Abstract
Low-frequency otoacoustic emissions (OAEs) are often concealed by acoustic background noise such as those from a patient’s breathing and from the environment during recording in clinics. When using electrocochleaography (ECochG or ECoG), such as cochlear microphonics (CMs), acoustic background noise do not contaminate the recordings. Our objective is to study the response pattern of CM waveforms (CMWs) to explore an alternative approach in assessing cochlear functions. In response to a 14-msec tone burst across several acoustic frequencies, CMWs were recorded at the ear canal from ten normal hearing subjects. A relatively long tone burst has a relatively narrow frequency band. The CMW amplitudes among different frequencies were compared. The CMW amplitudes among different frequencies were compared. Two features were observed in the response pattern of CMWs: the amplitude of CMWs decreased with an increase of stimulus frequency of the tone bursts; and such a decrease occurred at a faster rate at lower frequencies than at higher frequencies. Five factors as potential mechanisms for these features are proposed. Clinical applications such as hearing screening are discussed. Therefore, the response pattern of CMWs suggests that they may be used as an alternative to OAEs in the assessment of cochlear functions in the clinic, especially at low frequencies.
Keywords: ear canal electrode, cochlear microphonics (CMs), electrocochleography (ECoG or ECochG), otoacoustic emissions (OAEs), auditory brainstem responses (ABRs)
Introduction
There are inherent limitations associated with the measurement of otoacoustic emissions (OAEs) for assessing cochlear functions (Gorga et al., 1993; Harrison & Norton, 1999; Suckfull, Schneeweiss, Dreher, & Schorn, 1996; Tognola, Ravazzani, & Grandori, 1995; Whitehead, Lonsbury-Martin, & Martin, 1993). Therefore, an alternative clinical assessment approach would be beneficial. One possible alternative is the application of cochlear microphonics (CMs). Both OAEs and CMs are cochlear responses (Dallos, 1981; Ferraro & Durrant, 2002; Kusakari et al., 1988; Norton, Ferguson, & Mascher, 1989; Ponton, Don, & Eggermont, 1992; Sun & Shaver, 2009), and as such, with sufficient research, using CM measurements may become an alternative approach to OAE measurements for assessing cochlear functions (Riazi & Ferraro, 2008; Zhang, 2010; Zhang & Abbas, 1993).
The OAE has been well studied for clinical applications, and therefore it is commonly used in clinics to assess cochlear functions (Dreisbach & Siegel, 2005; Martin, Stagner, & Lonsbury-Martin, 2011; Sun & Shaver, 2009). For example, distortion product OAEs (DPOAEs) are used in clinics to assess cochlear function with an f2 frequency at 1000 Hz and above (Gorga et al., 1993; Harrison & Norton, 1999; Suckfull et al., 1996). However, OAEs are an acoustic version of cochlear responses, and inherent limitations are associated with acoustic signals. One major limitation is acoustic background noise. Acoustic background noise at lower frequencies is remarkable, and such noises may not be avoidable or controllable, as they include those from the patient’s own breathing. Not only is low-frequency noise much greater than high-frequency noise, it is also much greater than the actual low-frequency OAE signals. Therefore, low-frequency OAEs are almost always concealed by acoustic background noise in a typical recording under clinical settings (Andersson, Arlinger, & Jacobsson, 2000; Hussain, Gorga, Neely, Keefe, & Peters, 1998).
Due to this acoustic background noise limitation, the performance of OAE measurements suffers greatly when assessing low-frequency cochlear functions such as at 500 Hz in clinics (Gorga et al., 1993; Harrison & Norton, 1999; Suckfull et al., 1996). Consequently, the lowest frequency for measuring specific OAE frequencies, such as in DPOAEs, is typically set by manufacturers at 1000 Hz for f2 primary tones. As such, measurement of OAEs at 500 Hz is basically unworkable in clinics.
However, 500 Hz is an important frequency in clinical assessments as this frequency is included in many key hearing tests, such as the turning fork test, the pure tone average test, and the tone burst auditory brainstem response (ABR) test (Burkey, Lippy, Schuring, & Rizer, 1998; Chien et al., 2008; Vander Werff, Prieve, & Georgantas, 2009; Wang et al., 2011).
CMs, like OAEs, are also cochlear responses but are an electrical version rather than an acoustic version (Withnell, 2001). The recorded CM is the compound potential maintained by alternating currents coming from a large population of hair cells, and especially from the outer hair cells (Dallos & Cheatham, 1976; Keidel, 1962). Being an electrical signal, CMs are unaffected by acoustic background noise. Thus CM measurements would be especially valuable for low-frequency testing in consideration of the acoustic background noise limitation which is associated with OAE measurements.
Besides the assessment of low-frequency cochlear function, research on CMs is also valuable for other applications. For example, the CM may be a factor (a) in the diagnosis of Meniere’s disease (Ferraro & Durrant, 2006; Ge, Shea, & Orchik, 1997; Gibson & Beagley, 1976; Moriuchi & Kumagami, 1979; Zou, Zheng, Ren, & Nuttall, 2006), (b) in the assessment of auditory neuropathy disorders (Berlin, Hood, Morlet, Rose, & Brashears, 2003; Deltenre et al., 1999), and (c) in the improvement of sensitivity and specificity when diagnosing hearing-related disorders.
Due to the potentially useful applications of CMs in clinics as described above, interest in CMs has gradually been increasing. However, in humans, the number of studies investigating CM recordings and their use in clinical applications and in clinical procedures is quite limited (Ferraro & Durrant, 2002; Ferraro & Ruth, 1994), even though CMs have been extensively studied in animals (Dallos & Wang, 1974; H. Davis, 1958). Such a limitation may be due to technical complexity (Ferraro & Durrant, 2002), or may be due to lack of sufficient knowledge regarding the presentation and interpretation of CMs measured from a far-field electrode in clinics. The number of studies on ear canal recorded CM waveforms (CMWs) is even more limited. Hence, to increase our knowledge of CM measurements in humans, we studied the response pattern of ear canal recorded CMWs across several acoustic frequencies in normal hearing subjects to determine the clinical possibility of using CMWs to assess cochlear functions.
Method
Subjects
Ten volunteers with normal hearing aged 18 to 35 years participated in the study as approved by the university institutional ethics committee. Behavioral pure-tone air conduction thresholds were obtained at 250, 500, 1000, 2000, 4000, 6000, and 8000 Hz using the GSI-61 Audiometer (Grason-Stadler, Inc.). Hearing thresholds less than 25 dB HL from 250 through 8000 Hz were regarded as normal for this study.
Stimuli
We adopted the methods used in our previous reports (Zhang, 2010; Zhang & Abbas, 1997; Zhang et al., 2003). A Nicolet Viking IV-P system was used to generate the stimulus, which was a 14-msec tone burst with a 4-msec rise and fall time gated by the Blackman function. The frequencies of the tone burst were set at 500, 1000, 2000, and 6000 Hz. The stimuli were presented at 75 dB nHL at a rate of 22.7/sec. The acoustic stimuli originated from an insert earphone (Nicolet-TIP 300) were delivered to the ear canal through a sound delivery tube that ran through the center of an earplug. The earplug is a type of tip-trode combined with a recording electrode as described in the next paragraph. The intensity of the stimuli inside the ear canal was calibrated in situ before the measurement of the responses was performed.
Recording
The CMWs were collected from recording electrodes which were appropriately placed and with an impedance of less than 3,000 ohms. The inverting electrode (−) was placed in the ipsilateral ear canal using an ear canal electrode, the noninverting electrode (+) was placed at the high forehead (Fz) using a disc electrode, and the common ground electrode (G) was placed on the contralateral side (A2). The noninverting (+) and ground electrodes (G) are surface disk electrodes. The ear canal electrode is a type of tip-trode with a thin layer of gold foil surrounding a foam earplug which was expanded against the skin of the ear canal. A 20-msec recording window was used to record the responses. The amplification was set at 100,000x, and the band pass was enclosed between 5 Hz and 10000 Hz during real-time recording. The potential noise recorded due to this wide-band filtering was refiltered again offline if refiltering was shown to substantially improve data analysis. To increase the signal to noise ratio during recording, up to 2,000 sweeps were averaged into a final recorded trace.
The main problematic artifact in recording CMWs is electromagnetic interference coming from stimulation components and in particular from the earphone transducer. To avoid such artifacts, the stimulation components were shielded. For example, the transducer was encased in metal shielding which was electrically grounded. A negative control was also performed by clamping the sound delivery tube so that no acoustic stimuli could reach the ear canal, which confirmed that the recorded responses were artifact free.
Data Analysis
The amplitudes of the CMWs were measured from each peak to the following trough. The three peak-to-trough cycles immediately after the midtime point of the 14-msec period of CMWs were selected, and the amplitudes of the three cycles were averaged. In the present study, only one group was involved, and all data were pooled into this group. Therefore, no statistical comparison was needed as would be the case between two or more groups. The comparison among mean amplitudes of CMWs at different frequencies (500, 1000, 2000, and 6000 Hz) was performed with an ANOVA run in SPSS statistical software. The difference among amplitudes across these frequencies was considered significant if the p value was < .05.
Results
Appearance of 1000-Hz CMWs in Response to a 14-msec Tone Burst Stimulus With the Potential Issue of Artifacts
Figure 1 shows an example of a typical result from CMW recordings. The recording was performed using a tone burst at 1000 Hz. A pair of traces, A and B, were recorded. We show the results by overlapping these two traces along the baseline to show a clear difference between the two recordings. Trace A was recorded with sound delivery tube open so that the sound could reach the tympanic membrane and the inner ear. Trace B was recorded with the sound delivery tube clamped so that no sound could reach the tympanic membrane. Conducting these trace recordings allows us to rule out the possibility of artifacts which may come from the transducer and contaminate the recorded CMW response signals. The relatively flat trace recorded in trace B when the sound delivery tube was clamped suggests that the sinusoidal-like waveforms in trace A are not artifacts but are actual response signals.
Figure 1.
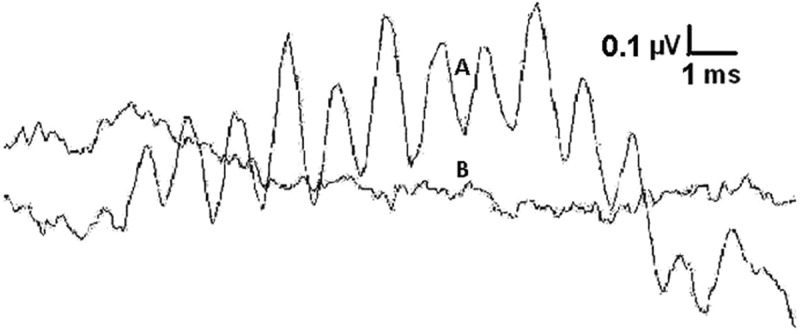
A typical recording of 1-kHz CMWs with a negative control
Note: Trace A appeared as sinusoidal-like waveforms recorded with an ear canal electrode in response to a 14-ms long 1-kHz tone burst at 75 dB nHL with sound delivery tube open. Trace B appeared as a relatively flat line recorded under the same settings with the sound delivery tube clamped. Scales: Horizontal bar = 1 msec. Vertical bar = 0.1 µ V).
Interestingly, the segment at the beginning of trace A does not appear to contain any remarkable sinusoidal waveforms although the 14-msec tone burst at the earphone transducer was turned on instantaneously at the start. This relatively quiet initial segment is regarded as a delay, and is the time period needed for the sound vibration to travel from the earplug to the hair cells through the external ear canal, middle ear, and basilar membrane. A 0.8 msec time period, to allow for sound to travel through the delivery tube, was already excluded from the recording time window.
The amplitude of the CMW in trace A can be easily recognized and measured although both traces do not appear completely smooth and are relatively noisy. This is most likely due to the high cutoff frequency of 10000 Hz which was set for the low-pass filter. Trace A is a recording of a 1000 Hz CMW. Although a lower cutoff frequency might have reduced the high-frequency noises, we did not set the cutoff frequency at 2000 Hz for this real-time recording due to the consideration of high-frequency CMWs. The highest frequency CMW we had planned to record was 6000 Hz. Thus, we set the low-pass cutoff frequency at 10000 Hz to reduce the aliasing effects when recording the 6000 Hz CMWs. We maintained the same cutoff frequency (i.e., at 10000 Hz) for the 1000 Hz recording to preserve the same conditions for all CMW frequencies to facilitate a more accurate comparison of the results. Under these identical conditions, the difference, if any, that might be observed could be attributed more clearly to the difference in frequencies which were used in the recordings. We did not observe any remarkable negative effects by keeping the cutoff frequency identical. In fact, at a high level of stimulus intensity (75 dB nHL), the CMWs appeared very clearly as shown in Figure 1. We did not experience any issues with recognizing the CMW signals from this noisy background and did not have problems in our subsequent data analysis.
Finally, in order to detect unexpected artifacts other than electromagnetic interference which might potentially be recorded, and in order to detect other signals which were unknown but might be potentially interesting, we set the low cutoff frequency at 5 Hz for the high-pass filter. This is shown by the slight drift from the baseline in the two traces. Presently, we have not detected any other signals of interest, and we have not found this drift to have caused any problems in our data analysis.
Replication of 2000-Hz CMWs in Response to a 14-msec Tone Burst Stimulus With the Potential Issue of Neural Components
Figure 2 shows another example of a typical recording of CMWs. The recording was conducted using a 2000 Hz stimulus frequency. The results of the 2000 Hz CMW recording show substantial similarities to the 1000 Hz CMW in Figure 1 as both have sinusoidal-like waveforms, and both contain a delay appearing at the beginning of the traces.
Figure 2.
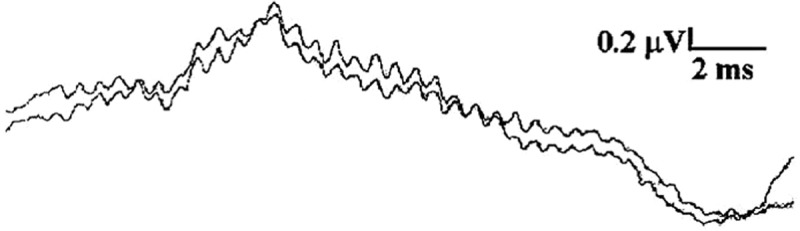
A typical recording of 2-kHz CMWs with replications
Note: Both traces were recorded under the same conditions with one recorded immediately after the first. The recording was performed with an ear canal electrode in response to a 14-ms long 2-kHz tone burst at 75 dB nHL. Scales: Horizontal bar = 2 ms. Vertical bar = 0.2 µ V).
However, differences do exist. Compared to the results of the 1000 Hz CMW measurement, the amplitudes of 2000 Hz CMWs are smaller, and the periods of the cycles are shorter. Interestingly, neural components can be seen in Figure 2 as an upward peak near the 1/3 point of the epoch (i.e., recording time window). Neural components, which typically are a compound action potential from a population of auditory nerve fibers, may become prominent during CMW recording (Ferraro & Durrant, 2002; Kusakari et al., 1988). A direct current component distorted by 5-Hz high-pass filter may appear in the recording as well. This can be observed in Figure 2 as a downward shift of the baseline at the end of the CMWs corresponding to the offset (time) of the tone burst stimulation. We did not find that these features affected the data analysis for our study.
The features observed and described above were verified to be real and reliable by recording a pair of traces with one trace recorded immediately after the first under the same settings. The results show that both traces have an initial delay. CMWs in both traces mimic the waveforms of tone burst stimulations. Therefore, the nearly identical appearances of these duplicated waveforms indicate that the recording is repeatable and reliable.
Response Pattern Based on Amplitudes of CMWs Across Several Acoustic Frequencies
As shown above in Figure 1 and Figure 2, the amplitudes of 1000 Hz CMWs are greater than that of 2000 Hz CMWs. Based on this trend, the amplitudes of 500 Hz CMWs should be greater than that of 1000 Hz CMWs, and the amplitudes of 6000 Hz CMWs should be smaller than that of 2000 Hz CMWs. This was true as shown in Figure 3. Figure 3 shows the mean amplitudes of CMWs in response to 14-msec tone burst stimuli at 75 dB nHL across four acoustic frequencies. The means of CMWs at 500 Hz and 6000 Hz are also displayed, although typical recordings for 500 Hz and 6000 Hz CMWs like those in Figures 1 and 2 are not shown. The typical example of a 500 Hz recording can be viewed in a separate report (Zhang, 2010), although it was measured with different test subjects. Amplitude is defined as the difference between the peaks and troughs as described in our earlier Methods section. Clearly, the amplitudes are different across different frequencies, and this difference is significant.
Figure 3.
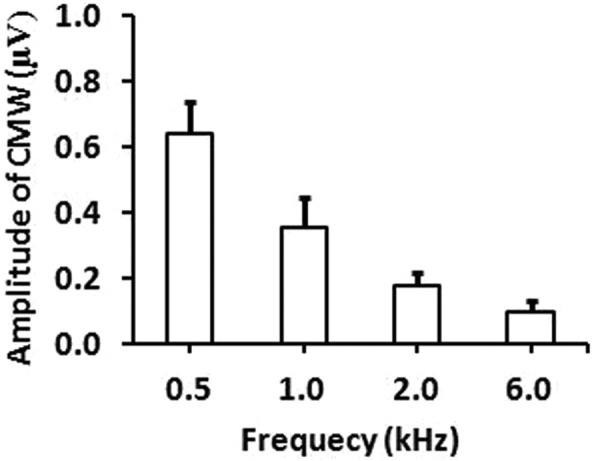
Response pattern based on amplitudes of CMWs across four acoustic frequencies
Note: The means of the amplitudes of CMWs (vertical axis) with standard error bars are plotted against the different frequencies (horizontal axis). The difference of amplitudes of the CMWs across four frequencies is significant (p < .05, ANOVA, repeated measures, n = 20).
With four data points for four frequencies, the pattern becomes more obvious than with just two data points, that is, the two frequencies in Figures 1 and 2. There are two features that can be observed in this pattern. One is that the amplitude decreases with an increase in frequency; the other is that the amplitude decreases faster across lower frequencies than across higher frequencies. This pattern can be expressed with the mathematical power equation or model: A = cFe with “A” as CMW amplitude (µV), “F” as frequency (kHz), “c” representing a coefficient factor, and “e” representing an exponent power factor. When c = 0.35, and e = –0.875, the model becomes a good approximate representation of the results in the present study (A = 0.35F–0.875).
Figure 4 shows the line derived from this model based on these four data points. The equation-based pattern line passes very closely to the four data points. With this equation, the correlation coefficient r = .98. Therefore, the mathematic model is a good representation of the response pattern based on the amplitudes of the measured CMWs across several acoustic frequencies.
Figure 4.
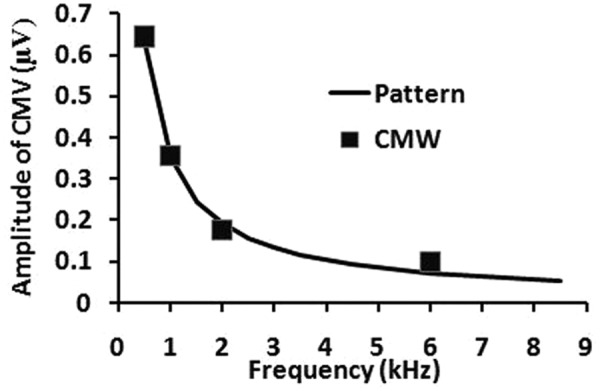
A model using an exponential equation to fit the response pattern
Note: The axis labels are identical to those in Figure 3. The solid line is the function derived from a model of an exponential equation. The line passes along the four CMW data points (solid squares) from Figure 3.
Discussions
CMWs Versus CMs
The use of the term “CMWs” may not be necessary as CMWs are covered under the concept of CMs. However, we use the term CMW in the hopes that some differences among various types of CMs measured through different recording configurations can be recognized, and that the reason for why we use one specific recording configuration rather than a different one can be better understood.
Three typical CM recording configurations are CMs evoked by a click, by a short tone burst (e.g., <5 msec), and by a relatively long tone burst (e.g., >14 msec). A click-evoked CM appears in only one or two period cycles and is represented by ringing of the basilar membrane and contains many frequencies (Arakawa, 1998; Dauman, Aran, Charlet de Sauvage, & Portmann, 1988). A short tone burst evoked CM appears in more period cycles than a click-evoked CM does, but does not contain sufficient period cycles to form a stable plateau (Arakawa, 1998; Dauman et al., 1988). It is more frequency specific as it contains fewer frequencies than a click-evoked CM does, but still contains more frequencies than a relatively long tone burst evoked CM. Therefore, frequency specificity using a shorter tone burst is poorer than using a relatively long tone burst. This will be further discussed in next subsection Frequency Specificity.
Besides the benefit of greater frequency specificity from longer tone burst evoked CMs, a second benefit lies in data analysis. Only a relatively longer tone burst evoked CM contains a sufficient number of period cycles to form a stable plateau. A stable plateau is a series of multiple sinusoidal CM waveforms between rising time and falling time. These CM waveforms have not only reached a maximum level of amplitude but also maintained relatively constant amplitude. Such a stable plateau can be seen in Figure 1 and Figure 2. In these two figures, well-formed CM waveforms can be observed in CMs within a stable plateau segment around 10 msecs long. A longer and more stable plateau with multiple CMs facilitates a more accurate extraction of amplitude values from the data.
Because these two benefits are associated with longer tone burst evoked CMs, we call them CMWs. In fact, CMWs are not new, and they were first recorded and mentioned in the 1930s in animal studies (Adrian, 1931; Wever & Bray, 1930). Later, CMWs were also measured in human studies by many investigators, for example by Ponton et al. using a tympanic membrane electrode (Ponton et al., 1992). We measured CMWs as well using an ear canal electrode before 2003 (Zhang et al., 2003).
Frequency Specificity
To explicitly view and understand the frequency specificity (or the range of frequencies) that a longer stimulus such as the 14-msec tone burst covers compared to a shorter one (6 msecs), we have run a frequency analysis to transform the tone bursts in the time domain into the frequency domain (power spectrum) via FFT. The difference in frequency specificity between the two tone burst stimuli can be observed in Figure 5. This figure shows the power spectra of both a 6-msec tone burst traditionally used in the clinic for measurement of tone burst ABRs and that of a 14-msec tone burst used in this study for measurement of tone burst CMWs. The bandwidth of the 6-msec tone burst (A) is much wider than that of the 14-msec tone burst (B). The narrower bandwidth of the 14-msec tone burst shows how much lower the spectral splatter is with the longer duration stimulus compared to the classical and shorter duration stimulus.
Figure 5.
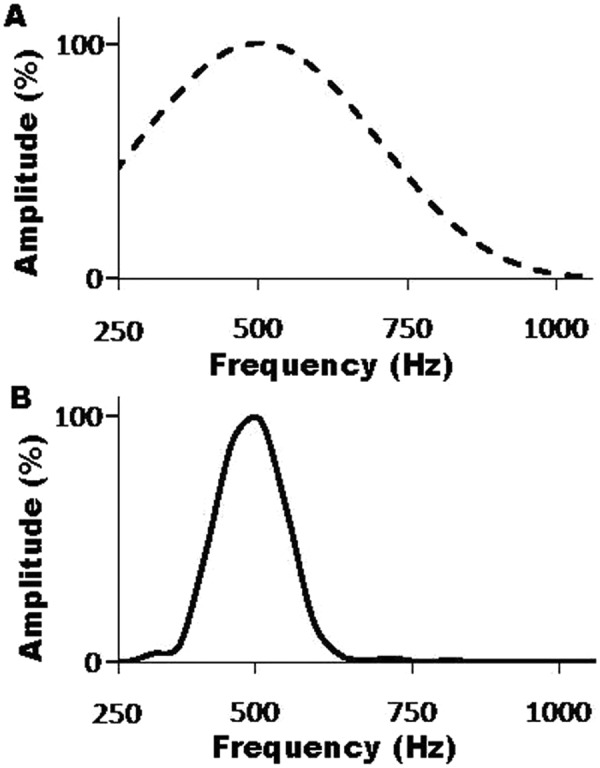
Power spectrum of the shorter and longer tone bursts
Note: Tone bursts in the time domain were transformed into the frequency domain (power spectrum) via FFT. A. Power spectrum of a 6-msec tone burst traditionally used in the clinic for measurement of tone burst ABRs. B. Power spectrum of a 14-msec tone burst used in this study for the measurement of tone burst CMWs. The bandwidth of the 6-msec tone burst (A) is much wider than that of the 14-msec tone burst (B).
The use of a frequency specific stimulus may allow us to assess the condition of a frequency specific cochlear region. This region is also called the best or characteristic frequency (BF or CF) region, and it (a) resonates to the specific stimulus frequencies the most, (b) has the largest displacement amplitude along the basilar membrane, and (c) generates the greatest responses due to its nonlinearity and active components. These three events are not activated anywhere outside the frequency specific cochlear region in response to the specific frequencies. Therefore, we agree that a narrower band stimulus is expected to excite a narrower cochlear region, allowing us to record a response from a narrower region. This is why measurements using tone burst ABRs instead of the click ABRs have been used in the clinic, and why we used tone burst CMs instead of click CMs. In addition, a previous report has shown that CM responses can indeed be recorded from a place-specific (i.e., frequency specific) cochlear region (Ponton et al., 1992).
Yet, despite the high level of frequency specificity present in CMW measurements, the number of clinical studies using CMWs in assessing frequency-related cochlear functions is much lower than the number of studies using OAEs, and also much lower than the number of studies on other CM types such as click-evoked CMs.
Recordings Using an Ear Canal Electrode
Although using a tympanic membrane electrode or an invasive intratympanic electrode in the clinic has allowed us to record a very robust response signal, an ear canal electrode which is less invasive and more convenient has also been successfully used to record electrocochleograms and its use has been well documented since 1983 (Chatrian, Wirch, Edwards, Lettich, & Snyder, 1984; Coats, 1986; Ferraro, Blackwell, Mediavilla, & Thedinger, 1994; Ghosh, Gupta, & Mann, 2002; Mori, Asai, Doi, & Matsunaga, 1987; Pappas, Pappas, Carmichael, Hyatt, & Toohey, 2000; Probst, 1983; Roland, Yellin, Meyerhoff, & Frank, 1995; Zhang et al., 2003). Therefore, we have also adopted the ear canal electrode in our study.
However, the electrocochleography approaches which have been used most often in the clinic have cancelled out CM responses in order to record summation potentials (Ferraro & Durrant, 2002). This is achieved by alternating stimulus polarities (Ferraro & Durrant, 2002). Both click and relatively long tone burst stimulations have been used to record direct current (dc) summation potentials, which are used for diagnosing Meniere’s disease (Ferraro & Durrant, 2002). However and importantly, in order to record and to present clearer tone burst evoked CMWs, the stimulus polarity must not be alternated. Therefore, reports of clinical studies to use ear canal electrodes to present CMWs are relatively fewer.
Nevertheless, CMWs can also be measured using an ear canal electrode (Zhang et al., 2003). Measurement of high-frequency CMW was attempted as well in the past (Davis & Zhang, 2005). One of the obvious attractive benefits of using ear canal electrodes is that placing an electrode into the ear canal is not difficult for the clinician and acceptable to the patient. However, the signals in electrocochleograms or in CMWs are smaller if measured at the ear canal than at other locations closer to the cochlea. Therefore, before ear canal recorded CMWs can be fully used in the clinic, more studies are needed. This study is one of the first attempts along this road. Our results show that recording a clear CMW is feasible, and therefore, such attempts are promising.
Reliability, Artifacts, and Consistency of the Response Patterns of the CMWs
The responses seem reliable as the results observed are based on real CMW recordings in a sufficient number of subjects. Both the disappearance of the CMWs after the sound delivery tube was clamped and the appearance of a delay before CMWs were evoked indicate that CMWs are a true response signal instead of an artifact. The CMWs are also reliable as the recordings show that the CMWs are replicable and that the replicates are almost identical.
As shown in Figure 3, two features as a response pattern can be observed. One is that the amplitude decreases with an increase in the frequency. The other is that the amplitude decreases faster at lower frequencies than at higher frequencies. Standard error exists in the results, which indicates that the means of other groups may not be the same as the means in this group of 20 ears (10 participants). However, although the means among different groups may potentially vary, we believe that this pattern with these two features will be consistent. Our belief is based on the difference in amplitudes which is obvious and statistically significant across these several acoustic frequencies in the results as well as based on five factors as described in the next subsection.
Model for the Pattern and Potential Mechanisms Underlying the Pattern
Using the mathematic model (A = cFe) as shown earlier in results to describe this response pattern is preliminary work. Although having the four data points located on different sides of the function line (fitting line/model line) as shown in Figure 4 is not problematic, there may be a better model for representing this pattern, although we do not yet know which model is most appropriate. The development of a best model also depends on how well we understand the mechanism of the pattern.
In our modeling, we have noticed a phenomenon where high-frequency CMs have lower amplitudes compared to low-frequency CMs. At present, we have not found any reports on humans or any clinical studies showing this phenomenon, and therefore, we have also not found any clinical research to interpret this phenomenon or behavior. As such, we do not know the details of the mechanisms underlying this phenomenon. However, after the pattern was modeled we found five interesting factors which may be relevant. We propose these factors as potential mechanisms for this phenomenon.
The first factor involves the cell’s electrical properties. The CM is a summation of alternating currents from a population of individual hair cells. At the cellular level in animal studies, the amplitude of CMs recorded from an intracellular electrode was ~12 mV at 500 Hz versus ~1 mV at 5000 Hz (>10x difference), i.e., the amplitude decreased as stimulus frequency increased (Palmer & Russell, 1986). Therefore, our result is consistent with theirs.
The second factor relates to map of cochlear length per frequency. According to the cochlear frequency map (Greenwood, 1990), at the location near 500 Hz, 1% of total longitudinal cochlear length represents as few as 23 frequencies, which translates into 0.043% of the total length per frequency. Conversely, near the 6000 Hz location, 1% of total cochlear length represents as many as 280 frequencies, which is only 0.003% of the total length per frequency. Therefore, a difference exceeding 10 magnitudes of length exists between these two values. Longer lengths have more contributors and thus larger CMs may be generated. Longer lengths also result in longer intervals between adjacent standing waves and result in less phase cancellation and so larger CMs may be summed.
The third factor is width and stiffness. The basilar membrane is wider (0.42-0.65 mm) at the apex and narrower (0.08-0.16 mm) at the base, and correspondingly, the base is stiffer than the apex (Oghalai, 2004). The displacement amplitude of basilar membrane can be expected to be greater with wider and more flexible items as shown in the next factor.
The fourth factor is displacement amplitude. The expectation above is consistent with findings in several reports. The displacement amplitude of basilar membrane was observed to be much greater at the apical region than at the basal region (Cooper & Rhode, 1997; Ren, He, & Gillespie, 2011). A larger response may be generated by a greater amplitude.
The fifth factor is the cells’ volume. There are more parallel rows of outer hair cells at the apex than at the base, and the size of individual hair cells is larger at the apex. Therefore, the volume of cells (combining more rows of cells and the larger sizes of individual cells) is greater at the apex, and thus greater CMs may be generated.
All of these five factors are just our proposal to explain as potential mechanisms for this phenomenon where high-frequency CMs have lower amplitudes compared to low-frequency CMs as shown in the model. Although these five factors seem to be reasonable, they remain to be confirmed. Thus, further research, including basic research besides clinical research, is needed.
Clinical Significance
The response pattern which is shown indicates that CMWs at lower frequencies are more robust than at higher frequencies. Such a pattern suggests that lower frequency CMWs may be useful for assessing lower frequency cochlear functions in the clinic. This may especially be true for 500 Hz measurements because OAEs below 1000 Hz are known to be difficult to measure in the clinic due to various noises. In fact, manufacturers set the lowest f2 at 1000 Hz for DPOAE measurements. Nevertheless, low frequencies such as 500 Hz are important frequencies in hearing assessment in the clinic. The response pattern here clearly shows that 500 Hz CMWs are robust. Therefore, CMW measurements can be an alternative test to OAE measurements in assessing low-frequency cochlear functions. The response pattern also indicates that CMWs at frequencies above 500 Hz are also measurable. Therefore, the CMWs at these frequencies can be a supplementary or complementary approach to OAE measurements.
The response pattern demonstrated in this study represents the data of normal hearing subjects. Therefore, for abnormal hearing subjects, we do not yet know the response pattern. However, we can assume that the response pattern in abnormal hearing subjects will be different from the pattern that is demonstrated in this study in normal hearing subjects. The pattern of normal subjects obtained in this study will provide useful information for the evaluation of patterns measured in abnormal hearing subjects in future.
There may be other areas in which the application of CM measurement can be developed. For example, one potential area is hearing screening. In hearing screening applications, especially in newborn populations, clinicians tried with automated ABR testing and later utilized screening OAEs to achieve shorter testing duration and more cost-effective procedures. However, OAEs are not used clinically to assess cochlear function in lower frequencies due to patients’ and environmental noise which interferes with recordings. Based on our work and experience as well as information in the literature, CM recordings have the potential to overcome this significant shortcoming. Hearing screening with low-frequency CMs can be achieved, and such a screening may take less than 1 minute. For example, the stimulus can be set to use a 14-msec tone burst at 500 Hz presented at an intensity of 75 nHL, and the acquisition can be set to use a rate of 22.7/sec which is much faster than that in OAE measurements. At these settings with 500 to 1,000 sweeps, a clear CM response can be obtained with a satisfactory signal to noise ratio. A software program can be developed to achieve an automated analysis, to display the results, and to create a report. To simplify the placement of the recording electrode, a concha electrode can be incorporated as an alternative approach to an ear canal electrode. The concha electrode is easy to apply as it only needs to be attached to the concha via clamps. The concha is the part of the pinna at the entrance of the ear canal. To use the concha electrode, neither is sticky tape needed to hold the electrode, nor is the snapping-style surface electrode needed. The concha electrode has been tested, and its detailed information has been reported (Zhang, 2010).
Conclusion
The study of CMWs recorded at the ear canal in response to a relatively lone tone burst (>14 msecs) for assessing cochlear function is a relatively underrepresented area in both CM studies and in cochlear function studies. Our findings support an indication that CMWs can be recorded at the ear canal in response to a relatively long tone burst. Two features were observed in the response pattern of CMWs: (a) the amplitude of CMWs decreased with an increase of stimulus frequency in the tone bursts; and (b) such a decrease occurred at a faster rate at lower frequencies than at higher frequencies. Such a response pattern suggests that CMWs may be used as an alternative to otoacoustic emissions (OAEs) in the assessment of cochlear functions in the clinic, especially at low frequencies.
Acknowledgments
The author wishes to thank the subjects who participated in the experiment for their time and effort; Jane De Pauw for effective editing and proofreading; Brianne Davis for participation in the study; Dr. Vicky Zhao, Mr. Brian Schmidt, and Dr. Melanie Campbell for their insightful discussions of the manuscript; and anonymous reviewers for their very valuable comments.
Footnotes
Declaration of Conflicting Interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Portions of this work were supported by grants from the Canada Foundation for Innovation (M.Z.), Glenrose Rehabilitation Hospital Foundation (M.Z.), and research funding from the University of Alberta (M.Z.).
References
- Adrian E. D. (1931). The microphonic action of the cochlea: An interpretation of Wever and Bray’s experiments. Journal of Physiology, 71, xxviii-xxix [Google Scholar]
- Andersson E., Arlinger S., Jacobsson S. (2000). Evaluation of OAE-recording as a complementary test method for adults with moderate to profound mental retardation. Scandinavian Audiology, 29(2), 120-126 [DOI] [PubMed] [Google Scholar]
- Arakawa K. (1998). [Summating potential evoked by long-tone burst stimuli in Meniere’s disease]. Nippon Jibiinkoka Gakkai Kaiho, 101(1), 53-62 [DOI] [PubMed] [Google Scholar]
- Berlin C. I., Hood L., Morlet T., Rose K., Brashears S. (2003). Auditory neuropathy/dys-synchrony: Diagnosis and management. Mental Retardation and Developmental Disabilities Research Reviews, 9, 225-231 [DOI] [PubMed] [Google Scholar]
- Burkey J. M., Lippy W. H., Schuring A. G., Rizer F. M. (1998). Clinical utility of the 512-Hz Rinne tuning fork test. American Journal of Otology, 19(1), 59-62 [PubMed] [Google Scholar]
- Chatrian G. E., Wirch A. L., Edwards K. H., Lettich E., Snyder J. M. (1984). Cochlear summating potential recorded from the external auditory meatus of normal humans. Amplitude-intensity functions and relationships to auditory nerve compound action potential. Electroencephalography and Clinical Neurophysiology, 59, 396-410 [DOI] [PubMed] [Google Scholar]
- Chien C. H., Tu T. Y., Shiao A. S., Chien S. F., Wang Y. F., Li A. C., Yang M. J. (2008). Prediction of the pure-tone average from the speech reception and auditory brainstem response thresholds in a geriatric population. ORL; Journal of Oto-Rhino-Laryngology and its Related Specialties, 70, 366-372 [DOI] [PubMed] [Google Scholar]
- Coats A. C. (1986). The normal summating potential recorded from external ear canal. Archives of Otolaryngology—Head & Neck Surgery, 112, 759-768 [DOI] [PubMed] [Google Scholar]
- Cooper N. P., Rhode W. S. (1997). Mechanical responses to two-tone distortion products in the apical and basal turns of the mammalian cochlea. Journal of Neurophysiology, 78, 261-270 [DOI] [PubMed] [Google Scholar]
- Dallos P. (1981). Cochlear physiology. Annual Review of Psychology, 32, 153-190 [DOI] [PubMed] [Google Scholar]
- Dallos P., Cheatham M. A. (1976). Production of cochlear potentials by inner and outer hair cells. Journal of the Acoustical Society of America, 60(2), 510-512 [DOI] [PubMed] [Google Scholar]
- Dallos P., Wang C. Y. (1974). Bioelectric correlates of kanamycin intoxication. Audiology, 13, 277-289 [DOI] [PubMed] [Google Scholar]
- Dauman R., Aran J. M., Charlet de Sauvage R., Portmann M. (1988). Clinical significance of the summating potential in Meniere’s disease. American Journal of Otology, 9(1), 31-38 [PubMed] [Google Scholar]
- Davis B., Zhang M. (2005). Electrocochleography to High-Frequency Toneburst with Ear Canal Electrode. Abstracts of the Association for Research in Otolaryngology, 28, 190 [Google Scholar]
- Davis H. (1958). A mechano-electrical theory of cochlear action. Annals of Otology, Rhinology and Laryngology, 67, 789-801 [DOI] [PubMed] [Google Scholar]
- Deltenre P., Mansbach A. L., Bozet C., Christiaens F., Barthelemy P., Paulissen D., Renglet T. (1999). Auditory neuropathy with preserved cochlear microphonics and secondary loss of otoacoustic emissions. Audiology, 38, 187-195 [DOI] [PubMed] [Google Scholar]
- Dreisbach L. E., Siegel J. H. (2005). Level dependence of distortion-product otoacoustic emissions measured at high frequencies in humans. Journal of the Acoustical Society of America, 117, 2980-2988 [DOI] [PubMed] [Google Scholar]
- Ferraro J. A., Blackwell W. L., Mediavilla S. J., Thedinger B. S. (1994). Normal summating potential to tone bursts recorded from the tympanic membrane in humans. Journal of the American Academy of Audiology, 5(1), 17-23 [PubMed] [Google Scholar]
- Ferraro J. A., Durrant J. D. (2002). Electrocochleography. In Katz J. (Ed.), Handbook of clinical audiology (5th ed., pp. 249-273). Philadelphia, PA: Lippincott Williams & Wilkins [Google Scholar]
- Ferraro J. A., Durrant J. D. (2006). Electrocochleography in the evaluation of patients with Meniere’s disease/endolymphatic hydrops. Journal of the American Academy of Audiology, 17(1), 45-68 [DOI] [PubMed] [Google Scholar]
- Ferraro J. A., Ruth R. A. (1994). Electrocochleography. In Katz J. (Ed.), Handbook of clinical audiology (5th ed., pp. 339-350). Philadelphia, PA: Lippincott Williams & Wilkins [Google Scholar]
- Ge N. N., Shea J. J., Jr., Orchik D. J. (1997). Cochlear microphonics in Meniere’s disease. American Journal of Otology, 18(1), 58-66 [PubMed] [Google Scholar]
- Ghosh S., Gupta A. K., Mann S. S. (2002). Can electrocochleography in Meniere’s disease be noninvasive? Journal of Otolaryngology, 31, 371-375 [DOI] [PubMed] [Google Scholar]
- Gibson W. P., Beagley H. A. (1976). Electrocochleography in the diagnosis of acoustic neuroma. Journal of Laryngology & Otology, 90(2), 127-139 [DOI] [PubMed] [Google Scholar]
- Gorga M. P., Neely S. T., Bergman B. M., Beauchaine K. L., Kaminski J. R., Peters J., . . .Jesteadt W. (1993). A comparison of transient-evoked and distortion product otoacoustic emissions in normal-hearing and hearing-impaired subjects. Journal of the Acoustical Society of America, 94, 2639-2648 [DOI] [PubMed] [Google Scholar]
- Greenwood D. D. (1990). A cochlear frequency-position function for several species–29 years later. Journal of the Acoustical Society of America, 87, 2592-2605 [DOI] [PubMed] [Google Scholar]
- Harrison W. A., Norton S. J. (1999). Characteristics of transient evoked otoacoustic emissions in normal-hearing and hearing-impaired children. Ear and Hearing, 20(1), 75-86 [DOI] [PubMed] [Google Scholar]
- Hussain D. M., Gorga M. P., Neely S. T., Keefe D. H., Peters J. (1998). Transient evoked otoacoustic emissions in patients with normal hearing and in patients with hearing loss. Ear and Hearing, 19, 434-449 [DOI] [PubMed] [Google Scholar]
- Keidel W. (1962). Mechanical frequency discrimination in the cochlea. Audiology, 1, 37-52 [Google Scholar]
- Kusakari J., Takeyama M., Kawase T., Takahashi K., Sasaki Y., Takasaka T. (1988). Studies with electrocochleography and auditory brainstem response in Ramsay Hunt syndrome. Acta oto-laryngologica. Supplementum, 446, 81-84 [DOI] [PubMed] [Google Scholar]
- Martin G. K., Stagner B. B., Lonsbury-Martin B. L. (2011). Evidence for basal distortion-product otoacoustic emission components. Journal of the Acoustical Society of America, 127, 2955-2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori N., Asai H., Doi K., Matsunaga T. (1987). Diagnostic value of extratympanic electrocochleography in Meniere’s disease. Audiology, 26(2), 103-110 [PubMed] [Google Scholar]
- Moriuchi H., Kumagami H. (1979). Changes of AP, SP and CM in experimental endolymphatic hydrops. Audiology, 22, 258-260 [Google Scholar]
- Norton S. J., Ferguson R., Mascher K. (1989). Evoked otoacoustic emissions and extratympanic cochlear microphonics recorded from human ears. Abstracts of the Association for Research in Otolaryngology, 12, 227(A). [Google Scholar]
- Oghalai J. S. (2004). The cochlear amplifier: Augmentation of the traveling wave within the inner ear. Current Opinion in Otolaryngology & Head and Neck Surgery, 12, 431-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A. R., Russell I. J. (1986). Phase-locking in the cochlear nerve of the guinea-pig and its relation to the receptor potential of inner hair-cells. Hearing research, 24(1), 1-15 [DOI] [PubMed] [Google Scholar]
- Pappas D. G., Jr., Pappas D. G., Sr., Carmichael L., Hyatt D. P., Toohey L. M. (2000). Extratympanic electrocochleography: Diagnostic and predictive value. American journal of otology, 21(1), 81-87 [DOI] [PubMed] [Google Scholar]
- Ponton C. W., Don M., Eggermont J. J. (1992). Place-specific derived cochlear microphonics from human ears. Scandinavian Audiology, 21(3), 131-141 [DOI] [PubMed] [Google Scholar]
- Probst R. (1983). Electrocochleography: Using extratympanic or transtympanic methods? ORL; Journal of Oto-Rhino-Laryngology and its Related Specialties, 45, 322-329 [DOI] [PubMed] [Google Scholar]
- Ren T., He W., Gillespie P. G. (2011). Measurement of cochlear power gain in the sensitive gerbil ear. Nature Communications, 2, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazi M., Ferraro J. A. (2008). Observations on mastoid versus ear canal recorded cochlear microphonic in newborns and adults. Journal of the American Academy of Audiology, 19(1), 46-55 [DOI] [PubMed] [Google Scholar]
- Roland P. S., Yellin M. W., Meyerhoff W. L., Frank T. (1995). Simultaneous comparison between transtympanic and extratympanic electrocochleography. American Journal of Otology, 16, 444-450 [PubMed] [Google Scholar]
- Suckfull M., Schneeweiss S., Dreher A., Schorn K. (1996). Evaluation of TEOAE and DPOAE measurements for the assessment of auditory thresholds in sensorineural hearing loss. Acta Oto-Laryngologica, 116, 528-533 [DOI] [PubMed] [Google Scholar]
- Sun X. M., Shaver M. D. (2009). Effects of negative middle ear pressure on distortion product otoacoustic emissions and application of a compensation procedure in humans. Ear and hearing, 30, 191-202 [DOI] [PubMed] [Google Scholar]
- Tognola G., Ravazzani P., Grandori F. (1995). An optimal filtering technique to reduce the influence of low-frequency noise on click-evoked otoacoustic emissions. British Journal of Audiology, 29, 153-160 [DOI] [PubMed] [Google Scholar]
- Vander Werff K. R., Prieve B. A., Georgantas L. M. (2009). Infant air and bone conduction tone burst auditory brain stem responses for classification of hearing loss and the relationship to behavioral thresholds. Ear and hearing, 30, 350-368 [DOI] [PubMed] [Google Scholar]
- Wang J., Tymczyszyn N., Yu Z., Yin S., Bance M., Robertson G. S. (2011). Overexpression of X-linked inhibitor of apoptosis protein protects against noise-induced hearing loss in mice. Gene Therapy, 18, 560-568 [DOI] [PubMed] [Google Scholar]
- Wever E. G., Bray C. (1930). Action currents in the auditory nerve response to acoustic stimulation. Proceedings of the National Academy of Sciences of the United States of America, 16, 344-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead M. L., Lonsbury-Martin B. L., Martin G. K. (1993). The influence of noise on the measured amplitudes of distortion-product otoacoustic emissions. Journal of speech and hearing research, 36, 1097-1102 [DOI] [PubMed] [Google Scholar]
- Withnell R. H. (2001). Brief report: The cochlear microphonic as an indication of outer hair cell function. Ear & Hearing, 22(1), 75-77 [DOI] [PubMed] [Google Scholar]
- Zhang M. (2010). Using concha electrodes to measure cochlear microphonic waveforms and auditory brainstem responses. Trends in Amplification, 14, 211-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Abbas P. J. (1993). Ontogeny of 2f1-f2 acoustic and microphonic distortion products. Journal of the Acoustical Society of America, 94(4 Pt. 2), 2331 [Google Scholar]
- Zhang M., Abbas P. J. (1997). Effects of middle ear pressure on otoacoustic emission measures. Journal of the Acoustical Society of America, 102(2 Pt 1), 1032-1037 [DOI] [PubMed] [Google Scholar]
- Zhang M., Paschall D., Chandler R., Reel L., Foster M. (2003). EcochG (Electrocochleography) to Toneburst with Ear Canal Electrode and Its Advantages. Abstracts of the Association for Research in Otolaryngology, 26, 190 [Google Scholar]
- Zou Y., Zheng J., Ren T., Nuttall A. (2006). Cochlear transducer operating point adaptation. Journal of the Acoustical Society of America, 119, 2232-2241 [DOI] [PubMed] [Google Scholar]


