Abstract
In vertebrates, perception of sound, motion, and balance is mediated through mechanosensory hair cells located within the inner ear. In mammals, hair cells are only generated during a short period of embryonic development. As a result, loss of hair cells as a consequence of injury, disease, or genetic mutation, leads to permanent sensory deficits. At present, cochlear implantation is the only option for profound hearing loss. However, outcomes are still variable and even the best implant cannot provide the acuity of a biological ear. The recent emergence of stem cell technology has the potential to open new approaches for hair cell regeneration. The goal of this review is to summarize the current state of inner ear stem cell research from a viewpoint of its clinical application for inner ear disorders to illustrate how complementary studies have the potential to promote and refine stem cell therapies for inner ear diseases. The review initially discusses our current understanding of the genetic pathways that regulate hair cell formation from inner ear progenitors during normal development. Subsequent sections discuss the possible use of endogenous inner ear stem cells to induce repair as well as the initial studies aimed at transplanting stem cells into the ear.
Keywords: stem cell therapy, inner ear, hair cell, regeneration
Introduction
Hearing loss is one of the most common disabilities in industrial nations. Approximately 12% to 17% of American adults (roughly 40 million people) report some degree of hearing loss (Lam, Lee, Gomez-Marin, Zheng, & Caban, 2006; Lee, Gomez-Marin, Lam, & Zheng, 2004, quick statistics by the National Institute on Deafness and Other Communication Disorders, 2010, http://www.nidcd.nih.gov/health/statistics/Pages/quick.aspx) and this number can be expected to increase as a result of increased life expectancy. Moreover, although a large number of children in the United States are diagnosed with conductive hearing loss due to otitis media, Eustachian tube dysfunction, or malformation of middle and external ear, between 2 and 3 out of every 1,000 children born in the United States have some degree of congenital sensorineural hearing loss (SNHL; Mehra, Eavey, & Keamy, 2009; Todd, 1994), which typically indicates damage to one or more components of the peripheral auditory pathway such as the cochlea and/or spiral ganglion neurons. SNHL is generally irreversible and can progress from mild hearing impairment to severe or profound deafness. The prevalence of hearing disorders combined with the lack of recovery makes the treatment of SNHL a major challenge in the area of otology and audiology.
Hearing aids can be used effectively for patients with moderate to severe hearing loss, however, only one out of five people who could benefit from a hearing aid actually wears one (Larson et al., 2000). For individuals with profound SNHL, a cochlear implant is the sole option for hearing rehabilitation. While the development of the cochlear implant has been remarkable, the prognosis for those individuals receiving an implant is still variable and, even with the best outcomes, normal hearing is not restored. Therefore patients with severe SNHL would welcome alternative strategies, and in particular, medical treatments for hearing rehabilitation.
In most cases of SNHL, the primary pathology is the loss of mechanosensory hair cells located within the sensory epithelium (the organ of Corti) of the cochlea. The survival and/or death of a hair cell can be influenced by exposure to a variety of factors, including loud sounds, environmental toxins, and ototoxic drugs. Aging, genetic background, and mutations in “deafness” genes also contribute to hair cell death. As will be discussed below, in mammals hair cells are only generated during a relatively brief period in embryogenesis. In the mature organ of Corti, once a hair cell dies, it is not replaced. Instead, the loss of a hair cell leads to rapid changes in the morphology of surrounding cells to seal the opening in the reticular lamina that results from the loss of a hair cell (Lenoir et al., 1999; Raphael, 2002).
In contrast with mammals, birds and all other vertebrates have been shown to add and/or regenerate hair cells and auditory function throughout their lives (Corwin, 1981, 1985; Corwin & Cotanche, 1988; Ryals & Rubel, 1988). Additional experiments demonstrated that these regenerated hair cells arise from a population of stem/progenitor cells that reside within the sensory epithelia. Similarly, whereas mammals lack the capacity to regenerate hair cells, other mammalian organs, such as skin, liver, and blood cells, are regularly regenerated. In each of these cases, the process of cell replacement is dependent on the presence of tissue-specific stem/progenitor cells that are characterized by two main properties: self-renewal and the ability to differentiate into tissue-specific cell types. These results, along with many other recent publications, have demonstrated the immense promise in the development of stem cells as therapeutic agents (Hanna, Saha, & Jaenisch, 2010; Saha & Jaenisch, 2009).
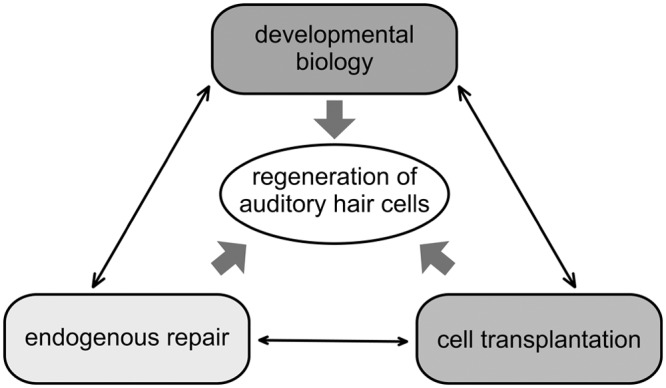
This review will focus on the current state of inner ear stem cell research from the viewpoint of their potential application in the treatment of inner ear disorders (Figure 1). First, we will discuss the anatomical development of the cochlea and introduce molecular factors that regulate inner ear developmental events that may have the potential to direct stem cells toward a hair cell fate. Next, we will discuss the phenotypic and functional features of potential stem/progenitor cells within the inner ears of both nonmammals and mammals. Finally, we will discuss existing studies in which stem cells have been transplanted into the cochlea to determine whether those cells can give rise to new hair cells or, alternatively, could be used to indirectly facilitate regenerative responses in other ways.
Figure 1.
Current approaches toward hair cell regeneration in the inner ear
Schema illustrates how mutually complementary studies promote and refine stem cell therapy for inner ear disease. Understanding of the developmental biology of sensory cells in the inner ear should provide molecular pathways for the generation of hair cells from stem/progenitor cells. Gene therapies and drug delivery systems designed for endogenous repair of inner ear sensory epithelia will affect the number of progenitor cells and their cell fate. Finally, cell transplantation into the inner ear appears to be relatively safe but has shown minor impact on reorganization of the sensory epithelia to date.
Anatomic Development of the Inner Ear
Over the past two decades, the development of innovative molecular biological techniques, in particular the creation of multiple transgenic and mutant mouse lines, has led to a remarkable increase in the number of studies examining inner ear development. As a result, our understanding of the genes and cellular interactions that regulate different aspects of inner ear morphogenesis has increased dramatically. At the same time, parallel studies of hair cell regeneration in model systems such as chicken and zebrafish have demonstrated that many of the molecular pathways required for the initial development of hair cells are recapitulated during a regenerative response. These results highlight the importance of increasing our understanding of the basic development of the inner ear. Therefore, in this section and the next, we will review the physical development of the inner ear and then discuss the present level of understanding of the molecular processes that regulate different aspects of inner ear development in the mouse, with a particular focus on factors that regulate multipotency and differentiation of inner ear sensory progenitors.
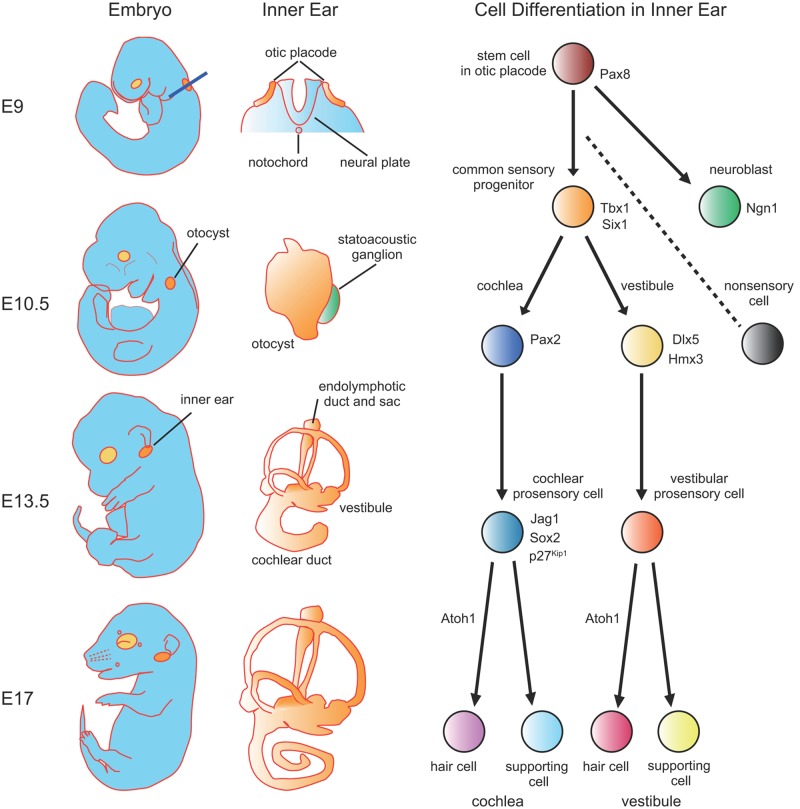
The inner ear begins as a placodal thickening (the otic placode) in the surface ectoderm that is induced by the adjacent dorsal hindbrain and the underlying mesoderm, around E8 in mice (Figure 2). Virtually all of the cells that will comprise the membranous labyrinth of the inner ear are derived from cells that were originally located in the placode. The only exceptions are melanocytes located in the stria vascularis and Schwann cells located in the spiral ganglion, both of which are derived from neural crest. Following its formation, the otic placode invaginates beneath the surface ectoderm to form the otic cup. As the invagination process continues, the lateral edges of the cup eventually converge and fuse to form a fluid filled cyst, referred to as the otocyst. Soon after the otocyst forms, a population of neuroblasts delaminate from the ventral anterior region and migrate into the adjacent mesoderm where they coalesce to form the auditory and vestibular ganglia. The specification of these otic neuroblasts represents the earliest cell fate decision that takes place in the inner ear (Fekete & Wu, 2002). Concomitant with the delamination of otic neuroblasts, gene expression studies suggest that the otocyst becomes regionally patterned along several different axes, also referred to as compartmentalization (Fekete & Wu, 2002). Soon after this period (E11), a narrow extension originating in the dorsomedial region of the otocyst begins to extend toward the brain to form the endolymphatic duct and sac. Beginning around the same time, the ventral region of the otocyst extends and coils to form the cochlear duct. Within the cochlear duct, as well as in other regions of the otocyst, cells become specified to develop as the sensory patches that will contain mechanosensory hair cells and nonsensory supporting cells. At this stage, cells within these developing sensory patches are referred to as the sensory progenitors or prosensory cells, because they are multipotent cells with the ability to give rise to different types of hair cells and supporting cells.
Figure 2.
Schematic depictions of the morphological events and cell fate decisions that occur during mammalian inner ear development
On the left anatomical development of the whole mouse embryo is illustrated with the primordia of the eye (light gray) and inner ear (dark gray) highlighted. Development of the membranous labyrinth at the corresponding embryonic stage are shown in the middle column. At E9, common inner ear stem cells form an ectodermal thickening, the otic placode, which is located adjacent to rhombomeres 5 and 6 of the neural plate. Blue line shows the cross section of the neural plate and the otic placode demonstrated in the middle column. By E10.5, the otic placode invaginates and closes to form an epithelial sac, the otocyst. Next, the otocyst undergoes morphogenetic changes, including the extension of the endolymphatic duct and sac in the posterior dorsal and the elongation of the cochlear duct in the anterior ventral. Finally, by this stage the rudiment of the statoacoustic ganglia has formed adjacent to the otocyst. At E13.5, the cochlear duct is less than a single turn and cells have reached the prosensory stage. By E17, the cochlear duct has grown nearly its complete length and sensory cell commitment is largely complete.
Immediately after specification of the cochlear prosensory domain, a subsequent important step is the regulation of the number of cells within this domain. This is particularly important in the organ of Corti where the number of hair cells and supporting cells is highly regular but apoptotic cell death is minimal. This suggests a crucial role for the regulation of cell number. Terminal mitosis within the cochlear prosensory cell population begins at the apex at E12.5 and then proceeds toward the base in a gradient. As is implied by the use of the word “terminal,” once prosensory cells become postmitotic, reentry into the cell cycle seems to be virtually impossible.
Following terminal mitosis, prosensory cells become committed to a specific cellular phenotype and then undergo cellular differentiation as a specific type of hair cell or supporting cell. In contrast to the gradient of terminal mitoses, cellular differentiation occurs initially in the base and then proceeds in a gradient toward the apex of the cochlea.
Molecular Development of the Inner Ear
At the otic placode stage, all of the cells within the placode express the transcription factors Pax8, Dlx5, and Pax2 (Noramly & Grainger, 2002). Pax8 is the first of these genes to be expressed, suggesting a possible role in otic induction. However, inner ear development is normal in Pax8 null mice (Mansouri, Chowdhury, & Gruss, 1998) indicating that the effects of Pax8 are either redundant with another gene or may be compensated for in response to deletion. In contrast with Pax8, which is broadly expressed throughout the formation of the otic vesicle, Pax2 becomes restricted to the ventral medial region by the otic vesicle stage (Nornes, Dressler, Knapik, Deutsch, & Gruss, 1990). Deleting Pax2 in the mouse inner ear leads to agenesis or severe malformation of the cochlea along with varying degrees of defects in the vestibular region of the inner ear and in the auditory and vestibular ganglia (Burton, Cole, Mulheisen, Chang, & Wu, 2004; Favor et al., 1996; Torres, Gomez-Pardo, & Gruss, 1996). Dlx5 becomes restricted to the dorsolateral compartment of otic vesicle and deletion affects the morphogenesis of sensory and nonsensory vestibular structures, characterized by the absence of one to three of the semicircular canals and a shortening of the endolymphatic duct. In addition, the utricle and saccule develop with slightly abnormal maculae (Acampora, Merlo, et al., 1999; Merlo et al., 2002). These results are consistent with roles for Pax2 and Dlx5 in the specification of ventral and dorsal structures, respectively, during the early formation of the otocyst. Unfortunately, similar insights regarding the role of Pax8 will require further studies.
As discussed, one of the first events that occurs following formation of the otocyst is the delamination of otic neuroblasts. The formation of these neuroblasts requires expression of the neurogenic basic-helix-loop-helix (bHLH) transcription factor Neurogenin1 (Ngn1) (Q. Ma, Anderson, & Fritzsch, 2000; Rubel & Fritzsch, 2002). Forced expression of Ngn1 is also sufficient to induce neuronal phenotypes within inner ear epithelial cells (Puligilla, Dabdoub, Brenowitz, & Kelley, 2010). While the factors that regulate expression of Ngn1 within the otocyst remain poorly understood, the transcription factor Tbx1, which is normally expressed in a complementary pattern to Ngn1 within the otocyst, appears to play a role in limiting the extent of Ngn1 expression. Deleting Tbx1 leads to an expansion of the Ngn1 expression domain (Jerome & Papaioannou, 2001; Raft, Nowotschin, Liao, & Morrow, 2004; Vitelli et al., 2003), whereas ectopically expressing Tbx1 suppresses Ngn1 and neuronal fates along with increasing the size of inner ear sensory structures (Funke et al., 2001).
Following neuroblast delamination, the remaining otocyst cells are thought to become compartmentalized. At least three studies have demonstrated specific defects in structures that arise from a specific region of the otocyst following deletion of a gene that is expressed within that region at the otocyst stage. Hmx3 and Dlx5 both mark the dorsolateral compartment of the otocyst (Acampora, Merlo, et al., 1999; Depew et al., 1999; Hadrys, Braun, Rinkwitz-Brandt, Arnold, & Bober, 1998; W. Wang, Van De Water, & Lufkin, 1998) and mice deficient for either of these genes show very similar semicircular canal malformations, structures that arise from this region. Otx1 is expressed in a posteroventrolateral domain of the otocyst that includes the presumptive lateral crista, and Otx1-null mutant mice lack the lateral crista as well as the associated lateral canal (Acampora, Avantaggiato, et al., 1999; Morsli et al., 1999). Finally, as discussed, deleting Pax2, which becomes restricted to the medial wall of the otocyst, results in agenesis of the entire auditory apparatus, but vestibular structures usually develop normally (Torres et al., 1996).
Recently, studies have identified two genes, Sox2 and Jagged1 (Jag1), that are expressed in the sensory progenitor domain prior to sensory cell formation and play key roles in the establishment of the prosensory cell population (Kiernan et al., 2005; Lewis, Frantz, Carpenter, de Sauvage, & Gao, 1998; Morrison, Hodgetts, Gossler, Hrabe de Angelis, & Lewis, 1999). A. E. Kiernan et al. (2005) reported that the prosensory cell population is absent in the inner ears of mice in which the transcription factor Sox2 is not expressed in the inner ear. Similarly, deleting Jag1, a ligand for Notch receptors, within the inner ear leads to a significant reduction in the number of hair cells and supporting cells suggesting a role similar to that of Sox2 (Kiernan, Xu, & Gridley, 2006). Analyses of prosensory cell markers indicated a significant or complete loss in both Sox2 and Jag1 mutants, supporting the hypothesis that prosensory cells are absent or significantly reduced in both mutants. However, recent studies in which all Notch signaling was eliminated from the inner ear through deletion of the Notch-effecter gene, Rbpj, indicated that the initial expression of Sox2 is largely normal (Basch, Ohyama, Segil, & Groves, 2011; Yamamoto, Chang, & Kelley, 2011). These results suggest that Sox2 probably acts upstream of Jag1 and Notch signaling in the formation of prosensory cells but that Notch is required for maintenance of the prosensory domain. Gain of function experiments in which either Sox2 or Notch signaling has been ectopically induced within nonsensory cells of the inner ear indicate that expression of either pathway is sufficient to induce the formation of ectopic prosensory cells (Dabdoub et al., 2008; Daudet & Lewis, 2005; Hartman, Reh, & Bermingham-McDonogh, 2010; Pan, Jin, Stanger, & Kiernan, 2010). Overall these results suggest that Sox2 and/or Notch signaling directs otocyst cells toward a prosensory fate.
As discussed, terminal mitosis plays a key role in the determination of the number of prosensory cells that will develop within the cochlea. Extensive previous research has identified a number of molecules that play a role in regulation of the cell cycle both during development and in cancer. The first of these to be examined in the cochlea was the cyclin-dependent kinase inhibitor, Cdkn1b (also known as p27kip1). Cdkn1b is initially expressed in a pattern that essentially matches, but slightly precedes, the pattern of terminal mitoses and deletion of Cdkn1b leads to prolonged proliferation and an overproduction of hair cells and supporting cells (Chen & Segil, 1999; Lowenheim et al., 1999). These results demonstrate the importance of cell cycle control and, in particular Cdkn1b, in regulating the number of prosensory cells. However, even in the absence of Cdkn1b, terminal mitosis of prosensory cells is only delayed by several days, indicating that other cell cycle regulators are also important (Sage et al., 2005). In fact, additional results have demonstrated similar roles for other cell cycle regulators, including Rb, Rbl2 (p130), Cdkn2d (Ink4d), Cdkn1a (p21cip1) and CyclinD in the inhibition or promotion of proliferation in both prosensory cells and differentiated hair cells and supporting cells (Laine et al., 2007; Laine, Sulg, Kirjavainen, & Pirvola, 2010; Mantela et al., 2005; Rocha-Sanchez et al., 2011).
The final key step in cochlear development to be discussed is the commitment of prosensory cells to a hair cell fate. A number of studies have demonstrated that the bHLH protein Atoh1 (formerly known as Math1) is a commitment factor that drives prosensory cells to develop as hair cells. All hair cells, both auditory and vestibular, are absent in Atoh1 mutant mice (Bermingham et al., 1999; Woods, Montcouquiol, & Kelley, 2004) and forced expression of Atoh1 is sufficient to induce a hair cell fate both within the prosensory domain (Jones, Montcouquiol, Dabdoub, Woods, & Kelley, 2006) and even in nonsensory regions of the cochlea (Zheng & Gao, 2000). These results indicate that Atoh1 is both necessary and sufficient for hair cell formation and suggest that Atoh1 acts at a very early time point in the molecular cascade that leads to hair cell formation.
It is important to consider that the number of cells that will develop as hair cells is not solely determined by Atoh1. Soon after Atoh1 expression is initiated within the prosensory population, Notch signaling is also activated as a result of the onset of expression of several Notch ligands within developing hair cells (Lanford, Shailam, Norton, Gridley, & Kelley, 2000; Murata, Tokunaga, Okano, & Kubo, 2006; Zine, Van De Water, & de Ribaupierre, 2000). Notch activation acts to prevent some Atoh1-positive cells from developing as hair cells, as disruption of Notch signaling in late embryonic or neonatal cochleae results in an increase in the number of cells that maintain Atoh1 expression and become committed to develop as hair cells (Lanford et al., 2000; Takebayashi et al., 2007; Yamamoto et al., 2006; Zheng, Shou, Guillemot, Kageyama, & Gao, 2000). This change occurs as a result of disrupting lateral inhibition between adjacent prosensory cells and is presumed to also play a role in forming the hair cell-supporting cell mosaic. In addition, several other factors have been shown to regulate Atoh1 expression within prosensory cells. The prosensory factor Sox2, a family of bHLH-related molecules known as inhibitors of differentiation (Ids), Ngn1, and Fibroblast Growth Factor 3 (Fgf3) also regulate hair cell number by modulating Atoh1 expression (Dabdoub et al., 2008; Jones et al., 2006; Millimaki, Sweet, Dhason, & Riley, 2007; Raft et al., 2007).
In summary, our understanding of inner ear development has expanded dramatically in the last 10 years, particularly in terms of identifying how signaling and transcriptional pathways are coordinated to direct cells from the otic placode stage to mature sensory epithelia. In particular, Atoh1 and Notch signaling have emerged as potential targets that could be modulated to direct stem cells toward a hair cell fate in potential regenerative therapies (Lin et al., 2011; G. P. Wang et al., 2010; Zhao et al., 2011).
General Features of Stem Cells
A stem cell is basically defined by two characteristics: self-renewal, the ability to give rise to new stem cells, and multipotency, the ability to differentiate into multiple cell types. Embryonic development is dependent on a population of stem cells that arise from the single fertilized egg. The initial cells within the early blastocyst are truly totipotent in that they give rise to both extraembryonic structures such as the placenta, as well as every cell type within the adult. This totipotency is lost after the first few cell divisioins, with embryonic stem cells (ESCs) isolated from the inner mass of the blastocyst retaining the capacity to contribute to the three germ layers of the embryo (ectoderm, mesoderm, and endoderm), but losing the capacity to generate extraembryonic structures (Hayashi & Surani, 2009; Surani, Hayashi, & Hajkova, 2007). As development continues, the ESCs within the inner cell mass continue to divide to give rise to additional cells that will become progressively restricted to a specific lineage and, in most cases, ultimately to a specific cell type. Once these cells are fully differentiated, they are no longer considered stem cells. However, as will be discussed below, stem cells are retained in some adult tissues.
Adult tissue-specific stem cells, also referred to as somatic stem cells, are small cell populations that reside in somatic tissues. These cells can self-renew and differentiate into specialized cell types. They function to maintain tissue homeostasis throughout life and can also play a role in tissue regeneration. Bone marrow contains a population of hematopoietic stem cells (HSCs), derived from early ESCs, that generate different blood cell types throughout life (Huang, Cho, & Spangrude, 2007). Tissue-specific stem cells have also been identified in a variety of tissues such as the brain, lung, and liver (C. F. Kim et al., 2005; Landgren & Curtis, 2010; Schwartz & Verfaillie, 2010). However, the regenerative ability of each of these stem cell populations is highly variable.
In 2006, stem cell research took a significant step forward when it was demonstrated that forcing the expression of only four genes, Sox2, Pou5f1, Klf4, and Myc, is sufficient to induce pluripotency in many different adult cell types, including easily obtained cells such as fibroblasts (Takahashi et al., 2007; Takahashi & Yamanaka, 2006). These induced pluripotent stem cells (iPSCs) offer several advantages over ESCs. In particular, ethical concerns are significantly reduced and because iPSCs can technically be created from any individual, cells derived from those iPSCs can be transplanted back into the same person without concern of immunological rejection. While there are differences between ESCs and iPSCs that we are only beginning to understand, the ability to generate iPSCs provides the potential to use fibroblasts from any individual to generate replacement cell types that could then be transplanted back into that person.
Stem Cells in Nonmammalian Inner Ears
As we have discussed, in contrast with mammals, all other vertebrates apparently retain the ability to generate hair cells throughout their adult lives. This phenomenon has been best studied in two different systems: the auditory epithelium of birds, referred to as the basilar or avian papilla, and the lateral line neuromasts in zebrafish. Both of these systems provide evidence for the presence of multipotent, self-renewing cells that retain the ability to develop as new hair cells and supporting cells. In the avian papilla, hair cell loss causes two different responses in surrounding supporting cells. In some cases, a subset of these cells will reenter the cell cycle and divide, giving rise to daughters that can differentiate as new hair cells and/or supporting cells (Corwin & Cotanche, 1988; Ryals & Rubel, 1988). In other cases, a surrounding supporting cell(s) will undergo a phenotypic change in which the cell directly develops into a hair cell with no intervening proliferation, a process referred to as transdifferentiation (Adler & Raphael, 1996; L. Li & Forge, 1997; Roberson, Alosi, & Cotanche, 2004). These observations suggest that the avian supporting cell population may be heterogenous with some cells terminally differentiated while others exist as stem/progenitor cells. The demonstration that individual birds may mount multiple hair cell regenerative events over the course of their lives (Oesterle & Rubel, 1993) suggests that at least some of the cells within the avian papilla must be stem cells. However, studies in which different mitotic tracers have been used to visualize the population of cells that reenter the cell cycle following repeated insults indicates that supporting cells that divide in response to one insult rarely divide in response to a second (Stone, Choi, Woolley, Yamashita, & Rubel, 1999), suggesting that a distinct stem cell population may not exist within the papilla. Instead, a large population of supporting cells may retain some limited stem cell-like abilities. The molecular signaling pathways that enable avian supporting cells to reenter the cell cycle and to differentiate as new hair cells remains to be determined, but gene profiling experiments have identified some potentially interesting candidates (Hawkins et al., 2003, 2007). Perhaps not surprisingly, many of the developmental signaling pathways and molecules discussed in the previous section are reactivated during regeneration including Pax, Notch, Atoh1, and Cdkn1b (Hawkins et al., 2007).
The zebrafish lateral line provides an intriguing model for the study of hair cell regeneration. The lateral line comprises a series of mechanosensitive clusters of hair cells, referred to as neuromasts, arrayed along the surface of the animal. As is the case for the avian papilla, damaged hair cells are replaced through a regenerative process. In addition, because the neuromasts are located on the surface of the animal rather than imbedded in the skull, direct visualization of regeneration is possible. Each neuromast contains three distinct cell types: hair cells, supporting cells that are located in direct contact with hair cells, and a second population of supporting cells, referred to as mantle cells that are located at the periphery of the neuromast. Time lapse videos of ongoing regeneration supports observations in the chick, indicating that lost hair cells can be replaced through either transdifferentiation of surrounding putative supporting cells or through proliferation of supporting or mantle cells (Hernandez, Moreno, Olivari, & Allende, 2006; E. Y. Ma, Rubel, & Raible, 2008). Interestingly, a recently published study has identified Phoenix as a gene that is required for supporting cell proliferation and, therefore, for hair cell regeneration, but that does not seem to play a role in other regenerative events (Behra et al., 2009). These results suggest that supporting cells can act as stem cells in hair cell epithelia of nonmammalian vertebrates. Moreover, the ability to visualize living lateral line neuromasts, along with an extensive battery of genetic and transgenic zebrafish lines should lead to significant insights in coming years.
Is There Any Evidence for Stem Cells in the Mammalian Inner Ear?
Functional regeneration does not occur in the mammalian inner ear. Does this necessarily mean that the sensory epithelia of the mammalian inner ear are devoid of regenerative ability and/or stem cells? This possibility was initially pursued in two studies in which the possibility of limited regeneration was examined in adult utricles from guinea pigs and humans (Forge, Li, Corwin, & Nevill, 1993; Warchol, Lambert, Goldstein, Forge, & Corwin, 1993). In an in vitro study, Warchol et al. (1993) treated explant cultures of the utricular sensory epithelia established from either adult guinea pigs or humans (obtained through labyrinthectomy surgery related to acoustic neuromas) with concentrations of aminoglycoside antibiotics that were sufficient to kill hair cells. Based on the regenerative process in the avian papilla, cellular proliferation was assayed using a mitotic tracer. A limited number of proliferative cells were observed within the epithelia within 2 to 4 days of the end of the aminoglycoside treatment and a small number of possibly regenerated hair cells were observed after 4 weeks. In an accompanying study, Forge et al. (1993) found evidence of a limited number of immature hair cells in the utricles of guinea pigs that had been treated with aminoglycosides in vivo. Subsequent work from several laboratories presented evidence for both limited proliferation and transdifferentiation in adult utricles in response to hair cell loss (Rubel, Dew, & Roberson, 1995; Stone, Oesterle, & Rubel, 1998). While the number of new hair cells generated is apparently insufficient for a recovery of function, the results are consistent with the presence of a small number of stem/progenitor cells within the adult utricular epithelia.
This conclusion was supported by demonstrating that a subset of isolated cells from an adult mouse utricle will proliferate and form spheres, a hallmark of stem cell identity (H. Li, Liu, & Heller, 2003). Moreover, the progeny of these spheres were shown to be multipotent with the ability to give rise to a variety of cell types, including mechanosensory hair cells when transplanted into an appropriate environment, such as the developing chick otocyst. Therefore, these cells, while small in number (representing only 0.025% of the cells within a utricle), posses the most important characteristics of stem cells, capacity for self-renewal and multipotency. More recently, a study was conducted to determine whether similar cells are present within the mouse cochlear epithelium. Using a similar technique, sphere-forming cells were isolated from neonatal cochleae of mice (Oshima et al., 2007). However, the number of cochlear cells with the ability to form spheres declines rapidly such that by the third postnatal week sphere-forming cells can no longer be isolated, suggesting that stem cells are no longer present in this epithelium. It is important to consider that in both of these studies, inner ear stem cells were identified based on function, rather than morphology. Whereas this method of identification is ultimately more relevant, it would be very useful to visualize the number and location of stem cells within different inner ear epithelia. In other systems, stem cells can be marked based on expression of specific cell surface markers. If similar markers could be identified for inner ear stem cells, it might be possible to trace these cells backwards in time to their point of origin within the otocyst, as well as to characterize their potentially unique pattern of gene expression. Whereas the identities of inner ear stem cells have not been confirmed, recent results provided some insights regarding their possible positions and developmental potential. As discussed, the cell cycle inhibitor, Cdkn1b is initially expressed in all cochlear prosensory cells, but shortly after birth expression is restricted to supporting cells (Chen & Segil, 1999). A transgenic mouse expressing GFP under control of the Cdkn1b promoter was used to isolate the supporting cell population from postnatal mouse cochleae by fluorescence-activated cell sorting (White, Doetzlhofer, Lee, Groves, & Segil, 2006). When these cells were cocultured with periotic mesenchymal cells, a subset were able to reenter the cell cycle and give rise to daughter cells that expressed the hair cell marker Myosin VI, suggesting that, at least in vitro, purified postnatal mammalian supporting cells can divide and transdifferentiate into hair cells through a combination of mitotic and nonmitotic mechanisms. In addition, the same study suggested that two specific types of supporting cells, pillar cells and Hensen’s cells, may possess a significantly greater potential to develop as hair cells. Whereas the ability of supporting cells to develop as hair cells in vitro was lost with age, the results were consistent with the hypothesis that in the early postnatal cochlea, some supporting cells retain stem cell characteristics.
A final study that may support the idea that some supporting cells retain “stem cell-like” characteristics is work in which an adenoviral vector forced the expression of Atoh1 in chemically damaged cochleae from adult guinea pigs (Izumikawa et al., 2005). Following 2 months of recovery, Atoh1-treated ears showed significantly better hearing by auditory brainstem response measurements by comparison with ears that received no treatment or infection with a virus that expressed only GFP. Scanning electron microscopy also showed numerous stereocilliary bundles in the Atoh1-treated ears. The authors speculated that Atoh1 treatment caused hair cell regeneration and improved hearing in deafened animals although they also mentioned that the ability of the tissue to respond to forced expression of Atoh1 and to reorganize the new hair cells generated by Atoh1 may depend on the mechanism of deafening, the timing of treatment after deafening, and the degree of differentiation of the surviving nonsensory cells. There are some significant issues to be considered regarding this study. One plausible alternative explanation for the improved hearing and stereocilliary bundle morphology is that Atoh1 acted as a protective factor for the hair cells during the apoptotic process that follows an ototoxic insult. While further studies are clearly required, these results could suggest the presence of progenitor or stem cell-like cells that retain some potential to develop as mechanosensory hair cells within the mature cochlea.
Another possible source of stem cells within the cochlea are bone marrow–derived cells that are present in adult mouse cochleae in a noninjured state (Lang et al., 2006; Okano et al., 2008). The results of these studies suggest that mesenchymal cells in the adult inner ear are derived continuously from HSCs in the bone marrow, and replaced slowly over a period of months. Although the majority of HSC-derived cells within the inner ear differentiate into fibrocytes or resident macrophages, the possibility that some of these HSCs could develop as replacement of hair cells remains an intriguing hypothesis. Hirose et al. (2005) reported that bone marrow-derived cells are attracted to the cochlear sensory epithelium when the cochlea is insulted by acoustic overstimulation. The same study also showed that the majority of these cells are inflammatory cells, such as macrophages. However, because inflammation and tissue-repair seem to be closely related processes, it might be possible to induce these HSCs to develop with alternate cell fates under some circumstances.
In summary, stem/progenitor cells are clearly present in inner ear sensory epithelia of nonmammalian vertebrates and these cells can generate a significant regenerative response. In contrast, in the mammalian inner ear, stem/progenitor cells are present in young epithelia, but their numbers decrease significantly (in the cochlea they apparently drop to zero) over time. HSCs can also be found in the mature inner ear, but to date these cells have not been found to develop as hair cells.
Can Stem Cells Develop as Functional Hair Cells?
As discussed, the ability to isolate, expand, and then direct stem cells toward a specific cell fate holds great potential for both clinical and basic auditory research. However, a key step in this process is the demonstration that an isolated stem cell can develop as a functional hair cell. Oshima et al. (2010) recently demonstrated that a stepwise protocol that mimics early embryonic development can be used to direct ESCs toward an inner ear, and, subsequently, a hair cell fate. ESC-induced hair cells generated in this manner express genes that are consistent with hair cell formation and develop a stereocilliary bundle-like protrusion that is consistent with a hair cell phenotype. Most importantly, electrophysiological studies demonstrate functional stereociliary bundles and mechanosensitivity in these ESC-derived hair cells. While these results offer definitive proof of the ability to drive ESCs to develop as functional hair cells in vitro, it is important to consider several caveats. First, the differentiation protocol required the use of an undefined, and potentially contaminating, population of chicken stromal cells and second, even with the inclusions of these cells, only 0.36% of the plated ESCs developed as hair cells. Clearly, techniques must be developed to significantly enhance the efficiency of hair cell induction and to purify the ESC-derived hair cells from surrounding nonhair cell ESCs and chicken stromal cells.
In the same study, Oshima et al. (2010) applied the same differentiation protocol to iPSCs derived from mouse fibroblasts. iPSC-derived hair cells appeared indistinguishable from ESC-derived hair cells, however, the efficiency of induction was reduced to 0.24%. Finally, S. J. Jeon et al. (2007) showed that endogenous bone marrow–derived stem cells can be induced to differentiate into cells with hair cell characteristics using a combination of treatment with growth factors to induce a neural progenitor phenotype followed by forced expression of the transcription factor, Atoh1. However, these cells were not characterized physiologically.
Overall, the results of these studies demonstrate that different types of stem cells can successfully be directed to a hair cell fate. However, the relative inefficiency of hair cell induction along with the requirement for unknown factors from chick stromal cells indicates that there is still much to be learned. The ability to generate significant numbers of hair cells from stem cells has the potential to contribute to breakthroughs in the search for additional factors that regulate hair cell development and function, as well as the identification of genetic, pharmacological, and environmental factors that may cause hair cell damage or malfunction.
Transplantation of Stem Cells to the Inner Ear
As discussed above, activation of an endogenous stem cell population within the inner ear would probably represent the ideal source for repair of the sensory epithelia. However, existing data suggest that the number of endogenous stem cells in a mature cochlea may be low to nonexistent. Moreover, the procedures/treatments needed to activate these cells remain unknown. Therefore, an alternative strategy might be to transplant exogenous stem cells into the inner ear (Figure 3). This approach would offer several advantages, such as the ability to activate and bias stem cells toward an inner ear or hair cell fate prior to transplantation, as well as several challenges, with the foremost being the difficulty in targeting transplanted cells to the correct portion of the cochlea. These issues will be discussed below.
Figure 3.
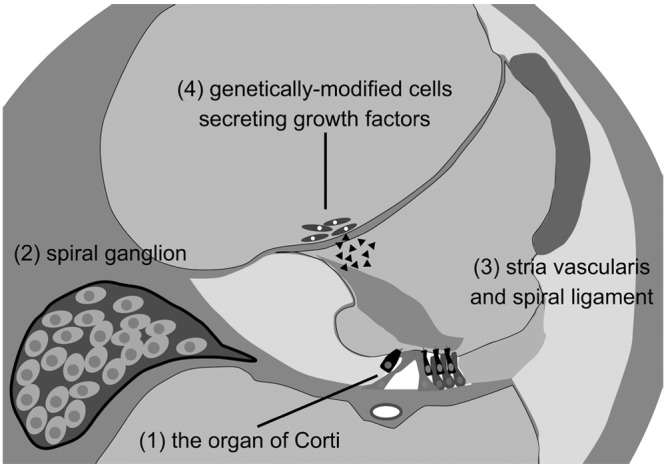
Targets for cell transplantation into the inner ear
(1) Delivering cells directly to the organ of Corti would be ideal but poses several challenges related to its small size and relative inaccessibility. (2) Transplantation of stem cells to the spiral ganglion appears to be a more promising approach. Increasing the number of neurons within the ganglion could benefit the efficacy of cochlear implant. (3) Stria vascularis and spiral ligament. A substantial percentage of congenital hearing loss is caused by mutations in genes related to ion transport, gap junctional communication, and endolymphatic homeostasis. Therefore, transplantation of stem cells that express wildtype alleles of some of these genes could be an option for the treatment of hearing loss. (4) A final strategy might be to transplant genetically engineered cells that produce and secrete factors that might protect hair cells or attract spiral ganglion peripheral axons into the scala vestibuli or scala media.
Beginning with a pioneering study by Ito, Kojima, and Kawaguchi (2001), numerous laboratories have transplanted different forms of stem cells into the inner ear (Han et al., 2010; Hildebrand et al., 2005; Parker et al., 2007; Sakamoto et al., 2004; Tateya et al., 2003). The results obtained to date have been varied. Whereas some of the transplanted cells have been shown to develop as potentially relevant cell types, including neurons, glia, and possibly hair cells and supporting cells, a far greater number of cells cannot be accounted for within several weeks of a transplant. Whether these cells die or leave the ear to ultimately settle elsewhere in the body is a crucial question that must be resolved. Moreover, assessing differentiation of specific cell types has relied heavily on cell type-specific markers, leaving open the issue of whether any of these cells are functional.
These results emphasize some of the issues that must be addressed before transplantation can be considered as a possible therapy for hair cell regeneration. Targeting of stem cells to the sensory epithelium represents a significant challenge. The hypothesis that stem cells might be targeted or attracted to sites of injury through the possible release of trophic factors, as has been reported in some instances (Kollet et al., 2003), has not been supported. Moreover, the physical structure of the organ of Corti may include some daunting impediments to stem cells. Hair cell function depends on the maintenance of an electrochemical gradient between the endolymphatic and perilymphatic spaces. The series of tight junctions between the lumenal surfaces of hair cells and supporting cells that form the reticular lamina normally acts as the boundary between these spaces. When a hair cell is lost, the reticular lamina is disrupted, leading to a potential breach. To prevent such a breach, supporting cells surrounding a lost hair cell expand their lumenal processes to form a phalangeal scar that is thought to seal the opening. It seems likely that this scar would prevent or limit transplanted stem cells from gaining access to the organ of Corti from the endolymph-containing scala media. In addition, endolymph contains unusually high levels of potassium, which could be toxic to cells transplanted within this compartment. The basal surfaces of the supporting cells are positioned on the basilar membrane, a basement membrane composed of various forms of collagen. This membrane would also seem to pose a significant impediment for stem cell access from the scala tympani.
In addition to the challenges listed above, it is also important to consider that hair cells are not homogenous along the length or width of the cochlea. Longitudinal gradients in cellular morphology correspond with changes in frequency tuning while inner and outer hair cells are located in specific positions along the medial-to-lateral axis of the duct. Finally, to provide meaningful recovery of function, new hair cells must attract and form functional synapses with tonotopically appropriate spiral ganglion neurons. Whereas each of these factors has the potential to serve as a significant hurdle in the use of stem cells to replace lost hair cells, it is important to consider that appropriate hair cell identities and synaptic connections occur autonomously during development, suggesting that necessary instructive cues for hair cell phenotype and synaptic morphology may still be present within the epithelium. This is certainly the case in birds, where regenerated hair cells develop with location-specific stereocilia and make connections with tonotopically appropriate afferent neurons (Park, Girod, & Durham, 2002). Further experiments are clearly required to determine the nature of these signals and whether similar signals persist in mammalian inner ears.
Alternative Uses of Stem Cells for Restoring Hearing in the Inner Ear
Another possible application for stem cells may be for the regeneration of spiral ganglion neurons. In humans, loss of spiral ganglion neurons is generally correlated with hearing loss (Zimmermann, Burgess, & Nadol, 1995) although there are reports of patients with significant numbers of spiral ganglion neurons despite long periods of profound deafness (Linthicum & Fayad, 2009; Teufert, Linthicum, & Connell, 2006). In addition, spiral ganglion function is disrupted in some patients with auditory neuropathy spectrum disorder (ANSD; Manchaiah, Zhao, Danesh, & Duprey, 2011; Starr, Picton, Sininger, Hood, & Berlin, 1996). Finally, the function and efficacy of a cochlear implant depends on the presence of residual spiral ganglion neurons although a strict correlation between ganglion number and outcome has not been established (Nadol, 1997). These observations suggest that functional recovery of spiral ganglion neurons has the potential to enhance hearing rehabilitation in a number of different clinical contexts.
A number of laboratories have successfully derived neurons from stem cells and demonstrated that these cells will extend neurites toward the organ of Corti and innervate hair cells both in vitro (T. S. Kim et al., 2005; Matsumoto et al., 2008; Shi, Corrales, Liberman, & Edge, 2007) and in vivo (Coleman et al., 2006; Corrales et al., 2006; Hu et al., 2005; Okano et al., 2005). However, to date none of these cells have been shown to form a functioning neuronal circuit or induce recovery of auditory function. These results, along with the comparatively easy surgical access to Rosenthal’s canal, the site of the spiral ganglion, suggests that stem cell-derived neurons could be used to reinnervate hair cells in ANSD patients with loss of spiral ganglion neurons or to form contacts with the electrodes of a cochlear implant. But, several important aspects of spiral ganglion anatomy must be considered. As discussed, tonotopically related gradients are present in spiral ganglion neurons although the roles of these gradients in auditory function are not completely clear. In addition, spiral ganglion neurons have a relatively unique bipolar morphology and any regenerated neurons would not only need to form appropriate connections with hair cells but would also need to establish appropriate contacts in specific target fields in the auditory brain stem.
The stria vascularis and spiral ligament could be additional targets for stem cell–based therapies. Mutations in genes coding for different junctional complexes within the stria vascularis or spiral ligament, lead to hearing loss (Nickel & Forge, 2008). In addition, an age-related loss of fibrocytes in the spiral ligament has been reported in the basal portion of the cochlea in C57Bl/6 mice (Hequembourg & Liberman, 2001), and acoustic overstimulation induces similar fibrocyte loss (Hirose & Liberman, 2003). Transplanting stem cells encoding a wild type version of one of these genes could lead to repopulation and reorganization of the cellular components of the stria vascularis or spiral ligament. However, virtually nothing is known about the mechanism(s) underlying differentiation of different cell types within these structures. Therefore, it remains to be determined whether disorders in these structures could be treated with stem cells.
A final way in which stem cell transplantation might be used to treat hearing loss is to use genetically modified stem cells as vectors to produce factors that promote regeneration or regrowth of specific structures within the inner ear. This concept would be especially well suited for promotion of endogenous regeneration or repair through a secreted paracrine or endocrine factor such as a hormone or growth factor. For instance, spiral ganglion neurons have been shown to grow toward a source of a neurotrophin such as BDNF or NT3 (Agerman et al., 2003; Liebl, Tessarollo, Palko, & Parada, 1997; Qun, Pirvola, Saarma, & Ylikoski, 1999; Tessarollo, Coppola, & Fritzsch, 2004), suggesting that a source of one of these factors could promote regrowth. Moreover, since the inner ear contains three fluid-filled compartments, secreted factors produced by transplanted cells could potentially diffuse throughout a particular compartment. Although the development of this type of stem cell technology is at an early stage (Hakuba et al., 2005; Kesser & Lalwani, 2009; Okano, Nakagawa, Kita, Endo, & Ito, 2006), it seems possible that such an approach could be used to improve the performance of cochlear implants in the future. Alternatively, genetically engineered stem cells secreting specific metabolic and/or humoral cues could be combined with a transplant of endogenous stem cells to augment regeneration in the inner ear.
Future Directions
Our understanding of the basic developmental pathways underlying hair cell formation has increased significantly in recent years. The ability to modify the mouse genome to delete specific genes either throughout the animal or in just a subset of tissues has become an indispensable tool in inner ear research. These technologies, along with a rapidly growing understanding of mutations underlying nonsyndromic hereditary deafness in humans, have significantly advanced our understanding of hair cell formation. However, the developmental regulation of many important aspects of hair cell formation and function remain unknown. For example, while the Atoh1 gene seems to be sufficient for hair cell formation, the downstream pathways that specify hair cell type (vestibular vs. auditory, inner vs. outer) are completely unknown. Similarly, the mechanisms that determine gradients in hair cell morphology along both the basal-to-apical and medial-to-lateral axes of the cochlea have not been determined. The specific factors that initiate Atoh1 expression are just now beginning to be identified (Shi, Cheng, Wang, & Edge, 2010) and it is still not clear which cells within the embryonic inner ear have the ability to develop as hair cells. The regulators that direct embryonic cells along a lineage that ultimately leads to formation of a hair cell are understood at only a basic level (Oshima et al., 2010). However, identifying these factors would have significant implications for specifying endogenous inner ear stem cells or their efficient differentiation of iPSCs as hair cell progenitors. Comparisons of the genetic pathways that are activated during the embryonic development of hair cells in mammals or hair cell regeneration in birds suggest that the molecular mechanisms are largely conserved (Cafaro, Lee, & Stone, 2007; Daudet et al., 2009). Yet mammals have lost the ability to reinitiate these programs. Are there mechanisms that suppress proliferation and regeneration of sensory cells in the mammalian inner ear, as is the case for other types of adult epithelia (Gurtner, Callaghan, & Longaker, 2007)? If so, what are they? And, can we manipulate these pathways safely? The discovery of adult stem cells in the inner ear, as well as the ability to manipulate ESCs and iPSCs has opened an entire new avenue for hair cell regeneration. But, a better understanding of the specific factors that specify cells as hair cells is a crucial next step. Transplanting stem cells into the inner ear indicates that simple injection of stem cells into the cochlea is insufficient to regenerate a substantial number of hair cells or reconstitute a functional sensory epithelium. Clearly we must be able to exert more specific control over the fates of any cell types prior to introducing them into the ear. An important first step will be to define inner ear, stem and progenitor cells based on immunocytological markers, localization, clonal activity, and cell-type potency. In addition, as discussed, we believe that alternative strategies for the use of stem cells, such as for the regeneration of spiral ganglion neurons or in combination with conventional therapies such as cochlear implantation, should also be considered. Recent progress in the fields of development, regeneration, and stem cell biology has been more rapid and remarkable than at any other time. Although there is still much work to be done, we believe that the future for stem cell therapy is bright.
Acknowledgments
The authors would like to thank Thomas Coate for reading an earlier version of this article and to apologize to any colleague whose work was not discussed or cited as a result of space limitations.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by funds from the Intramural Program at the National Institute on Deafness and Other Communication Disorders (NIDCD).
References
- Acampora D., Avantaggiato V., Tuorto F., Barone P., Perera M., Choo D., . . . Simeone A. (1999). Differential transcriptional control as the major molecular event in generating Otx1-/- and Otx2-/- divergent phenotypes. Development, 126, 1417-1426 [DOI] [PubMed] [Google Scholar]
- Acampora D., Merlo G. R., Paleari L., Zerega B., Postiglione M. P., Mantero S., . . . Levi G. (1999). Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development, 126, 3795-3809 [DOI] [PubMed] [Google Scholar]
- Adler H. J., Raphael Y. (1996). New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neuroscience Letters, 205(1), 17-20 [DOI] [PubMed] [Google Scholar]
- Agerman K., Hjerling-Leffler J., Blanchard M. P., Scarfone E., Canlon B., Nosrat C., Ernfors P. (2003). BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development, 130, 1479-1491 [DOI] [PubMed] [Google Scholar]
- Basch M. L., Ohyama T., Segil N., Groves A. K. (2011). Canonical Notch signaling is not necessary for prosensory induction in the mouse cochlea: Insights from a conditional mutant of RBPjkappa. Journal of Neuroscience, 31, 8046-8058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behra M., Bradsher J., Sougrat R., Gallardo V., Allende M. L., Burgess S. M. (2009). Phoenix is required for mechanosensory hair cell regeneration in the zebrafish lateral line. PLoS Genetics, 5, e1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham N. A., Hassan B. A., Price S. D., Vollrath M. A., Ben-Arie N., Eatock R. A., . . . Zoghbi H. Y. (1999). Math1: An essential gene for the generation of inner ear hair cells. Science, 284, 1837-1841 [DOI] [PubMed] [Google Scholar]
- Burton Q., Cole L. K., Mulheisen M., Chang W., Wu D. K. (2004). The role of Pax2 in mouse inner ear development. Developmental Biology, 272, 161-175 [DOI] [PubMed] [Google Scholar]
- Cafaro J., Lee G. S., Stone J. S. (2007). Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Developmental Dynamics, 236, 156-170 [DOI] [PubMed] [Google Scholar]
- Chen P., Segil N. (1999). p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development, 126, 1581-1590 [DOI] [PubMed] [Google Scholar]
- Coleman B., Hardman J., Coco A., Epp S., de Silva M., Crook J., Shepherd R. (2006). Fate of embryonic stem cells transplanted into the deafened mammalian cochlea. Cell Transplant, 15, 369-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales C. E., Pan L., Li H., Liberman M. C., Heller S., Edge A. S. (2006). Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: Growth of processes into the organ of Corti. Journal of Neurobiology, 66, 1489-1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin J. T. (1981). Postembryonic production and aging in inner ear hair cells in sharks. Journal of Comparative Neurology, 201, 541-553 [DOI] [PubMed] [Google Scholar]
- Corwin J. T. (1985). Perpetual production of hair cells and maturational changes in hair cell ultrastructure accompany postembryonic growth in an amphibian ear. Proceedings of the National Academy of Sciences of the United States of America, 82, 3911-3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin J. T., Cotanche D. A. (1988). Regeneration of sensory hair cells after acoustic trauma. Science, 240, 1772-1774 [DOI] [PubMed] [Google Scholar]
- Dabdoub A., Puligilla C., Jones J. M., Fritzsch B., Cheah K. S., Pevny L. H., Kelley M. W. (2008). Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proceedings of the National Academy of Sciences of the United States of America, 105, 18396-18401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N., Gibson R., Shang J., Bernard A., Lewis J., Stone J. (2009). Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Developmental Biology, 326(1), 86-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N., Lewis J. (2005). Two contrasting roles for Notch activity in chick inner ear development: Specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development, 132, 541-551 [DOI] [PubMed] [Google Scholar]
- Depew M. J., Liu J. K., Long J. E., Presley R., Meneses J. J., Pedersen R. A., Rubenstein J. L. (1999). Dlx5 regulates regional development of the branchial arches and sensory capsules. Development, 126, 3831-3846 [DOI] [PubMed] [Google Scholar]
- Favor J., Sandulache R., Neuhauser-Klaus A., Pretsch W., Chatterjee B., Senft E., . . . Schughart K. (1996). The mouse Pax2(1Neu) mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proceedings of the National Academy of Sciences of the United States of America, 93, 13870-13875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete D. M., Wu D. K. (2002). Revisiting cell fate specification in the inner ear. Current Opinion in Neurobiology, 12(1), 35-42 [DOI] [PubMed] [Google Scholar]
- Forge A., Li L., Corwin J. T., Nevill G. (1993). Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science, 259, 1616-1619 [DOI] [PubMed] [Google Scholar]
- Funke B., Epstein J. A., Kochilas L. K., Lu M. M., Pandita R. K., Liao J., . . . Morrow B. E. (2001). Mice overexpressing genes from the 22q11 region deleted in velo-cardio-facial syndrome/DiGeorge syndrome have middle and inner ear defects. Human Molecular Genetics, 10, 2549-2556 [DOI] [PubMed] [Google Scholar]
- Gurtner G. C., Callaghan M. J., Longaker M. T. (2007). Progress and potential for regenerative medicine. Annual Review of Medicine, 58, 299-312 [DOI] [PubMed] [Google Scholar]
- Hadrys T., Braun T., Rinkwitz-Brandt S., Arnold H. H., Bober E. (1998). Nkx5-1 controls semicircular canal formation in the mouse inner ear. Development, 125(1), 33-39 [DOI] [PubMed] [Google Scholar]
- Hakuba N., Hata R., Morizane I., Feng G., Shimizu Y., Fujita K., . . . Gyo K. (2005). Neural stem cells suppress the hearing threshold shift caused by cochlear ischemia. NeuroReport, 16, 1545-1549 [PubMed] [Google Scholar]
- Han Z., Yang J. M., Chi F. L., Cong N., Huang Y. B., Gao Z., Li W. (2010). Survival and fate of transplanted embryonic neural stem cells by Atoh1 gene transfer in guinea pigs cochlea. NeuroReport, 21, 490-496 [DOI] [PubMed] [Google Scholar]
- Hanna J. H., Saha K., Jaenisch R. (2010). Pluripotency and cellular reprogramming: Facts, hypotheses, unresolved issues. Cell, 143, 508-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. H., Reh T. A., Bermingham-McDonogh O. (2010). Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proceedings of the National Academy of Sciences of the United States of America, 107, 15792-15797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. D., Bashiardes S., Helms C. A., Hu L., Saccone N. L., Warchol M. E., Lovett M. (2003). Gene expression differences in quiescent versus regenerating hair cells of avian sensory epithelia: Implications for human hearing and balance disorders. Human Molecular Genetics, 12, 1261-1272 [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Bashiardes S., Powder K. E., Sajan S. A., Bhonagiri V., Alvarado D. M., . . . Lovett M. (2007). Large scale gene expression profiles of regenerating inner ear sensory epithelia. PLoS One, 2, e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Surani M. A. (2009). Resetting the epigenome beyond pluripotency in the germline. Cell Stem Cell, 4, 493-498 [DOI] [PubMed] [Google Scholar]
- Hequembourg S., Liberman M. C. (2001). Spiral ligament pathology: A major aspect of age-related cochlear degeneration in C57BL/6 mice. Journal of the Association for Research in Otolaryngology, 2(2), 118-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez P. P., Moreno V., Olivari F. A., Allende M. L. (2006). Sub-lethal concentrations of waterborne copper are toxic to lateral line neuromasts in zebrafish (Danio rerio). Hearing Research, 213(1-2), 1-10 [DOI] [PubMed] [Google Scholar]
- Hildebrand M. S., Dahl H. H., Hardman J., Coleman B., Shepherd R. K., de Silva M. G. (2005). Survival of partially differentiated mouse embryonic stem cells in the scala media of the guinea pig cochlea. Journal of the Association for Research in Otolaryngology, 6, 341-354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K., Discolo C. M., Keasler J. R., Ransohoff R. (2005). Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. Journal of Comparative Neurology, 489, 180-194 [DOI] [PubMed] [Google Scholar]
- Hirose K., Liberman M. C. (2003). Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. Journal of the Association for Research in Otolaryngology, 4, 339-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Wei D., Johansson C. B., Holmstrom N., Duan M., Frisen J., . . . Ulfendahl M. (2005). Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Experimental Cell Research, 302(1), 40-47 [DOI] [PubMed] [Google Scholar]
- Huang X., Cho S., Spangrude G. J. (2007). Hematopoietic stem cells: Generation and self-renewal. Cell Death & Differentiation, 14, 1851-1859 [DOI] [PubMed] [Google Scholar]
- Ito J., Kojima K., Kawaguchi S. (2001). Survival of neural stem cells in the cochlea. Acta Oto-Laryngologica, 121(2), 140-142 [DOI] [PubMed] [Google Scholar]
- Izumikawa M., Minoda R., Kawamoto K., Abrashkin K. A., Swiderski D. L., Dolan D. F., . . . Raphael Y. (2005). Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nature Medicine, 11, 271-276 [DOI] [PubMed] [Google Scholar]
- Jeon S. J., Oshima K., Heller S., Edge A. S. (2007). Bone marrow mesenchymal stem cells are progenitors in vitro for inner ear hair cells. Molecular and Cellular Neuroscience, 34(1), 59-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome L. A., Papaioannou V. E. (2001). DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nature Genetics, 27, 286-291 [DOI] [PubMed] [Google Scholar]
- Jones J. M., Montcouquiol M., Dabdoub A., Woods C., Kelley M. W. (2006). Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. Journal of Neuroscience, 26, 550-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesser B. W., Lalwani A. K. (2009). Gene therapy and stem cell transplantation: Strategies for hearing restoration. Advances in Oto-Rhino-Laryngology, 66, 64-86 [DOI] [PubMed] [Google Scholar]
- Kiernan A. E., Pelling A. L., Leung K. K., Tang A. S., Bell D. M., Tease C., . . . Cheah K. S. (2005). Sox2 is required for sensory organ development in the mammalian inner ear. Nature, 434, 1031-1035 [DOI] [PubMed] [Google Scholar]
- Kiernan A. E., Xu J., Gridley T. (2006). The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genetics, 2(1), e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. F., Jackson E. L., Woolfenden A. E., Lawrence S., Babar I., Vogel S., . . . Jacks T. (2005). Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell, 121, 823-835 [DOI] [PubMed] [Google Scholar]
- Kim T. S., Nakagawa T., Kita T., Higashi T., Takebayashi S., Matsumoto M., . . . Ito J. (2005). Neural connections between embryonic stem cell-derived neurons and vestibular hair cells in vitro. Brain Research, 1057(1-2), 127-133 [DOI] [PubMed] [Google Scholar]
- Kollet O., Shivtiel S., Chen Y. Q., Suriawinata J., Thung S. N., Dabeva M. D., . . . Lapidot T. (2003). HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. Journal of Clinical Investigation, 112, 160-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine H., Doetzlhofer A., Mantela J., Ylikoski J., Laiho M., Roussel M. F., . . . Pirvola U. (2007). p19(Ink4d) and p21(Cip1) collaborate to maintain the postmitotic state of auditory hair cells, their codeletion leading to DNA damage and p53-mediated apoptosis. Journal of Neuroscience, 27, 1434-1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine H., Sulg M., Kirjavainen A., Pirvola U. (2010). Cell cycle regulation in the inner ear sensory epithelia: Role of cyclin D1 and cyclin-dependent kinase inhibitors. Developmental Biology, 337(1), 134-146 [DOI] [PubMed] [Google Scholar]
- Lam B. L., Lee D. J., Gomez-Marin O., Zheng D. D., Caban A. J. (2006). Concurrent visual and hearing impairment and risk of mortality: The National Health Interview Survey. Archives of Ophthalmology, 124(1), 95-101 [DOI] [PubMed] [Google Scholar]
- Landgren H., Curtis M. A. (2010). Locating and labeling neural stem cells in the brain. Journal of Cellular Physiology, 226(1), 1-7 [DOI] [PubMed] [Google Scholar]
- Lanford P. J., Shailam R., Norton C. R., Gridley T., Kelley M. W. (2000). Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. Journal of the Association for Research in Otolaryngology, 1, 161-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H., Ebihara Y., Schmiedt R. A., Minamiguchi H., Zhou D., Smythe N., . . . Schulte B. A. (2006). Contribution of bone marrow hematopoietic stem cells to adult mouse inner ear: Mesenchymal cells and fibrocytes. Journal of Comparative Neurology, 496, 187-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson V. D., Williams D. W., Henderson W. G., Luethke L. E., Beck L. B., Noffsinger D., . . . Rappaport B. Z. (2000). Efficacy of 3 commonly used hearing aid circuits: A crossover trial. NIDCD/VA Hearing Aid Clinical Trial Group. Journal of the American Medical Association, 284, 1806-1813 [DOI] [PubMed] [Google Scholar]
- Lee D. J., Gomez-Marin O., Lam B. L., Zheng D. D. (2004). Trends in hearing impairment in United States adults: The national health interview survey, 1986-1995. Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 59, 1186-1190 [DOI] [PubMed] [Google Scholar]
- Lenoir M., Daudet N., Humbert G., Renard N., Gallego M., Pujol R., . . . Vago P. (1999). Morphological and molecular changes in the inner hair cell region of the rat cochlea after amikacin treatment. Journal of Neurocytology, 28, 925-937 [DOI] [PubMed] [Google Scholar]
- Lewis A. K., Frantz G. D., Carpenter D. A., de Sauvage F. J., Gao W. Q. (1998). Distinct expression patterns of notch family receptors and ligands during development of the mammalian inner ear. Mechanisms of Development, 78, 159-163 [DOI] [PubMed] [Google Scholar]
- Li H., Liu H., Heller S. (2003). Pluripotent stem cells from the adult mouse inner ear. Nature Medicine, 9, 1293-1299 [DOI] [PubMed] [Google Scholar]
- Li L., Forge A. (1997). Morphological evidence for supporting cell to hair cell conversion in the mammalian utricular macula. International Journal of Developmental Neuroscience, 15, 433-446 [DOI] [PubMed] [Google Scholar]
- Liebl D. J., Tessarollo L., Palko M. E., Parada L. F. (1997). Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice. Journal of Neuroscience, 17, 9113-9121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin V., Golub J. S., Nguyen T. B., Hume C. R., Oesterle E. C., Stone J. S. (2011). Inhibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. Journal of Neuroscience, 31, 15329-15339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthicum F. H., Jr., Fayad J. N. (2009). Spiral ganglion cell loss is unrelated to segmental cochlear sensory system degeneration in humans. Otology & Neurotology, 30, 418-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenheim H., Furness D. N., Kil J., Zinn C., Gultig K., Fero M. L., . . . Zenner H. P. (1999). Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proceedings of the National Academy of Sciences of the United States of America, 96, 4084-4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma E. Y., Rubel E. W., Raible D. W. (2008). Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. Journal of Neuroscience, 28, 2261-2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Anderson D. J., Fritzsch B. (2000). Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. Journal of the Association for Research in Otolaryngology, 1(2), 129-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchaiah V. K., Zhao F., Danesh A. A., Duprey R. (2011). The genetic basis of auditory neuropathy spectrum disorder (ANSD). International Journal of Pediatric Otorhinolaryngology, 75, 151-158 [DOI] [PubMed] [Google Scholar]
- Mansouri A., Chowdhury K., Gruss P. (1998). Follicular cells of the thyroid gland require Pax8 gene function. Nature Genetics, 19(1), 87-90 [DOI] [PubMed] [Google Scholar]
- Mantela J., Jiang Z., Ylikoski J., Fritzsch B., Zacksenhaus E., Pirvola U. (2005). The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development, 132, 2377-2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Nakagawa T., Kojima K., Sakamoto T., Fujiyama F., Ito J. (2008). Potential of embryonic stem cell-derived neurons for synapse formation with auditory hair cells. Journal of Neuroscience Research, 86, 3075-3085 [DOI] [PubMed] [Google Scholar]
- Mehra S., Eavey R. D., Keamy D. G., Jr. (2009). The epidemiology of hearing impairment in the United States: Newborns, children, and adolescents. Otolaryngology-Head & Neck Surgery, 140, 461-472 [DOI] [PubMed] [Google Scholar]
- Merlo G. R., Paleari L., Mantero S., Zerega B., Adamska M., Rinkwitz S., . . . Levi G. (2002). The Dlx5 homeobox gene is essential for vestibular morphogenesis in the mouse embryo through a BMP4-mediated pathway. Developmental Biology, 248, 157-169 [DOI] [PubMed] [Google Scholar]
- Millimaki B. B., Sweet E. M., Dhason M. S., Riley B. B. (2007). Zebrafish Atoh1 genes: Classic proneural activity in the inner ear and regulation by Fgf and Notch. Development, 134, 295-305 [DOI] [PubMed] [Google Scholar]
- Morrison A., Hodgetts C., Gossler A., Hrabe de, Angelis M., Lewis J. (1999). Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mechanisms of Development, 84, 169-172 [DOI] [PubMed] [Google Scholar]
- Morsli H., Tuorto F., Choo D., Postiglione M. P., Simeone A., Wu D. K. (1999). Otx1 and Otx2 activities are required for the normal development of the mouse inner ear. Development, 126, 2335-2343 [DOI] [PubMed] [Google Scholar]
- Murata J., Tokunaga A., Okano H., Kubo T. (2006). Mapping of notch activation during cochlear development in mice: Implications for determination of prosensory domain and cell fate diversification. Journal of Comparative Neurology, 497, 502-518 [DOI] [PubMed] [Google Scholar]
- Nadol J. B., Jr. (1997). Patterns of neural degeneration in the human cochlea and auditory nerve: Implications for cochlear implantation. Otolaryngology-Head & Neck Surgery, 117, 220-228 [DOI] [PubMed] [Google Scholar]
- Nickel R., Forge A. (2008). Gap junctions and connexins in the inner ear: Their roles in homeostasis and deafness. Current Opinion in Otolaryngology & Head and Neck Surgery, 16, 452-457 [DOI] [PubMed] [Google Scholar]
- Noramly S., Grainger R. M. (2002). Determination of the embryonic inner ear. Journal of neurobiology, 53(2), 100-128 [DOI] [PubMed] [Google Scholar]
- Nornes H. O., Dressler G. R., Knapik E. W., Deutsch U., Gruss P. (1990). Spatially and temporally restricted expression of Pax2 during murine neurogenesis. Development, 109, 797-809 [DOI] [PubMed] [Google Scholar]
- Oesterle E. C., Rubel E. W. (1993). Postnatal production of supporting cells in the chick cochlea. Hearing Research, 66, 213-224 [DOI] [PubMed] [Google Scholar]
- Okano T., Nakagawa T., Endo T., Kim T. S., Kita T., Tamura T., . . . Ito J. (2005). Engraftment of embryonic stem cell-derived neurons into the cochlear modiolus. NeuroReport, 16, 1919-1922 [DOI] [PubMed] [Google Scholar]
- Okano T., Nakagawa T., Kita T., Endo T., Ito J. (2006). Cell-gene delivery of brain-derived neurotrophic factor to the mouse inner ear. Molecular Therapy: The journal of the American Society of Gene Therapy, 14, 866-871 [DOI] [PubMed] [Google Scholar]
- Okano T., Nakagawa T., Kita T., Kada S., Yoshimoto M., Nakahata T., Ito J. (2008). Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. Journal of Neuroscience Research, 86, 1758-1767 [DOI] [PubMed] [Google Scholar]
- Oshima K., Grimm C. M., Corrales C. E., Senn P., Martinez Monedero R., Geleoc G. S., . . . Heller S. (2007). Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. Journal of the Association for Research in Otolaryngology, 8(1), 18-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K., Shin K., Diensthuber M., Peng A. W., Ricci A. J., Heller S. (2010). Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell, 141, 704-716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W., Jin Y., Stanger B., Kiernan A. E. (2010). Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proceedings of the National Academy of Sciences of the United States of America, 107, 15798-15803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D. L., Girod D. A., Durham D. (2002). Avian brainstem neurogenesis is stimulated during cochlear hair cell regeneration. Brain Research, 949(1-2), 1-10 [DOI] [PubMed] [Google Scholar]
- Parker M. A., Corliss D. A., Gray B., Anderson J. K., Bobbin R. P., Snyder E. Y., Cotanche D. A. (2007). Neural stem cells injected into the sound-damaged cochlea migrate throughout the cochlea and express markers of hair cells, supporting cells, and spiral ganglion cells. Hearing Research, 232(1-2), 29-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puligilla C., Dabdoub A., Brenowitz S. D., Kelley M. W. (2010). Sox2 induces neuronal formation in the developing mammalian cochlea. Journal of Neuroscience, 30, 714-722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qun L. X., Pirvola U., Saarma M., Ylikoski J. (1999). Neurotrophic factors in the auditory periphery. Annals of the New York Academy of Sciences, 884, 292-304 [DOI] [PubMed] [Google Scholar]
- Raft S., Koundakjian E. J., Quinones H., Jayasena C. S., Goodrich L. V., Johnson J. E., . . . Groves A. K. (2007). Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development, 134, 4405-4415 [DOI] [PubMed] [Google Scholar]
- Raft S., Nowotschin S., Liao J., Morrow B. E. (2004). Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development, 131, 1801-1812 [DOI] [PubMed] [Google Scholar]
- Raphael Y. (2002). Cochlear pathology, sensory cell death and regeneration. British Medical Bulletin, 63, 25-38 [DOI] [PubMed] [Google Scholar]
- Roberson D. W., Alosi J. A., Cotanche D. A. (2004). Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. Journal of Neuroscience Research, 78, 461-471 [DOI] [PubMed] [Google Scholar]
- Rocha-Sanchez S. M., Scheetz L. R., Contreras M., Weston M. D., Korte M., McGee J., Walsh E. J. (2011). Mature mice lacking Rbl2/p130 gene have supernumerary inner ear hair cells and supporting cells. Journal of Neuroscience, 31, 8883-8893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel E. W., Dew L. A., Roberson D. W. (1995). Mammalian vestibular hair cell regeneration. Science, 267, 701-707 [DOI] [PubMed] [Google Scholar]
- Rubel E. W., Fritzsch B. (2002). Auditory system development: Primary auditory neurons and their targets. Annual Review of Neuroscience, 25, 51-101 [DOI] [PubMed] [Google Scholar]
- Ryals B. M., Rubel E. W. (1988). Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science, 240, 1774-1776 [DOI] [PubMed] [Google Scholar]
- Sage C., Huang M., Karimi K., Gutierrez G., Vollrath M. A., Zhang D. S., . . . Chen Z. Y. (2005). Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science, 307, 1114-1118 [DOI] [PubMed] [Google Scholar]
- Saha K., Jaenisch R. (2009). Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell, 5, 584-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T., Nakagawa T., Endo T., Kim T. S., Iguchi F., Naito Y., . . . Ito J. (2004, March). Fates of mouse embryonic stem cells transplanted into the inner ears of adult mice and embryonic chickens. ACTA Oto-laryngologica Supplement, 48-52 [DOI] [PubMed] [Google Scholar]
- Schwartz R. E., Verfaillie C. (2010). Hepatic stem cells. Methods in Molecular Biology, 640, 167-179 [DOI] [PubMed] [Google Scholar]
- Shi F., Cheng Y. F., Wang X. L., Edge A. S. (2010). Beta-catenin up-regulates Atoh1 expression in neural progenitor cells by interaction with an Atoh1 3’ enhancer. Journal of Biological Chemistry, 285, 392-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Corrales C. E., Liberman M. C., Edge A. S. (2007). BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. European Journal of Neuroscience, 26, 3016-3023 [DOI] [PubMed] [Google Scholar]
- Starr A., Picton T. W., Sininger Y., Hood L. J., Berlin C. I. (1996). Auditory neuropathy. Brain, 119, 741-753 [DOI] [PubMed] [Google Scholar]
- Stone J. S., Choi Y. S., Woolley S. M., Yamashita H., Rubel E. W. (1999). Progenitor cell cycling during hair cell regeneration in the vestibular and auditory epithelia of the chick. Journal of Neurocytology, 28, 863-876 [DOI] [PubMed] [Google Scholar]
- Stone J. S., Oesterle E. C., Rubel E. W. (1998). Recent insights into regeneration of auditory and vestibular hair cells. Current Opinion in Neurology, 11(1), 17-24 [DOI] [PubMed] [Google Scholar]
- Surani M. A., Hayashi K., Hajkova P. (2007). Genetic and epigenetic regulators of pluripotency. Cell, 128, 747-762 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131, 861-872 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663-676 [DOI] [PubMed] [Google Scholar]
- Takebayashi S., Yamamoto N., Yabe D., Fukuda H., Kojima K., Ito J., Honjo T. (2007). Multiple roles of Notch signaling in cochlear development. Developmental Biology, 307, 165-178 [DOI] [PubMed] [Google Scholar]
- Tateya I., Nakagawa T., Iguchi F., Kim T. S., Endo T., Yamada S., . . . Ito J. (2003). Fate of neural stem cells grafted into injured inner ears of mice. NeuroReport, 14, 1677-1681 [DOI] [PubMed] [Google Scholar]
- Tessarollo L., Coppola V., Fritzsch B. (2004). NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. Journal of Neuroscience, 24, 2575-2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufert K. B., Linthicum F. H., Jr., Connell S. S. (2006). The effect of organ of corti loss on ganglion cell survival in humans. Otology & Neurotology, 27, 1146-1151 [DOI] [PubMed] [Google Scholar]
- Todd N. W. (1994). At-risk populations for hearing impairment in infants and young children. International Journal of Pediatric Otorhinolaryngology, 29(1), 11-21 [DOI] [PubMed] [Google Scholar]
- Torres M., Gomez-Pardo E., Gruss P. (1996). Pax2 contributes to inner ear patterning and optic nerve trajectory. Development, 122, 3381-3391 [DOI] [PubMed] [Google Scholar]
- Vitelli F., Viola A., Morishima M., Pramparo T., Baldini A., Lindsay E. (2003). TBX1 is required for inner ear morphogenesis. Human Molecular Genetics, 12, 2041-2048 [DOI] [PubMed] [Google Scholar]
- Wang G. P., Chatterjee I., Batts S. A., Wong H. T., Gong T. W., Gong S. S., . . . Raphael Y. (2010). Notch signaling and Atoh1 expression during hair cell regeneration in the mouse utricle. Hearing Research, 267(1-2), 61-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Van De, Water T., Lufkin T. (1998). Inner ear and maternal reproductive defects in mice lacking the Hmx3 homeobox gene. Development, 125, 621-634 [DOI] [PubMed] [Google Scholar]
- Warchol M. E., Lambert P. R., Goldstein B. J., Forge A., Corwin J. T. (1993). Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science, 259, 1619-1622 [DOI] [PubMed] [Google Scholar]
- White P. M., Doetzlhofer A., Lee Y. S., Groves A. K., Segil N. (2006). Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature, 441, 984-987 [DOI] [PubMed] [Google Scholar]
- Woods C., Montcouquiol M., Kelley M. W. (2004). Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nature Neuroscience, 7, 1310-1318 [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Chang W., Kelley M. W. (2011). Rbpj regulates development of prosensory cells in the mammalian inner ear. Developmental Biology, 353, 367-379 [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Tanigaki K., Tsuji M., Yabe D., Ito J., Honjo T. (2006). Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. Journal of Molecular Medicine (Berlin), 84(1), 37-45 [DOI] [PubMed] [Google Scholar]
- Zhao L. D., Guo W. W., Lin C., Li L. X., Sun J. H., Wu N., . . . Yang S. M. (2011). Effects of DAPT and Atoh1 overexpression on hair cell production and hair bundle orientation in cultured Organ of Corti from neonatal rats. PLoS One, 6, e23729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. L., Gao W. Q. (2000). Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nature Neuroscience, 3, 580-586 [DOI] [PubMed] [Google Scholar]
- Zheng J. L., Shou J., Guillemot F., Kageyama R., Gao W. Q. (2000). Hes1 is a negative regulator of inner ear hair cell differentiation. Development, 127, 4551-4560 [DOI] [PubMed] [Google Scholar]
- Zimmermann C. E., Burgess B. J., Nadol J. B., Jr. (1995). Patterns of degeneration in the human cochlear nerve. Hearing Research, 90, 192-201 [DOI] [PubMed] [Google Scholar]
- Zine A., Van De, Water T. R., de Ribaupierre F. (2000). Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development, 127, 3373-3383 [DOI] [PubMed] [Google Scholar]