Abstract
Background
Gelatin tannate, a gelatin powder containing tannic acids, is commonly employed as an intestinal astringent. Neither information nor animal model exist to confirm its efficacy or unravel mechanisms of action.
Objective
To evaluate the action of gelatin tannate in murine dextran sodium sulphate (DSS)-induced acute colitis.
Methods
Mice were exposed to DSS and received gelatin tannate by gavage. At sacrifice, colon histological degree of inflammation was assessed. Stool samples were cultured for microbiological analysis. Colon samples were analysed by two-photon confocal microscopy and atomic force microscopy. Elisa was performed on murine serum to assess lipopolysaccharide and peptidoglycan levels.
Results
Gelatin tannate treatment reduced disease activity, bodyweight loss, and preserved colonic length. It produced a decrease in the amount of enterobacteria and enterococci. At confocal microscopy, intestinal samples from healthy and treated mice displayed similar structure in mucus layer thickness and composition; samples from placebo group had no mucus layer or a thinner stratus. Atomic force microscopy confirmed these findings. Treated mice showed lower blood LPS levels vs. control.
Conclusions
Gelatin tannate decreased the severity of colitis. Acting as a gut barrier enhancer, it re-establishes gut homeostasis by recovering intestinal permeability and mucus layer integrity in gut mucosa and by modulating microbiota composition.
Keywords: DSS-acute colitis, gelatin tannate, gut barrier, gut microbiota, intestinal mucus
Introduction
The intestinal barrier represents a functional unit responsible for two main tasks crucial for survival of the individual, allowing nutrient absorption,and defending the body from penetration of unwanted, often dangerous, macromolecules. The gut mucosa is a multilayer system consisting of an external ‘anatomical’ barrier and an inner ‘functional’ immunological barrier.1 Commensal gut microbiota, a mucous layer, and the intestinal epithelial monolayer constitute the anatomical barrier. The deeper, inner layer consists of a complex network of immune cells organized in a specialized and compartmentalized system known as gut-associated lymphoid tissue. The interaction of these components sustains the maintenance of the delicate equilibrium of intestinal homeostasis and gut permeability. Many factors can alter this balance, including modifications of the mucus layer, alterations in the gut microflora, and epithelial damage, leading to increased intestinal permeability and translocation of luminal contents (i.e. lipopolysaccharide, peptidoglycan) to the underlying mucosa. The integrity of these structures is necessary for the maintenance of normal intestinal barrier function and dysregulation of any of the aforementioned components have been implicated in the pathogenesis of inflammatory bowel disease (IBD), and also of many other gastrointestinal disorders, including infectious enterocolitis, irritable bowel syndrome, small intestinal bowel overgrowth, and allergic food intolerance.2–4
Inflammatory bowel diseases, such as Crohn’s disease and ulcerative colitis, are chronic, relapsing inflammatory disorders of the digestive tract resulting from a loss of homeostasis between the intestinal immune system and the intestinal microbiota in genetically predisposed individuals.5 Several lines of evidence suggest that an increased intestinal permeability due to the disruption of the intestinal barrier plays a central role in their pathogenesis.6,7
The mucus layer is the first physical barrier that bacteria meet within the intestinal tract. It provides protection by shielding the epithelium from microorganisms and harmful antigens, while acting as a lubricant for intestinal motility. It consists of two layers: an inner layer, firmly adherent to the epithelial cells, and an outer nonattached layer.8 These mucus layers are organized around the highly glycosylated MUC2 mucin, forming a large net-like polymer that is secreted by the goblet cells. The inner mucus layer is dense and does not allow bacteria to penetrate, thus keeping the epithelial cell surface free from bacteria. This compartmentalization seems to be fundamental for the homeostasis in the highly colonized colon. Break and loss of the barrier formed by the inner mucus layer trigger inflammation and development of colon cancer, as shown by dextran sodium sulphate (DSS)-induced model of murine colitis.9–11
DSS-induced colitis represents a T-cell-independent, chemically induced model of epithelial damage and acute inflammation, primarily driven by innate immune responses. It has been recently shown that DSS directly affects gut epithelial cells of the basal crypts, disturbing the integrity of the mucosal barrier.12 It exerts its action by causing a fast alteration in the mucus permeability and a disruption of the mucus biophysical structure. Thus, it allows bacteria to enter and penetrate the inner mucus layer. Once bacteria come in contact with the epithelial cells, enter the crypts, and are taken up by epithelial cells, gut-associated lymphoid tissue is triggered to react against also relatively harmless commensal bacteria and to cause an inflammation reaction.13 Several studies focused on the clinical improvement of acute DSS-induced colitis by using probiotics and antibiotics in order to modulate the commensal microflora.14–19 On the other hand, this model is a useful way to evaluate those factors that can modulate the anatomical and functional health of the gut barrier, by acting not only on the gut microbiota, but also on the recovering of the mucus layer.
In this scenario, gelatin tannate, a combination of tannic acid (penta-m-digallolyl glucose), and gelatine could be able to create a protective film, forming bonds with the mucin and, thus, protecting the gut from the aggressive penetration of commensal bacteria. Tannins are widely distributed throughout the plant kingdom. Since tannic acid is one of the principal tannins, the term tannin is ordinarily used as a synonym for tannic acid. Gelatin is a collagen derivate, which is ingested as a powder insoluble at gastric acidic pH and becomes a gelatin with the increase of pH to over 5.5.20
Gelatin tannate is commonly employed as an intestinal astringent and present in the market with various names, such as tanagel, gelenterum, or tasectan.21 This complex passes through the stomach unaltered to dissolve into gelatine and tannic acid once in the gut, where it may exert its action by restoring the physiological function of the intestine. Furthermore, the well-known astringent properties of tannins allow the precipitation of proinflammatory mucoproteins from the intestinal mucus and their elimination through the faeces.22,23 Nevertheless no information exists regarding in vivo studies and no animal models have been used to confirm its efficacy or unravel further mechanisms of action.
In the present study, we evaluated the therapeutic effect and mechanisms of action of gelatin tannate using the DSS-induced acute colitis mouse model. Gelatin tannate significantly ameliorated disease severity. This was assessed through clinical and histological parameters. Gelatin tannate reduced not only the disease activity index (DAI) and the loss of bodyweight, but also the histological score used to evaluate the colitis. In addition to these findings, we assessed a modulation of the microflora composition, a restoring of mucus layer and, consequently, of gut permeability. These findings confirmed the efficacy of gelatin tannate from a mechanistic point of view. Gelatin tannate seems to act as a protective film and shows how the mucus layer recovering, beside the gut microbiota modulation, can be a useful weapon to re-establish the physiological intestinal homeostasis after an acute injury.
Materials and methods
Gelatin tannate
Gelatin tannate (Gelenterum; ACRAF, Italy) is composed of tannic acid and gelatin. The gelatin used is a type-A or acid gelatin with a dissolution pH of 5.5 and a Bloom gel strength of 40–60 g (viscosity of 27 mP). Gelatin is a pure natural protein, easily digested, with no cholesterol, odour, or flavour. Gelatin contains 84–90% collagen protein and 1–2% mineral salts, and the rest is water. The protein is composed of 18 amino acids (25.5% glycine, 8.7% alanine, 2.5% valine, 3.2% leucine, 1.4% isoleucine, 0.1% cysteine, 1% methionine, 2.2% phenylalanine, 18% proline, 14% hydroxyproline, 0.4% serine, 1.9% threonine, 0.5% tyrosine, 6.6% aspartic acid, 11.4% glutamic acid, 8.1% arginine, 4.1% lysine, 0.8% histidine).
Experimental acute DSS colitis
C57/BL6 mice received 2,5% DSS in drinking water ad libitum for 7 days. They were treated with 1 or 10 mg gelatin tannate or saline, given once daily by gavage, starting day 3 of DSS and for 4 consecutive days. Mice were monitored for bodyweight changes compared to baseline and DAI, a score calculated on the base of presence of blood in faeces, consistency of faeces, and bodyweight loss. At the end of the experiment (day 9), animals were sacrificed, serum was collected, colonic length measured, and then colons were processed for frozen sampling and histology assessment. Faeces were collected and processed for direct culture in selected media just before starting experiments, at day 5, and at sacrifice. As a further control a group of mice received gelatin tannate by gavage at the same time points, but without DSS challenge. Five mice per group were evaluated in three repeated experiments.
Histological assessment
Colon was fixed in 4% formalin and embedded in paraffin. A well-established histological score was used for histological inflammatory assessment.24
Gut microbiota assessment
Fresh stool pellets were collected from mice from each group at days 0 and 5 and at the end of the experiment. The specimens were then weighed and homogenized in 1×phosphate-buffered saline (PBS) to normalize for dry weight of faeces. The homogenized samples were plated in 10-fold-diluted onto trypticase soy agar (Becton Dickinson) and onto MacConkey agar (BioMérieux). All plates were incubated at 37℃ for 24 h. The colonies were subsequently counted and the results are given as colony-forming units (CFU)/g dry matter. Single colonies were selected for characterization of bacteria by MALDI-TOF mass spectrometry (Bruker).
Two photon microscopy and Atomic force microscopy
At sacrifice, samples of colon were snap frozen. Then, they were analysed using a two-photon emission confocal microscopy in order to evaluate the thickness of the mucus layer. Moreover, we measured the elastic property of tissue by atomic force spectroscopy, as an indirect assessment of the presence and quality of mucus layer.
Elisa for LPS and peptidoglycan assessment
Murine serum was analysed in order to assess lipopolysaccharide (LPS; LAL Chromogenic Endpoint assay; Hycult Biotech) and peptidoglycan (human peptidoglycan ELISA kit; Cusabio Biotech) levels following manufacturer’s instructions. Spectrophotometric lecture at 450/540 or 579 nm was estimated by Spectramas Plus 384 (Molecular Devices). The SoftMax PRO program was used for data interpolation.
Statistical analysis
All statistical analyses were performed with the statistical software package STATA version 12.0 (Stata Corporation, College Station, TX, USA). Each result was calculated as the mean ± SEM. Statistical significances between paired and unpaired values were performed using the Student’s t-test. p < 0.05 were considered statistically significant.
Results
Gelatin tannate ameliorates DSS acute colitis in mice
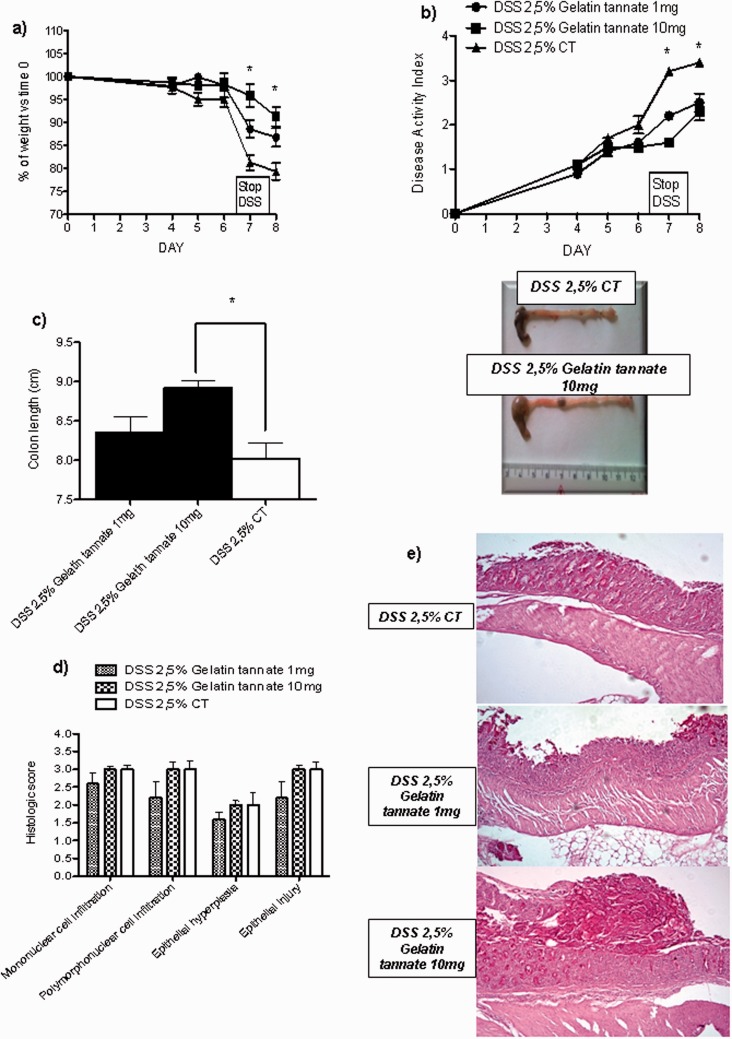
Gelatin tannate significantly reduced the DAI and the loss of bodyweight in treated mice compared to controls in a dose-dependent manner, being 10 mg more efficacious than 1 mg dose (both p < 0.05). No difference in mortality was observed. At sacrifice, colon length was measured and a histology assessment by a well-established histological score was performed. Gelatin tannate-treated mice showed a longer colon and a lower histological score, compared to controls (both p < 0.05). Finally, gelatine tannate did not significantly modify expression of MUC2 by real-time PCR (data not shown); however, its use was associated to the recovery of an amorphous layer overlaying the inflamed intestinal mucosa (Figure 1).
Figure 1.
Gelatine tannate ameliorates DSS colitis in mice.
Mice receiving 2.5% DSS in drinking water ad libitum were treated with 1 or 10 mg gelatin tannate or saline, given orally by gavage starting on day 3. Mice were monitored for bodyweight changes compared to baseline (a) and disease activity index (b). At sacrifice, colon length was measured (c) and histology assessment by a well-established score was performed (d). At microscopy (e), samples from gelatin tannate-treated mice showed an amorphous layer overlaying the inflamed mucosa probably due to the action of gelatin tannate.
Gelatin tannate modulates gut microbiota composition in stool samples in colitic mice
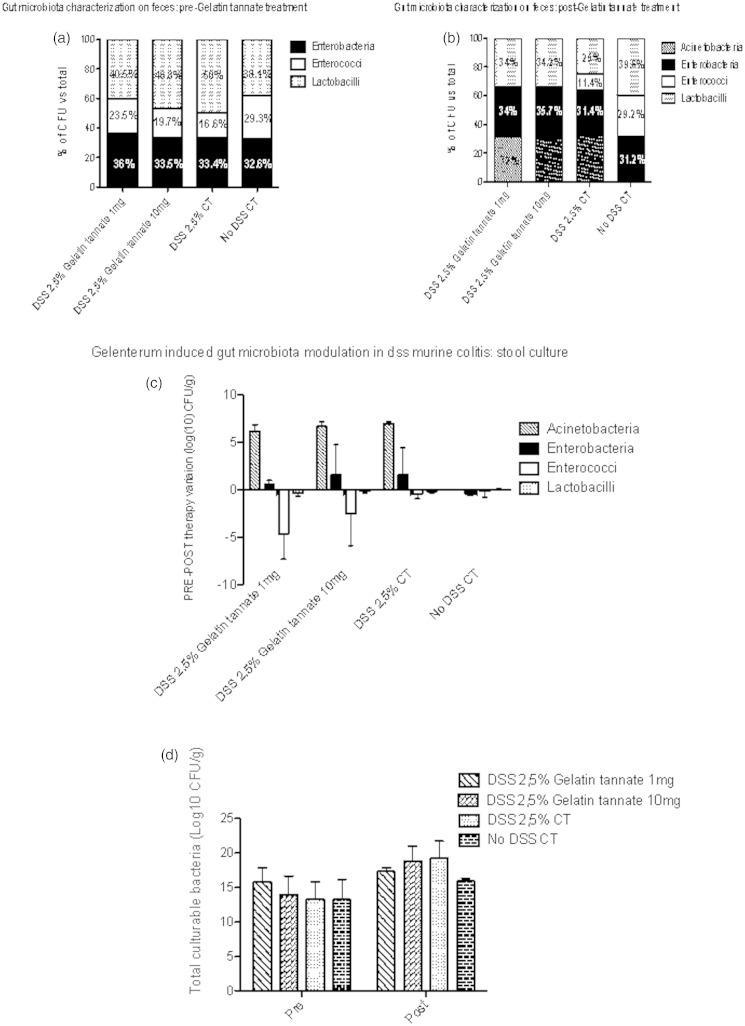
To evaluate the impact of gelatin tannate on gut microbiota of treated mice stool samples were cultured for microbiological analysis. There was a decrease in the amount of enterobacteria and enterococci in treated mice (p < 0.05). In DSS-treated mice we observed an increase of total culturable bacteria expressed as log10 CFU/g faeces. In particular, we noticed the presence of acinetobacteria, which, conversely, were absent in healthy mice (Figure 2).
Figure 2.
Gelatine tannate modulates gut microbiota composition in stool samples in colitic mice.
Stool samples from mice were collected before treatment with gelatin tannate (a) and at the end of the experiment (b) in all groups. Selective culture media were used for acinetobacteria, enterobacteria, enterococci, and lactobacilli. Number of bacteria are expressed as % of colony-forming units (CFU) on total number. Variation pre–post gelenterum is expressed as difference in log10 CFU (c). Total number of counted CFU in all groups, before and after treatment with gelatin tannate is also shown (d).Values are mean ± SEM.
Gelatin tannate preserves intestinal mucus layer appearance in mice
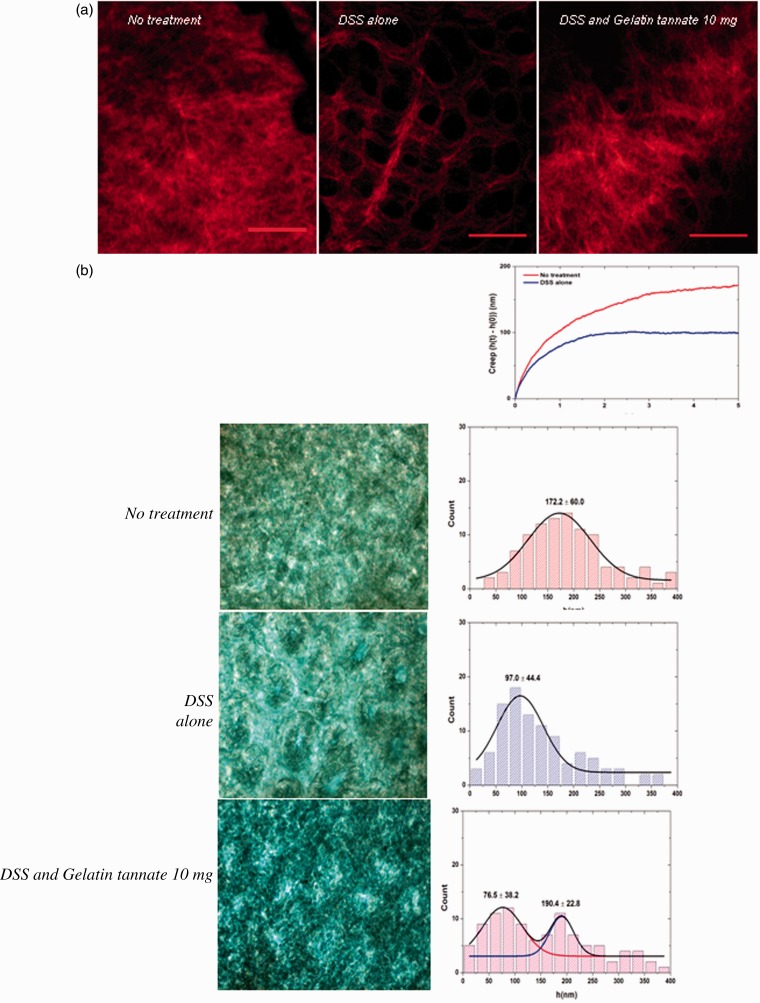
At confocal microscopy, intestinal samples from healthy and treated mice displayed similar structure in mucus layer thickness and composition, while samples from placebo group had no mucus layer or a thinner stratus. Healthy control mice treated only with gelatin tannate did not show any significant modification in mucus layer appearance. Atomic force microscopy confirmed these findings, by assessing the elastic property as an indirect assessment of the mucus layer. Colon samples from healthy and treated mice showed similar elastic property while samples from placebo group had a lower elasticity (Figure 3).
Figure 3.
Gelatin tannate preserves intestinal mucus layer appearance in mice.
At sacrifice, samples of colon were snap frozen. They were then analysed using a two-photon emission confocal microscopy (a) and atomic force spectroscopy (b).
Gelatin tannate reduces LPS levels in peripheral blood of colitic mice
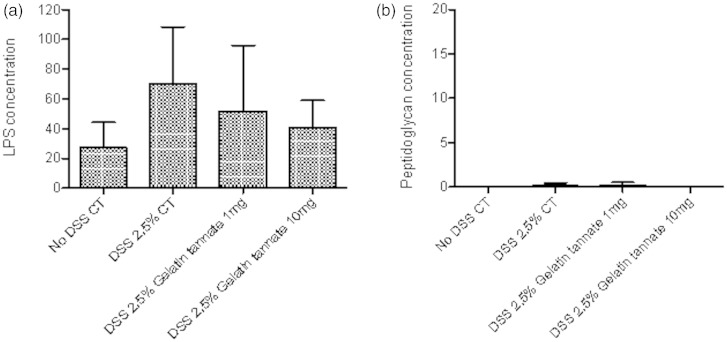
At sacrifice, serum samples were taken and then analysed for LPS and peptidoglycan content in all groups. Treated colitic mice showed lower blood LPS levels when compared to control, even if this reduction was not statistically significant. No difference was found in peptidoglycan level among groups (Figure 4).
Figure 4.
Gelatin tannate reduces LPS levels in peripheral blood of colitic mice.
At sacrifice, serum samples were taken and then analysed for LPS (a) and peptidoglycan (b) content in all groups.
Discussion
In the present study, we evaluated the therapeutic effect and mechanisms of action of gelatin tannate using the DSS-induced acute colitis mouse model. Gelatin tannate significantly reduced the disease activity index (DAI) and the loss of bodyweight in treated mice compared to controls in a dose-dependent manner, 10 mg being more efficacious than 1 mg. Neither difference in mortality was observed. At sacrifice, gelatin tannate-treated mice showed a longer colon and a lower histological score, compared to controls, both signs of a better outcome of colitis, although histology assessment did not differ significantly among treated groups. In addition to these findings, we assessed a modulation of the microflora composition, but mainly a restoring of mucus layer and perhaps, as suggested by the re-establishment of normal values of LPS in serum, of gut permeability.
Indeed, in specific culture, acinetobacteria, enterobacteria, enterococci, and lactobacilli grew at different concentration from stool as well as from intestinal mucosa (data not shown). Furthermore, acinetobacteria were clearly upregulated within the faeces of DSS colitic mice compared to noncolitic mice, and this paper is the first to report this finding.
Gelatin tannate-treated mice showed a lower concentration of enterobacteria and enterococci while no significant differences were found for the other strains. At confocal microscopy, intestinal samples from healthy and treated mice displayed a similar structure in mucus layer thickness and composition, while samples from placebo group had no mucus layer or a thinner stratus. Atomic force microscopy confirmed these findings, by assessing the elastic property as an indirect assessment of the mucus layer. Colon samples from healthy and treated mice showed similar elastic property while samples from placebo group had a lower elasticity. Finally, lower blood LPS levels in treated mice compared to controls further demonstrated the re-established shield activity of the mucus layer.
On the base of the experimental design, gelatin tannate has shown an ability to ameliorate acute colitis in mice, by reducing the direct damage due to DSS exposure and also promoting the early phase of recovery. In fact, mice started to receive gelatin tannate at the third day of DSS, when the colitis was already established.13 Despite the clinical improvement of colitis, the histological assessment did not correlate with the reduced bodyweight loss and disease activity index and did not show a significant difference between groups. Assessing whether gelatin tannate has a role in recovering from acute colitis in mice would require further investigation, which is beyond the aim of the present paper.
The mucus layer is the first physical barrier that bacteria meet within the intestinal tract. It consists of two layers: an inner layer, firmly adherent to the epithelial cells and about approximately 50 µm thick, and an outer layer, a nonattached layer that is usually approximately 100 µm thick in mouse.8 In physiological conditions, the sterile inner mucus layer is converted into the outer layer, which is the habitat of the commensal flora. These mucus layers are organized around the highly glycosylated MUC2 mucin. After secretion, the MUC2 mucin network is hydrated and expanded in volume and forms together with other secreted proteins, a well-organized, stratified inner mucus layer.25 This layer is dense, firmly attached to the epithelium and is insoluble in chaotropic salts.13 The protein composition is similar in these two mucus layers as formed from a common source of secreted material. The normal bacterial flora resides in the loose mucus, whereas the inner attached mucus is impervious to bacteria and functions as a protective filter for the epithelial cell surface.26 This compartmentalization seems to be fundamental for the homeostasis in the highly colonized colon. The importance of the mucus barrier was firstly demonstrated in Muc2−/− mice where bacteria are in direct contact with the epithelial cells and are also found deep in the crypts as well as inside epithelial cells. Break and loss of the barrier formed by the inner mucus layer trigger bacterial invasion, inflammation, and development of colon cancer, as shown by the DSS-induced colitis mouse model.9–11
In IBD patients, increased intestinal epithelial permeability precedes clinical relapse by as much as 1 year, suggesting that a permeability defect is an early event in disease exacerbation.27 The hypothesis that abnormal intestinal barrier function is a genetic trait involved in the pathogenesis of IBD is further supported by the observation that clinically asymptomatic first-degree relatives of Crohn’s disease patients may have increased intestinal permeability.27 While a primary defect of the intestinal barrier function may be involved in the early steps of the pathogenesis of IBD, the production of cytokines, including interferon γ and tumour necrosis factor α secondary to the inflammatory process, serve to perpetuate the increased intestinal permeability by reorganizing tight-junction proteins ZO-1, junctional adhesion molecule 1, occludin, and claudin 1 4.28 In this manner, a vicious cycle is created in which barrier dysfunction allows further leakage of luminal contents, thereby triggering an immune response that in turn promotes further leakiness.3
In this scenario, gelatin tannate, thanks to its chemical structure, seems to substitute mucus function impairment and re-establish the gut barrier permeability and homeostasis by acting as a gut barrier enhancer and modulating gut microbiota composition. This could explain the clinical and histological amelioration of acute colitis showed respectively by the improvement in bodyweight loss, DAI, and colon length and by the reduced histological inflammatory score. In DSS colitis, the disruption of the mucus biophysical structure allows commensal bacteria to penetrate the inner mucus layer and to activate immune cells. The abnormal response towards normally harmless bacteria leads to an inflammatory state and colitis. As indicated directly by two-photon microscopy and indirectly by atomic force microscopy, gelatin tannate acts as a ‘mucus-like’ shield for the damaged gut barrier, by creating a protective film over it. This reduces the bacterial leakage and allows the gut mucosal healing process to take place. Furthermore, it has been shown that gelatin tannate reduces the proinflammatory effects of LPS in human intestinal epithelial cells. It is able to inhibit the intercellular adhesion molecule-1 (ICAM-1) expression in LPS-stimulated Caco-2 cells29. ICAM-1 is induced on a wide variety of cells by inflammatory stimuli such as LPS. Together with this, adding gelatin tannate at different concentrations induces a dose-dependent inhibition of interleukin 8 and tumour necrosis factor α released by LPS-stimulated Caco-2 cells. Finally, tannic acid is a polyphenolic compound, which includes gallic acid that has been shown to exert potent antioxidant effects. We can speculate that the observed evidence could depend also on this important characteristic of gelatin tannate. On the base of these data, gelatin tannate could represent a helpful tool for blocking the vicious circle that is involved in the altered permeability and exacerbation of IBD.
One limitation of our study is that it was performed in an acute and not chronic model of colitis, which could better represent the pathophysiological alterations observed in IBD. Further investigations are needed to evaluate a possible therapeutic role of gelatin tannate in this disease. However, our model is well recognized for studying initial intestinal barrier impairment and the mechanism of mucosal healing; this study evaluates the beneficiary effect of intestinal permeability modulation, and its primary defect is an early and acute event in IBD exacerbation. Another limit of our study is that we evaluated, for methodological reasons, only four different bacterial families. It will be interesting in the future to assess a hypothetical complete modulation of gut microbiota.
Taken together, our data suggest that not only the microbiota modulation, but also the recovering of gut mucus layer in the course of acute colitis, can decrease the clinical disease severity in mice. The translational therapeutic implications of these concepts are obvious and open new horizons to novel, targeted, and more effective options for patients with colitis and impaired gut permeability.
Funding
This work was supported with an unrestricted grant by the Fondazione in Ricerca in Medicina, ONLUS, Bologna, Italy and the Centro di Ricerche Sperimentali, Catholic University of the Sacred Heart, Rome, Italy.
Conflict of interest
FS and and AG have been consultants for Acraft-Angelini, Ancona, Italy. The other authors declare no conflict of interest.
References
- 1. Scaldaferri F, Pizzoferrato M, Gerardi V, et al. The gut barrier: new acquisitions and therapeutic approaches. J Clin Gastroenterol 2012; 46(Suppl): S12–S17. [DOI] [PubMed] [Google Scholar]
- 2. Camilleri M, Madsen K, Spiller R, et al. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 2012; 24: 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol 2012; 42: 71–78. [DOI] [PubMed] [Google Scholar]
- 4. Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 2011; 91: 151–175. [DOI] [PubMed] [Google Scholar]
- 5. Bamias G, Corridoni D, Pizarro TT, et al. New insights into the dichotomous role of innate cytokines in gut homeostasis and inflammation. Cytokine 2012; 59: 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yacyshyn BR, Meddings JB. CD45RO expression on circulating CD19+B cells in Crohn’s disease correlates with intestinal permeability. Gastroenterology 1995; 108: 132–137. [DOI] [PubMed] [Google Scholar]
- 7. Schmitz H, Barmeyer C, Fromm M, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999; 116: 301–309. [DOI] [PubMed] [Google Scholar]
- 8. Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA 2011; 108(Suppl 1): 4659–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velcich A, Yang W, Heyer J, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002; 295: 1726–1729. [DOI] [PubMed] [Google Scholar]
- 10. Johansson ME, Phillipson M, Petersson J, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 2008; 105: 15064–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van der Sluis M, De Koning BA, De Bruijn AC, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006; 131: 117–129. [DOI] [PubMed] [Google Scholar]
- 12. Wirtz S, Neufert C, Weigmann B, et al. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2007; 2: 541–546. [DOI] [PubMed] [Google Scholar]
- 13. Johansson ME, Gustafsson JK, Sjoberg KE, et al. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One 2010; 5: e12238–e12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dai C, Zheng CQ, Meng FJ, et al. VSL#3 probiotics exerts the anti-inflammatory activity via PI3k/Akt and NF-kappaB pathway in rat model of DSS-induced colitis. Mol Cell Biochem 2013; 374: 1–11. [DOI] [PubMed] [Google Scholar]
- 15. Liu WS, Chen MC, Chiu KH, et al. Amelioration of dextran sodium sulfate-induced colitis in mice by Rhodobacter sphaeroides extract. Molecules 2012; 17: 13622–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong CC, Zhang L, Li ZJ, et al. Protective effects of cathelicidin-encoding Lactococcus lactis in murine ulcerative colitis. J Gastroenterol Hepatol 2012; 27: 1205–1212. [DOI] [PubMed] [Google Scholar]
- 17. Hakansson A, Branning C, Molin G, et al. Blueberry husks and probiotics attenuate colorectal inflammation and oncogenesis, and liver injuries in rats exposed to cycling DSS-treatment. PLoS One 2012; 7: e33510–e33510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garrido-Mesa N, Utrilla P, Comalada M, et al. The association of minocycline and the probiotic Escherichia coli Nissle 1917 results in an additive beneficial effect in a DSS model of reactivated colitis in mice. Biochem Pharmacol 2011; 82: 1891–1900. [DOI] [PubMed] [Google Scholar]
- 19. Huang TY, Chu HC, Lin YL, et al. Minocycline attenuates experimental colitis in mice by blocking expression of inducible nitric oxide synthase and matrix metalloproteinases. Toxicol Appl Pharmacol 2009; 237: 69–82. [DOI] [PubMed] [Google Scholar]
- 20. Esteban Carretero J, Durban Reguera F, Lopez-Argueta Alvarez S, et al. A comparative analysis of response to vs. ORS +gelatin tannate pediatric patients with acute diarrhea. Rev Esp Enferm Dig 2009; 101: 41–48. [DOI] [PubMed] [Google Scholar]
- 21. Grujic-Vasic J, Bosnic T, Jovanovic M. The examining of isolated tannins and their astringent effect. Planta Med 1986(6): 548–548. [DOI] [PubMed] [Google Scholar]
- 22. Bheemachari J AK, Joshi NH, Suresh DK, et al. Antidiarrheal evaluation of Ficus racemosa Linn. latex. Acta Pharm Sci 2007; 49: 133–138. [Google Scholar]
- 23. Souza SM, Aquino LC, Milach AC Jr, et al. Antiinflammatory and antiulcer properties of tannins from Myracrodruon urundeuva Allemao (Anacardiaceae) in rodents. Phytother Res 2007; 21: 220–225. [DOI] [PubMed] [Google Scholar]
- 24. Garrett WS, Gallini CA, Yatsunenko T, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe 2010; 8: 292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kitajima S, Takuma S, Morimoto M. Histological analysis of murine colitis induced by dextran sulfate sodium of different molecular weights. Exp Anim 2000; 49: 9–15. [DOI] [PubMed] [Google Scholar]
- 26. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009; 9: 799–809. [DOI] [PubMed] [Google Scholar]
- 27. Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut 2007; 56: 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang F, Schwarz BT, Graham WV, et al. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology 2006; 131: 1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frasca G, Cardile V, Puglia C, et al. Gelatin tannate reduces the proinflammatory effects of lipopolysaccharide in human intestinal epithelial cells. Clin Exp Gastroenterol 2012; 5: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]