Abstract
Introduction
Fructose can trigger or worsen symptoms in irritable bowel syndrome (IBS) patients. The aim of this study was to determine the prevalence of symptomatic fructose malabsorption in IBS patients and to test whether the patient's characteristics can help to detect a fructose malabsorption.
Materials and methods
Ninety Rome III IBS patients (predominant diarrhoea (IBS-D): 31%, predominant constipation (IBS-C): 18%, mixed type (IBS-M): 51%) were included prospectively. After exclusion of a small intestinal bacterial overgrowth by a glucose breath test, fructose malabsorption was assessed by a five-hour breath test, with symptom monitoring, after a 25 g load of fructose. An increase of more than 20 ppm of hydrogen (H2) or methane (CH4) levels in the exhaled air led to the diagnosis of malabsorption.
Results
Fructose test was abnormal in 20/90 patients among whom only 35% were intolerant, with a simultaneous rise of H2/CH4 levels and the onset of abdominal discomfort or diarrhoea. IBS characteristics were not predictive even if young (p = 0.031) and male IBS patients (p = 0.029) were at higher risk of malabsorption. At variance, 18 additional patients experienced intestinal symptoms during the test despite normal fructose absorption.
Discussion
After a 25 g fructose load, symptomatic fructose malabsorption and intolerance without malabsorption were detected in 22% and 28% of IBS patients respectively.
Keywords: Fructose malabsorption, irritable bowel syndrome, FODMAPs, breath tests
Introduction
Irritable bowel syndrome (IBS) is a chronic disorder characterised by recurrent abdominal pain or discomfort and transit disturbances which are worse during exacerbations of episodes of pain exacerbations.1 Depending on the type of transit disturbances, IBS can be categorised as predominant diarrhoea (IBS-D), predominant constipation (IBS-C), or mixed type (IBS-M). Current knowledge suggests that IBS is a multifactorial disorder, including visceral hypersensitivity of varying degrees, intestinal motor disturbances and brain-gut axis dysfunction. More recent research has also shown intestinal dysbiosis, altered intestinal permeability and low-grade intestinal inflammation.2 Stress, mood disorders and life events influence the patient’s capacity to cope with symptoms.3
A link between food intake and the occurrence or the exacerbation of symptom has been confirmed by prospective surveys4,5 while the deleterious role of some fermentable oligo-, di- and mono-saccharides or polyols (FODMAPs), particularly fructose, was recently highlighted6 while fructose is increasingly incorporated in beverages, dairy products and canned, baked or processed foods worldwide. When poorly absorbed, these carbohydrates are fermented and have an osmotic effect, promoting gas production and gut distension. Malabsorption is worsened by the concomitant intake of polyols. This relationship between FODMAP intake and symptom onset has led to dietary recommendations, including a significant reduction in fructose intake.
However, the prevalence of symptomatic fructose malabsorption in western European IBS patients remains poorly documented. Moreover, the percentage of IBS patients who experience abdominal symptoms in cases of malabsorption and the predictive value of IBS symptom pattern to detect symptomatic fructose malabsorption remain to be assessed.
The aims of this prospective study were to determine the prevalence of a symptomatic fructose malabsorption in IBS patients according to Rome III criteria, following a fructose load, and to assess whether particular clinical characteristics of these patients could be considered predictors of symptomatic fructose malabsorption.
Materials and methods
Patients
All consecutive patients diagnosed with IBS according to Rome III criteria1, without organic digestive disease or coeliac disease and referred to our Department for IBS management were included in the study. To meet the Rome III criteria, the patients must suffer from recurrent abdominal pain or discomfort at least 3 days per month in the last 3 months associated with 2 or more of the following: 1) improvement with defecation 2) onset associated with a change in frequency of stool 3) onset associated with a change in form (appearance) of stool.
Methods
Clinical assessment
When visiting our unit, all patients were asked to complete the Rome III questionnaire whereas IBS subtype was determined with the Bristol stool scale. Prior to the test, IBS severity was assessed by the IBS symptom severity score (IBS-SSS). In addition, a five-point Likert scale was used to assess symptom frequency. A score of 0 signified ‘no symptoms', 1 corresponded to ‘infrequent symptoms' (less than once a week), 2 to ‘relatively frequent symptoms' (at least once a week), 3 to ‘frequent symptoms' (several times a week) and 4 to ‘very frequent symptoms' (daily or almost daily). Patients were also asked about their abdominal tolerance to food with a high fructose content according to the list published by Gibson and Shepherd.6 No diet restrictions were proposed prior to considering the patient as a true IBS patient.
Levels of anxiety and/or depression were assessed using the hospital anxiety and depression scale (HAD),7 and the validated Gastrointestinal Quality of Life Index (GIQLI) assessed quality of life.8
Breath tests
Each patient underwent two breath tests. First, a glucose breath test was carried out in order to rule out small intestinal bacterial overgrowth (SIBO), which is a condition that may promote false positive results of sugar breath tests.9,10 If negative, a fructose test was then performed after a 25 g fructose load (10% solution). If the glucose breath test was positive, the patient was treated, and then underwent the fructose test if the glucose test was negative.
Before both breath-tests, patients were instructed to follow a diet for 48 h. This diet strictly excluded the following food products: bread, biscuits, cheese, fruits, vegetables, dairy products, fermented drinks, sodas, honey, marmalade, candy, chocolate, ice cream and pastry. Moreover, the evening before each test, patients were given a dinner exclusively composed of rice and meat which are rapidly absorbed. The aim of this regimen was to reduce intestinal gas production that could distort the forthcoming breath tests. In order to reduce the possible metabolic effects of oral flora,11 a careful mouth-wash with a 20% chlorexidine solution was performed just prior to the test and smoking and exercise were not allowed prior to or during the test.
End-alveolar breath samples were collected using a two-bag system consisting of a mouthpiece, a T-valve and two collapsible bags. The first bag collected the dead space air and the second bag the alveolar air. A breath sample was taken from the second bag using a 20 ml syringe and immediately analysed. The concentrations of carbon dioxide (CO2), hydrogen (H2) and methane (CH4) in the breath samples were determined simultaneously with a gas chromatograph (Quintron Breathtracker SC) and plotted graphically by a trained technician. The chromatograph was calibrated with a CO2, H2 and CH4 reference mixture in compressed air (Quingas). Desiccants in the drying tubes were changed if their colour changed. Results were expressed as parts per million (1 ppm = approximately 0.05 µmol/l for H2 and CH4).
Glucose breath test
Both H2 and CH4 were measured before the glucose load in order to verify compliance with the instructed diet. Then patients ingested 75 g of glucose dissolved in 250 ml of sterile water. End-alveolar breath samples of expired air were collected every 15 min for 2 h. The test was considered positive if one of the following results occurred: (a) peak of H2 or CH4 above 20 ppm, (b) increase of H2 or CH4 levels above 10 ppm in two samples by comparison with individual baseline levels, (c) baseline H2 or CH4 levels above 20 ppm despite good compliance with the diet. When the glucose breath test was positive, patients received an antibiotic treatment with quinolone or metronidazole for 10 days per month over two successive months. A second glucose breath test was then performed. The fructose breath test was only carried out in patients in whom the control glucose test was negative.
Fructose breath test
The fructose breath test was carried out using the same gas analyser as the glucose breath test. Patients ingested 25 g of fructose dissolved in 250 cc of sterile water then end alveolar breath samples were collected every 30 min for 5 h. Both H2 and CH4 levels were calculated. The test was considered positive and defined a fructose malabsorption in case of a rise of H2 and/or CH4 levels above 20 ppm.12 Moreover, during the test, symptoms such as abdominal pain, bloating, and diarrhoea were closely monitored and were collected by the laboratory technician every 30 min of the breath test. Only the occurrence of gastro-intestinal symptoms was then analysed and defined fructose intolerance but not fructose malabsorption. Patients were asked whether the symptoms occurring during the test reproduced their spontaneous IBS symptoms.
Statistics
A Chi square test was used for the qualitative analyses and Mann-Witney and Wilcoxon tests were used to analyse quantitative variables with Graphpad Prism 5 software. A univariate analysis was carried out with the results of the fructose breath test using Statview software. To determine the factors associated with a positive fructose breath test, a multivariate analysis using a step-by-step logistic regression was performed, with 95% confidence intervals and a level of significance of 0.05. The positive or negative result of the fructose breath test was defined as a dependent variable.
Results
Patients
Ninety consecutive IBS patients were included. Patients’ characteristics are reported in Table 1. Eighteen percent of patients had IBS-C, 31% IBS-D and 51% IBS-M. There were no differences for clinical characteristics between the three IBS sub-groups. In no case did the history of symptoms onset suggest a clear-cut association between the onset or the worsening of symptoms and the intake of nutrients with a high fructose content listed by Gibson and Shepherd.6,9
Table 1.
Population characteristics
| Characteristics | Population |
|---|---|
| Patients | 90 |
| Age (years) | 44.3 ± 1.7 |
| Weight (kg) | 70.4 ± 1.9 |
| Height (m) | 1.7 ± 0.01 |
| BMI | 25.3 ± 0.7 |
| Male gender | 19 (21.1%) |
| Gastroenteritis before occurrence of symptoms | 11 (12.2%) |
| Stress before symptoms occurred | 57 (63.3%) |
| IBS-SSS | 285.0 ± 9.9 |
| HADS anxiety | 9.2 ± 0.5 |
| HADS depression | 4.8 ± 0.4 |
| Frequent abdominal pain | 37 (41.1%) |
| Frequent bloating | 53 (58.9%) |
| Frequent diarrhoea | 28 (31.1%) |
| Frequent constipation | 16 (17.8%) |
| GIQLI score | 81.8 ± 2.4 |
| Fructose intolerance | 27 (30.0%) |
BMI: body mass index; IBS: irritable bowel syndrome; IBS-SSS: IBS-symptom severity score; HADS: Hospital Anxiety Depression Score; GIQLI: Gastrointestinal Quality of Life Index.
Symptoms were considered as frequent with VAS (Visual Analogue Scale) 3–4 on Likert scale (moderate to severe symptoms). Results are presented as mean ± standard error of the mean and number (percentage).
All patients had a negative glucose test except one in whom the glucose test was initially positive then became negative after two months of a sequential antibiotic treatment. This patient then underwent the fructose test.
Fructose breath test
The fructose test was positive in 20 patients (22%). In all of these patients, the test was positive due to an increase in H2 while no CH4 increase occurred in any patient. Among these 20 patients, H2 levels higher than 20 ppm in the end-alveolar samples were observed after a mean delay of 80 min while the mean delay for the H2 peak onset was 114 min. Among patients with a positive fructose breath test, 25% were IBS-C patients, 30% were IBS-D and 45% were IBS-M.
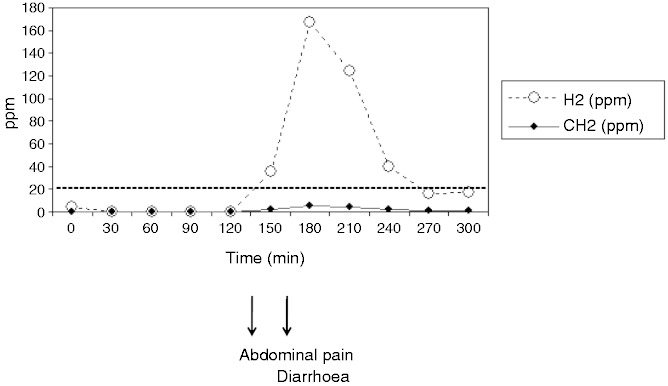
Figure 1 is an example of the result of a five-hour breath test. Twenty-five patients (27.8%) experienced symptoms during the test and were considered as fructose intolerant. In most cases, symptoms were the same as the spontaneous symptoms leading to patients seeking medical advice. Thirty-five percent of the patients with an abnormal fructose breath test had symptoms during the test with an abdominal pain in 89%, a diarrhoea in 44% while an objective abdominal distension was observed in 11%. In contrast, 18 patients (26%) with a normal fructose breath test had abdominal symptoms after fructose ingestion. These symptoms included abdominal pain in 11/18 (61%), diarrhoea in 10/18 (56%) and bloating in 3/18 (17%). In these 18 cases of poor tolerance to fructose without malabsorption, symptoms occurred after a mean delay of 62 min after fructose intake, but their early onset within the first 30 min of the test was reported by 44% of the patients.
Figure 1.
Example of the result of a five-hour breath test. H2: hydrogen; CH4: methane.
There were no significant differences for either prevalence or pattern of symptoms between patients with positive or negative fructose breath tests. In particular, there was no difference on the time of symptom occurrence during the test.
Comparison of patient characteristics as a function of fructose breath test result according to univariate and multivariate analyses
Characteristics of patients with positive and negative fructose breath tests are shown in Table 2. The univariate analysis showed that patients with symptomatic fructose malabsorption were younger (p = 0.031), and more often males (p = 0.029) compared to those without fructose malabsorption according to the breath test. There was no other characteristic significantly different between the two groups.
Table 2.
Univariate analysis of conditions and symptoms associated with the detection of a fructose malabsorption by a fructose breath test after a 25 g fructose load
| Characteristics | Fructose absorbers (n = 70) | Fructose malabsorbers (n = 20) | p |
|---|---|---|---|
| Age (years) | 46.3 ± 2.0 | 37.3 ± 2.9 | 0.03 |
| Weight (kg) | 70.0 ± 2.3 | 71.6 ± 2.9 | 0.72 |
| Height (m) | 1.7 ± 0.01 | 1.7 ± 0.02 | 0.02 |
| BMI | 25.5 ± 0.8 | 24.8 ± 1.1 | 0.66 |
| Male gender | 11 (15.7%) | 8 (40.0%) | 0.03 |
| Acute gastroenteritis prior symptoms | 8 (11.4%) | 3 (15.0%) | 0.70 |
| Stress before symptoms | 46 (65.7%) | 11 (55.0%) | 0.70 |
| IBS-SSS | 281.0 ± 11.8 | 304.0 ± 16.2 | 0.36 |
| HADS anxiety | 9.2 ± 0.5 | 9.3 ± 1.0 | 0.90 |
| HADS depression | 4.6 ± 0.4 | 5.3 ± 0.8 | 0.40 |
| Frequent abdominal pain | 27 (38.6%) | 10 (50.0%) | 0.44 |
| Frequent bloating | 41 (58.6%) | 12 (60.0%) | 1.00 |
| Frequent diarrhoea | 22 (31.4%) | 6 (30.0%) | 1.00 |
| Frequent constipation | 11 (15.7%) | 5 (25.0%) | 0.34 |
| GIQLI Score | 83.2 ± 2.5 | 77.1 ± 5.9 | 0.29 |
| Fructose intolerance | 18 (25.7%) | 7 (35.0%) | 0.11 |
BMI: body mass index; IBS: irritable bowel syndrome; IBS-SSS: IBS-symptom severity score; HADS: Hospital Anxiety Depression Score; GIQLI: Gastrointestinal Quality of Life Index.
Symptoms were considered frequent when patients scored their frequency 3 or 4 on a Likert scale. Results are presented as mean ± standard error of the mean and number (percentage).
All characteristics with a p-value <0.03 were included in the multivariate analysis (Table 3). Gender, age, symptoms during the test and the GIQLI score were all included. Height was not included in the logistic regression model because it co-varied with gender (p < 0.0001). The results of the multivariate analysis showed that only gender (p = 0.04) and age (p = 0.009) were associated with positive fructose breath test.
Table 3.
Multivariate analysis of conditions and symptoms associated with a positive fructose breath test after a 25 g fructose load
| Variable | Odds ratio (95% CI) | p value |
|---|---|---|
| Age | −2.59 (0.90–0.97) | 0.009 |
| Gender | 2.01 (1.03–11.96) | 0.04 |
| Symptoms during the test | 1.07 (0.59–5.87) | 0.28 |
| GIQLI | −1.76 (0.95–1.00) | 0.08 |
CI: confidence interval; GIQLI: Gastrointestinal Quality of Life Index.
Correlation between the results of the fructose test and the anxiety and depression levels
Fructose intolerance was neither more frequent in IBS patients with abnormal anxiety or depression levels (p = 0.29 and p = 0.7532) (Tables 4 and 5).
Table 4.
Relationship between anxiety and fructose intolerance
| Fructose intolerance | Normal HADS (anxiety score) | Abnormal HADS (anxiety score) |
|---|---|---|
| Yes | 18 | 7 |
| No | 39 | 26 |
HADS: Hospital Anxiety Depression Score. Anxiety was pathological when the overall score of the seven questions testing anxiety was equal or higher than 11/21.
Table 5.
Relationship between depression and fructose intolerance
| Fructose intolerance | Normal HADS (depression score) | Abnormal HADS (depression score) |
|---|---|---|
| Yes | 23 | 2 |
| No | 61 | 4 |
HADS: Hospital Anxiety Depression Score. Depression was pathological when the overall score of the seven questions testing depression was equal or higher than 11/21.
Discussion
The dietary recommendation to avoid or at least to reduce the amount of fructose within the diet appears logical for IBS patients with a demonstrated symptomatic fructose malabsorption associated with the onset of intestinal symptoms after fructose intake.
The first aim of this prospective study was to assess the prevalence of a symptomatic fructose malabsorption in all types of IBS patients fulfilling Rome III criteria. Therefore, the experimental protocol was carefully designed to avoid any methodological bias which could interfere with the interpretation of the test. First, we carefully excluded IBS patients with SIBO, a pathological condition during which the colonic bacteria population migrates proximally into the small intestine, gaining access to sugars. This shift in fermentation might lead to a abnormal breath test.13 The second major methodological point was the limitation of the fructose intake to 25 g. Indeed, several recent studies have highlighted that 25 g was the highest amount of fructose absorbed by healthy volunteers.14,15 According to these studies, a fructose load of 50 g induces false positive results in 40–60% of cases.14,15 The collection of breath samples was carried out according to previous methodological recommendations in order to ensure end-alveolar breath samples.16 We measured both H2 and CH4 levels as it has been reported that false negative results occur in 14% of cases when CH4 is not measured.17,18
Nevertheless, one limitation of this study was the absence of demonstration of production of H2 or CH4 with lactulose prior to patient inclusion in the study. However, lactulose does not seem to be a good test to define a non-hydrogen producer.19 We used a five-hour test duration that allowed for increasing the prevalence of fructose malabsorption by 10%. Wilder-Smith et al.20 have underlined the importance of a five-hour test duration. In their data, they would have missed 16% of fructose intolerance with only a three-hour duration test.
Another potential limitation of this study is the use of a glucose breath test for SIBO detection. The gold standard for the diagnosis of SIBO remains the culture of small bowel aspirates,13 even if such aspirates cannot detect a bacterial overgrowth in the distal jejunum or in the proximal ileum. The main reported disadvantage of the sugar breath test, particularly when lactulose is used, is an overestimation of the prevalence of SIBO. However, in the present study, we found only one abnormal glucose breath test in this sample of 90 patients.13 This low prevalence of small intestinal bacterial overgrowth is in accordance with the results of Posserud et al., reporting a 4% prevalence with the results of small bowel aspirate cultures.13 The ingestion of fructose alone during the test could be criticised. However, one could argue that simultaneous consumption of glucose and fructose during the test resembles normal food intake and thus more likely reflects conditions in daily life. In addition, fructose malabsorption appears to be partially modulated by the amount of glucose concomitantly ingested and the fructose/glucose ratio of a meal. Indeed glucose reduces fructose malabsorption via the upregulation of the facilitative transporter Glucose Transporter 2 (GLUT2).21
Our first result is that a fructose malabsorption was observed in 22% of this sample of IBS patients, without any differences according to the IBS sub-type. This result is significantly lower than the figures of the major Wilder-Smith et al.’s study20 or that of the Barrett et al. trial22 in which the patients were tested with 35 g of fructose. In fact Barret et al. did not report a different prevalence of fructose malabsorption between IBS patients (45%) and healthy volunteers (34%)22 after 35 g of fructose intake. Moreover, in the Wilder-Smith et al. study, fructose malabsorption was defined as an increase of >20 ppm for H2 and >10 ppm for CH4, over baseline, in succession. Usually an increase of >20 ppm is used for CH4 levels.12 This methodological difference could also explain the higher fructose malabsorption prevalence in Wilder-Smith et al.'s study.20
Our second aim was to assess the percentage of IBS patients with symptomatic fructose malabsorption. One important result of our study was to demonstrate the lack of correlation between the onset of symptoms during the test and impaired fructose absorption: less than half of the malabsorbers were intolerant to fructose. This point is very relevant for clinical practice, reinforcing the need for a diagnostic test including a concomitant symptoms analysis prior to discussing an exclusion diet. In these patients with both intolerance and malabsorption, symptoms always occurred when H2 levels rose above 20 ppm or at the peak of H2 rise. Therefore, we can speculate that in these patients, the symptom onset is related to the malabsorption.
Furthermore, 18 patients, without malabsorption, always experienced symptoms during the first hour of the test. The occurrence of symptoms during a sugar test is not rare in patients with functional gastro-intestinal disorders, even in the absence of sugar malabsorption. For instance, several studies based on the lactose breath test have demonstrated the lack of a relationship between no lactase and the onset of abdominal symptoms.23,24 In our patients who were intolerant to fructose, one possible explanation could be that the symptoms were triggered by other gases (such as CO2) which could not be detected with the device we used. Symptoms could be also promoted by visceral hypersensitivity and/or be related to the osmotic effect of fructose rather than the fermentation process leading to H2 or CH4 production. Tolerance to the sugar is partly conditioned by gastric emptying, a key factor for the progressive arrival of fructose in the small intestine. The early onset of symptoms in these intolerant patients could be explained by an osmotic effect in relation to gastric emptying.
Our last aim was to determine whether a particular profile of fructose malabsorber did exist. None of the clinical symptoms were predictive of fructose malabsorption and the patients with symptomatic fructose malabsorption did not show a more altered psychological profile. However, in this study, young male IBS patients had a greater incidence of fructose malabsorption. Therefore, a breath test could be particularly indicated for the diagnosis of fructose malabsorption in these patients.
To conclude, our study demonstrates that the prevalence of symptomatic malabsorption of a 25 g fructose load was 22% in IBS patients and lower than that previously reported. Moreover, this 25 g load of fructose triggered bothersome intestinal symptoms in 28% of the patients who were not always malabsorbers. Different mechanisms are implicated in a poor tolerance to fructose. Therefore, further studies are now warranted to determine whether a low-fructose regimen is effective in intolerant to fructose IBS patients, with and without malabsorption.
Acknowledgements
Contributions to the study: G Gourcerol, AM Leroi and P Ducrotté designed the research study. C Melchior, G Gourcerol, AM Leroi and P Ducrotté performed the research. C Melchior, G Gourcerol, AM Leroi and P Ducrotté analysed the data. C Melchior, G Gourcerol, AM Leroi and P Ducrotté wrote the paper. The authors are indebted to Gregory Mosni for technical help and to Richard Medeiros, Rouen University Hospital Medical Editor, for editing the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 2. Ford AC, Talley NJ. IBS in 2010: Advances in pathophysiology, diagnosis and treatment. Nat Rev Gastroenterol Hepatol 2010; 8: 76–78. [DOI] [PubMed] [Google Scholar]
- 3. Choung RS, Locke GR, 3rd, Zinsmeister AR, et al. Psychosocial distress and somatic symptoms in community subjects with irritable bowel syndrome: A psychological component is the rule. Am J Gastroenterol 2009; 104: 1772–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ragnarsson G, Bodemar G. Pain is temporally related to eating but not to defaecation in the irritable bowel syndrome (IBS). Patients' description of diarrhea, constipation and symptom variation during a prospective 6-week study. Eur J Gastroenterol Hepatol 1998; 10: 415–421. [DOI] [PubMed] [Google Scholar]
- 5. Shepherd SJ, Parker FC, Muir JG, et al. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: Randomized placebo-controlled evidence. Clin Gastroenterol Hepatol 2008; 6: 765–771. [DOI] [PubMed] [Google Scholar]
- 6. Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol 2010; 25: 252–258. [DOI] [PubMed] [Google Scholar]
- 7. Cho HS, Park JM, Lim CH, et al. Anxiety, depression and quality of life in patients with irritable bowel syndrome. Gut Liver 2011; 5: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Slim K, Bousquet J, Kwiatkowski F, et al. [First validation of the French version of the Gastrointestinal Quality of Life Index (GIQLI)]. Gastroenterol Clin Biol 1999; 23: 25–31. [PubMed] [Google Scholar]
- 9. Gibson PR, Newnham E, Barrett JS, et al. Review article: Fructose malabsorption and the bigger picture. Aliment Pharmacol Ther 2007; 25: 349–363. [DOI] [PubMed] [Google Scholar]
- 10. Nucera G, Gabrielli M, Lupascu A, et al. Abnormal breath tests to lactose, fructose and sorbitol in irritable bowel syndrome may be explained by small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2005; 21: 1391–1395. [DOI] [PubMed] [Google Scholar]
- 11. Thompson DG, O'Brien JD, Hardie JM. Influence of the oropharyngeal microflora on the measurement of exhaled breath hydrogen. Gastroenterology 1986; 91: 853–860. [DOI] [PubMed] [Google Scholar]
- 12. Romagnuolo J, Schiller D, Bailey RJ. Using breath tests wisely in a gastroenterology practice: An evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol 2002; 97: 1113–1126. [DOI] [PubMed] [Google Scholar]
- 13. Posserud I, Stotzer PO, Bjornsson ES, et al. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 2007; 56: 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frieling T, Kuhlbusch-Zicklam R, Kalde S, et al. Fructose malabsorption: How much fructose can a healthy subject tolerate? Digestion 2011; 84: 269–272. [DOI] [PubMed] [Google Scholar]
- 15. Rao SS, Attaluri A, Anderson L, et al. Ability of the normal human small intestine to absorb fructose: Evaluation by breath testing. Clin Gastroenterol Hepatol 2007; 5: 959–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol 2000; 95: 3503–3506. [DOI] [PubMed] [Google Scholar]
- 17. Knudsen CD, Di Palma JA. Carbohydrate challenge tests: Do you need to measure methane? South Med J 2012; 105: 251–253. [DOI] [PubMed] [Google Scholar]
- 18. De Lacy Costello BP, Ledochowski M, Ratcliffe NM. The importance of methane breath testing: A review. J Breath Res 2013; 7: 024001–024001. [DOI] [PubMed] [Google Scholar]
- 19. Bate JP, Irving PM, Barrett JS, et al. Benefits of breath hydrogen testing after lactulose administration in analysing carbohydrate malabsorption. Eur J Gastroenterol Hepatol 2010; 22: 318–326. [DOI] [PubMed] [Google Scholar]
- 20. Wilder-Smith CH, Materna A, Wermelinger C, et al. Fructose and lactose intolerance and malabsorption testing: The relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther 2013; 37: 1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones HF, Ross N, Butler RN, et al. Intestinal fructose transport and malabsorption in humans. Am J Physiol Gastrointest Liver Physiol 2011; 300: G202–G206. [DOI] [PubMed] [Google Scholar]
- 22. Barrett JS, Irving PM, Shepherd SJ, et al. Comparison of the prevalence of fructose and lactose malabsorption across chronic intestinal disorders. Aliment Pharmacol Ther 2009; 30: 165–174. [DOI] [PubMed] [Google Scholar]
- 23. Tomba C, Baldassarri A, Coletta M, et al. Is the subjective perception of lactose intolerance influenced by the psychological profile? Aliment Pharmacol Ther 2012; 36: 660–669. [DOI] [PubMed] [Google Scholar]
- 24. Yang J, Deng Y, Chu H, et al. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2012; 11: 262–268. [DOI] [PubMed] [Google Scholar]