Abstract
Background
Oesophageal adenocarcinoma (OAC) incidence is rising rapidly and prognosis remains poor. Endoscopic surveillance of Barrett’s oesophagus (BO) remains controversial.
Objective
A nested case–control study was undertaken to evaluate risk factors for progression of BO to OAC, potentially guiding surveillance efforts.
Methods
The Health Improvement Network database includes general practitioner consultations from 5 million UK subjects. BO subjects with 1-year minimum of follow up were followed until development of OAC or end of time on database. Demographic variables (age, gender, smoking, body mass index) and data on medication considered negatively (aspirin/nonsteroidal anti-inflammatory drugs/proton pump inhibitors) or positively associated (lower oesophageal sphincter-relaxing and asthma drugs) with OAC development were studied. Cox regression analysis-derived hazard ratios with 95% confidence intervals estimated the relative risk for OAC progression.
Results
A total of 3749 BO subjects were studied: 55 developed OAC during 17,743 patient years of follow up, a progression rate of 0.3% per annum. There was 96.7% of the cohort who took proton-pump inhibitors, with no association observed. Increasing age (1.03, 95% CI 1.01–1.05, p = 0.005), male gender (3.06, 95% CI 1.50–6.24, p = 0.002), and having ever smoked (2.36, 95% CI 1.13–4.93, p = 0.023) were associated with progression to OAC, (although smoking lost association on multivariate analysis). Increasing number of drugs used for asthma (2.91, 95% CI 1.10–7.68, p= 0.0314) was also associated.
Conclusion
In this nested case–control study of BO, male gender, increasing age, and increasing use of asthma drugs were associated with progression to OAC.
Keywords: Asthma, Barrett’s oesophagus, lower oesophageal sphincter-relaxing drugs, male gender, oesophageal adenocarcinoma
Introduction
The incidence of oesophageal adenocarcinoma (OAC) is rapidly rising across the Western world, particularly in the UK,1 which has the highest incidence in the world.2 The 5-year survival of patients developing OAC remains dismal at 12%.3 Barrett’s oesophagus (BO), a sequela of chronic gastro-oesophageal reflux disease (GORD), is a premalignant precursor of OAC. Endoscopic surveillance of BO has therefore been widely adopted. The annual risk of progression from BO to OAC has been reported to be between 0.2–2%.4,5 However, a more recent study suggested this was an overestimate, with the annual progression rate among 11,028 BO patients reported to be 0.12%.6 Since the annual OAC progression rate is a key factor in determining the cost effectiveness of BO endoscopic surveillance in mathematical models,7 the value of surveillance has been questioned.
Case–control studies of OAC have identified a number of associations, but studies of the risk factors associated with the progression of BO to OAC are limited by the inherent selection bias involved in studying subjects undergoing endoscopic surveillance rather than unselected cohorts.8,9 An unselected study of risk factors for progression to OAC among BO patients has not been previously performed in the UK.
We have therefore examined a retrospective cohort of BO subjects from a UK primary care database and examined risk factors for the development of oesophageal cancer (OC) to potentially guide surveillance efforts and suggest other interventions to lower the risk of OAC in BO.
Methods
The Health Improvement Network
BO subjects were identified from The Health Improvement Network (THIN) database. THIN database contains computerized and anonymized longitudinal records from 326 UK general practice (GP) surgeries, covering 5 million patients that are regionally and demographically representative of the UK population (http://csdmruk.cedegim.com/).. One of the strengths of the database lies in the computerized prescribing practices of the GPs involved, ensuring that all drugs prescribed will be recorded. Demographic and lifestyle information (e.g. sex, age, smoking status, height, and weight) are subject to Quality and Outcomes Framework criteria, ensuring a very high level of data entry. Socioeconomic status was derived at the postcode level (Townsend score, 2001 census data) and recorded in quintiles.
Subjects
BO subjects with a minimum of 1 year of follow up, and, when applicable, a minimum of 1 year between a diagnosis of BO and OC were included. This design aims to prevent confounding from a simultaneous diagnosis of OAC and BO. Study subjects were followed from the first coded entry of BO/Barrett’s ulcer until end of database registration, death, end of data capture, or diagnosis of OC. Those subjects developing OC became the cases and those who did not formed the control group. The earliest codings of BO were recorded in 1988 and the latest in 2004, with end of data capture in 2006.
Data extracted
Patient demographics (sex, age, period of BO follow up, proportion developing OC, socioeconomic status, body mass index (BMI) and smoking status) were extracted. Both smoking status and BMI data were extracted from closest to the first recorded entry code for BO, avoiding confounding from subjects’ lifestyle changing as a result of a diagnosis of a potential premalignant condition.
Use of drugs, by class, was initially examined as having ever or never been prescribed. In subjects developing OC, drug prescription was censored from examination 1 year before first coding of OC to prevent confounding from drugs that may have been prescribed for cancer symptoms. Drug classes examined include those potentially negatively associated with OAC (aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), proton-pump inhibitors (PPIs), statins, and angiotensin-converting enzyme inhibitors (ACE-Is)), and those potentially implicated in the aetiology of OAC by their side effect of relaxation of the lower oesophageal sphincter (LOS; tricyclic antidepressants/anticholinergics, benzodiazepines, calcium-channel antagonists, β-agonists, nitrates, and theophyllines), and those used for asthma/chronic asthma including inhaled steroids.
Oesophageal cancer verification and morphology
As the database does not record OC morphology, verification of OC and subtype was sought. The ‘free text entry’ of the THIN database was examined for each OC patient, and enquiries were made of the subjects’ GPs or by death certification (anonymized process through EPIC-UK) to verify the morphology. Cases proven to be squamous cell carcinoma were excluded.
Ethical approval was obtained (London MREC: 06/MRE02/93).
Statistical methods
SPSS version 19.0.0 (IBM, USA) was used to perform Cox regression analysis, deriving hazard ratios (HR) with 95% confidence intervals with subsequent correction for age, gender, and smoking, to evaluate the association of risk factors for the development of OAC. The potential interaction between age and gender was examined.
To establish for dose–response relationships, the number of scripts prescribed during the time period on the database was extracted, providing prescription density. Prescription density data was separated into quintiles and further Cox regression analysis performed. Cumulative use of both drugs associated with LOS relaxation and drugs used to treat asthma, including inhaled β-agonists, inhaled steroids, combined steroid and β-agonist inhalers, and oral theophyllines were also examined using Cox regression analysis, comparing by categories and trend across the categories.
Results
Subjects and validation
A total of 3752 subjects with BO were eligible for inclusion, following exclusion of 34 subjects with less than 1 year of follow up or those developing OC within 1 year of the first BO coding.
Of the 58 identified OC subjects, 53 (91%) were successfully verified as having OC. Of the 53 verified with OC, 38 free-text entries were available, confirming 31 cases of adenocarcinoma. In the remaining seven free-text entries, two received chemotherapy consistent with treatment of OAC, one poorly differentiated, one small cell cancer (a published complication in BO),10 and three squamous cell carcinoma (excluded). Examination of 16 death certificates confirmed the presence of OC in 15 (one revealing OAC). Six subjects with free-text comments were also (dual) assessed via death certification, with no contradicting information. Fifteen enquiries were made to general practitioners via EPIC-UK: nine confirmed adenocarcinoma, five cancer/carcinoma, and one did not respond.
Oesophageal cancer risk
With exclusion of three cases of squamous call carcinoma after the verification process, 3749 subjects with BO were included, providing 17,743 subject years of follow up (median 4 years (interquartile range (IQR) 3–6 years). The median age was 63 years (IQR 52–72) years, and 2731 were male (63%). Fifty-five subjects developed OAC, a progression rate of 0.3% per annum.
Risk factors for oesophageal cancer
Table 1 shows the demographic characteristics, lifestyle factors, and drug use within the study period among BO subjects who did and did not progress to OAC.
Table 1.
Demographic characteristics, lifestyle factors, and drug use in subjects with Barrett’s oesophagus who developed oesophageal adenocarcinoma and those who did not
| Barrett’s oesophagus without progression (n = 3694) | Barrett’s oesophagus progressing to oesophageal adenocarcinoma (n = 55) | |
|---|---|---|
| Age | 63 (52–72) | 67 (59–73) |
| Male | 2325 (63) | 46 (84) |
| Body mass index | 25.9 (23.7–28.3) | 25.8 (23.7–28.5) |
| Smoking (ever) | 2037 (55) | 33 (60) |
| PPI | 3569 (97) | 55 (100) |
| Aspirin | 1194 (32) | 19 (35) |
| NSAID | 2526 (68) | 38 (69) |
| COX-2 | 408 (11) | 3 (5) |
| Statin | 1124 (30) | 18 (33) |
| ACE-I | 1067 (29) | 18 (33) |
| Nitrates | 776 (21) | 19 (35) |
| Inhaled β‐agonist | 1080 (29) | 22 (40) |
| Inhaled steroids | 702 (19) | 18 (33) |
| Inhaled β‐agonist and steroids | 259 (7) | 8 (15) |
| Theophylline | 161 (4) | 6 (11) |
Values are median (interquartile range) or n (%).
ACE-I, angiotensin-converting enzyme inhibitor; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton-pump inhibitor.
Demographic and lifestyle factors
Table 2 shows the results of univariate and multivariate analyses for factors associated with progression to OAC, initially correcting for age and gender and then also smoking status. Male gender was associated with progression to OAC (HR 3.06, 95% CI 1.50–6.24, p = 0.002), with 84% of those developing OAC compared with 63% of those remaining with BO. Increasing age (HR (for each year: 1.03, 95% CI 1.01–1.05, p = 0.005) was associated with developing OAC, with a median age of 67 years (IQR 59–73 years) among those developing OC, compared with a median age of 63 years (IQR 52–72 years) among those who did not progress. No interaction was identified between age and gender (data not shown).
Table 2.
Estimation of risk of developing oesophageal adenocarcinoma from Barrett’s oesophagus on univariate and multivariate analysis
| Univariate analysis |
Corrected for age and gender |
Corrected for age, gender, and smoking |
||||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Increasing age | 1.03 (1.01–1.05) | 0.005 | 1.04 (1.02–1.06) | <0.0001 | – | – |
| Male | 3.06 (1.50–6.24) | 0.002 | 3.80 (1.84–7.84) | <0.0001 | – | – |
| Smoking status (ever vs. never) | 2.36 (1.13–4.93) | 0.023 | 1.99 (0.94–4.19) | 0.071 | – | – |
| Increasing body mass index (kg m−2) | 0.97 (0.91–1.04) | NS | 0.99 (0.92–1.06) | NS | 0.97 (0.90–1.06)* | NS |
| Aspirin | 1.08 (0.62–1.89) | NS | 0.81 (0.46–1.43) | NS | 0.73 (0.38–1.41)* | NS |
| NSAIDs | 1.02 (0.58–1.81) | NS | 0.89 (0.50–1.59) | NS | 0.69 (0.37–1.31)* | NS |
| COX-2 inhibitors | 0.46 (0.14–1.47) | NS | 0.49 (0.15–1.56) | NS | 0.61 (0.19–1.96)* | NS |
| Statin | 1.04 (0.59–1.82) | NS | 0.94 (0.53–1.65) | NS | 0.82 (0.43–1.56)* | NS |
| Nitrates | 1.76 (1.01–3.08) | 0.046 | 1.47 (0.84–2.57) | 0.18 | 1.01 (0.51–1.98)* | NS |
| Inhaled β‐agonist | 1.51 (0.88–2.59) | NS | 1.53 (0.89–2.62) | NS | 1.27 (0.68–2.38)* | NS |
| Inhaled steroids | 1.95 (1.11–3.42) | 0.02 | 2.00 (1.14–3.51) | 0.016 | 2.11 (1.12–3.97) | 0.021 |
| Inhaled β‐agonist and steroids | 2.20 (1.04–4.65) | 0.04 | 2.11 (1.00–4.46) | 0.051 | 2.54 (1.17–5.51) | 0.018 |
| Theophyllines | 2.52 (1.07–5.89) | 0.034 | 2.16 (0.92–5.08) | 0.077 | 2.31 (0.90–5.93) | 0.082 |
NS: p > 0.1, not significant.
Smoking status was not recorded in 333 subjects (8.8%): 320 of the group who did not develop OAC (8.7%) and 13 of the OAC group (23.6%). There were 2037 (55%) in the BO-only group and 33 (60%) in the OAC group who had ever smoked. Having smoked doubled the risk for progression to OAC on univariate analysis (HR 2.36, 95% CI 1.13–4.93, p = 0.023), but there was no significant association when corrected for age and gender (HR 1.99, 95% CI 0.94–4.19, p = 0.07).
BMI data was not available from the database in 744 subjects (19.8%): 733 of the group who did not develop OAC (19.8%) and 11 of the OAC group (20%). There was no association between increasing BMI and progression to OC on univariate and multivariate analyses. Furthermore, no association was seen when analysed by categorizing BMI ≤25 kg/m2, overweight (BMI 25.1–30 kg/m2), and obese (BMI >30 kg/m2; data not shown). There was also no association with socioeconomic status as determined by Townsend quintile (p = 0.49 for trend; data not shown).
Drug therapy
Nitrate use was associated with progression to OAC, but lost significance when corrected for age, gender, and smoking (Table 2), and by prescription density. PPI use was very common among all subjects (Table 1) and no association was thus observed. No association was seen between developing OAC and the following drug classes: aspirin, NSAIDs, COX-2 inhibitors, and statins (Table 2). There was also no association with iron preparations, anticholinergics, ACE-I, calcium-channel antagonists, tricyclic antidepressants, benzodiazepines, or nicorandil (data not shown).
The use of drugs associated with the treatment of asthma/chronic asthma was more prevalent among subjects developing OAC than among subjects who did not develop OAC: inhaled β-agonists, 40 vs. 29%; inhaled steroids, 33 vs. 19%; combined inhaled steroid and β-agonist, 15 vs. 7%; and theophyllines 11 vs. 4%. The use of both inhaled steroids (HR 2.11, 95% CI 1.12–3.97, p = 0.021) and steroid and β-agonist combination inhalers (HR 2.54, 95% CI 1.17–5.51, p = 0.018) was associated with progression to OAC on both univariate and multivariate analysis (Table 2). The association of OAC development with theophylline use was no longer significant (HR 2.31, 95% CI 0.90–5.93, p = 0.082) when corrected for age, gender, and smoking. Use of inhaled β-agonists was not associated with developing OC.
Prescription density analysis (corrected age, gender, and smoking)
The fourth quintile of increasing inhaled steroid use was associated with developing OAC (2.78, 95% CI 1.15–6.77, p = 0.024) and a significant trend with increasing prescription density through the quintiles (p = 0.028 for trend) (Figure 1). Examining prescription density for combined inhaled steroid and β-agonist revealed an association with developing OAC (Figure 2), reaching significance in the fourth and fifth quintiles (HR 3.79, 95% CI 1.16–12.39, p = 0.027; and HR 3.58, 95% CI 1.09–11.80, p = 0.036, respectively) with an increasing association across the quintiles (p = 0.005 for trend). While theophyllines on an ever/never basis were only associated with progression to OAC in univariate analysis (HR 2.52, 95% CI 1.07–5.89, p = 0.034), the analysis of prescription density exhibited a HR 4.89 (95% CI 1.17–20.37, p = 0.029) in the fourth quintile.
Figure 1.

Estimation of the risk of developing oesophageal adenocarcinoma from Barrett’s oesophagus by prescription density of inhaled steroids in quintiles, corrected for age, gender, and smoking.
Values are hazard ratios and 95% confidence intervals. p = 0.028 for trend.
Figure 2.

Estimation of the risk of developing oesophageal adenocarcinoma from Barrett’s oesophagus by prescription density of inhaled combined β-agonist and steroid in quintiles, corrected for age, gender, and smoking.
Values are hazard ratios and 95% confidence intervals. p = 0.005 for trend.
Cumulative drug use of drugs for asthma and those with a side effect of lower oesophageal sphincter relaxation
Increasing number of drugs used for asthma showed an increasing association with progression to OAC (HR 2.91, 95% CI 1.10–7.68, p = 0.031 for the use of all three examined drugs) following correction for age, gender, and smoking status (Figure 3).
Figure 3.
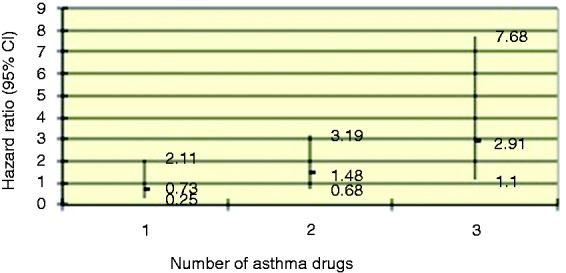
Estimation of the risk of developing oesophageal adenocarcinoma from Barrett’s oesophagus by increasing number of drugs prescribed for the treatment of asthma, corrected for age, gender, and smoking.
Values are hazard ratios and 95% confidence intervals. p = 0.031 for the use of three or more drugs; p = 0.061 for trend.
Figure 4 illustrates an increasing risk with drugs with a side effect of LOS relaxation, with the HR increasing and reaching significance with three or more drugs (HR 2.31, 95% CI 1.09–4.88, p = 0.029, p = 0.009 for the trend). Given the association seen with cumulative asthma/chronic asthma drugs, β-agonists and theophylllines were subsequently removed from the cumulative LOS analysis, with loss of association.
Figure 4.
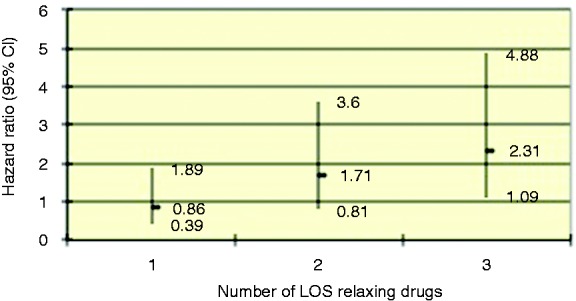
Estimation of the risk of developing oesophageal adenocarcinoma from Barrett’s oesophagus by increasing number of drugs with a side-effect profile of relaxation of the lower oesophageal sphincter, corrected for age and gender.
Values are hazard ratios and 95% confidence intervals. p = 0.009 for trend.
Discussion
Previous studies of BO and progression to OAC have several inherent limitations including small numbers of subjects studied, restriction to endoscopic surveillance patients with their inherent biases, and relatively short follow-up periods. This study aimed to overcome some of these issues by following subjects from a long-standing population-based general practice database rather than a BO surveillance programme. The 0.3% per annum conversion rate from BO to OAC is 3-fold higher than more recent data,6 which may be explained by both the greater incidence of OAC in the UK as compared with the rest of the world,2 and also the inclusion of subjects in the database who are not necessarily under endoscopic surveillance. Unfortunately, the THIN database will rarely record histological confirmation of BO (intestinal metaplasia) or the presence/degree of dysplasia. This has removed the possibility of using dysplasia, notably high-grade, as an endpoint for disease progression, which is of increasing importance with newer modalities of treatment becoming more widely accepted and available. Moreover, Barrett’s segment length is not recorded. The cohort would be expected to include many subjects with short segments of BO, since these are much more common than longer segments.11 Despite these inherent weaknesses of a general practice database study of BO, it is extremely unlikely that such a diagnosis would be coded by a GP unless prompted by an endoscopic/histological report of BO (British Society of Gastroenterology standard of practice). The length of follow up generated, with almost 18,000 subject-years, median follow up per subject of 4 years, and verification of OAC are strengths compared with many other studies of BO progression.
OAC is more common in men and a number of potential explanations have been suggested including work-place exposure to potential carcinogens,12 the influence of sex hormones,13,14 and the influence of increasing BMI.15 In the present study, there was a >3-fold increased risk of men developing OAC. However, no association was found between increasing BMI and progression to OAC suggesting that the effect of gender at this stage of oncogenesis is unlikely to be related to visceral obesity. Increasing BMI may therefore be associated with OAC through influencing the frequency and severity of GORD and thus development of BO, rather than development of OAC in BO subjects.
Of the common OC morphologies, squamous cell carcinoma has been considered to be most influenced by smoking16 and lower socioeconomic status.1 Our simplified analysis of smoking status (never vs. ever) was designed to overcome some of the limitations of the database in the recording of smoking status at random rather than set intervals (resulting in inability to produce pack-year data). Having ever smoked more than doubled the risk of developing OAC from BO on univariate analysis. Similar findings were reported from the Northern Ireland Barrett’s Oesophagus Registry.17 On a practical note, the results of the present study and others on the influence of smoking in OAC suggest that strenuous efforts should be made to encourage BO patients to stop smoking as a readily modifiable risk factor for OAC. There was no association between OAC progression and socioeconomic status as determined by Townsend quintile in the present study, a finding in keeping with an observational UK study of OAC.1
PPI use was very common among all subjects and no association was thus observed, limiting any interpretation from this study. Neither aspirin nor NSAIDs were associated with development of OAC in this study (ever vs. never usage, and prescription density analysis; data not shown). Meta-analyses have shown a reduced risk of OAC with aspirin and/or NSAIDs.18,19 Further modelling suggested that those who benefited most from aspirin/NSAIDs were those with frequent reflux symptoms.20 While aspirin/NSAIDs have been shown to reduced risk of neoplastic progression from BO,21 analysis of the UK National Barrett’s Oesophagus Registry failed to show an impact of aspirin upon the development of OAC and dysplasia.22 NSAID and aspirin use is not solely by prescription, with widespread over-the-counter use (not recorded in THIN), resulting in a likely underestimation, potentially explaining the lack of a significant association observed. The results of the AspECT randomized control trial, examining aspirin in BO, will shed further light.23
None of the medications that have a side effect of reducing LOS pressure were individually associated with neoplastic progression. When analysed by increasing number of drugs (one, two, and three or more), there was a significant trend for increasing relative risk of neoplastic progression seen. This finding is in keeping with previous case–control studies,24,25 with the underlying mechanism suggested to be through promoting gastro-oesophageal reflux. However, when β-agonists and theophyllines were removed from analysis of LOS-relaxing drugs, negating any confounding from asthma/chronic asthma, no association was seen. There is clearly potential for confounding to occur in the associations between asthma medication use, gastro-oesophageal reflux symptoms and BO.26 Asthma drugs may be associated with developing OAC through GORD causing asthma. While asthma and GORD has proven linkage, little data exists for COPD leading to GORD; however, we are unable to confidently distinguish those patients having such drugs for asthma and those using them for COPD in the database, although such drug use is associated with bronchoreversibilty and would naturally assume usage is amongst those subjects with chronic asthma. Alternatively asthma drugs, through their effect on LOS pressure, may worsen gastro-oesophageal reflux and thereby increase OAC risk. Theophyllines have been shown to be associated with OAC in a meta-analysis,27 but in the present study, although there was an increased hazard ratio, this failed to reach statistical significance. In prescription density analysis, the fourth quintile reached statistical significance, but not across the quintiles (data not shown). Inhaled steroids, while not affecting LOS pressures, doubled the risk of progression to OAC when comparing ever vs. never usage, and also with increasing prescription density suggesting a dose–response effect, or more likely an association with increasing disease severity. The fifth quintile of prescription density was not significantly associated with OAC, both with inhaled steroids and theophyllines, but this may be due to those with a greater severity of asthma or chronic asthma developing life-threatening complications of pulmonary disease, rather than OC, shortening their time on the database. Furthermore, combined inhalers containing both inhaled steroid and β-agonist doubled the risk of neoplastic progression from BO not only on ever vs. never analysis but also increasing across all quintiles, reaching significance in both the fourth and fifth quintiles. Finally, there was an association between increasing number of different drugs used for asthma and OAC development, reaching significance when three drugs were used. We were unable to definitively distinguish whether the association between asthma drugs and progression to OAC relates to the side effects of the drugs on LOS pressure promoting reflux, or asthma in BO patients being associated with a higher risk of OAC. However, the latter seems the more plausible explanation given the association of inhaled steroids with progression to OAC. Controlling for smoking during the analysis removes one potential source of confounding in this association. Patients with BO and asthma, chronic or acute, should be regarded from the present study as being at increased risk of developing OAC.
There are inherent limitations in performing a retrospective nested case–control study from GP-entered databases. While all prescriptions issued are accurately recorded (computer-generated scripts), it cannot be guaranteed that it is dispensed or taken by the patient. In some cases (e.g. β-agonist inhalers), multiple devices may be obtained but not used. Deriving prescription density data not only enables identification of dose–response effects, but also reduces confounding from infrequent users of medication. Furthermore, over-the-counter medication and drugs prescribed at other institutions will not be recorded. While these limitations exist, their impact is likely to be uniform among all patients, so bias is unlikely to affect the analysis. Finally, we were unable to derive data on symptom patterns of GORD, dietary data, or the prevalence of Helicobacter pylori, all of which may contribute to the development of OAC.
Progression to OAC from BO is more common among men and with increasing age. There is some evidence of smoking being associated with progression to OAC but this association was not significant on multivariate analysis. LOS-relaxing drugs do not appear to be associated with OAC development once drugs for asthma are excluded. The association of inhaled steroids with OAC development strongly suggests that it is the pathophysiolology of asthma/chronic asthma or the severity of gastro-oesophageal reflux necessary to cause asthma, rather the drugs themselves that are associated with progression to OAC.
Funding
We are grateful to ‘The Upper GI Blues’, our local support group for patients with upper gastrointestinal malignancies, for raising funds to help obtain the data and the Sandwell General Hospital Postgraduate Trustees for funding the queries for the validation of oesophageal cancer diagnosis and morphology. We are also grateful to CSD Medical Research UK for their advice on analysis of the THIN database and extraction of smoking status records
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1. Cooper SC, Day R, Brooks C, et al. The influence of deprivation and ethnicity on the incidence of oesophageal cancer in England. Cancer Causes Control 2009; 20: 1459–1467. [DOI] [PubMed] [Google Scholar]
- 2. El-Serag HB. The epidemic of esophageal adenocarcinoma. Gastrenterol Clin N Am 2002; 31: 421–440. [DOI] [PubMed] [Google Scholar]
- 3. Rachet B, Maringe C, Nur U, et al. Population-based cancer survival trends in England and Wales up to 2007: an assessment of the NHS cancer plan for England. Lancet Oncol 2009; 10: 351–369. [DOI] [PubMed] [Google Scholar]
- 4. Spechler SJ. The frequency of esophageal cancer in patients with Barrett’s esophagus. Acta Endoscopica 1992; 22: 541–544. [Google Scholar]
- 5. Eckardt VF, Kanzler G, Bernhard G. Life expectancy and cancer risk in patients with Barrett’s esophagus: a prospective controlled investigation. Am J Med 2001; 111: 33–37. [DOI] [PubMed] [Google Scholar]
- 6. Hvid-Jensen F, Pederson L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med 2011; 365: 1375–1383. [DOI] [PubMed] [Google Scholar]
- 7. Barbiere JM, Lyratzopoulos G. Cost-effectiveness of endoscopic screening followed by surveillance for Barrett’s esophagus: a review. Gastroenterology 2009; 137: 1869–1876. [DOI] [PubMed] [Google Scholar]
- 8. El-Serhag HB, Aguirre TV, Davis S, et al. Proton pump inhibitiors are associated with reduced incidence of dysplasia in Barrett’s oesophagus. Am J Gastroenterol 2004; 99: 1877–1883. [DOI] [PubMed] [Google Scholar]
- 9. Nguyen DM, El-Serhag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009; 7: 1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Markogiannakis H, Theodorou D, Toutouzas KG, et al. Small cell carcinoma arising in Barrett’s esophagus: a case report and review of the literature. J Med Case Rep 2008; 2: 15–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic. Gastrointest Endosc 2005; 61: 226–231. [DOI] [PubMed] [Google Scholar]
- 12. Jansson C, Johansson AL, Bergdahl IA, et al. Occupational exposures and risk of oesophageal and gastric cardia cancers among male Swedish construction workers. Cancer Causes Control 2005; 16: 755–764. [DOI] [PubMed] [Google Scholar]
- 13. Cooper SC, Croft S, Day R, et al. Patients with prostate cancer are less likely to develop oesophageal adenocarcinoma; could anti-androgen therapy have a protective role? Cancer Causes Control 2009; 20: 1363–1368. [DOI] [PubMed] [Google Scholar]
- 14. Chandanos E, Lagergren J. The mystery of male dominanace in oesophageal cancer and the potential protective role of oestrogen. Eur J Cancer 2009; 45: 3149–3155. [DOI] [PubMed] [Google Scholar]
- 15. Anderson LA, Watson RGP, Murphy SJ, et al. Risk factors for Barrett’s oesophagus and oesophageal adenocarcinoma: results from the FINBAR study. World J Gastroenterol 2007; 13: 1585–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaughan TL, Davis S, Kristal AS, et al. Obesity, alcohol and tobacco as risk factors for cancers of the oesophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomark Prev 1995; 4: 85–92. [PubMed] [Google Scholar]
- 17. Coleman HG, Bhat S, Johnston BT, et al. Tobacco smoking increases the risk of high-grade dysplasia and cancer among patients with Barrett’s esophagus. Gastroenterology 2012; 142: 233–240. [DOI] [PubMed] [Google Scholar]
- 18. Corley DA, Kerlikowske K, Verma R, et al. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003; 14: 47–56. [DOI] [PubMed] [Google Scholar]
- 19. Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces the risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastreonterology 2012; 142: 442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pandeya N, Webb PM, Sadeghi S, et al. Gastro-oesophageal reflux symptoms and the risks of oesophageal cancer: are the effects modified by smoking, NSAIDs or acid suppressants? Gut 2010; 59: 31–38. [DOI] [PubMed] [Google Scholar]
- 21. Vaughan TL, Dong LM, Blount PL, et al. Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett’s oesophagus: a prospective study. Lancet Oncol 2005; 6: 945–952. [DOI] [PubMed] [Google Scholar]
- 22. Gatenby PA, Ramus JR, Caygill CP, Winslet MC, Watson A. Aspirin is not chemopreventive for Barrett’s adenocarcinoma of the oesophagus in multicentre cohort. Eur J Cancer Prev 2009; 18: 381–384. [DOI] [PubMed] [Google Scholar]
- 23. Jankowski J, Moayyedi P. Re: cost effectiveness of aspirin chemoprevention for Barrett’s esophagus. J Natl Cancer Inst 2004; 96: 885–887. [DOI] [PubMed] [Google Scholar]
- 24. Lagergren J, Bergström R, Adami H-O, et al. Association between medications that relax the lower esophageal sphinter and risk for esophageal adenocarcinoma. Ann Intern Med 2000; 133: 165–175. [DOI] [PubMed] [Google Scholar]
- 25. Corley DA, Levin TR, Habel LA, et al. Barrett’s esophagus and medication that relax the lower esophageal sphincter. Am J Gastroenterol 2006; 101: 937–944. [DOI] [PubMed] [Google Scholar]
- 26. Ladanchuk TC, Johnston BT, Murray LJ, et al. FINBAR study group. Risk of Barrett’s oesophagus, oesophageal adenocarcinoma and reflux oesophagitis and the use of nitrates and asthma medications. Scand J Gastroenterol 2010; 45: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 27. Alexandre L, Broughton T, Loke Y, et al. Meta-analysis: risk of esophageal adenocarcinoma with medications which relax the lower esophageal sphincter. Dis Esophagus 2011; 25: 535–544. [DOI] [PubMed] [Google Scholar]


