Abstract
The dietary carbohydrate fructose can be incompletely absorbed in the small intestine and is sometimes associated with gastrointestinal symptoms that include motility disturbances and abdominal pain. Fructose malabsorption has been well documented in variable but similar proportions of healthy and populations with functional gastrointestinal disorders. Recent work into the expression of the main intestinal fructose transporter proteins highlight that our understanding of the mechanistic basis for fructose malabsorption and how it differentiates in gastrointestinal patients is incomplete. Until we have further mechanistic insight, restricting dietary fructose intake and other poorly absorbed short-chain carbohydrates and polyols remains an efficacious approach for managing functional gastrointestinal symptoms.
Keywords: Malabsorption, fructose transporters, FODMAP, GLUT5, GLUT2
There is ample evidence to support that fructose malabsorption plays a role in inducing gastrointestinal symptoms in patients with functional gastrointestinal disorders (FGID).1 The underlying mechanism of fructose absorption is thought to be due to an individual’s transporter function capability capacity, dictated by expression of the respective transporters. In this issue, a study by Wilder-Smith et al.2 provides insight into the mechanism of fructose malabsorption and for the first time assesses whether a direct correlation exists between the expression of the main intestinal fructose transporter proteins and patients with FGID.
Fructose is a dietary monosaccharide slowly absorbed across the intestinal epithelium by carrier-mediated facilitated diffusion, which is an energy-independent process (Figure 1). The fructose carrier is a member of the glucose transport (GLUT) family of genes encoding for facilitative sugar transporters, of which there are two absorptive pathways. GLUT5 is a high-affinity fructose transporter found in the apical membrane on the luminal surface of small intestinal epithelial cells, is low-capacity and glucose-independent, and depends on a concentration gradient.3 GLUT2 also has a facultative role and is a high-capacity, glucose-dependent fructose co-transporter. In addition to fructose, GLUT2 also actively transports glucose and galactose. GLUT2 is essentially located in the basolateral membrane; however, an apical GLUT2 pathway also exists. When glucose is transported by the Na+/glucose co-transporter (SGLT1), GLUT2 activation and insertion is promoted into the apical membrane.4 Although less substantiated, there have been suggestions of other regulatory pathways including GLUT85 and GLUT7–GLUT126 also being involved in fructose absorption, for example in allowing accommodation of large amounts of fructose.
Figure 1.
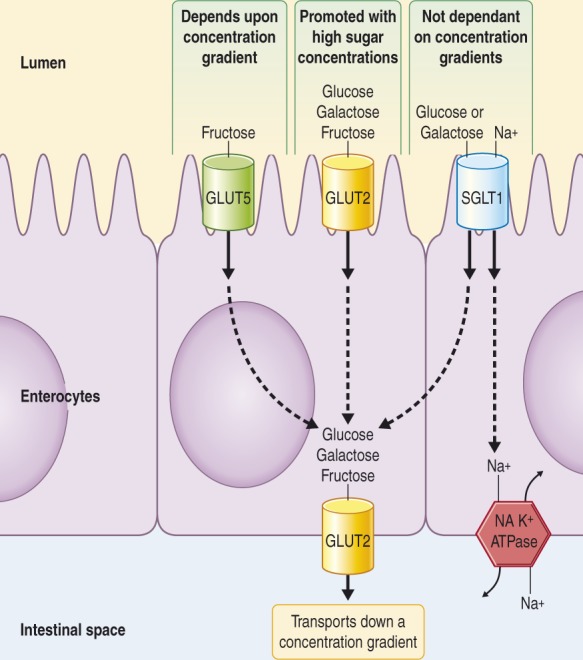
The main transporters involved with fructose absorption.
SGLT1 and GLUT5 are both located in the apical membrane of the small intestinal epithelium, where SGLT1 is the sodium/glucose-galactose transporter and GLUT5 is specific for fructose movement. GLUT2 is a high-capacity pathway for the absorption of glucose, galactose, and fructose essentially located in the basolateral membrane; however, apical GLUT2 is rapidly induced with high dietary carbohydrate intake.
When fructose is incompletely absorbed (malabsorption), it can exert osmotic effects in the intestinal lumen, increasing water delivery and undergoing rapid fermentation by bacteria with consequent gas production. Although the prevalence of malabsorption is similar between subjects with FGID and healthy individuals,7,8 the clinical ramifications are different. Malabsorption can become problematic for patients with FGID because their heightened visceral hypersensitivity leads to the increased gas and fluid content of the bowel being associated with bloating, distension, excessive flatus, and motility disturbances.9,10 The mechanism underlying adult fructose malabsorption is unclear, although it has been hypothesized that a cause could be a change in the expression or activation of the transporter proteins.
Research into specific fructose transport and absorption has mostly been conducted in animal model studies and has shown that changes in GLUT expression correlates with altered fructose intestinal absorption.11,12 Recent human data from infant13,14 and diabetic15 studies have supported this, where a reduced expression of GLUT5 were associated with high rates of fructose malabsorption. Moreover, fructose malabsorption is not associated with GLUT2 or GLUT5 mutations.3
Factors that may influence the degree of absorption include rapid small intestinal transit time, small intestinal microbiota (bacterial overgrowth), developmental patterns, and varying glucocorticoid and thyroid hormonal roles.6,16,17 High-fructose dietary intake has been shown to transiently up-regulate GLUT5 and GLUT2, with increases in mRNA and protein levels.18 Intestinal fructose absorption is facilitated by co-ingestion with glucose due to the solvent drag and passive diffusion by the GLUT2 mechanism.1,8 Increasing GLUT5 expression by the presence of luminal fructose or sucrose, co-ingestion of amino acids, or altering the insertion of GLUT2 into the apical membrane, such as in the case of diabetes, can also increase fructose uptake.19 Sorbitol ingestion impedes fructose absorption.20
The study in this issue provides the first evidence in human adults directly assessing expression of the main fructose transporters, GLUT5 and GLUT2 protein and mRNA in small intestinal tissue in adults with symptomatic fructose malabsorption in comparison with controls.2 Eleven patients met the Rome III criteria for irritable bowel syndrome and reported increased symptoms during a positive fructose breath test. The control group (n = 15) were made up of age- and sex-matched asymptomatic subjects with a negative fructose breath test. The authors found that levels of mRNA in the duodenal mucosa did not differ significantly between the patient groups. This conflicts with previous thoughts that depressed or absent GLUT5 or GLUT2 protein levels could result in failure to transport fructose normally. The authors acknowledge that differences in membrane abundance, cellular activation or subcellular localization and expression of the transporters along different sites of the small intestine should be examined before excluding a role of the fructose transporters.
Given these results can be reproduced in larger samples, explanations other than differing GLUT5 and GLUT2 expression are needed for the manifestation of fructose malabsorption. For example, there may be other avenues of fructose absorption. The molecular effects of fructose on GLUT5 message and protein levels are not fully understood, for example there may be differences in the cis-acting elements and trans-acting factors.3 Additional proteins may exist that are necessary for proper functioning of the GLUT5 transporter within the enterocyte membrane.3 Because fructose absorption is stimulated by the presence of glucose in a dose-dependent fashion, there is a possibility of additional fructose transport mechanisms such as a transepithelial, phosphorylation-dependent glucose transport system.13
Breath hydrogen tests have been advocated for the assessment of dietary fructose malabsorption.8 One of the uncertainties regarding the diagnosis of malabsorption are that there are reasons other than inefficient fructose absorption behind a positive breath test result, such as rapid small bowel transit, excessive hydrogen-consuming bacteria, too few hydrogen-producing bacteria, or inadequate bacterial sugar fermentation.1,20 There are also issues in the heterogeneity of methodologies used (e.g. ingested dose and concentration of fructose used), and gastrointestinal symptoms have poor correlation with breath hydrogen levels21 and can appear independent of carbohydrate malabsorption.22 Another issue is the cut-off value used for hydrogen/methane concentrations, especially given the known variability of methane production and possibility of inadequate hydrogen production.23,24 Of note, this study employed a low cut off (>10 ppm from baseline) for methane production (although the authors did not state how many of their subjects were diagnosed as fructose malabsorbers according to hydrogen or methane criteria), which may lead to overestimating the prevalence of fructose malabsorption. Malabsorption has been shown to range from 5 g to more than 50 g,8 and might be dose- and concentration-related.25 Reasons for the absorptive capacity of fructose varying widely within the population remain unknown. Recently introduced semiquantification of breath hydrogen results has allowed for insight into degree of malabsorption.23 This offers the opportunity for future mechanistic studies to focus on those depicting convincing malabsorption only, and to assess the differences between the likely distinct populations with low- and high-absorptive capacity.23
The patients in the current study were more likely to get symptoms from the fructose ingestion, rather than from fructose malabsorption,2 suggesting mechanism(s) other than or additional to their fermentation may be responsible for fructose induced symptoms in FGID. For example, it has been hypothesized that other poorly absorbed short-chain carbohydrates may affect the enteric nervous system via local osmolytic effects or the involvement of inflammatory mediators.22 Undoubtedly the enteric nervous system has a complex role in responding to any food stimuli by varying secretion, motility, blood flow, and mucosal growth.26
At 50 g, a dose well below the average daily fructose intake in the USA, about 60–80% of adults experience some form of malabsorption.13 The main dietary sources of fructose include honey, apples, pears, and the increased use of high-fructose corn syrup. Other poorly absorbed short-chain carbohydrates and sugar alcohols have a similar fate to fructose in the distal small bowel and colon. Their effects are additive, distending the intestinal lumen by changing the volume of its contents.20 Dietary restriction of these fermentable short-chain carbohydrates (also referred to as the low FODMAP diet) is a proven efficacious approach that is effective in the majority of patients with FGID, as recently reviewed.26 Regardless of actual fermentation or breath hydrogen response, there is likely to be symptoms induced by fructose and the other FODMAPs because of their osmotic effect and slow absorption.22
Contrary to previous hypotheses, the study by Wilder-Smith et al. demonstrates that fructose intolerance with malabsorption may not be secondary to changes in the duodenal expression of the fructose transporters or their production. Despite GLUT5 and GLUT2 being established as the primary fructose transporters, the connection between a biological mechanism for fructose transport and malabsorption remains elusive. These data add further support to the concept that the ability to absorb fructose in patients with FGID are not abnormal overall and that the sensitivity of the bowel to the change in luminal conditions induced by fructose malabsorption is the key difference rather than the malabsorption itself.
References
- 1. Skoog SM, Bharucha AE. Dietary fructose and gastrointestinal symptoms: a review. Am J Gastroenterol 2004; 99: 2046–2050 [DOI] [PubMed] [Google Scholar]
- 2. Wilder-Smith. Fructose transporters GLUT5 and GLUT2 expression in adult patients with fructose intolerance. United Eur Gastroenterol J 2013 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 3. Wasserman D, Hoekstra JH, Tolia V, et al. Molecular analysis of the fructose transporter gene (GLUT5) in isolated fructose malabsorption. J Clin Invest 1996; 98: 2398–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kellett GL, Brot-Laroche E. Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes 2005; 54: 3056–3062 [DOI] [PubMed] [Google Scholar]
- 5. DeBosch BJ, Chi M, Moley KH. Glucose transporter 8 (GLUT8) regulates enterocyte fructose transport and global mammalian fructose utilization. Endocrinology 2012; 153: 4181–4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Douard V, Ferraris RP. Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab 2008; 295: 227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rumessen JJ. Functional bowel disease: Malabsorption and abdominal distress after ingestion of fructose, sorbitol, and fructose-sorbitol mixtures. Gastroenterology 1988; 95: 694–700 [DOI] [PubMed] [Google Scholar]
- 8. Rumessen JJ, Gudmand-Hoyer E. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut 1986; 27: 1161–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barrett JS, Gearry RB, Muir JG, et al. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther 2010; 31: 874–882 [DOI] [PubMed] [Google Scholar]
- 10. Ong DK, Mitchell SB, Barrett JS, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol 2010; 25: 1366–1373 [DOI] [PubMed] [Google Scholar]
- 11. Castello A, Guma A, Sevilla L, et al. Regulation of GLUT5 gene expression in rat intestinal mucosa: regional distribution, circadian rhythm, perinatal development and effect of diabetes. Biochem J 1995; 309: 271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barone S, Fussell SL, Singh AK, et al. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J Biol Chem 2009; 284: 5056–5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Douard V, Ferraris RP. The role of fructose transporters in diseases linked to excessive fructose intake. J Physiol 2013; 591: 401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones HF, Butler RN, Moore DJ, et al. Developmental changes and fructose absorption in children: effect on malabsorption testing and dietary management. Nutr Rev 2013; 71: 300–309 [DOI] [PubMed] [Google Scholar]
- 15. Dyer J, Wood IS, Palejwala A, et al. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol 2002; 282: 241–248 [DOI] [PubMed] [Google Scholar]
- 16. Barrett JS, Irving PM, Shepherd SJ, et al. Comparison of the prevalence of fructose and lactose malabsorption across chronic intestinal disorders. Aliment Pharmacol Ther 2009; 30: 165–174 [DOI] [PubMed] [Google Scholar]
- 17. Nucera G, Gabrielli M, Lupascu A, et al. Abnormal breath tests to lactose, fructose and sorbitol in irritable bowel syndrome may be explained by small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2005; 21: 1391–1395 [DOI] [PubMed] [Google Scholar]
- 18. Cui XL, Jiang L, Ferraris RP. Regulation of rat intestinal GLUT2 mRNA abundance by luminal and systemic factors. Biochim Biophys Acta 2003; 1612: 178–185 [DOI] [PubMed] [Google Scholar]
- 19. Latulippe ME, Skoog SM. Fructose malabsorption and intolerance: effects of fructose with and without simultaneous glucose ingestion. Crit Rev Food Sci Nutr 2011; 51: 583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gibson PR. Review article: Fructose malabsorption and the bigger picture. Aliment Pharmacol Ther 2007; 25: 349–363 [DOI] [PubMed] [Google Scholar]
- 21. Hoekstra JH, van Kempen AA, Bijl SB, et al. Fructose breath hydrogen tests. Arch Dis Child 1993; 68: 136–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yao CK, Tan HL, van Langenberg DR, et al. Dietary sorbitol and mannitol: food content and distinct absorption patterns between healthy individuals and patients with irritable bowel syndrome. J Hum Nutr Diet 2013 [Epub ahead of print] [DOI] [PubMed]
- 23. Barrett JS, Kalubovila U, Irving PM, et al. Semiquantitative assessment of breath hydrogen testing. J Gastroenterol Hepatol 2013; 28: 1450–1456 [DOI] [PubMed] [Google Scholar]
- 24. Bate JP, Irving PM, Barrett JS, et al. Benefits of breath hydrogen testing after lactulose administration in analysing carbohydrate malabsorption. Eur J Gastroenterol Hepatol 2006; 22: 318–326 [DOI] [PubMed] [Google Scholar]
- 25. Choi YK, Johlin FC, Summers RW, et al. Fructose intolerance: an under-recognized problem. Am J Gastroenterol 2003; 98: 1348–1353 [DOI] [PubMed] [Google Scholar]
- 26. Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol 2012; 107: 657–666 [DOI] [PubMed] [Google Scholar]


