Abstract
Difficulty understanding speech in background noise, even with amplification to restore audibility, is a common problem for hearing-impaired individuals and is especially frequent in older adults. Despite the debilitating nature of the problem the cause is not yet completely clear. This review considers the role of spatial processing ability in understanding speech in noise, highlights the potential impact of disordered spatial processing, and attempts to establish if aging leads to reduced spatial processing ability. Evidence supporting and opposing the hypothesis that spatial processing is disordered among the aging population is presented. With a few notable exceptions, spatial processing ability was shown to be reduced in an older population in comparison to young adults, leading to poorer speech understanding in noise. However, it is argued that to conclude aging negatively effects spatial processing ability may be oversimplified or even premature given potentially confounding factors such as cognitive ability and hearing impairment. Further research is required to determine the effect of aging and hearing impairment on spatial processing and to investigate possible remediation options for spatial processing disorder.
Keywords: spatial processing, aging, simultaneous stream segregation, speech understanding in noise
Introduction
Difficulty understanding speech in complex environments, such as in the presence of background noise, is a frequent complaint among the aging population (Committe on Hearing Bioacoustics, and Biomechanics [CHABA], 1988; Pichora-Fuller, 1997). The Committee on Hearing Bioacoustics and Biomechanics (CHABA, 1988) suggested difficulty understanding speech should be considered one of the most incapacitating elements of hearing impairment given its potential to cause feelings of isolation and effect relationships. Identifying and understanding the cause of this reported difficulty is vital if successful management is to be accomplished.
The effects of aging on the human auditory system are both numerous and complex. Research has shown that as people age deterioration occurs on many fronts; hearing sensitivity declines, dynamic range is reduced, speech understanding in noise is compromised, and cognitive processing slows, to name just a few. Effective communication in complex listening environments requires the peripheral auditory systems, central auditory pathways, and cognitive systems to all function effectively (Schneider, Pichora-Fuller, & Daneman, 2010). If the process is impeded at any one point, then the ability to understand speech breaks down (Schneider et al., 2010). Spatial processing is one skill that plays an important role in understanding speech in complex listening environments, and this review will consider whether deteriorating spatial processing may in fact be a cause of the difficulty understanding speech in noise that is so frequently reported by older adults.
What Is Spatial Processing?
Spatial processing ability can be defined as the ability to selectively attend to sounds arriving from one direction while simultaneously suppressing sounds arriving from another. To do this effectively, minute interaural intensity differences (IID) and interaural time differences (ITD) must be interpreted and used to segregate the simultaneous streams of speech (Dubno, Ahlstrom, & Horwitz, 2002). The role of spatial processing ability in teasing apart competing messages has been acknowledged since the groundbreaking article from Cherry (1953). Spatial processing is usually calculated in research studies as the difference in scores obtained on two test conditions of a speech reception in noise task, where the only difference between conditions is whether there is spatial separation between where the target speech originates and where the noise originates (Ahlstrom, Horwitz, & Dubno, 2009; Allen, Carlile, & Alais, 2008; Arbogast, Mason, & Kidd, 2005; Brown, Cameron, Martin, Watson, & Dillon., 2010; Cameron & Dillon, 2007; Cameron & Dillon, 2009; Dubno et al., 2002; Dubno, Ahlstrom, & Horwitz, 2008; Gelfand, Ross, & Miller, 1988; Kim, Frisina, & Frisina, 2006). Spatial processing ability has been referred to in the literature by a number of terms over the years, including, but not limited to, spatial release from masking, spatial hearing, spatial stream segregation, spatial advantage, and in limited contexts binaural advantage. In this review, we will use the term spatial processing exclusively.
Research examining the spatial processing ability of normal-hearing young adults has demonstrated the importance of access to spatial cues. Brown et al. (2010) used the Listening in Spatialized Noise–Sentences Test (LiSN-S; Cameron & Dillon, 2009) to examine the benefit 120 normal-hearing adolescents and young adults gained from spatial processing. The LiSN-S is an adaptive speech-in-noise test conducted under headphones in a simulated anechoic auditory environment. The test includes four different conditions (shown in Figure 1) in which the perceived spatial location of the distracting speech, as well as the pitch and timbre characteristics of the speakers, are manipulated. By calculating the spatial advantage, which is the difference between the speech reception threshold (SRT) in the condition known as the same voice ± 90° condition (shown in the bottom left quadrant of Figure 1) and the same voice 0° condition (shown in the top left quadrant of Figure 1) the researchers were able to isolate how much performance on the task improved due to access to spatial cues.
Figure 1.
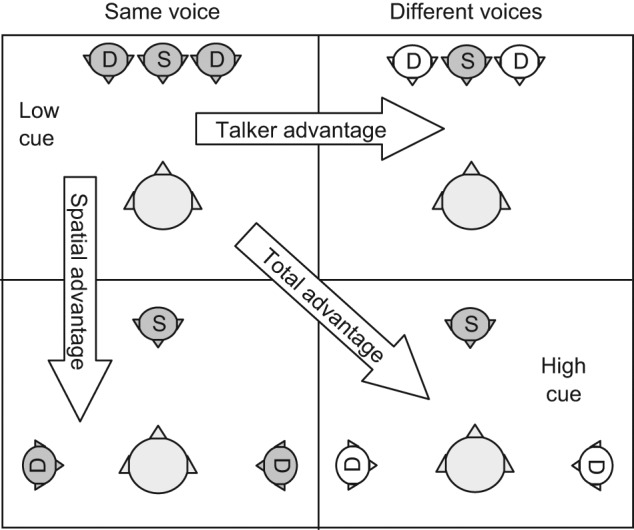
The four conditions of the LiSN-S, and the three difference scores (advantage measures) that can be derived from them
Note: The signal, S, always comes from the front, whereas the distractors, D, come from the front or the sides, in different conditions.
Brown et al. (2010) demonstrated a 12-dB improvement in performance, referred to as spatial advantage, once adult-like performance was reached. In other words, participants were able to understand the target sentences at a 12-dB worse signal-to-noise ratio (SNR) once the signal and the noise were separated and they could use spatial processing to assist them with the task. These results are consistent with the earlier findings of Allen et al. (2008) who demonstrated that young adults gained between 10.7-dB and 12.8-dB benefit from spatial cues. This is despite using a completely different protocol and test material than that reported in Brown et al. (2010). In both cases, little additional improvement in performance was observed when the participants were able to use not only spatial processing but also pitch differences between the voices to help separate their target message from the competing speech (Allen et al., 2008; Brown et al., 2010). This demonstrates the important role spatial processing can play in successful speech understanding in noise. Previous research has also shown that the importance of spatial processing increases in situations where greater amounts of informational masking (i.e., the maskers and target speech are highly similar) are present (Noble & Perrett, 2002).
What Is Spatial Processing Disorder?
Spatial processing disorder is defined as a reduced ability to selectively attend to sounds arriving from one direction while simultaneously suppressing sounds arriving from another Cameron & Dillon (2011). Cameron and Dillon (2008) demonstrated the detrimental effect that spatial processing disorder can have on the ability of normal-hearing children to understand speech in noise. It should be acknowledged that though it could be argued that spatial processing disorder may be a specific type of CAPD, this review will not consider how spatial processing disorder may fit into a CAPD framework, instead focusing on its potential impact on speech understanding for older adults.
Aims
Given the large benefit that can be gained from successful spatial processing and the fact that spatial processing disorder has already been implicated as a cause of difficulty understanding speech in complex listening environments for one population, the role of spatial processing in understanding speech in noise deserves careful consideration. This article provides a comprehensive review of what is known regarding spatial processing ability in older adults. We consider the evidence supporting sspatial processing disorder as a major cause of difficulty hearing in noise and identify possible future directions for research from here.
What Age-Related Changes Could Lead to Spatial Processing Disorder?
Before examining the research concerning how aging affects spatial processing ability, it is important to understand what physiological changes within the auditory system could lead to a change in spatial processing ability. Many years of research have linked aging with physiological changes in the peripheral auditory system, central auditory pathways, and at the cortical level (Schmiedt, 2010). As it is not within the scope of this article to discuss all the changes in the auditory system that have been shown to occur with aging, discussion is restricted to the physical changes that may affect spatial processing ability. For a comprehensive review of age-related changes in the auditory system, please refer to Gates and Mills (2005).
For spatial processing to occur, the IID or ITD, or possibly both IID and ITD, must be accurately transmitted and interpreted at different points throughout the auditory pathway. The peripheral hearing system, especially the cochlea, must be sensitive enough to detect this timing and intensity information. Probably the most commonly discussed effect of aging on the cochlea is the loss of outer hair cells predominantly at the basal end (Gates & Mills, 2005). Loss of outer hair cells, though commonly seen in the older adults, is thought to be a result of accumulated exposure to noise as well as aging rather than the aging process in isolation (Gates & Mills, 2005). Loss of outer hair cells has been linked with the reduction in high-frequency thresholds, commonly referred to as presbycusis, which is frequently seen among the older adults (Schmiedt, 2010). Reduced functioning of the OHCs leads to reduced frequency resolution. For complex, broadband sounds, if there is a greater-than-normal spread of excitation in each cochlea, the ability to detect IID at each frequency will likely deteriorate because energy in one frequency will affect the excitation of nerve fibers across a greater-than-normal frequency range.
The inner hair cells are also susceptible to effects of aging. Inner hair cells of the cochlea each have approximately 10 to 20 dendritic connections. The afferent fibers from these dendritic connections are largely responsible for transmitting the temporal information of signals away from the inner ear (Gates & Mills, 2005). Animal studies have demonstrated that aging can result in loss and shrinkage of these afferent fibers leading to less accurate temporal resolution as there are fewer fibers firing (Schmiedt, 2010). Gates and Mills (2005) explain that this degradation then results in asynchronous activity in the auditory nerve. Therefore, it becomes apparent that age-related degeneration in the cochlea has the potential to affect both the spectral and temporal resolution of the auditory system.
Due to our reliance on animal and postmortem studies for detailed information regarding the central auditory nervous system (CANS), less research has been undertaken concerning age-related changes at this level. However, the potential impact of changes in the CANS on spatial processing should not be disregarded as it is in the CANS that the information from both ears is first combined, providing the first point at which IID and ITD can be interpreted (Moore, 1991). Postmortem studies conducted in the 1960s provided support for the theory that aging results in a loss of cells in the cochlear nucleus, inferior colliculus, and medial geniculate body (Hansen & Reske-Nielsen, 1965; Kirikae, 1969). Ferraro and Minckler (1977) examined the lateral leminiscus at postmortem in 15 people and found that nucleus size was also reduced with advanced age. The observed changes in the inferior colliculus and lateral leminiscus have the potential to result in disordered spatial processing ability as binaural interaction occurs at these locations (Moore, 1991).
What Does Research Show About Spatial Processing and Aging?
While the above overview demonstrates that physical changes associated with aging occur in parts of the auditory system that are involved in transmitting or analyzing spatial information in both speech and nonspeech sounds, this in itself is not evidence of reduced spatial processing ability in older adults. A number of researchers have, however, sought to measure spatial processing ability in this population (see summary Table 1). Early attempts by Warren, Wagener, and Herman (1978) compared results from a speech repetition task presented under headphones so that the target words and distracting speakers were perceived as coming from the centre of the head, with one presented dichotically where the distracting speakers were perceived as coming from ±15° and ±30° azimuth. Their findings indicated that older adults benefited less from access to spatial cues than young adults. Since then there have been many variations in the protocols used, though all have been based around the basic theory of delivering a speech test in spatially separated noise and manipulating the spatialization to determine the amount of benefit gained from spatial processing. Table 1 provides an overview of the findings from each study, which are discussed in detail below.
Table 1.
Summary of Research Studies That Provide Information Regarding Spatial Processing Ability and Aging
| Study | Speech type | Noise type | Masker-symmetry | Effect of age and hearing impairment | Type of control for hearing impairment |
|---|---|---|---|---|---|
| Warren, Wagener, and Herman (1978) | Monosyllabic words | 4 voices speaking prose passages | Symmetrical | Significant effect of age | Nil |
| Gelfand, Ross, and Miller (1988) | High predictability SPIN sentences | 12 speaker babble | Asymmetrical | No significant effect of age between the normal hearing groups | Nil |
| Hearing impairment group showed significantly poorer spatial processing ability | |||||
| Divenyi and Haupt (1997) | Sentences | Babble | Asymmetrical and Symmetrical conditions | Significant effect of age between groups, for some measures of spatial processing, when hearing impairment controlled for | Flat amplification and statistical |
| Dubno, Ahlstrom, and Horwitz (2002) | HINT sentences | HINT-shaped noise | Asymmetrical | Significant effect of age Significant effect of hearing loss |
Audiometric thresholds matched between groups up to 2 kHz Comparison of spatial benefit when low pass filtering applied to speech as well as without filtering |
| Arbogast, Mason, and Kidd (2005) | CRM sentences | 1. CRM sentences 2. Random broadband Gaussian noise shaped to avoid spectrum of CRM sentences 3. 8 bands of noise overlapping target sentences. |
Asymmetrical | Significant effect of hearing loss | Age matched hearing impaired group |
| Significant effect of masker type Significant effect of age for hearing-impaired group only |
Flat amplification for hearing impaired participants | ||||
| Divenyi, Stark, and Haupt (2005) | Sentences | 12 speaker babble | Asymmetrical and symmetrical conditions | Significant effect of age between groups | Flat amplification and statistical |
| Kim, Frisina, and Frisina (2006) | HINT sentences | Spectrally shaped noise | Asymmetrical | Significant effect of age | No control reported |
| Murphy, Daneman, and Schneider (2006) | One act plays with 2 voices | 12 speaker babble | Symmetrical | Significant effect of age when loudspeakers separated by 45° | Flat amplification for older participants |
| Dubno, Ahlstrom, and Horwitz (2008) | Low pass filtered HINT sentences | Low pass filtered steady state HINT-shaped noise | Symmetrical vs. asymmetrical | No significant age effect for symmetrical or asymmetrical conditions | Use of low pass filtered stimuli to minimize effects of different thresholds in high frequencies |
| Marrone, Mason, and Kidd (2008) | CRM sentences | CRM sentences | Symmetrical | Significant but weak effect of age in both reverberant and anechoic conditions | Flat amplification for hearing impaired subjects and no compensation for older normal hearers |
| Significant effect of hearing in both reverberant and anechoic conditions | Statistical | ||||
| Ahlstrom, Horwitz, and Dubno (2009) | Sentences | Multitalker babble | Asymmetrical | Up to 5 dB of benefit Hearing impaired older adults still gain a small amount of benefit from access to spatial cues | Tested while aided to NAL prescription |
| Cameron, Glyde, and Dillon (2011) | Sentences | 2 children stories | Symmetrical | No significant effect of age among adults | Use of normal hearers up to 8 kHz |
Note: All reported age effects are in the direction of poorer spatial processing ability with increasing age. All reported hearing effects are in the direction of poorer spatial processing with greater hearing impairment.
As part of a larger test battery, Divenyi and Haupt (1997) assessed spatial processing ability in older adults using simulated free-field presentation of the Speech Perception in Noise Test (SPIN; Kalikow, Stevens, & Elliot, 1977) By statistically controlling for hearing impairment, they demonstrated that an age effect remained for 4 of the 10 measures of spatialization. Interestingly, an age effect was evident only in measures assessing what they refer to as sentence-level processing rather than more basic word-level processing, suggesting that, even with reduced spatial processing ability, basic levels of speech reception can still be successfully accomplished. The fact that only tasks which required greater levels of processing were affected suggests that central pathways may be implicated in the decline in spatial processing.
A subsequent longitudinal study by the same research group demonstrated that over as little as a 5-year period, there was significant deterioration in the spatial processing ability of older adults (Divenyi, Stark, & Haupt, 2005). Deterioration in hearing thresholds during this period was also observed; however, the researchers ascertained statistically that the degree of spatial processing decline could not be completely accounted for by the change in pure-tone threshold levels.
Kim et al. (2006), as part of a study investigating the relationship between distortion product otoacoustic emissions and speech understanding in noise, investigated the spatial processing ability of normally hearing adults aged from 18 to 75 years. The Hearing in Noise Test (HINT; Nilsson, Soli, & Sullivan, 1994) sentences were presented in spectrally shaped noise which varied between conditions based on whether the noise originated from the same direction as the speech or from the left or right of the listener. They reported a significant deterioration in spatial processing ability with aging.
These findings are reinforced by the work of Murphy, Daneman, and Schneider (2006) who also found supporting evidence for the fact that spatial processing ability is reduced with aging. In a series of free-field experiments, these researchers showed reduced spatial processing ability in a group of 36 normally hearing older adults compared with a group of 36 normally hearing young adults when listening to one-act plays spoken by two voices. The researchers acknowledge that, despite both groups of listeners being classed as normal hearers, defined as pure-tone thresholds ≤25 dB HL between 250 Hz and 3 kHz in both ears with an exception of ≤35 dB HL at no more than one frequency in this range, the thresholds of the two ages groups could not be considered equivalent. Given that frequencies above 3 kHz were up to 40 dB worse for the older adults, the potential effects of hearing impairment must be considered. Murphy and colleagues attempted to address questions of audibility by presenting the speech test at levels that proved equally difficult for each participant to understand individual words and demonstrated that the age effect was still present.
Each of the studies discussed up to this point offer support for the hypothesis that aging results in a reduction in spatial processing ability which, in turn, could offer an explanation for why older adults struggle to understand speech in noise. However, one cannot discount the possibility that the link between age and spatial processing ability may be correlational and a third factor may be at play. Although Murphy et al. (2006) attempted to control for hearing impairment by increasing the presentation levels of the test stimuli, this does not guarantee that hearing impairment is no longer contributing to the poor spatial processing abilities observed in the study. In fact, as presbycusis affects the high frequencies, using a non-frequency-specific increase in amplification, such as that used by Murphy et al. (2006), is unlikely to provide equal access to speech information across the frequency spectrum (Divenyi & Haupt, 1997). More worryingly, no attempt was made by Kim et al. (2006) to ensure the speech stimuli were equally audible for the older adults in their sample who may well have had a high-frequency hearing loss as their hearing was only measured to 4 kHz.
Even if the method of increasing the intensity levels of the test stimuli employed by Murphy et al. (2006) does, by chance, provide the older adults with audible speech at all frequencies, it is not accurate to claim that this controls for hearing impairment as the effects of hearing impairment are not limited to decreased audibility. Even after adjusting for audibility, the effects of hearing impairment will still have been evident in the form of reduced dynamic range, loss of frequency selectivity, and loss of fine temporal resolution. By controlling for hearing impairment statistically, Divenyi and Haupt (1997) and Divenyi et al. (2005) were able to better differentiate between the effect of aging and the effects of hearing impairment; therefore, it is likely that the reduction in spatial processing ability that they report is actually attributable to age. It is worth noting that in the process of controlling for hearing impairment in their analysis, the researchers did conclude that greater degree of hearing impairment was also correlated with poorer spatial processing ability (Divenyi & Haupt, 1997). However, one could not say which effect of hearing impairment (whether it be loss of audibility, loss of frequency selectivity, or reduced temporal resolution) causes this decline.
Dubno et al. (2002) attempted not only to identify whether older adults had reduced spatial processing abilities compared with young adults but also to identify whether this was caused by inability to use IID or ITD. As previously mentioned, IIDs are strongest for high frequencies whereas ITDs are most prominent in the low-frequency range (Dubno et al., 2002). By systematically filtering the speech stimuli with either low-pass filters or high-pass filters, the researchers were able to control whether the participants had access to IID or ITD to aid in their speech understanding. Dubno and colleagues demonstrated that spatial processing ability in their normally hearing older adult participants was reduced relative to the younger adult group regardless of what cues were available, and furthermore, that poorer ability to use IID had the greatest effect on their performance.
As was the case in the work of Murphy et al. (2006), the older “normal-hearing” participants in the experiments conducted by Dubno and colleagues had poorer high-frequency thresholds than the young normally hearing group leading us to question whether the reported poorer ability to use IID may have been caused by the presence of a high-frequency hearing loss in the older adults. Dubno et al. (2002) dispute that poorer hearing thresholds are an adequate explanation of the differences in spatial processing between the groups as these differences were still present even when all information above 2 kHz had been filtered out. They offer an alternative explanation that functional changes in the base of the cochlea, that occur with aging, have affected the way interaural differences can be used. However, one could argue that the functional changes in the base of the cochlea are also hearing-loss related. Regardless of the exact cause of the differences in spatial processing ability between the age groups, it is clear that this research further supports the hypothesis that older adults have disordered spatial processing.
A follow-up study was conducted by the same researchers in 2008 in an attempt to provide further information regarding the relative contribution of IID and ITD to the decline in spatial processing ability. Dubno et al. (2008) used a free-field setup similar to their earlier study but this time removed IID in one condition by presenting an equivalent masker to the ear that would normally experience an advantage because of the head shadow effect. Though performance on speech in noise tasks was reduced by approximately 3 dB when access to IID was removed, the size of this reduction did not differ significantly between young and older adults (Dubno et al., 2008). This would suggest that older and younger adults gain about equal benefit from the presence of IID. There was still a small but not significant difference between the age groups in spatial processing ability when both ITD and IID were accessible.
Ahlstrom et al. (2009) suggested another method of disentangling the effects of hearing impairment and aging which offers additional information regarding the spatial processing ability of older adults. These researchers measured the spatial processing ability of older adults with a mild to moderately severe sensorineural hearing impairment. To minimize the impact of reduced audibility, hearing aids programmed to match NAL-NL1 targets were fitted and all testing took place in the free field. This method of amplification should provide better audibility than that obtained through a non-frequency-specific increase in stimulus intensity as it takes into consideration individual hearing levels at each frequency. With hearing aids in situ, this group of older adults were able to understand frontal speech in the presence of multitalker babble presented from one side with up to 5-dB worse SNR compared with when the speech and babble were presented from the same frontal speaker; thus they had a 5-dB benefit from spatial cues. However, this should not be interpreted further than to say that, with hearing aids in situ, older adults do demonstrate some spatial processing ability.
As Ahlstrom and colleagues (2009) offer no normal-hearing control group or a younger hearing-impaired control group, it is difficult to draw too many conclusions from this finding. First, if we were to attempt to compare these results with the 10.7 dB to 12.8 dB of benefit reported in normal-hearers by Allen et al. (2008), then the assumption could be made that the spatial processing ability of the older adults is reduced. However, this would be an inappropriate comparison to make as the studies use different protocols including a different target stimulus and distracting noise. Second, because the masker was only presented from one side, the spatial processing ability displayed may be no more than the attending to the ear with the better SNR.
It is clear from the studies reviewed above that there is a large body of work supporting the hypothesis that aging is correlated with poorer spatial processing ability; nevertheless, there are also contradictory findings which must be considered. Gelfand et al. (1988) investigated speech-recognition ability in noise in the free field with three groups of normal-hearing adults and a group of hearing-impaired older adults. Using SPIN sentences presented in babble they demonstrated that normal-hearing adults aged above 55 years required more favorable signal-to-babble ratios than young normal-hearing adults in both the co-located and spatially separated conditions. However, they did not see any significant change in spatial processing ability with age within the normal-hearing participants (Gelfand et al., 1988). Spatial processing ability was, however, reduced in the hearing-impaired group relative to the normal-hearing groups. Given this finding, which on first glance seems completely in opposition with the studies discussed previously, one must question whether what other researchers have interpreted as an age effect could actually be occurring because of the link between age and declining peripheral hearing.
More recent articles provide support for the findings of Gelfand et al. (1988). Cameron, Glyde, and Dillon (2011) used the LiSN-S to investigate spatial processing in normally hearing adolescents and adults aged 12 to 60 years and, among the adults, found no effect of age on spatial processing ability. Marrone, Mason, and Kidd (2008) provide further evidence supporting the hypothesis that age is not the major cause of declining spatial processing ability in older adults. Comparing a group of young normal-hearing adults, young hearing-impaired adults, older normal-hearing adults, and older hearing-impaired adults, Marrone and colleagues found a much stronger correlation between hearing loss and spatial processing ability than age and spatial processing ability. In fact, the authors point out that the reported age effect could be a result of the differing high-frequency hearing thresholds between their younger and older adults (Marrone et al., 2008).
It is unknown whether the small size of the age effect reported by Marrone et al. (2008) may have been related to the use of a different response task to the vast majority of the previously discussed studies. Participants were required to respond by selecting a color and number from a closed set of responses which was displayed on a screen (Marrone et al., 2008). It is possible that this task was less cognitively taxing than requiring the participant to repeat sentences heard and that if the task had been more cognitively demanding that an effect of age may have been found, but we will touch more on cognition shortly.
In another study using the same target stimuli and response protocol as that used by Marrone et al. (2008), Arbogast et al. (2005) reported that increasing hearing loss was correlated with decreased spatial processing abilities when speech masking was used, but in a group of age-matched normal-hearing controls they failed to find any effect of age. Within their hearing-impaired group, increasing age was correlated with reduced benefit from spatial cues (Arbogast et al., 2005). The authors offer no explanation for this finding, and, as they report that increasing age was not significantly correlated with worsening hearing thresholds in quiet, it is unlikely that the correlation between age and spatial processing ability is being confounded by the potential role of hearing impairment. One factor that may have played a role is that of cognition.
This section of the review has highlighted the lack of consensus in the literature regarding the effect of aging on spatial processing. These contradictory findings may be due, in part, to the different protocols and participant samples used; however, it is unlikely this explains all of the contradictory results. If we are to gain a clearer picture of the effect of aging on spatial processing more central factors, cognition in particular, must also be considered.
Could Changes in Cognition Affect Spatial Processing?
Understanding speech in noise requires more than simply identifying the correct stream of speech sounds (Schneider, Daneman, Murphy, & See, 2000). Even when considering a task in which listeners are only required to repeat a target sentence, listeners must attend to the relevant signal, process the speech sounds quickly enough so that they are ready to identify the next word spoken, have adequate knowledge of language to map the speech sounds to words in their lexical maps, and hold the words from the beginning of the sentence in working memory until the sentence has ended. They may also be employing top-down processes such as auditory closure and contextual clues to fill in words they are unsure of. Each of these processes—working memory, attention, processing speed, auditory closure, and language skills—are all components of cognition, and cognition has been shown to decline with increasing age (Lunner, 2003). So with this in mind, could the changes in spatial processing ability with increasing age that have been reported by many researchers actually be the effect of declining cognition, effects that therefore are evident only in cognitively demanding tasks?
It is possible that a person’s cognitive abilities may affect their spatial processing score. However, as we are unaware of any work directly examining the effect of cognition on spatial processing ability, we can only make inferences based on more general research concerning the effects of cognition on speech understanding in noise. It is beyond the scope of this article to review all the literature regarding the effects of cognition on speech understanding and, as such, discussion will be limited to a couple of key examples. The potential impact of the degradation hypothesis (Lindenberger & Baltes, 1994), a popular hypothesis regarding the impact of cognition on speech understanding tasks, will also be considered.
Lunner (2003) investigated whether sentence reception in noise was affected by working memory capacity in 72 hearing-impaired adults. Using a visual working memory task and processing speed task, he demonstrated, as expected, that poorer performance was observed for both the working memory and processing speed tasks with increasing age. A correlation between decreased speech understanding in noise and poorer cognitive ability was observed even once the effect of hearing impairment and age were partialled out (Lunner, 2003). This result could be explained by what is known as the degradation hypothesis (Lindenberger & Baltes, 1994).
According to the degradation hypothesis, the presence of a cochlear hearing impairment or background noise will make listening more cognitively demanding and cognitive resources have to be diverted away from working memory to be used for effortful listening instead. If the degradation hypothesis is accurate, then one could hypothesize that perhaps a person with a poor working memory, in addition to a peripheral hearing impairment, may have less cognitive resources available to divert to effortful listening and subsequently less resources available to direct toward analyzing the binaural cues used in spatial processing which may in turn result in poorer spatial processing.
Humes et al (1994), however, did not find evidence to support the idea that declining cognition is responsible for poorer speech understanding in noise. Fifty older adults were tested on a range of speech understanding in noise measures, the Weschler Adult Intelligence Scale–Revised (WAIS-R; Weschler, 1981) and Weschler Memory Scale–Revised (WMS-R; Weschler, 1987). It was found that each of the measures of cognition accounted for minimal amounts of the variation in the participants’ ability to understand speech in noise with hearing thresholds accounting for the vast majority of the variation. This finding is supported by the work of Jerger, Jerger, Oliver, and Pirozzolo (1989). Unfortunately, the results of these studies do not shed much light on whether cognition is likely to affect spatial processing results in the older adults, but they could be interpreted as providing evidence that is contrary to the degradation hypothesis. Daneman and Merikle (1996) offer a different interpretation of results such as those found by Humes et al. (1994), suggesting that the measures of cognition used may not be taxing enough to place sufficiently large demands on the cognitive system.
What is discussed here is just a small subset of the articles investigating the effects of cognition on speech understanding in noise and more comprehensive overviews are available elsewhere (e.g., Pichora-Fuller & Singh, 2006). The purpose of our brief review here is to emphasize that cognition remains an unknown factor in relation to spatial processing ability. Given that it is widely accepted that cognition deteriorates with age and such changes may affect spatial processing, this should be considered a possible factor when assessing spatial processing and aging.
Summary
It is clear that the vast majority of findings suggest that older hearing-impaired adults are likely to experience reduced spatial processing ability to some degree. Though the size of this reduction in spatial processing ability varies between studies, it does seem to be evident to some extent irrespective of masker symmetry, type of masker, whether the masker is energetic or informational in nature, and speech signal (see Table 1). What is not clear is whether the decline in spatial processing is due to changes in the auditory system associated with sensorineural hearing impairment, age-related cognitive changes, a combination of both, or whether it is simply another age-related decline that is distinct from cognition and hearing impairment. So, is it enough to know that older hearing-impaired adults are prone to spatial- processing difficulties? No. Without knowing whether it is aging or hearing impairment that correlates with poor spatial processing ability, we cannot know how best to attempt to address the difficulties it causes.
What Further Research Is Needed?
Given the lack of consensus in the literature regarding whether spatial processing disorder is a major cause of difficulty hearing in noise for older adults, further research is needed. Specifically, this research should seek to address some of the limitations evident in the existing body of literature. First, if the relationships between hearing impairment and spatial processing ability and between age and spatial processing ability are going to be better understood, then a study which better differentiates the two factors is needed. Hearing impairment needs to be controlled for both in terms of improving audibility, through the use of frequency specific amplification, and in the way the data are analyzed. A number of the studies discussed in this review attempted to employ either one method or the other but neither is sufficient on their own as hearing impairment has far wider effects than audibility and, conversely, if audibility is not considered at all, then optimum performance is not being obtained.
Second, the research must be specific as to which aspects of speech perception in spatially separated noise are being addressed. Age and/or cognition both may have different effects on speech perception, spatial release from masking with asymmetrical maskers and spatial release from masking with symmetrical maskers. The first of these abilities, speech perception, requires skills such as auditory closure and working memory. The second, spatial release from masking with asymmetrical maskers, can be achieved by simply attending to the ear with a better signal-to-noise ratio. The third, spatial release from maskers with symmetrical maskers, requires the information at the two ears to be combined.
Future research in the area of spatial processing and aging should also seek to establish the cognitive status of participants so that cognition can be examined as a contributing factor to any observed decline in spatial processing. This is not to say that researchers should use poor scores on cognitive measures as an exclusion criterion. Rather, the sample should be as representative of the older population as possible. If the cognitive system does have a maximum load as hypothesized by many researchers (e.g., Lindenberger & Baltes, 1994), it may well have effects on spatial processing ability. Ideally the cognitive measures used should have been shown to be sensitive to small cognitive declines and tax a wide range of cognitive skills as more basic measures, such as measures of digit span, may not be sensitive enough to cognitive decline because they do not stress both storage and processing simultaneously (Daneman & Merikle, 1996).
It is also important to remember that, depending on how research participants are recruited, the relationship between age and cognition may be stronger in some research studies than in others. If researchers recruit only those elderly people who seem most capable of performing the experimental tasks, it is likely that the relationships between age and cognition will be weaker than in the general population.
Ensuring the results are as generalizable as possible is another important goal for future research in this field. As such, more research is needed that does not seek to limit itself to a sample of “normal hearing” older adults as normal hearing is not in fact the norm for this age group. One study of Australian adults reports that 64% of adults aged 71 years or older have a hearing impairment (Wilson, 1997). Using normal-hearing older adults is, in effect, using a sample that have above-average hearing abilities for their age, and we cannot know whether they may likewise have above average spatial processing ability, cognition, or any other attribute that is affected by age. Future studies should also avoid free-field testing as this will make the results of studies more replicable. Free-field setups are not always easily duplicated from one laboratory to another and potentially confounding factors such as head movement may corrupt results (Wilber, 2002). However, it is important to bear in mind that the use of headphone testing could be considered less realistic than free-field testing.
With a relatively large body of literature suggesting that older hearing-impaired adults do have poor spatial processing ability (regardless of whether they attribute this to aging or hearing impairment), it is surprising that little research exists that attempts to link spatial processing ability to participant’s real-life performance in noise. Whether such research is carried out through the use of self-report questionnaires, such as the Speech, Spatial and Qualities of Hearing Questionnaire (SSQ; Gatehouse & Noble, 1994), or through the use of tests that more closely mimic everyday situations, it is a necessary step if the question of a causal link from spatial processing disorder to the problems reported by older adults is going to be answered.
Furthermore, if future research into the effects of aging on spatial processing suggests that older adults have poorer spatial processing ability, then it would be appropriate to turn our research focus to the question of what we can do to remediate this problem. Cameron and Dillon (2011) developed auditory training software called the LiSN & Learn (Cameron & Dillon, 2011) to provide deficit-specific remediation to normal-hearing children with spatial processing disorder. Nine children aged 6 through 11 years who were diagnosed with the LiSN-S as having spatial processing disorder trained with the LiSN & Learn software for a period of 12 weeks. At the end of the training, retesting on the LiSN-S showed that each of the children had normal spatial processing abilities. Given this finding future research investigating whether deficit-specific remediation of spatial processing disorder could be effective for older adults is warranted.
This review has highlighted the fact that current literature concerning spatial processing disorder and aging is at times contradictory; however, the majority of articles show the spatial processing ability of older adults is reduced in comparison with young adults. However, to conclude aging per se negatively effects spatial processing ability may be oversimplified or even premature given potentially confounding factors such as hearing impairment. This may well prove to be an important focus for future research, with a long-term view of developing remediation strategies to improve older adults’ speech understanding in noise.
Acknowledgments
The authors acknowledge the financial support of the HEARing CRC, established and supported under the Australian Government’s Cooperative Research Centres Program.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research was financially supported by the HEARing CRC, established and supported under the Australian Government;s Cooperative Research Centres Program. by the HEARing CRC, established and supported under the Australian Government’s Cooperative Research Centres Program.
References
- Ahlstrom J. B., Horwitz A. R., Dubno J. R. (2009). Spatial benefit of bilateral hearing aids. Ear and Hearing, 30, 203-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen K., Carlile S., Alais D. (2008). Contributions of talker characteristics and spatial location to auditory streaming. Journal of the Acoustical Society of America, 123, 1562-1570 [DOI] [PubMed] [Google Scholar]
- Arbogast T. L., Mason C. R., Kidd G., Jr. (2005). The effect of spatial separation on information masking of speech in normal-hearing and hearing-impaired listeners. Journal of the Acoustical Society of America, 117, 2169-2180 [DOI] [PubMed] [Google Scholar]
- Brown D., Cameron S., Martin J., Watson C., Dillon H. (2010). The North American Listening in Spatialized Noise–Sentence test (NA LiSN-S): Normative data and test-retest reliability study for adolescents and young adults. Journal of the American Academy of Audiology, 21, 629-641 [DOI] [PubMed] [Google Scholar]
- Cameron S., Dillon H. (2007). Development of the Listening in Spatialized Noise–Sentences Test (LISN-S). Ear and Hearing, 28(2), 196-211 [DOI] [PubMed] [Google Scholar]
- Cameron S., Dillon H. (2008). The Listening in Spatialized Noise–Sentences Test (LISN-S): Comparision to the prototype LISN and results from children with either a suspected (central) auditory processing disorder or a confirmed language disorder. Journal of the American Academy of Audiology, 19, 377-391 [DOI] [PubMed] [Google Scholar]
- Cameron S., Dillon H. (2009). Listening in Spatialized Noise–Sentences test (LiSN-S) (Version 1.014) [Computer software] Murten, Switzerland: Phonak Communications AG [Google Scholar]
- Cameron S., Dillon H. (2011). LiSN & Learn (Version 1.0) [Computer software]. Sydney, Australia: National Acoustic Laboratories [Google Scholar]
- Cameron S., Dillon H. (2011). Development and evaluation of the LiSN & Learn auditory training software for deficit-specific remediation of binaural processing deficits in children: Preliminary findings. Journal of the American Academy of Audiology, 22(10):678-96 [DOI] [PubMed] [Google Scholar]
- Cameron S., Glyde H., Dillon H. (2011). Listening in Spatialized Noise–Sentences test: Normative and retest reliability data for adolescents and adults up to 60 years of age. Journal of the American Academy of Audiology, 22(10):697-709 [DOI] [PubMed] [Google Scholar]
- Cherry E. C. (1953). Some experiments on the recognition of speech with one and two ears. Journal of the Acoustical Society of America, 25, 975-979 [Google Scholar]
- Committe on Hearing Bioacoustics, and Biomechanics. (1988). Speech understanding and aging. Journal of the Acoustical Society of America, 83, 859-895 [PubMed] [Google Scholar]
- Daneman M., Merikle P. M. (1996). Working memory and language comprehension: A meta-analysis. Psychonomic Bulletin & Review, 3, 422-433 [DOI] [PubMed] [Google Scholar]
- Divenyi P. L., Haupt K. M. (1997). Audiological correlates of speech understanding deficits in elderly listeners with mild-to-moderate hearing loss. I. Age and lateral asymmetry effects. Ear and Hearing, 18(1), 42-61 [DOI] [PubMed] [Google Scholar]
- Divenyi P. L., Stark P. B., Haupt K. M. (2005). Decline of speech understanding and auditory thresholds in the elderly. Journal of the Acoustical Society of America, 118, 1089-1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubno J. R., Ahlstrom J. B., Horwitz A. R. (2002). Spectral contributions to the benefit from spatial separation of speech and noise. Journal of Speech, Language, and Hearing Research, 45, 1297-1310 [DOI] [PubMed] [Google Scholar]
- Dubno J. R., Ahlstrom J. B., Horwitz A. R. (2008). Binaural advantage for younger and older adults with normal hearing. Journal of the American Academy of Audiology, 51, 539-556 [DOI] [PubMed] [Google Scholar]
- Ferraro J. A., Minckler J. (1977). The human lateral leminscus and its nuclei. Brain and Language, 4, 277-294 [DOI] [PubMed] [Google Scholar]
- Gatehouse S., Noble W. (2004). The Speech, Spatial and Qualities of Hearing Scale (SSQ). International Journal of Audiology, 43, 85-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates G. A., Mills J. H. (2005). Presbycusis. The Lancet, 366, 1111-1120 [DOI] [PubMed] [Google Scholar]
- Gelfand S. A., Ross L., Miller S. (1988). Sentence reception in noise from one versus two sources: Effects of aging and hearing loss. Journal of the Acoustical Society of America, 83(1), 248-256 [DOI] [PubMed] [Google Scholar]
- Hansen C., Reske-Nielsen E. (1965). Pathological studies in presbycusis. Archive of Otolaryngology Head and Neck Surgery, 82, 115-132 [DOI] [PubMed] [Google Scholar]
- Humes L. E., Watson B. U., Christensen L. A., Cokely C. G., Halling D. C., Lee L. (1994). Factors associated with individual differences in clinical measures of speech recognition among the elderly. Journal of Speech and Hearing Research, 37, 465-474 [DOI] [PubMed] [Google Scholar]
- Jerger J., Jerger S., Oliver T., Pirozzolo F. (1989). Speech understanding in the elderly. Ear and Hearing, 10(2), 79-88 [DOI] [PubMed] [Google Scholar]
- Kalikow D. N., Stevens K. N., Elliot L. L. (1977). Development of a test of speech intelligibility in noise using sentence materials with controlled word predictability. Journal of the Acoustical Society of America, 61, 1337-1351 [DOI] [PubMed] [Google Scholar]
- Kim S., Frisina R. D., Frisina D. R. (2006). Effects of age on speech understanding in normal hearing listeners: Relationship between the auditory efferent system and speech intelligibility in noise. Speech Communication, 48, 855-862 [Google Scholar]
- Kirikae I. (1969). Auditory function in advanced age with reference to histological changes in the central auditory system. International Journal of Audiology, 8, 221-230 [Google Scholar]
- Lindenberger U., Baltes P. B. (1994). Sensory functioning and intelligience in old age: A strong connection. Psychology & Aging, 9, 339-355 [DOI] [PubMed] [Google Scholar]
- Lunner T. (2003). Cognitive function in relation to hearing aid use. International Journal of Audiology, 42(Suppl. 1), S49-S58 [DOI] [PubMed] [Google Scholar]
- Marrone N., Mason C. R., Kidd G., Jr. (2008). The effects of hearing loss and age on the benefit of spatial separation between multiple talkers in reverberant rooms. Journal of the Acoustical Society of America, 124, 3064-3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. (1991). Anatomy and physiology of binaural hearing. International Journal of Audiology, 30, 125-134 [DOI] [PubMed] [Google Scholar]
- Murphy D. R., Daneman M., Schneider B. A. (2006). Why do older adults have difficulty following conversations? Psychology & Aging, 21(1), 49-61 [DOI] [PubMed] [Google Scholar]
- Nilsson M., Soli S. D., Sullivan J. A. (1994). Development of the hearing in noise test for the measurement of speech reception thresholds in quiet and noise. Journal of the Acoustical Society of America, 95, 1085-1099 [DOI] [PubMed] [Google Scholar]
- Noble W., Perrett S. (2002). Hearing speech against spatially separate competing speech vs competing noise. Perception & Psychophysics, 64, 1325-1336 [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller M. K. (1997). Language comprehension in older adults. Journal of Speech and Language Pathology and Audiology, 21, 125-142 [Google Scholar]
- Pichora-Fuller M. K., Singh G. (2006). Effects of age on auditory and cognitive processing: Implications for hearing aid fitting and audiologic rehabilitation. Trends in Amplification, 10(1), 29-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt R. A. (2010). The physiology of cochlear presbycusis. In Gordon-Salant S., Frisina R. D., Popper A. N., Fay R. R. (Eds.), The aging auditory system (pp. 9-38). New York, NY: Springer [Google Scholar]
- Schneider B. A., Pichora-Fuller M. K., Daneman M. (2010). Effects of senscent changes in audition and cognition of spoken language comprehension. In Gordon-Salant S., Frisina R. D., Popper A. N., Fay R. R. (Eds.), The aging auditory system (pp. 167-210). New York, NY: Springer [Google Scholar]
- Schneider B. A., Daneman M., Murphy D. R., See K. S. (2000). Listening to discourse in distracting settings: The effects of aging. Psychology and Aging, 15(1), 110-125 [DOI] [PubMed] [Google Scholar]
- Warren L. R., Wagener J. W., Herman G. E. (1978). Binaural analysis in the aging auditory system. Journal of Gerontoloy, 33, 731-736 [DOI] [PubMed] [Google Scholar]
- Weschler D. (1981). WAIS-R manual: Weschler Adult Intelligence Scale–Revised. New York, NY: Pyschological Corporation [Google Scholar]
- Weschler D. (1987). Weschler Memory Scale–Revised manual. New York, NY: Psychological Corporation [Google Scholar]
- Wilber L. A. (2002). Transducers for audiologic testing. In Katz J. (Ed.), Handbook of clinical audiology (pp. 88-95). Baltimore, MD: Lippincott Williams & Wilkins [Google Scholar]
- Wilson D. (1997). Hearing in South Australia: Disability, impairment and quality-of-life. Adelaide, SA: University of Adelaide Press [Google Scholar]


