complications of endoscopy or bleeding. Various costs are weighted by their probability of occurrence estimated based on clinical experience. The overall outcome of the decision analysis is phrased in terms of a threshold value.3,4 Such threshold analysis answers the question, at what threshold of probability value does one management option gain superiority over its alternative? The decision tree is applied as a bedside tool to three different clinical scenarios associated with gastrointestinal bleeding and its management through gastrointestinal endoscopy. Such usage implies that the probabilities and costs, which enter the analysis, represent only crude estimates based on the physician’s judgment and clinical experience. Rather than rely on a detailed calculation, the analysis provides a comparison of different management options by orders of magnitude.
Results
GI endoscopy in a high-risk cardiac patient
The gastroenterology service was consulted by cardiology for endoscopic work up of a 61-year-old man with chronic iron-deficient anaemia. The patient denied any abdominal symptoms, haematemesis, haematochezia, or melena. Because of colon cancer in his father at age 60, the patient had already undergone four negative colonoscopies in the past, the last one done 6 years ago. He was admitted to the hospital for work up of unstable angina, coronary artery disease with a 95% occlusion of his left anterior descending artery, and severe congestive heart failure. The cardiology service requested an upper and lower endoscopy to find and treat a potential GI bleeding source, before the patient was subjected to coronary stenting and long-term anticoagulation. The gastroenterology service felt that, without prior cardiac treatment, dual endoscopy, including bowel preparation and conscious sedation, would constitute an inordinate risk. It was recommended to defer the endoscopy work up until after his cardiac status had improved.
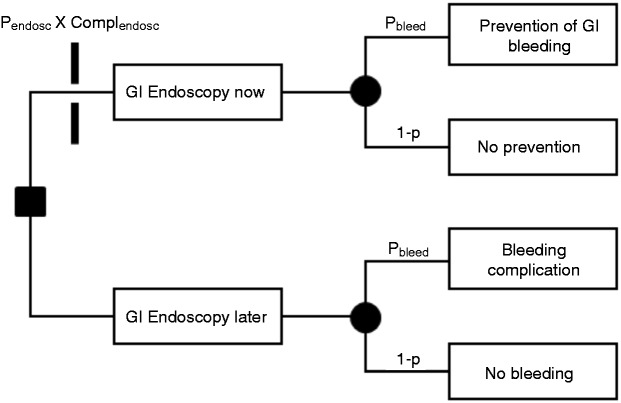
The decision tree of Figure 1 depicts the two choices of whether to perform the endoscopy now or later. If performed now, endoscopy could result in a serious complication (Complendosc) as represented by the toll bar blocking the upper branch. On the side of benefit, early endoscopy could find and treat a bleeding source with a probability pbleed and, thus, prevent future GI bleeding from anticoagulation. The alternative, with a probability 1 – pbleed, is that no bleeding source is found and no prevention is achieved. A later endoscopy, as represented by the lower main branch, would not incur the risk of endoscopy complication and, hence, no toll bar is blocking its passage. The potential outcomes of the lower branch are essentially the same as those of the upper branch, that is, bleeding complication or none. However, the bleeding complication (Complbleed) while on anticoagulation would be expensive in terms of suffering, health expenditures, and potential life loss as compared with timely prevention of bleeding in the upper branch, which would be essentially cost free.
Figure 1.
Decision tree of whether to perform endoscopy now or later for a patient with gastrointestinal bleeding.
For ‘endoscopy now’ to be preferred over ‘endoscopy later’, the overall expected costs of ‘endoscopy now’ need to be less than those of ‘endoscopy later’:
Rearranging probabilities and costs to appear on two separate sides of the equation leads to:
This outcome means that for ‘endoscopy now’ to be the preferred option, the probability ratio (of endoscopy over bleeding complication) needs to be smaller than the inverse cost ratio (of bleeding over endoscopy complication).
Table 1 again summarizes the four parameters that enter the decision analysis, that is, probability of complication associated with endoscopy or bleeding and the cost of endoscopy or bleeding. In the present context, the term ‘cost’ does not refer to monetary expenditures, but loss in health through medical complication. For instance, the gastroenterologist estimated the risk for major complication during endoscopy to be pendosc = 10%, while the probability of finding a treatable bleeding lesion in an otherwise asymptomatic patient with previously negative endoscopies was estimated as pbleed = 1%. Costs could be measured in terms of a life-threatening event or lost life. Loss of life seemed more closely associated with an endoscopic complication (Complendosc = 0.6 life) before cardiac treatment than with gastrointestinal bleeding (Complbleed = 0.2 life) after cardiac treatment. Bleeding complications were also deemed less deadly, because the patient could still undergo endoscopy or arterial embolization by interventional radiology, should he develop future gastrointestinal bleeding even while on anticoagulation. With
and
the probability ratio becomes greater than the inverse cost ratio, and the conditions from above for early endoscopy are not being met. This preference for late endoscopy remains stable under multiple assumptions, because the probability ratio always stays greater than 1 and the cost ratio never increases above 1, even when considering the most extreme scenarios.
Table 1.
Four decision parameters affecting early endoscopy
| Cause of complication | Probability of complication | Cost of complication | |
|---|---|---|---|
| Endoscopy | pendosc | Complendosc | |
| Bleeding | pbleed | Complbleed | |
| Decision criterion | < |
Immediate vs. deferred emergency endoscopy for acute GI bleeding
A 62-year-old man was admitted by the emergency department to the medical intensive care unit for 1-day symptoms of lightheadedness, orthostasis, and maroon-coloured stool. The initial blood work revealed a 25-point drop in his haematocrit. The gastroenterology service was requested to initiate immediate endoscopic work up, because concerns were raised about an ongoing brisk bleeding. The gastroenterologist on call felt that, prior to any endoscopy, the patient needed to be haemodynamically stable first.5
This is a scenario frequently encountered by gastroenterologists. Its underlying structure is very similar to the one of the first scenario shown in Figure 1. Instead of a time period measured in weeks or months between GI endoscopy now and later, in the current scenario the time period would be measured in hours. The outcome options of the upper branch would be prevention of complication from ongoing active bleeding vs. no such prevention. The outcome options of the lower branch would be the occurrence vs. nonoccurrence of complication from ongoing active bleeding.
Early endoscopy can result in an increased risk of oxygen desaturation in up to 80% of patients.6,7 A large 20–50% fraction of critically ill patients undergoing endoscopy develops cardiopulmonary complications.8–10 In bleeding patients, a high ASA score has been identified as the predictor variable with the greatest risk for mortality.11,12 Although 2–10% of patients can die from their acute gastrointestinal bleeding, such mortality is mostly associated with cardiopulmonary complications or multiorgan failure rather than exsanguination.13,14 Based on such available data from the literature, one can try to estimate the probabilities and cost of complications associated with the present scenario.
For immediate endoscopy to be the preferred option, again, the probability ratio (of endoscopy over bleeding complication) needs to be smaller than the inverse cost ratio (of bleeding over endoscopy complication). On one hand, the probability or risk of a life-threatening complication in an unstable patient in the short term exceeds the risk of continued bleeding (i.e. pendosc/pbleed > 1). On the other hand, blood can be transfused and the magnitude or costs of complication from continued bleeding is, in most instances, lower than those of any complication-associated failing to adequately resuscitate the patient and stabilize his vitals (i.e. Complbleed/Complendosc < 1). This expected outcome violates the condition for immediate endoscopy to be the preferred option, and deferred endoscopy appears the more prudent choice.
Early vs. late repeat banding of oesophageal varices
The guidelines for endoscopic band ligation of oesophageal varices suggest that banding sessions should be repeated at 2-week intervals until the varices have become completely obliterated.15,16 A short surveillance interval with early repeat banding carries the advantage of being able to prevent recurrent variceal bleeding. Because of multiple residual oesophageal ulcerations from the previous endoscopy, which have failed to heal within the short surveillance interval, early repeat banding also carries the potential disadvantage of being unable to efficaciously place new variceal bands and waste an endoscopy. Another option is to extend the interval between consecutive banding sessions to 8 weeks and to give the oesophageal mucosa more time to heal.
The decision tree of whether to repeat banding sooner rather than later again resembles the one shown in Figure 1. The upper and lower main branch relate to early and late oesophagogastroduodenoscopy (OGD) for repeat banding, with early and late OGD referring to a surveillance intervals of ≤2 or ≥8 weeks, respectively. The toll bar on the upper branch marks the product of probability and costs associated with a premature and wasted OGD. The two outcomes of the upper main branch are prevention vs. no prevention of recurrent variceal bleeding.
For early endoscopy to be the preferred option, again, the probability ratio (of wasted endoscopy over recurrent variceal bleeding) needs to be smaller than the inverse cost ratio (of recurrent variceal bleeding over wasted endoscopy). It is safe to assume that the cost of repeat bleeding with subsequent hospital admission, blood transfusion, and endoscopy would be always more expensive than the costs of a single (wasted) OGD. The cost ratio Complbleed/Complendosc may vary between 3- and 10-fold. The probably of interval bleeding from ruptured oesophageal bleeding depends on whether one is dealing with primary or secondary prophylaxis of variceal bleeding. Such probability would be much lower for primary prophylaxis in varices that have never bled before than for secondary prophylaxis in varices that have bled only recently. Assuming a scenario of primary prophylaxis, the probability of wasting an endoscopy is much greater than the probability of bleeding, with the probability ratio pendosc/pbleed varying between 10- and 30-fold. Late repeat banding would therefore be the preferred option for such scenario. In a scenario of secondary prophylaxis, however, the probability of recurrent variceal bleeding may become as big as the probability of wasting an endoscopy, and early repeat banding would therefore become the preferred option.
Discussion
Three typical clinical scenarios of gastrointestinal bleeding are presented, where performing endoscopy later rather than sooner represents the preferred management option. The management of gastrointestinal bleeding in patients with serious cardiac problems should be deferred until resolution of their reduced cardiovascular status. It is also recommended that, even in acute bleeding, endoscopy is deferred until the patient has become haemodynamically stable. Lastly, for nonemergency treatment of oesophageal varices bleeding, a long rather than short interval between consecutive banding sessions appears more beneficial. Other such situations abound in clinical practice.
All three scenarios were phrased in terms of a threshold analysis using a simple decision tree to compare the two management alternatives.3,4 The potential outcomes were limited to costs associated with the two options. Instead of using precise monetary values, the costs were expressed as estimates of lost life. Rather than look at the probability and cost values individually, they were analysed jointly as two comparative probability and cost ratios. With hardly any mathematical manipulation involved, the assessment of competing management alternatives became reduced to a simple comparison of the two ratios. In spite of this seeming simplicity, however, the outcome of the analysis is insightful for clinical decision making. Its simplicity also makes it easy to apply such reasoning at the bedside.
Multiple studies have been devoted to the issue of endoscopy timing in the management of gastrointestinal bleeding.17–19 A general consensus has emerged that, in acute gastrointestinal bleeding, endoscopy should be performed within 24 h of presentation. Although the outcomes of these studies have provided a framework and resulted in guidelines for the general management of gastrointestinal bleeding, they cannot provide the answers to all specific problems that arise in clinical decision making centred on individual patients. In the routine management of sick patients, medical decisions cannot be deferred and often need to be made more instantaneously based on imprecise data or subjective estimates. The aim of the present analysis was to provide a practical tool of how to apply such estimates utilizing the technique of medical decision analysis. Since the three scenarios served primarily to illustrate the applicability of the threshold analysis, the results must not be considered cast in stone. For instance, it is conceivable that under circumstances different from those of the 1st and 2nd scenarios, the probability of a complications associated with endoscopy becomes smaller than the probability of exsanguination from gastrointestinal bleeding. The probability ratio may then drop below the cost ratio, with early endoscopy turning into the preferred option.
In summary, the present article serves to show how threshold analysis can be used as a simple bedside tool to solve seemingly complex decisions associated with management of gastrointestinal bleeding. The transparency of the analysis makes it easy to check the assumptions built into the decision process. Such analysis suggests that, in gastrointestinal bleeding, fast is not always good and that haste makes waste.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author declares that there is no conflict of interest.
References
- 1. Rockey DC. Gastrointestinal bleeding. Gastroenterol Clin North Am 2005; 34: 581–588 [DOI] [PubMed] [Google Scholar]
- 2. Marek TA. Gastrointestinal bleeding. Endoscopy 2011; 43: 971–977 [DOI] [PubMed] [Google Scholar]
- 3. Pauker SG, Kassirer JP. The threshold approach to clinical decision making. N Engl J Med 1980; 302: 1109–1117 [DOI] [PubMed] [Google Scholar]
- 4. Sonnenberg A. Decision analysis in clinical gastroenterology. Am J Gastroenterol 2004; 99: 163–169, 400 [DOI] [PubMed] [Google Scholar]
- 5. Hwang JH, Fisher DA, Ben-Menachem T, et al. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc 2012; 75: 1132–1138 [DOI] [PubMed] [Google Scholar]
- 6. Hill DB, Stokes BD, Gilinsky NH. Arterial oxygen saturation during emergency esophagogastroduodenoscopy: the effects of nasal oxygen. J Clin Gastroenterol 1994; 18: 284–286 [DOI] [PubMed] [Google Scholar]
- 7. Yen D, Hu SC, Chen LS, et al. Arterial oxygen desaturation during emergent nonsedated upper gastrointestinal endoscopy in the emergency department. Am J Emerg Med 1997; 15: 644–647 [DOI] [PubMed] [Google Scholar]
- 8. Lipper B, Simon D, Cerrone F. Pulmonary aspiration during emergency endoscopy in patients with upper gastrointestinal hemorrhage. Crit Care Med 1991; 19: 330–333 [DOI] [PubMed] [Google Scholar]
- 9. Rudolph SJ, Landsverk BK, Freeman ML. Endotracheal intubation for airway protection during endoscopy for severe upper GI hemorrhage. Gastrointest Endosc 2003; 57: 58–61 [DOI] [PubMed] [Google Scholar]
- 10. Rehman A, Iscimen R, Yilmaz M, et al. Prophylactic endotracheal intubation in critically ill patients undergoing endoscopy for upper GI hemorrhage. Gastrointest Endosc 2009; 69: e55–e59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barkun A, Sabbah S, Enns R, et al. The Canadian Registry on Nonvariceal Upper Gastrointestinal Bleeding and Endoscopy (RUGBE): endoscopic hemostasis and proton pump inhibition are associated with improved outcomes in a real-life setting. Am J Gastroenterol 2004; 99: 1238–1246 [DOI] [PubMed] [Google Scholar]
- 12. Marmo R, Del Piano M, Rotondano G, et al. Mortality from nonulcer bleeding is similar to that of ulcer bleeding in high-risk patients with nonvariceal hemorrhage: a prospective database study in Italy. Gastrointest Endosc 2012; 75: 263–272 [DOI] [PubMed] [Google Scholar]
- 13. Laine L, Peterson WL. Bleeding peptic ulcer. New Engl J Med 1994; 331: 717–727 [DOI] [PubMed] [Google Scholar]
- 14. Sung JJ, Tsoi KK, Ma TK, et al. Causes of mortality in patients with peptic ulcer bleeding: a prospective cohort study of 10,428 cases. Am J Gastroenterol 2010; 105: 84–89 [DOI] [PubMed] [Google Scholar]
- 15. Qureshi W, Adler DG, Davila R, et al. ASGE Guideline: the role of endoscopy in the management of variceal hemorrhage, updated July 2005. Gastrointest Endosc 2005; 62: 651–655 [DOI] [PubMed] [Google Scholar]
- 16. Toubia N, Arun J, Sanyal AJ. Portal hypertension and variceal hemorrhage. Med Clin N Am 2008; 92: 551–574 [DOI] [PubMed] [Google Scholar]
- 17. Spiegel BM, Vakil NB, Ofman JJ. Endoscopy for acute nonvariceal upper gastrointestinal tract hemorrhage: is sooner better? A systematic review. Arch Intern Med 2001; 161: 1393–1404 [DOI] [PubMed] [Google Scholar]
- 18. Tsoi KK, Ma TK, Sung JJ. Endoscopy for upper gastrointestinal bleeding: how urgent is it? Nat Rev Gastroenterol Hepatol 2009; 6: 463–469 [DOI] [PubMed] [Google Scholar]
- 19. Targownik LE, Murthy S, Keyvani L, et al. The role of rapid endoscopy for high-risk patients with acute nonvariceal upper gastrointestinal bleeding. Can J Gastroenterol 2007; 21: 425–429 [DOI] [PMC free article] [PubMed] [Google Scholar]