Abstract
Background and objectives
Faecal calprotectin is a valuable noninvasive marker for inflammatory bowel disease (IBD). The aim of our study was to determine the correlation between six different calprotectin assays and compare their performance for diagnosis and follow up of IBD.
Methods
Thirty-one patients with suspected IBD and 31 patients in follow up were included. We determined calprotectin by means of three rapid immmunochromatographic tests, two enzyme-linked immunosorbent assays, and one automated fluoroimmunoassay. Results were correlated with endoscopic and histological findings.
Results
Although all methods correlated significantly, slopes and intercepts differed extensively, with up to 5-fold quantitative differences between assays. Sensitivity and specificity for diagnosis of IBD were 82–83 and 84–89%, respectively. For follow up, sensitivity in detecting mild ulcerative colitis was 71–100%. In moderate-to-severe ulcerative colitis, sensitivity was 100% for all assays. Specificity was 67–86% in both subgroups. In Crohn’s disease, only moderate-to-severe disease could be differentiated from remission, with sensitivity 83–86% and specificity 75% for all tests.
Conclusions
All calprotectin assays showed comparable clinical performance for diagnosis of IBD. For follow up, performance was acceptable, except for mild Crohn’s disease. Because of the large quantitative differences, further efforts are needed to standardize calprotectin assays.
Keywords: Calprotectin, Crohn’s disease, diagnosis, follow up, inflammatory bowel disease, ulcerative colitis
Introduction
Ileocolonoscopy with histopathological sampling is generally considered the gold standard in the diagnosis and follow up of inflammatory bowel disease (IBD).1 However, endoscopy procedures and the required bowel preparation are expensive, time consuming, and inconvenient to the patient.
Several biochemical parameters in plasma and stool have been investigated for their ability to detect bowel inflammation, of which faecal calprotectin (FC) has shown the best test performance until now.2,3 Calprotectin is a protein that is released by neutrophils in the bowel in case of inflammation, as is the case in IBD. In patients suspected for IBD, a negative FC indicates a low chance of having bowel inflammation (high negative predictive value), whereas an elevated FC needs further investigation with endoscopy and histology. A recent meta-analysis calculated that calprotectin screening might reduce the need for endoscopic procedures in adults with suspected IBD in up to 67%.4
In patients with known IBD, FC is high when active inflammation is present and low in case of remission. Recent promising results were obtained with FC to monitor disease activity in IBD patients, performing better than traditional inflammatory markers such as C-reactive protein.2,5
Calprotectin is often measured with enzyme-linked immunosorbent assays (ELISA). Since ELISA is time consuming and mostly suited for analysing samples in batch, faster and more user-friendly techniques have been developed. However, very few data are available on the comparability of these different methods. The goal of this study was to evaluate six different calprotectin tests and to determine their performance for diagnosis and follow up of IBD patients.
Materials and methods
Study population
Sixty-four adult patients (≥16 years old), referred for colonoscopy to the gastroenterology department of our hospital, provided a stool sample for calprotectin measurement. Patients were prospectively included based on the indication for the endoscopy, being either clinical suspicion of IBD or the follow up of known IBD. Demographic characteristics of the study population are shown in Table 1. The patients with suspected IBD (n=33) were selected based on one or more of the following criteria: chronic diarrhoea and/or abdominal pain, iron deficiency anaemia, unexplained weight loss, or a family history of IBD. Two of these patients had to be excluded: in one case, the histopathological and endoscopic evaluation was inconclusive; in the second patient, the consistency of the stool was extremely watery and calprotectin was below the detection limit of all assays. This resulted in a population of 31 included patients with suspected IBD. The study population of known IBD patients undergoing follow-up endoscopy consisted of 31 patients, of which 17 had ulcerative colitis (UC) and 14 had Crohn's disease (CD). There were no exclusion criteria concerning medical history or drug intake, because this information was not available for most of our study patients.
Table 1.
Demographic characteristics of the study populations
| Study group | n | M/F | Age (years) |
||
|---|---|---|---|---|---|
| Median | Range | IQR | |||
| Suspected IBD | 31 | 10/21 | 36 | 16–75 | 30–45 |
| Follow up IBD | 31 | 17/14 | 50 | 24–83 | 42–60 |
F, female; IBD, inflammatory bowel disease; M, male.
Colonoscopy
Bowel preparation was done at the outpatient clinic with electrolyte or polyethylene glycol solutions. Ileocolonoscopies were performed by five experienced gastroenterologists. Endoscopic disease activity was evaluated by means of the Mayo endoscopic subscore6 for UC and the simple endoscopic score for CD (SES-CD).7
During endoscopy, biopsy specimens targeted at the most severely affected areas and from the edge of ulcers were obtained. If no lesions were present, biopsies were collected from random sites. Routine histology was performed by three experienced pathologists.
Calprotectin measurement
From each patient, a faecal sample was obtained on the day of the endoscopy. To minimize interference by the bowel preparation solution, the first stool that was obtained after starting the bowel preparation was collected. Sample collection was coordinated by the nurses of the outpatient clinic.
We determined FC by means of three rapid immunochromatographic assays (Bühlmann Quantum Blue, Eurospital Calfast, Biotec Certest), two ELISAs (Eurospital and Calprolab), and one automated immunoassay (Phadia EliA). The tests’ characteristics are shown in Table 2.
Table 2.
Characteristics of the six tested calprotectin assays
| Assay (manufacturer) | Method | Extraction device | Measurement range (µg/g) |
|---|---|---|---|
| Calprest (Eurospital, Trieste, Italy) | ELISA | Eurospital extraction device | 15–500 |
| Calprolab (Calpro, Oslo, Norway) | ELISA | Roche faecal extraction device | 25–2500 |
| Calprotectin EliA (Phadia/Thermo-Fisher, Uppsala, Sweden) | Enzyme fluoroimmunoassay | Roche faecal extraction device | 15–3000 |
| Calfast (Eurospital, Trieste, Italy) | Quantitative immunochromatography (automated reading) | Eurospital extraction device | 50–300 |
| Calprotectin Quantum Blue (Bühlmann, Schönenbuch, Switzerland) | Quantitative immunochromatography (automated reading) | Roche faecal extraction device | 30–300 |
| Certest (Biotec, Zaragoza, Spain) | Semi-quantitative immunochromatography (visual reading) | Certest extraction device | 50–200 |
ELISA, enzyme-linked immunosorbent assay.
The rapid tests are based on lateral flow immunochromatography, resulting in a line on the test strip if the analyte is present in the sample. Bühlmann Quantum Blue and Eurospital Calfast produce quantitative results, as the density of the test line is measured by an automated reader. Biotec Certest is a semi-quantitative test, with visual interpretation of the test line(s), resulting in a value <50, 50–200, or >200 µg/g. ELISA is a quantitative method on a microtitre plate. In our study, ELISAs were performed manually and optical densities were read afterwards by a PhD plate reader (BioRad). EliA from Phadia is a recently developed quantitative enzyme fluoroimmunoassay performed on a automated analyser (Immunocap 250).
All analyses were performed in the laboratory of the Imelda Hospital strictly according to the manufacturer’s instructions. Stool samples were stored at 2–8℃ for a maximum of 1 week. Afterwards, stool extraction was completed using commercial extraction devices recommended by the manufacturer, as shown in Table 2. Since each assay has its own extraction buffer and extraction procedure, it was not possible to assess the performance of all assays using a common faecal extract. Extracts were centrifuged and stored for a maximum of 3 months at –20℃ before analysis. Only Biotec Certest had to be performed immediately after extraction, according to the manufacturer’s instructions.
Three samples of the diagnostic test group could not be analysed with Eurospital Calfast and Calprest assays, because of the limited amount of available test kits.
Statistics
Correlation between the different assays was calculated using nonparametric Passing Bablok regression analysis. Only those results within the measurement range of the assay were included. The degree of correlation between assays was determined by the nonparametric Spearman rank correlation coefficient. The significance level was determined by the associated p-value set at <0.05.
Receiver operating characteristic (ROC) analyses were performed to determine the optimal cut offs that provided the highest sum of sensitivity and specificity. The following performance characteristics were calculated: sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios.
All statistical analyses and graphics were performed using GraphPad Prism version 4, except the Passing Bablok regression analysis which was performed using Medcalc Software version 12.
Ethical considerations
All patients participated at a voluntary basis and provided informed consent. The study was approved by the local ethics committee of the Imelda Hospital (registration number B689201112774).
Results
Correlation between assays
Method comparison was performed for the five quantitative assays: Bühlmann Quantum Blue, Calprolab, Eurospital Calfast, Eurospital Calprest, and Phadia EliA. The Passing Bablok regression and Spearman rank analysis characteristics are summarized in Table 3. Significant correlations (p<0.05) between all assays were obtained. Correlation coefficients ranged from 0.65 to 0.93. However, the slopes and/or intercepts differed extensively, with up to 5-fold quantitative differences between assays.
Table 3.
Passing Bablok analysis and Spearman rank correlation between the different assays
| Method X | Method Y | n | Passing Bablok regression equation | Spearman rank correlation coefficient | p-value |
|---|---|---|---|---|---|
| Calprest | QB BM | 37 | y=2.39x–19 | 0.91 | <0.001 |
| Calprest | Phadia EliA | 24 | y=1.67x–80 | 0.70 | <0.001 |
| Calprest | Calprolab | 29 | y=2.91x–108 | 0.85 | <0.001 |
| Calprest | Calfast | 27 | y=0.76x+19 | 0.82 | <0.001 |
| Calfast | QB BM | 31 | y=5.37x–266 | 0.69 | <0.001 |
| Calfast | Phadia EliA | 26 | y= 1.66x–71 | 0.65 | <0.001 |
| Calfast | Calprolab | 31 | y=4.85x–227 | 0.75 | <0.001 |
| Calprolab | QB BM | 33 | y=1.12x+51 | 0.93 | <0.001 |
| Calprolab | Phadia EliA | 32 | y=0.31x+6 | 0.65 | <0.001 |
| QB BM | Phadia EliA | 28 | y= 0.34x–15 | 0.77 | <0.001 |
n, number of samples available for regression analysis (results within measurement range of both tests); QB BM, Quantum Blue Bühlmann
Clinical performance
Diagnosis
For the 31 enrolled patients with suspected IBD, a diagnosis of UC and CD was confirmed by ileocolonoscopy and histology in five and seven patients, respectively. This resulted in a prevalence of 39% IBD. In the 19 other patients, IBD could be excluded. No discrepancies between endoscopy and histology were observed. In our patients without IBD, the following other organic diseases were diagnosed: nonsteroidal anti-inflammatory drug enteropathy (n=1), adenoma (n=1), and infectious colitis (n=1).
For all tested assays, a cut off of 50 µg/g faeces is recommended by the manufacturer, except for Calfast (70 µg/g). We performed a ROC curve analysis (data not shown) to determine the cut off that provided the highest sum of sensitivity and specificity. This led to an optimal cut off for Bühlmann Quantum Blue of 75 µg/g and for Phadia EliA of 15 µg/g. For the other assays, the optimal cut offs were identical to the one from the manufacturer. Sensitivity, specificity, predictive values, and likelihood ratios at the calculated cut offs are summarized in Table 4.
Table 4.
Diagnostic accuracy of the different calprotectin assays
| Cut off (µg/g) | IBD (n) | Non-IBD (n) | Median FC in non-IBD (µg/g) | Median FC in IBD (µg/g) | Ratio median FC IBD/non-IBDa | Sensitivity | Specificity | PPV | NPV | LR+ | LR– | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calprolab | 50 | 12 | 19 | <25 | 239 | 10 | 83 | 89 | 83 | 89 | 7.9 | 0.19 |
| QB BM | 50 | 12 | 19 | <30 | 425 | 14 | 83 | 68 | 63 | 87 | 2.7 | 0.24 |
| 75 | 83 | 84 | 77 | 89 | 5.28 | 0.20 | ||||||
| Calfast | 70 | 11 | 17 | <50 | 132 | 3 | 82 | 88 | 82 | 88 | 6.9 | 0.21 |
| Calprest | 50 | 11 | 17 | 38 | 149 | 4 | 82 | 88 | 82 | 88 | 6.9 | 0.21 |
| Phadia EliA | 50 | 12 | 19 | <15 | 183 | 12 | 75 | 95 | 90 | 86 | 14.3 | 0.26 |
| 15 | 83 | 84 | 77 | 89 | 5.3 | 0.20 | ||||||
| Certest | 50 | 12 | 19 | <50 | >200 | / | 83 | 84 | 77 | 89 | 5.3 | 0.20 |
If median FC was outside the measurement range, the detection limit was used to calculate the ratio IBD/non-IBD.
FC, faecal calprotectin; IBD, inflammatory bowel disease; LR+, positive likelihood ratio; LR–, negative likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; QB BM, Quantum Blue Bühlmann.
At the optimal cut offs, the assays were clinically (positive/negative) discordant in only two of the 31 patients (6.5%) and these discrepancies were only found in the non-IBD patients (false-positives). None of the patients with other organic disease (n=3) had an elevated calprotectin level with any of the assays.
In addition, we determined the discriminatory power of the tests by dividing the median calprotectin value in the IBD patients by the median value in the non-IBD patients. As shown in Table 4, this resulted for Bühlmann Quantum Blue, Phadia EliA, Calprolab ELISA, Calprest ELISA, and Calfast in the following ratios: 14, 12, 10, 4, and 3, respectively.
Follow up
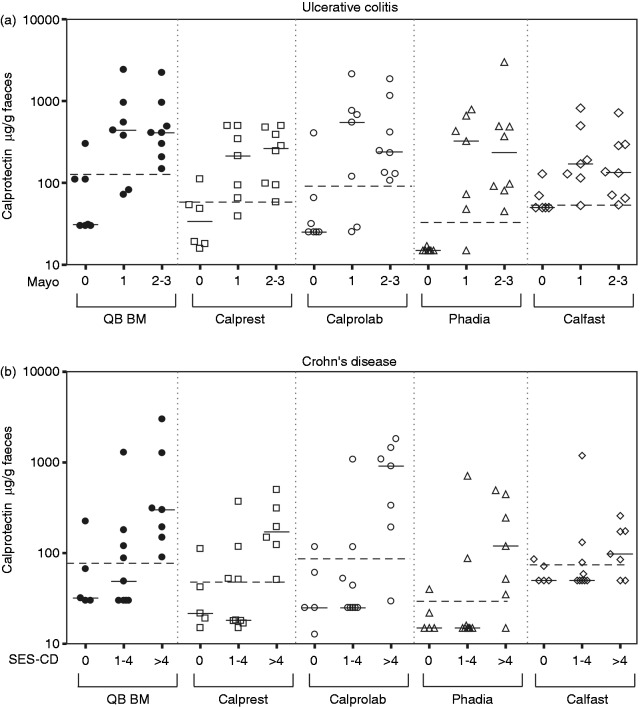
Data from patients with UC and CD were analysed separately. We added the data from the newly diagnosed IBD patients (from the subpopulation with suspected IBD) to those from the known IBD patients to obtain a larger sample size. As such, 21 patients with CD and 22 patients with UC were included in the analysis. Results are shown in Figure 1.
Figure 1.
Faecal calprotectin measured by different assays in patients grouped by Mayo endoscopic subscore for ulcerative colitis (a) and simple endoscopic score for Crohn’s disease (b).
Solid lines indicate the median values of each group; dashed lines indicate the optimal cut offs determined by ROC curve analysis. QB BM, Bühlmann Quantum Blue.
None of the manufacturers proposes cut offs for identifying relapse in patients with IBD. Analogous to the evaluation of calprotectin in the diagnostic setting, we calculated optimal cut offs for detecting endoscopic disease activity by ROC analysis (data not shown).
Crohn’s disease
Median SES-CD score in the subgroup with CD was 3 (range 0–15, IQR 1–7). We defined three subgroups according to the corresponding endoscopic disease activity. Endoscopic remission was defined as a SES-CD score of 0, mild endoscopic disease activity as SES-CD 1–4, and moderate-to-severe endoscopic disease activity as SES-CD>4. These groups consisted of five, nine, and seven patients, respectively. Of the patients with active inflammation, 10/16 (63%) had ileal disease, 3/16 (19%) colonic disease, and 3/16 (19%) ileocolonic disease. None of our patients in endoscopic remission had any histological disease activity. For all patients with endoscopic disease activity (SES-CD≥1), pathologists reported the presence of active inflammation.
The results of the calprotectin assays for the assessment of endoscopic disease activity in CD are listed in Table 5. Optimal cut offs for the quantitative assays ranged from 29 to 89 µg/g. Performance of calprotectin in detecting mild disease activity (SES-CD ≤4) was low for all methods, with a sensitivity between 22 and 44%. For patients with a higher disease activity level (SES-CD >4), sensitivity increased for the different assays to 83–100%. All the assays displayed a specificity of 80%, except for Certest which only reached a specificity of 40% at a cut off of 50 µg/g. This assay showed a specificity of 100% at the higher cut off of 200 µg/g, but this resulted in substantially lower sensitivities.
Table 5.
Performance of the calprotectin assays for detecting disease activity in Crohn’s disease
| Assay | Cut off (µg/g) | SES-CD 1–4 (n=9) vs. SES-CD 0 (n=5) | SES-CD >4 (n=7, except Calprest/Calfast n=6) vs. SES-CD 0 (n=5) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | NPV | PPV | LR+ | LR– | Sensitivity | Specificity | NPV | PPV | LR+ | LR– | ||
| Calprolab | 89 | 22 | 80 | 36 | 67 | 1.1 | 1.0 | 86 | 80 | 80 | 86 | 4.3 | 0.18 |
| QB BM | 77.5 | 44 | 80 | 44 | 80 | 2.2 | 0.7 | 100 | 80 | 100 | 88 | 5 | 0 |
| Phadia EliA | 28.5 | 22 | 80 | 36 | 67 | 1.1 | 1.0 | 86 | 80 | 80 | 86 | 4.2 | 0.21 |
| Calfast | 75.5 | 33 | 80 | 40 | 75 | 1.7 | 0.8 | 83 | 80 | 80 | 83 | 3.3 | 0.2 |
| Calprest | 46.5 | 44 | 80 | 44 | 80 | 2.2 | 0.7 | 100 | 80 | 100 | 86 | 5 | 0 |
| Certest | 50 | 33 | 40 | 25 | 50 | 0.56 | 1.7 | 100 | 40 | 100 | 70 | 1.7 | 0 |
| 200 | 22 | 100 | 42 | 100 | / | 0.8 | 71 | 100 | 71 | 100 | / | 0.3 | |
IBD, inflammatory bowel disease; LR+, positive likelihood ratio; LR–, negative likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; QB BM, Quantum Blue Bühlmann; SES-CD, simple endoscopic score for Crohn’s disease.
Ulcerative colitis
As for CD, patients with UC were divided into three subgroups: endoscopic remission (Mayo endoscopic subscore 0, n=7), mild endoscopic disease activity (Mayo 1, n=7), and moderate-to-severe endoscopic disease activity (Mayo 2 or 3, n=8). No discrepancies were found between endoscopy and histology regarding the absence/presence of disease activity.
Table 6 summarizes the performance characteristics of the different tests in UC. Optimal cut offs for the quantitative assays ranged from 31 to 129 µg/g. A Mayo score of 2–3 could be differentiated from remission with a sensitivity of 100% by all the assays, whereas the sensitivities to distinguish Mayo 1 from remission were between 71 and 100%. The high sensitivities of Certest (at a cut off of 50 µg/g) and Calfast were associated with lower specificities (57% and 67%, respectively) in comparison to the other assays.
Table 6.
Performance of the calprotectin assays for detecting disease activity in ulcerative colitis
| Assay | Cut off (µg/g) | Mayo 1 (n=7) vs. Mayo 0 (n=7, except Calprest/Calfast n=6) |
Mayo 2–3 (n=8) vs. Mayo 0 (n=7, except Calprest/Calfast n=6) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | NPV | PPV | LR+ | LR– | Sensitivity | Specificity | NPV | PPV | LR+ | LR– | ||
| Calprolab | 86 | 71 | 86 | 75 | 83 | 5 | 0.33 | 100 | 86 | 100 | 89 | 6.0 | 0 |
| QB BM | 129 | 71 | 86 | 75 | 83 | 5 | 0.33 | 100 | 86 | 100 | 89 | 6.0 | 0 |
| Phadia | 31 | 86 | 86 | 86 | 86 | 6 | 0.17 | 100 | 86 | 100 | 89 | 6.0 | 0 |
| Calfast | 50 | 100 | 67 | 100 | 88 | 3 | 0 | 100 | 67 | 100 | 80 | 3.0 | 0 |
| Calprest | 56 | 86 | 83 | 83 | 86 | 5 | 0.17 | 100 | 83 | 100 | 89 | 6.0 | 0 |
| Certest | 50 | 100 | 57 | 100 | 70 | 2.3 | 0 | 100 | 57 | 100 | 73 | 2.3 | 0 |
| 200 | 57 | 86 | 67 | 80 | 4.0 | 0.5 | 75 | 86 | 75 | 86 | 5 | 0.3 | |
LR+, positive likelihood ratio; LR–, negative likelihood ratio; Mayo, Mayo endoscopic subscore; NPV, negative predictive value; PPV, positive predictive value; QB BM, Quantum Blue Bühlmann.
Discussion
The aim of this study was to determine the correlation between the most widely used calprotectin assays and to compare their clinical performance for diagnosis and follow up of IBD.
In the first part of our study, we evaluated the quantitative correlation between the different tests. Correlation coefficients were moderate to excellent. However, when performing regression analysis, significant differences (up to 5-fold) were observed, even though manufacturers propose identical cut offs. For that reason, analysing the same sample with different calprotectin assays will result in very different values for FC.
These results are in line with a recent study from the UK National External Quality Assessment Service that revealed up to 3.8-fold differences between ELISAs from different manufacturers.8 Studies comparing the Bühlmann Quantum Blue rapid test with an ELISA kit found correlation coefficients between 0.56 and 0.94. Slopes of the regression curves varied from 1.0 to 2.24 and intercepts from –11 to –40.9–12
Some of the variability in our study might have been caused by the different extraction devices and procedures, as was shown in the study of Whitehead et al.8 However, we also observed major quantitative differences between assays using the same extraction device.
In the second part of our study, we assessed the diagnostic accuracy of FC for the diagnosis of IBD among patients with a suspect clinical history. The optimal cut offs determined by ROC curve analysis resulted in highly comparable performance characteristics for all assays. A recent meta-analysis of prospective studies in adults found sensitivity, specificity, positive predictive value, and negative predictive value of 93, 96, 91, and 97%, respectively.4 The majority of the pooled results were obtained with Phical or Calprest ELISA. In our study, a slightly lower performance was found. False-positive FC results have been attributed to several causes (e.g. infections, malignancies, and several drugs).4 However, in our two patients having a false-positive result with all assays, further investigation revealed no explanation for their abdominal symptoms or elevated calprotectin. In three patients, another organic abdominal disease than IBD was diagnosed, but they all had a low FC.
Two newly diagnosed IBD patients had FC levels below the limit of detection, with 3/5 and 5/5 assays. These two false-negative results were from patients with mild, strictly ileal CD. It has been shown in previous studies that this type of disease can result in low calprotectin levels despite detectable endoscopic inflammation.13–15 A false-negative calprotectin can also be caused by very liquid stool samples.16 In this regard, we rejected one extremely aqueous stool sample in which the calprotectin concentration was below the detection limit for all assays, thereby affecting the performance of all assays equally. Although the influence of the bowel preparation on the first stool sample is expected to be minimal, a small dilution effect on the other samples cannot be completely excluded. This could have slightly lowered the sensitivity, negative predictive value, and optimal cut offs of all calprotectin tests to the same extent.
Besides specificity and sensitivity, we determined the calprotectin value in IBD patients relative to non-IBD patients for each of the investigated assays. The highest discriminatory power between IBD and non-IBD samples was achieved with Bühlmann Quantum Blue, Phadia EliA, and Calprolab ELISA, with ratios that were ≥10.
In the last part of our study, we compared the different assays in their ability to detect endoscopic disease activity in the follow up of IBD patients. In CD, mild endoscopic disease activity (SES-CD ≤4) could not be reliably differentiated from remission with any of the assays. Sixty-six per cent (6/9) of these patients only showed mild ileal disease, which results in low calprotectin levels, as already discussed. However, a SES-CD score >4 could be identified with all the assays, except Certest, with good sensitivities. In UC, sensitivities for mild disease were moderate to good and substantially better than in mild Crohn’s disease. All assays had a very good sensitivity in detecting moderate-to-severe UC.
Nine studies on the performance of calprotectin in the follow up of IBD patients were reviewed by Lewis et al.;2 sensitivity and specificity ranged from 70 to 93% and from 70 to 92%, respectively. Another recent prospective study conducted in our centre, in which samples were sent to another laboratory for FC measurement by PhiCal ELISA, included a large cohort of CD patients (n=87). Sensitivities and specificities of 60 and 79%, respectively, for a SES-CD score of ≥2 were observed. In the same study, 39 patients with UC were included, with sensitivities and specificities of 71 and 100% for Mayo scores of 1–3.17 Our performance characteristics are thus in line with these observations.
Our optimal cut offs for detecting endoscopic disease activity in IBD varied widely from one assay to another. In general, they were rather low compared to other publications. In the review by Lewis et al.,2 cut offs between 50 and 200 µg/g were used by the different authors. The study by D’Haens et al.17 proposed a cut off of 250 µg/g. We hypothesize that mean disease activity of our study population was lower than in other studies, and more patients had only very mild inflammation. However, our (sub)populations were rather small, which is the main limitation of our study. Therefore, more extensive studies are needed on the different FC assays, and their cut offs for follow up in IBD have to be established in larger cohorts.
Overall, the evaluated immunochromatography tests are easier and faster to perform than the ELISA tests and are acceptable alternatives to an ELISA for diagnostic determinations. For purposes of follow up, a quantitative test with a high measuring range is preferred. The Certest is only semiquantitative, whereas the two quantitative rapid tests in our study only have a low measurement range. However, Bühlmann recently developed a high range version of the Quantum Blue assay, with an extended measurement range (100–1800 µg/g), specifically for follow up samples.
Since the immunochromatographic fast tests have short turnaround times and can be performed on one individual sample at the time, they would be practical and cost-effective in laboratories with small amounts of samples. ELISA is more suited for large laboratories, where large amounts of samples can be analysed in batch, which is more time- and cost-effective.
The Phadia automated EliA is a new method that may combine the fast and easy manipulation of the rapid tests with the precision and high measurement range of an ELISA test. However, the measurement results and optimal cut offs that were obtained in this study were remarkably lower than those from other assays. The cut off for diagnosis was optimal at a level of 15 µg/g. This is as low as the detection limit of the assay, which is analytically unacceptable. Further studies with larger study populations are needed to evaluate the performance of this new assay and to establish its cut offs.
In conclusion, we found a acceptable performance of all calprotectin tests. However, large quantitative differences between the assays were observed. This variation makes it impossible to use methods interchangeably and enforces the urgent need for a further standardization.
Acknowledgements
Calprotectin test kits were provided for free or at reduced prices by all manufacturers. The authors would like to thank Bühlmann, Phadia/Thermofisher, Eurospital, Calpro, and Biotest for this support.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1. Bernstein CN, Fried M, Krabshuis JH, et al. World Gastroenterology Organization practice guidelines for the diagnosis and managment of IBD in 2010. Inflamm Bowel Dis 2010; 16: 112–114 [DOI] [PubMed] [Google Scholar]
- 2. Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology 2011; 140: 1817–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centre for Evidence-based Purchasing. Evidence review: value of calprotectin in screening out irritable bowel syndrome, CEP09026. London: NHS Purchasing and Supply Agency, 2009.
- 4. Van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 2010; 341: c3369–c3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Vos M, Dewit O, D’Haens G, et al. Fast and sharp decrease in calprotectin predicts remission by infliximab in anti-TNF naïve patients with ulcerative colitis. J Crohns Colitis 2012; 6: 557–562 [DOI] [PubMed] [Google Scholar]
- 6. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987; 317: 1625–1629 [DOI] [PubMed] [Google Scholar]
- 7. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004; 60: 505–512 [DOI] [PubMed] [Google Scholar]
- 8. Whitehead S, French J, Brookes M, et al. Between-assay variability of faecal calprotectin enzyme-linked immunosorbent assay kits. Ann Clin Biochem 2013; 50: 53–61 [DOI] [PubMed] [Google Scholar]
- 9. Kolho KL, Turner D, Veereman-Wauters G, et al. Rapid test for fecal calprotectin levels in children with Crohn’s disease. J Pediatr Gastroenterol Nutr 2012; 55: 436–439 [DOI] [PubMed] [Google Scholar]
- 10. Coorevits L, Baert F, Vanpoucke H. Faecal calprotectin: comparative study of the Quantum Blue rapid test and an established ELISA method. Clin Chem Lab Med 2012; 51: 825–831 [DOI] [PubMed] [Google Scholar]
- 11. Dolci A, Panteghini M. Comparative study of a new quantitative rapid test with an established ELISA method for faecal calprotectin. Clin Chim Acta 2012; 413: 350–351 [DOI] [PubMed] [Google Scholar]
- 12. Wassell J, Wallage M, Brewer E. Evaluation of the Quantum Blue rapid test for faecal calprotectin. Ann Clin Biochem 2012; 49: 55–58 [DOI] [PubMed] [Google Scholar]
- 13. Sipponen T, Savilahti E, Kolho KL, et al. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis 2008; 14: 40–46 [DOI] [PubMed] [Google Scholar]
- 14. Sipponen T, Karkkainen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther 2008; 28: 1221–1229 [DOI] [PubMed] [Google Scholar]
- 15. Jones J, Loftus EV, Pannaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2008; 6: 1218–1224 [DOI] [PubMed] [Google Scholar]
- 16. Vestergaard TA, Nielsen SL, Dahlerup JF, et al. Fecal calprotectin: assessment of a rapid test. Scand J Clin Lab Invest 2008; 68: 343–347 [DOI] [PubMed] [Google Scholar]
- 17. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012; 18: 2218–2224 [DOI] [PubMed] [Google Scholar]