Abstract
T wave “memory” is a peculiar variety of cardiac remodeling caused by a transient change in the course of ventricular depolarization (due to ventricular pacing, rate-dependent intraventricular block, ventricular preexcitation or tachyarrhythmias with wide QRS complexes). It is usually manifested by inverted T waves that appears when normal ventricular activation is restored. This phenomenon is cumulative and occurs earlier if the ventricular myocardium has previously been exposed to the same conditioning stimuli. In this article the different conditions giving rise to “classical” T wave memory development are reviewed and also “another” type of T wave memory is described. It is also shown that cardiac memory may induce not only negative (pseudo-primary) T waves but also a reversal of primary and pseudo-primary T waves leading to “normalization” of ventricular repolarization. The knowledge of these dissimilar consequences of T wave memory is essential to assess the characteristics of ventricular repolarization.
Keywords: Cardiac electrophysiology, electrocardiography, cardiac pacing, cardiac arrhythmias, memory induced T wave.
INTRODUCTION
In 1969, Chaterjee and co-workers described for the first time the relationship between transient periods of abnormal ventricular depolarization caused by artificial stimulation of the heart and the T wave abnormalities subsequently found in non paced beats [1]. Almost a decade after Chaterjee’s observation, Denes et al. showed that deep symmetrical T-wave inversion was present in right and mid-precordial leads during normal ventricular conduction in 19 of 23 patients with intermittent left bundle branch block (LBBB) [2]. However, the mechanism of these phenomena did not attract the interest of researchers and no further systematic studies were performed until the early 1980s, when Mauricio Rosenbaum and colleagues published their seminal articles on the so-called “cardiac memory” [3, 4]. In fact, they demonstrated that a temporary change in the sequence of ventricular depolarization induced by different conditions (i.e. ventricular pacing, rate-dependent intraventricular block, ventricular preexcitation or tachyarrhythmias with wide QRS complexes) can be followed by T wave changes that become apparent once ventricular activation returns to normal. Furthermore, they showed that this phenomenon is cumulative and occurs earlier if the ventricular myocardium has previously been exposed to the same conditioning stimulus.
As will be shown later, the most outstanding feature of “memory-induced” abnormal T waves is that their spatial polarity reproduces with remarkable accuracy that of the preceding conditioning aberrant QRS complexes.
For several years cardiac memory research focused on the inverted T waves that appeared after transient anomalous ventricular activation caused by right ventricular apical pacing or LBBB as they were identical to those found in myocardial ischemia or cardiomyopathies. Subsequently, Kalbfleisch et al. [5] reported that unusually peaked positive T waves were present in most precordial leads after radiofrequency (RF) ablation of postero-septal or left sided accessory pathways. More recently, our group demonstrated that pacing induced transient changes in the ventricular depolarization sequence may cause normalization of primary and pseudo-primary negative T waves [6]. Therefore, ventricular repolarization “memory” may give rise to diverse electrocardiographic changes. In this chapter we will describe these multiple manifestations of T wave “memory” and discuss the underlying mechanisms of this intriguing phenomenon.
THE “CLASSICAL” T WAVE “MEMORY”
As mentioned above, cardiac “memory” usually refers to inverted T waves appearing after a period of abnormal ventricular activation once the conditioning stimuli cease and normal ventricular depolarization resumes. This may be caused by several mechanisms as described below.
INTERMITTENT LEFT BUNDLE BRANCH BLOCK
Figure (1) depicts a distinctive example of T wave “memory” in a patient with intermittent LBBB. In each ECG lead (left panel) the first beat shows normal ventricular conduction, and the second exhibits a typical LBBB pattern. The most outstanding finding is that the T waves in the normally conducted beats are symmetrically negative in those leads where the aberrant QRS complex were negative (i.e. leads III, aVF, V1,V2 and V3 ) while it remains positive in those leads in which the LBBB gave rise to a positive QRS complex (leads I, II, aVL, V5 and V6). Thus, as can be seen even more clearly in the vectorcardiogram (right panel), the polarity of “memory-induced” abnormal T waves accurately tracks that of the conditioning aberrant QRS complexes.
Fig. (1).
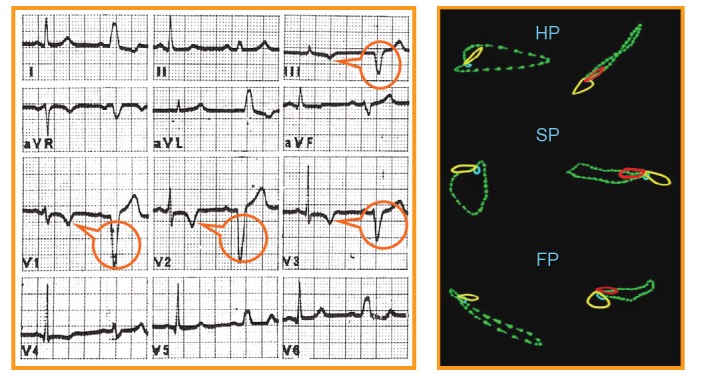
A typical example of “memory-induced” T waves in a patient with intermittent LBBB (electrocardiogram on left and vectorcardiogram on right). The red loop of the T wave at right (superimposed to the vectorcardiographic QRS loop of the LBBB) corresponds to the yellow loop of T wave on left (normal ventricular conduction). This composition highlights that the T wave spatial direction follows that of the QRS aberrancy. See text for full description.
A noteworthy fact is that the changes due to ventricular repolarization “memory” are totally masked by the secondary T waves induced by the conduction disturbance and that they only become apparent when normal ventricular depolarization resumes. Although this is virtually the rule, in some patients showing T wave “memory” caused by an intermittent LBBB, a small terminal negative deflection of the T wave may be observed in leads V1 to V3. Furthermore, Shvilkin has described that the T waves of the LBBB tend to be of lesser amplitude when the conduction block has been long lasting due to the effect of T wave cardiac memory superimposed on the secondary T wave changes caused by the abnormal depolarization sequence [7].
The morphologic characteristics of the negative “memory-induced” T waves (also known as “pseudo-primary” T waves) are very similar to that of primary inverted T waves, due to coronary heart disease or myocardial diseases. Thus, the differentiation of these is of extreme clinical importance. Besides the clinical picture, the identification of the etiology of such alteration from a strictly electrocardiographic point of view should be based on the comparison between the polarity of the abnormal T waves and that of the transient QRS aberrancy in each electrocardiographic lead. In fact, the accurate concordance between the polarity of T waves following normal ventricular conduction and the polarity of the conditioning aberrant QRS complex is strongly supportive of the pseudo-primary character of the abnormal T waves. On the other hand, when the T wave polarity departs from that of the conditioning QRS aberrancy, the presence of a genuine primary T wave abnormality should be suspected.
RIGHT VENTRICULAR APICAL PACING
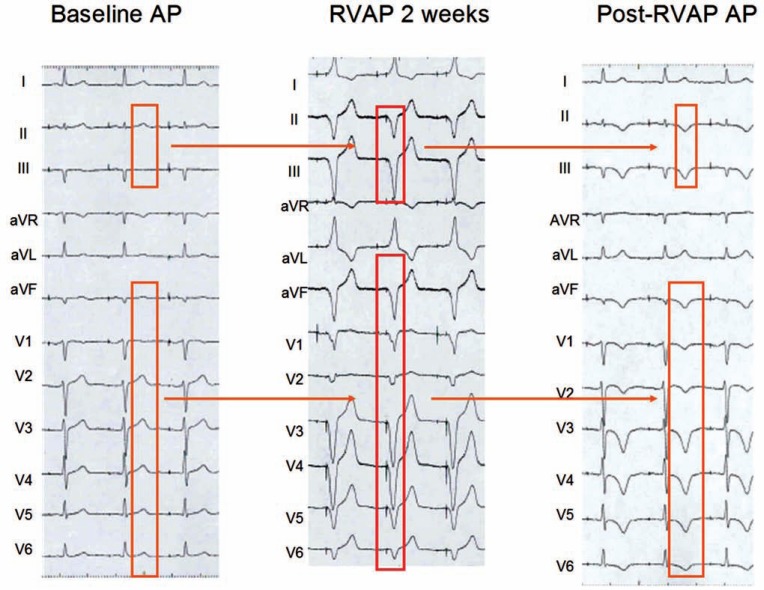
The “postponed effect” of a relatively prolonged period of right ventricular apical pacing on the T wave is depicted in (Fig. 2). Ventricular stimulation gave rise to wide, negative QRS complexes in the inferior ECG leads as well as in all precordial leads. Consequently, as could be anticipated, the post pacing T waves were symmetrically negative in these leads once normal ventricular activation was restored.
Fig. (2).
Induction of ventricular repolarization memory by right ventricular apical pacing (RVAP) in a patient with symptomatic sinus node dysfunction and a dual chamber pacemaker. During AAI pacing (AP), ventricular repolarization is normal. RVAP during DDD stimulation with a short A-V interval (88% of beats were ventricular paced during a one month period) caused aberrant QRS complexes with negative polarity in all ECG leads, except I, aVR and aVL. One week later, negative T waves were apparent during AAI pacing. Note the strict concordance between the polarity of these T waves and that of the QRS complexes induced by right ventricular pacing. Some noticeable differences can be observed when compared to (Fig. 1).
It is important to analyze the differences in the direction of the “memory-induced” T wave changes observed in Figures 1 (intermittent LBBB) and 2 (transient right apical ventricular pacing). First, as the conditioning QRS complex axis in the frontal plane was directed more superiorly during right ventricular apical pacing than during LBBB, the “memory-induced” T waves were negative in leads II, III and aVF in (Fig. 2) but only in leads III and aVF in (Fig. 1). Second, as the conditioning QRS complexes showed a negative polarity in all precordial leads during right apical pacing, the “memory-induced” T waves were also negative in all precordial leads in (Fig. 2), but only in V1 to V3 in (Fig. 1), where the LBBB caused negative QRS complexes).
WIDE QRS COMPLEX TACHYCARDIAS
“Memory-induced” negative and symmetrical T waves may be also observed after termination of ventricular or supraventricular tachycardias showing wide QRS complexes.
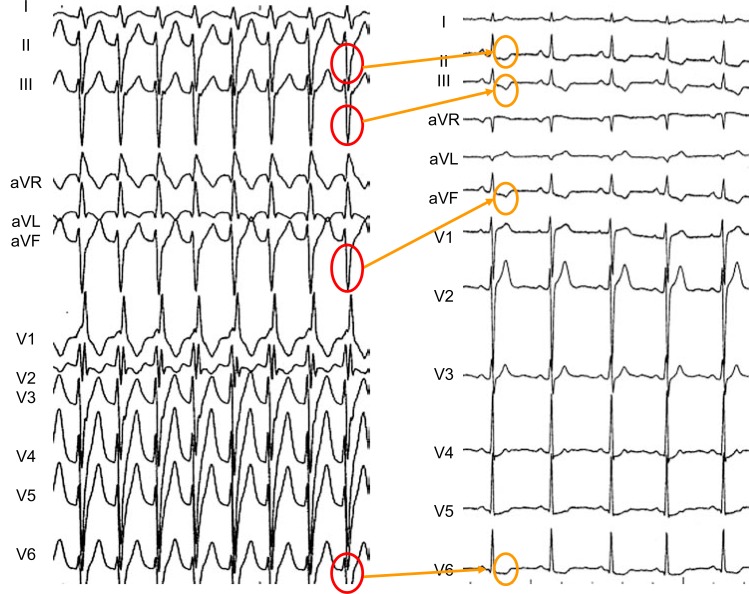
Fig. (3) shows an example of “memory-induced” abnormal T waves recorded after a self-limited episode of a fascicular ventricular tachycardia. Post tachycardia, the T waves in sinus rhythm were negative in those ECG leads in which the conditioning aberrant QRS complexes had been predominantly negative (II, III, aVF and V4 to V6).
Fig. (3).
Fascicular ventricular tachycardia (left) showing a pattern of “right bundle branch block and left anterior hemiblock”. The wide QRS are predominantly negative in leads II, III, aVF and V3 to V6. After spontaneous termination of the arrhythmia, abnormal “memory-.induced” T waves are observed in leads II, III, aVF and V4 to V6. Note that post- tachycardia T waves are positive in leads V1 and V2, in which the conditioning QRS complex was also positive. This is an example of short-term T wave memory, appearing after a short period of abnormal ventricular depolarization.
OTHER FORMS OF T WAVE MEMORY
Ventricular Preexcitation
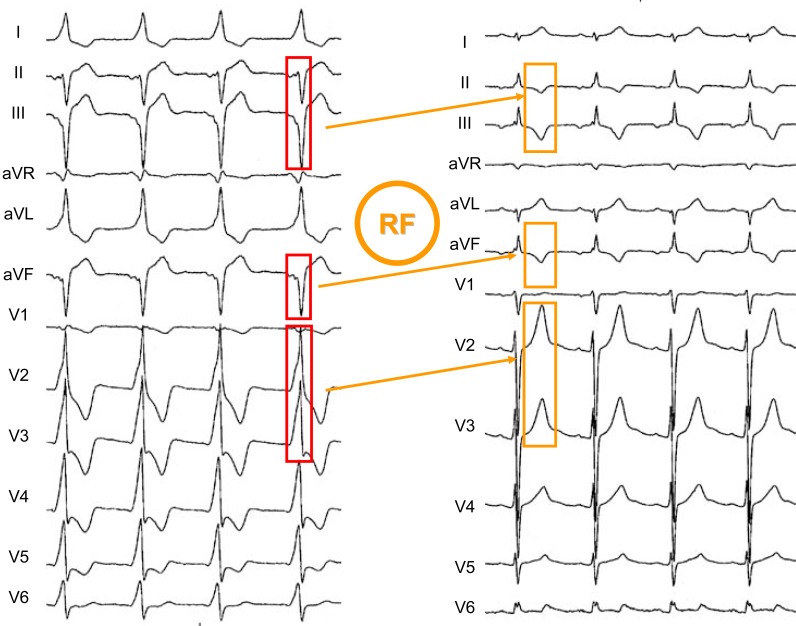
Fig. (4) shows a typical example of T wave “memory” observed after ablation of an infero-paraseptal AV accessory pathway. The preexcited QRS complexes were negative in the inferior leads and positive in leads I, aVL and V2 to V6. Concordant with the preexcited QRS complexes, negative T waves appeared after RF ablation of the accessory A-V pathway in leads II, III and aVF (“classical” cardiac memory) but in other ECG leads prominent positive T waves were present, particularly in those showing the more conspicuous positive conditioning preexcited QRS complexes. This example demonstrates, as shown by Kalbfleisch and coworkers [5], that a repetitive abnormal depolarization process giving rise to positive QRS complexes may induce subsequent tall peaked positive T waves whenever normal ventricular activation resumes.
Fig. (4).
An example of “memory-induced” T waves observed after radiofrequency (RF) ablation of an infero-paraseptal accessory A-V pathway. In the inferior leads, the “classic” T wave memory (symmetrically, negative T waves) are seen after elimination of ventricular pre-excitation (which caused the wide negative QRS complexes). On the other hand, in leads V2 and V3 tall peaked positive memory-induced T waves where the conditioning pre-excited QRS complexes were markedly positive.
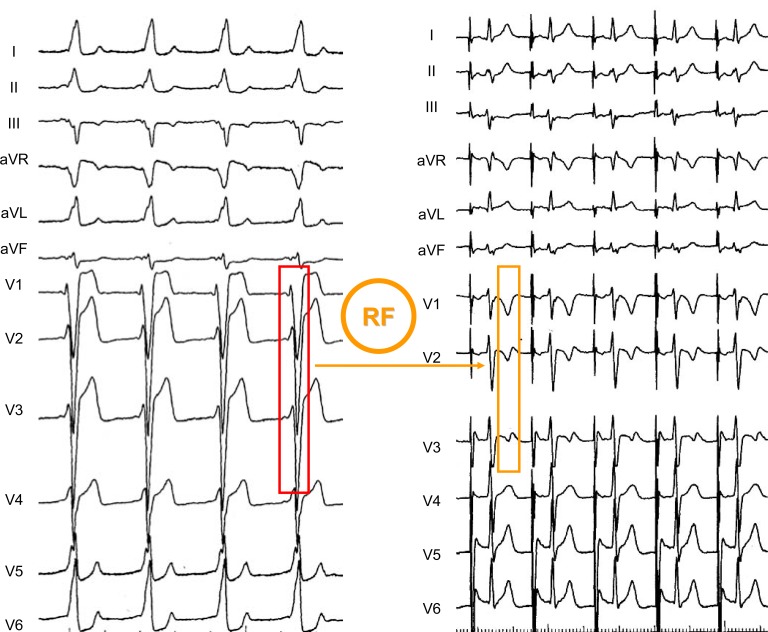
After RF ablation of a right lateral A-V accessory pathway, a quite different pattern of “memory-induced” ventricular repolarization was observed (compared Fig. (5) to Fig. (4)). The conditioning preexcited QRS complexes were predominantly negative in leads V1 to V3 and positive in leads II and aVF. Accordingly, disappearance of ventricular preexcitation was accompanied by negative T waves only in the right precordial leads whilst positive T waves were observed in leads II and aVF.
Fig. (5).
“Memory-induced” T waves observed after radiofrequency (RF) ablation of a right lateral AV accessory pathway. The spatial direction of post-ablation T waves is clearly different from that observed in Fig. (4), due to the different anatomical location of the accessory pathway.
Outflow Tract Ventricular Pacing, Tachycardias and Extrasystoles
Outflow tract right ventricular pacing as well as ventricular extrasystoles and tachycardias arising from the right and left ventricular outflow tracts (including the aortic cusps and the pulmonary valve), give rise to a peculiar “LBBB- like” pattern. In fact, the axis of the QRS complex in the frontal plane is invariably directed downwards and sometimes rightwards. Therefore, the inferior leads always show prominent R waves, whereas the precordial transition of the QRS complex is variable and may be found within the right precordial leads, which may exhibit slurred and even predominant R waves. Consequently, “memory” T waves induced by these conditions will invariably have positive polarity in leads II, III and aVF, whilst their morphological characteristics in the precordial leads will depend on the electrocardiographic pattern of the conditioning ventricular beats.
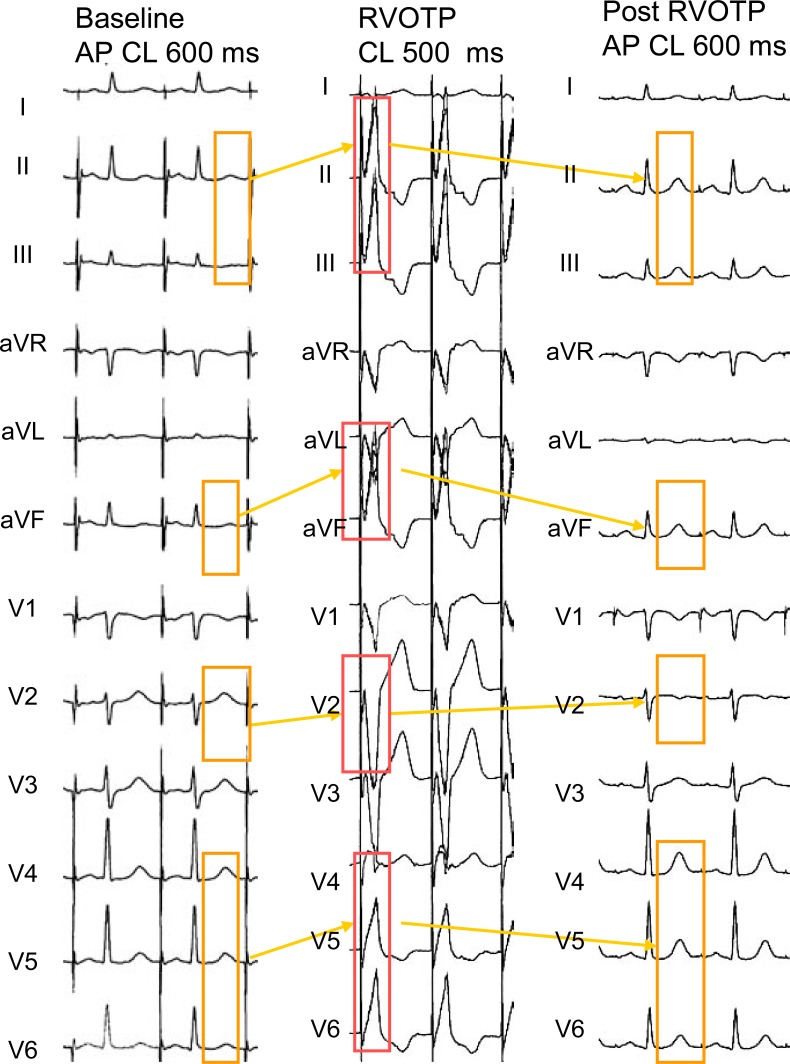
Fig. (6) shows the changes in the T waves observed after a 15 min period of right ventricular outflow tract pacing in a patient with normal ventricular conduction and repolarization. The post-pacing T waves become clearly more positive in the inferior leads and from V4 to V6, in which the conditioning paced QRS complexes had been positive. On the other hand, the post-pacing T waves became more negative in lead V1, inverted in lead V2 and less positive in lead V3, in accordance with the negative polarity of the precedent paced QRS complexes in those leads.
Fig. (6).
“Memory-induced” T wave changes after a 15 min period of right ventricular outflow tract pacing (RVOTP). AP: atrial pacing. See text for full description.
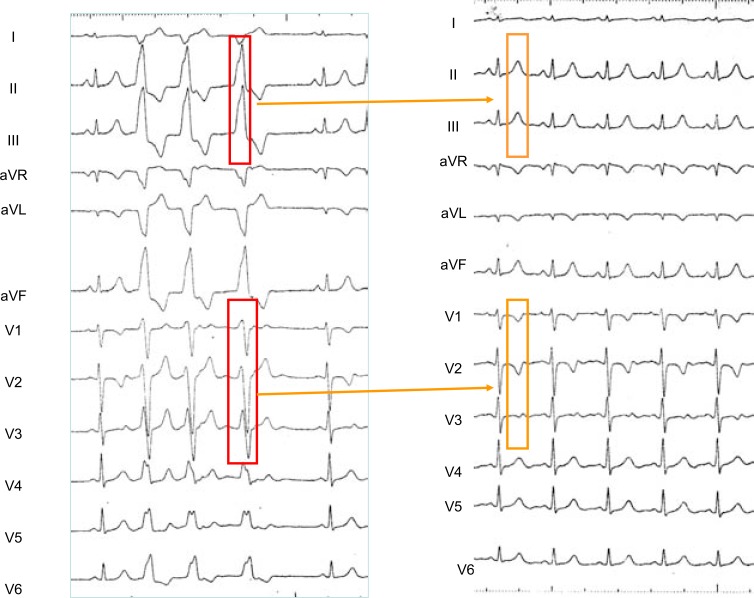
An example of “memory-induced” T waves in a patient with self-limited episodes of ventricular tachycardia and extrasystoles arising from the left aortic cusp is presented in (Fig. 7). The “memory-induced” T waves precisely tracked the spatial orientation of the conditioning ectopic QRS complexes.
Fig. (7).
Left panel: 12 simultaneous ECG leads obtained in a patient with incessant self-limited episodes of ventricular tachycardia with a “LBBB like” pattern before ablation of the ectopic focus). Ventricular ectopy shows tall R waves in the inferior leads and its precordial transition is situated in lead V3. Right Panel: ECG recorded after ablation of the focus found situated in the left aortic sinus of Valsalva, 1cm below the left coronary artery ostium. The “memory-induced” T waves spatial orientation exactly reproduces that of the QRS complexes of ventricular tachycardia.
Cardiac Memory Leading to Normalization of Primary and Pseudo-primary T Waves
Recently, we reported that transient periods of ventricular pacing (even lasting only 15 minutes) can lead to a “postponed” reversal of primary or pseudo-primary T wave abnormalities [6]. To obtain this effect, pacing has to be performed from specific ventricular sites in order to evoke QRS complexes whose polarity is opposite to that of the abnormal T waves. Thus, memory-induced normalization of altered ventricular repolarization can be achieved by pacing from the right ventricular outflow tract in patients with negative T waves in inferior and left precordial leads or from the base of the left ventricle (via the coronary sinus) in patients with abnormal T waves in most precordial leads. It is conceivable that identical changes in ventricular repolarization may be caused by spontaneous aberrant QRS complexes whenever they meet the above mentioned morphologic characteristics.
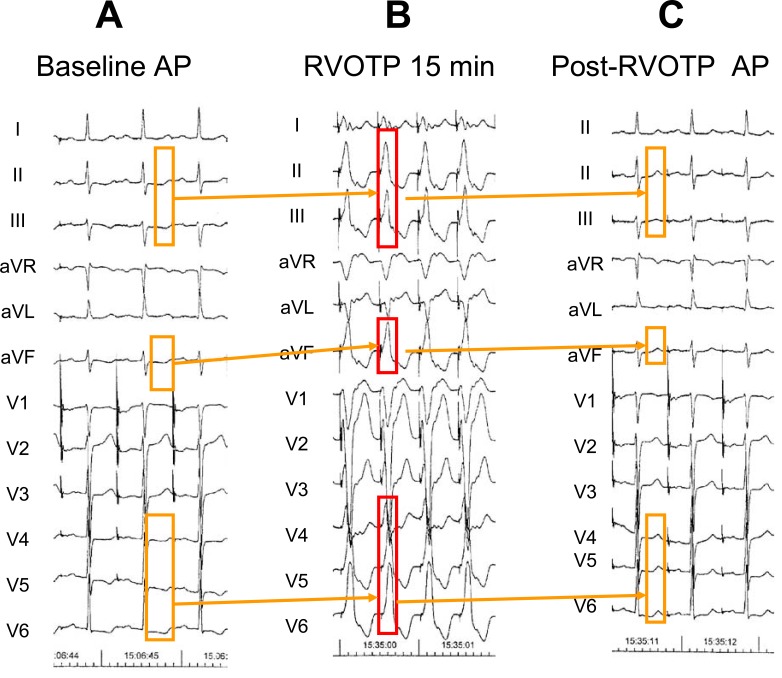
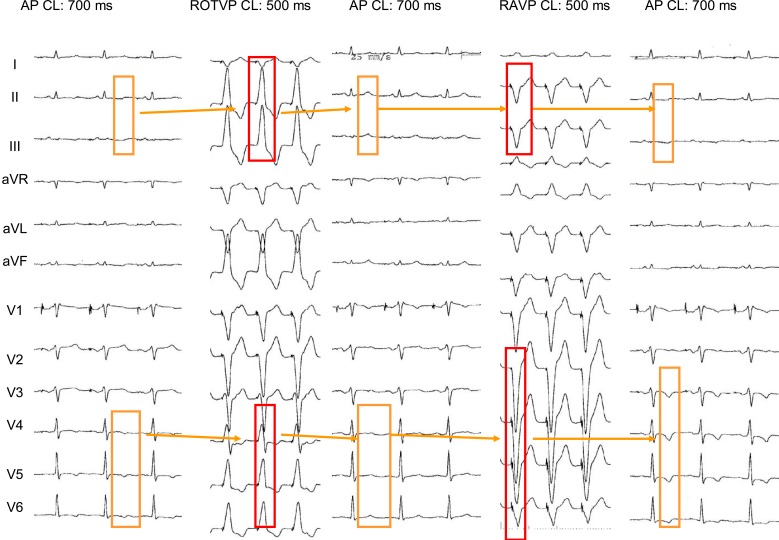
Fig. (8) illustrates the reversal of inferior and lateral primary abnormal T waves in a 70-year old patient with idiopathic dilated cardiomyopathy after a 15 min period of right ventricular outflow tract pacing.
Fig. (8).
Short term “memory-induced” normalization of infero-lateral inverted T waves in a 70-year old patient with idiopathic dilated cardiomyopathy. A: Baseline ECG recording obtained during atrial pacing(AP) at cycle length (CL) of 700 ms showing inverted T waves in leads II,III, aVF and V4 to V6. B: the right ventricle was paced for 15 minutes from the right ventricular outflow tract (RVOTP) to obtain wide positive QRS complexes in the ECG leads where the T waves were abnormal. C: after cessation of ventricular pacing, the T wave clearly tracked the electrical forces of the previous paced QRS complexes. This resulted in positive T waves in leads II, III, aVF and V4 to V6. Note that the T waves turned negative in lead V1 and less positive in lead V2, following the direction of the precedent negative conditioning QRS complexes in those leads.
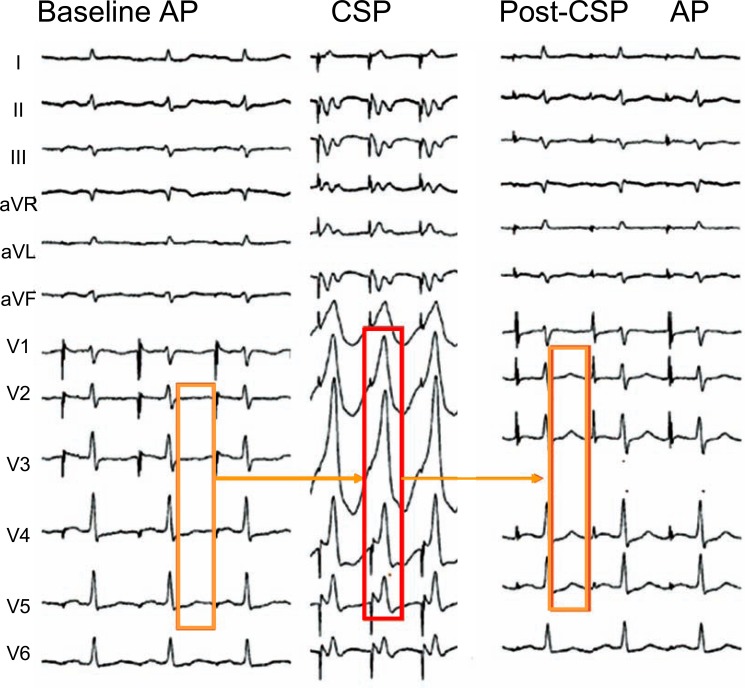
In Fig. (9), temporary left ventricular pacing from the coronary sinus causing predominantly positive QRS complexes in all precordial leads led to upright post-pacing T waves across the precordium, by the “delayed” effect of the altered ventricular excitatory wave on repolarization.
Fig. (9).
Memory-induced normalization of primary abnormal T waves in precordial leads in a 57 year old patient with idiopathic dilated cardiomyopathy and syncope. The baseline ECG recorded during atrial pacing (AP) at a cycle length (CL) of 750 ms showed abnormal negative or flat T waves in leads V1 to V6 (A). After a 15-minute period of left ventricular pacing (VP) at a CL of 500 ms (B), the T wave became less negative in lead V1 and ”normalized” in V2-V6, tracking the predominant electrical forces of the previously paced QRS complexes (C).
“Dual” Cardiac Memory
Fig. (10) shows an example of “dual” cardiac memory in a patient with primary T wave abnormalities in inferior and left precordial leads, in whom a 15 min period of pacing was performed separately from two different sites of the right ventricle. Remarkably, T wave normalization was obtained after cessation of right ventricular pacing from the outflow tract while exacerbation of the abnormal T waves occurred following pacing from the right ventricular apex. This indicates than the electrocardiographic configuration of the T wave may be modulated virtually at will whenever appropriate conditioning QRS complexes are transiently evoked.
Fig. (10).
“Dual” cardiac memory induced by pacing from two different sites of the right ventricle in a 57 year old patient suffering from idiopathic dilated cardiomyopathy. A: During baseline atrial pacing (AP) at a cycle length (CL) of 700 ms, low voltage QRS complexes, incomplete right bundle branch block and negative T waves in leads II, III, aVF, and V4 to V6 were recorded. B: wide positive QRS complexes were induced in leads II, III, aVF and V4 to V6 by right ventricular outflow tract pacing (RVOTP) during a 15 min period. C: after cessation of RVOTP, T waves turned positive in inferior and left precordial leads. Please note that the T waves also became less positive in leads V2 and V3 and negative in leads V1 and aVL, in which the paced QRS complexes had been negative. D: once baseline conditions resumed, a 15-min period of right ventricular apical pacing was performed, after which the T waves turned negative (E) in all precordial leads (even more negative than at baseline in V4 to V6).
All the previous examples demonstrate that transient alterations in the direction of ventricular depolarization may cause “memory-induced” changes in ventricular repolarization, leading not only to the classically described abnormal (inverted and symmetrical) T waves but also to peaked positive T waves or even to partial or total normalization of abnormal T waves. Moreover, pseudo-normalization or pseudo-worsening of repolarization can occur following transient events that alter the sequence of ventricular activation such as rate-dependent intraventricular blocks, frequent ventricular ectopy, wide QRS complex paroxysmal tachycardias, intermittent ventricular pre-excitation or temporary ventricular pacing. The corollary of all these observations is that an accurate clinical interpretation of the electrocardiographic characteristics of the T wave can only be made taking into account the patient’s previous patterns of ventricular activation.
The Genesis of the T Wave and the Mechanisms Underlying “Memory-induced” Changes of Ventricular Repolarization
The Mechanisms Underlying the Normal T Wave
The cellular and electrophysiological bases of the T wave are still controversial [8, 9]. The ECG represents summation of the action potentials of the ventricular myocytes and there are gradients of action potential duration (APD) from apex to base, left to right ventricle and epicardium to endocardium which allow the repolarization sequence to run in the opposite direction from depolarization. Under normal conditions, the concordant polarity of the T wave and the R wave in the surface ECG indicates that the repolarization sequence proceeds in the opposite direction to that followed by the depolarization process [10-12]. This has been attributed to electrotonic interactions operating during ventricular excitatory process, by which repolarization lasts longer at sites where depolarization begins and is shorter at sites where depolarization ends [13, 14] a behavior that yields regional repolarization gradients.
The Mechanisms of Secondary and “Memory-induced” T Waves
As the direction of ventricular repolarization depends on the course of ventricular depolarization, any shift of the latter results in an instantaneous modification of the T waves, whose spatial orientation tends to be opposite to that of abnormal QRS complexes (secondary T waves). During the period of abnormal depolarization, gradual changes in ventricular repolarization develop that remain masked by the secondary T wave changes and are only unveiled once normal ventricular activation is restored. Therefore, the electrocardiographic manifestations of “memory-induced” T wave reflect a change in the sequence of ventricular repolarization that tracks the spatial direction of the main electrical forces of the previously abnormal depolarization process. Thus, any repetitive modification in the pattern of ventricular activation induces cumulative regional changes in the repolarization process that modify the ventricular repolarization gradient.
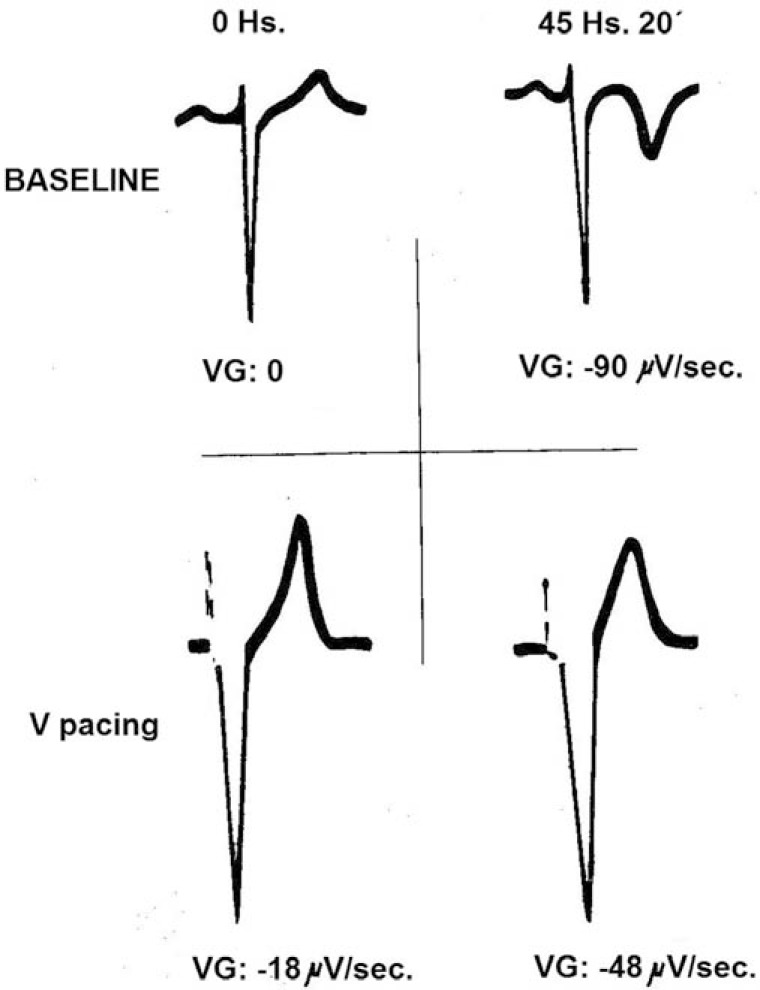
“Memory-induced” negative T waves, as seen in (Figs. 1 to 3), imply that the transient shift of ventricular activation caused by LBBB, right ventricular apex pacing, or wide QRS tachycardias induces regional changes in the course of the repolarization process that lengthens in some areas where it was shorter and vice versa. This depends not only on the electrotonic influence of surrounding tissues but also on the activation time of ventricular myocardium [15]. For example, during right ventricular apical pacing, activation starts from the endocardial surface of the apical region and end in the basal and epicardial portions of the ventricles giving rise to depolarizing electrical forces oriented posteriorly, superiorly, and to the left. Therefore, repolarization lengthens in the apical endocardium but shortens near the base. Because of that, once normal ventricular activation is restored, negative T waves appear in the ECG leads in which the paced QRS complexes had been negative (inferior and most precordial leads) indicating a significant change in the normal ventricular repolarization gradient. Rosenbaum and coworkers [2] measured the local ventricular repolarization gradient in leads in which a residual effect of right apical ventricular pacing were apparent. They found significant changes in the ventricular repolarization gradient during right ventricular pacing once the “memory-induced” T waves were apparent (Fig. 11). Thus, when the altered sequence of ventricular depolarization lasts for the time needed to evoke its “deferred” effect on the course of ventricular repolarization, the morphologic characteristics of the T waves during the conditioning abnormal QRS complexes results from a balance between two opposite forces. One, causing the secondary T wave changes, occurs instantaneously and its magnitude is proportional to the area of the conditioning QRS complex but of opposite direction. The other, develops after a variable period of time and shows the same direction than the conditioning QRS forces. This suggests that an altered ventricular activation sequence causes not one, but two contrary adaptation in ventricular repolarization, both of which are secondary, although one (memory-induced) appears as “primary”.
Fig. (11).
Changes in ventricular repolarization gradient (VG) during right ventricular (V) pacing measured in microvolts/s in lead V2 at the beginning of pacing (Baseline) and 45 hr and 20 min after continuous stimulation. Modified from reference 2.
Costard-Jackle et al. performed an elegant study to determine the mechanism underlying T wave “memory” in isolated Langerdoff-perfused rabbit heart preparations [15]. They analyzed the relationship between ventricular activation time, monophasic action potential duration (from 12 to 20 different epicardial sites) and repolarization time during three consecutive changes in the pacing site (atria, right ventricle and atria, respectively). During baseline atrial pacing, ventricular action potential duration was inversely related to the activation time (sites with earlier activation repolarized later). After a 2-hour period of ventricular pacing, resumption of atrial pacing demonstrated loss of the inverse relationship between the action potential duration and the sequence of ventricular activation (sites with earlier activation repolarized earlier). The authors concluded that modulation of ventricular repolarization dependent on the activation sequence would account for the T wave abnormalities observed after periods of aberrant ventricular conduction, ectopic ventricular activity, or ventricular pacing.
Memory-induced “normalization” of inverted T waves can be explained by the same electrophysiological phenomena stated above. During ventricular tachycardias or pacing from the right ventricular outflow tract the main depolarization forces are oriented inferiorly and to the left, giving rise to a repolarization process following the same direction. Thus, post- right ventricular outflow tract tachycardia and pacing are accompanied by positive “memory-induced” T waves in inferior and left precordial leads (see Figs. (5 to 8)). Similarly, during pacing or tachycardias from the basal region of the left ventricle, as well as during left ventricular pre-excitation, the main depolarization forces are directed anteriorly, and the post-pacing or post-tachycardia repolarization sequence is oriented in the same direction resulting in positive “memory-induced” T waves across the precordium (see Figs. (3 and 9)).
Temporal Development and the Mechanisms Involved in Short-term and Long-term T Wave “Memory”
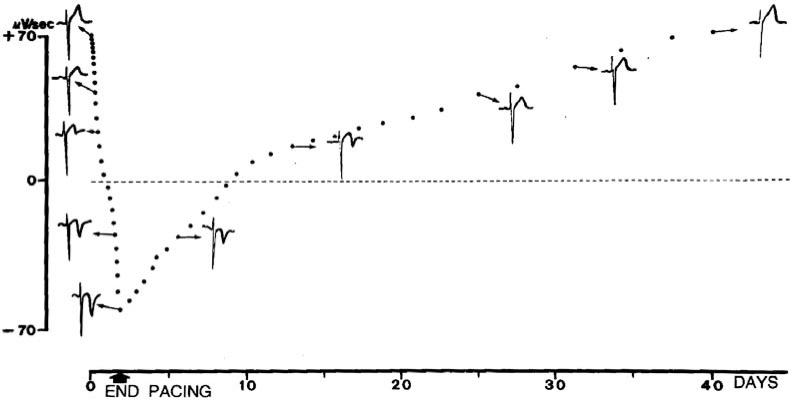
In their seminal studies, Rosenbaum et al. [1, 2] analyzed the progression and regression of the “memory-induced” T wave changes by right ventricular apical pacing (Fig. 12). They noted that these changes develop gradually during the first 30 to 60 hr of pacing and suggested that the time needed to reach a plateau is about one or two weeks. Regression of the T wave to its control configuration took between 8 and 38 days. Thus, complete normalization of ventricular repolarization after exposure to a prolonged period of conditioning altered ventricular activation takes much longer than the development of memory-induced T wave changes. However, the normalization is also dependent on the magnitude of the change of the T was achieved during the pacing period.
Fig. (12).
Development and regression of “memory-induced” T wave changes obtained by right ventricular apical pacing in lead V2. Modified from reference 2.
Further studies led to recognition of two different types of temporarily related “memory-induced” ventricular repolarization changes, which are thought to be caused by different cellular and molecular mechanisms [16-22].
So called short-term T wave “memory” develops after minutes to hours of continuous abnormal ventricular activation and disappears rather rapidly. On the other hand, long-term T wave “memory” is fully expressed after days or weeks of altered ventricular depolarization and vanishes in a slow and gradual fashion.
The primary stimulus giving rise to short-term electrical cardiac memory seems to be an abnormal regional ventricular stretch caused by the asynchronous activation of left ventricular walls [21]. The abnormal regional stretch of ventricular walls is critical for the initiation of short-term memory as a consequence of an increased in local synthesis and liberation of angiotensin II. This causes augmented trafficking and internalization of a macromollecular complex composed of the AT I angiotensin II receptor and the potassium channel protein KV4,3/KChIP2C carrying the IKto current, thus modulating the transmural gradient of this current [22]. In fact, Ricard et al. [20] demonstrated that angiotensin II receptor blockade or angiotensin converting enzyme inhibition reduces the development of short- term cardiac memory.
Furthermore, an increment in the calcium current ICaL is a relevant cofactor for the initiation of the memory mechanism since in a canine model, short-term as well as long-term T wave “memory” induced by ventricular pacing was attenuated by ICaL blockers [23]. The increased intracellular calcium concentration might act as a secondary messenger which, in turn, could activate changes in the nuclear transcription factors and thus, reduce the density of the potassium (IKto and IKr) and calcium (ICaL) currents thought to play a major role in long-term T wave memory. Patberg et al. [24] demonstrated that pretreatment with saralasin (an angiotensin II receptor blocker) or nifedipine (a calcium channel blocker) prevented the reduction of nuclear CREB (cAMP response element binding) protein induced in whole cell lysates of dogs undergoing ventricular pacing for 2 hours.
The effect of short-term and long-term ventricular repolarization memory on QTc interval is dissimilar. An abbreviation of the QTc interval was observed in patients who developed “memory-induced” T waves indicating that ventricular repolarization was shorter after a brief period of altered depolarization. Janse et al. [25] reported that left ventricular repolarization time and epicardial monophasic action potential at 90% repolarization shortened after induction of short-term cardiac memory in dogs. Furthermore, Wecke et al. [5] found that the QTc interval shortened after one day of continuous right ventricular apex pacing but lengthened after one to eight weeks of pacing.
The interaction between short-term and long-term T wave “memory” is intriguing. The permanent form of ventricular pre-excitation is a very attractive model of long-term T wave “memory” which, as described above, is well apparent after radiofrequency ablation of the accessory pathways. In this clinical model, a brief period (15 minutes) of ventricular pacing from an selected site that induces positive QRS complexes in the electrocardiographic leads showing pseudo-primary T waves may be enough to obtain a post-pacing transient normalization of ventricular repolarization. Therefore, the mechanism involved in short-term T wave “memory” is able to overcome the changes underlying long-term memory, an unexpected finding that deserves further investigations.
ACKNOWLEDGEMENTS
This work was supported by FICE (Fundación de Investigaciones Cardiológicas Einthoven), a grant of FONCYT (PICT Bicentenario 2010 # 2591).
FINAL REMARKS
It is well known that a transient change in the sequence of ventricular depolarization (due to intermittent ventricular pacing, rate-dependent bundle branch block, ventricular preexcitation and tachyarrhythmias with aberrant QRS complexes) is usually succeeded by inverted T waves that become apparent once ventricular activation returns to normal (“memory-induced” or “pseudo-primary” T waves). Morphologically, the most outstanding feature of “memory-induced” abnormal T waves is that their spatial polarity tracks that of the preceding conditioning aberrant QRS complexes. Following this concept, it was recently demonstrated that brief periods of pacing performed from specific ventricular sites can normalize or improve T wave abnormalities. The knowledge of the postponed consequences of abnormal ventricular activation is clinically important for evaluation of T wave characteristics and has potential implications for management of patients. In fact, “memory-induced” inverted T waves, examined without taking into account the background of ventricular depolarization, may be erroneously taken as indicative of a nonexistent severe coronary artery disease. On the contrary, “memory-induced” reversal of intrinsically abnormal T waves may preclude the electrocardiographic diagnosis of an underlying myocardial or ischemic heart disease. So called “short-term” and “long-term” T wave memories are caused by different, but interrelated, cellular and molecular mechanisms.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Chaterjee K, Harris A, Davies G, Leathman A. Electrocardiographic changes subsequent to artificial ventricular depolarization. Br Heart J. 1969;31:770–9. doi: 10.1136/hrt.31.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denes P, Pick A, Miller RH, Pietras RJ, Rosen KM. Characteristic precordial repolarization abnormality with intermittent left bundle-branch block. Ann Intern Med. 1978;89(1):55–7. doi: 10.7326/0003-4819-89-1-55. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum MB, Blanco HH, Elizari MV, Lázzari JO, Davidenko JM. Electrotonic modulation of the T wave and cardiac memory. Am J Cardiol. 1983;50:213–22. doi: 10.1016/0002-9149(82)90169-2. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbaum MB, Blanco HH, Elizari MV, Lázzari JO, Vetulli HM, editors. Frontiers of Cardiac Electrophysiology. The Hague: Martinus Nijhoff; 1983. Electrotonic modulation of ventricular repolarization and cardiac memory.In: Rosenbau MB. pp. 67–99. [Google Scholar]
- 5.Kalbfleisch SJ, Sousa J, El-Atassi R, Calkins H, Langberg J, Morady F. Repolarization abnormalities after catheter ablation of accessory atrioventricular connections with radiofrequency current. J Am Coll Cardiol. 1991;18:1761–6. doi: 10.1016/0735-1097(91)90518-e. [DOI] [PubMed] [Google Scholar]
- 6.Chiale PA, Pastori JD, Garro HA , et al. Reversal of primary and pseudo-primary T wave abnormalities by ventricular pacing.A novel manifestation of cardiac memory. J Interv Card Electrophysiol. 2010;28:23–33. doi: 10.1007/s10840-010-9473-9. [DOI] [PubMed] [Google Scholar]
- 7.Shvilkin A, Bojovic B, Vajdic B , et al. http://www.ournals.elsevierhealth.com/periodicals/ hrthm/article/S1547-5271(10)00507-2/abstract - article-footnote-1. Vectorcardiographic and electrocardiographic criteria to distinguish new and old left bundle branch blockhttp://www.journals. elsevierhealth. com/periodicals/hrthm/ article/S1547-5271(10)00507-2/abstract - article-footnote-1. Heart Rhythm. 2010;7:1085–92. doi: 10.1016/j.hrthm.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Opthof T, Coronel R, Janse MJ. Repolarization gradients in the intact heart. Circ Arrhythmias Electrophysiol. 2009;2:89–96. doi: 10.1161/CIRCEP.108.825356. [DOI] [PubMed] [Google Scholar]
- 9.Patel C, Burke JF, Patel H , et al. Cellular basis of the T wave.A century of controversy. . Circ Arrhythmia Electrophysiol. 2009;2:80–8. doi: 10.1161/CIRCEP.108.791830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson FN, MacLeod AG, Becker PS. The T deflection of the electrocardiogram. Trans Assoc Am Physicians. 1931;46:29–38. [Google Scholar]
- 11.Noble D, Cohen I. The interpretation of the T wave of the electrocardiogram. Cardiovasc Res. 1978;12:13–27. doi: 10.1093/cvr/12.1.13. [DOI] [PubMed] [Google Scholar]
- 12.Franz MR, Bargheer K, Rafflenbeul W, Haverich A, Lichtlen PR. Monophasic action potential mapping in human subjects with normal electrocardiograms: direct evidence for the genesis of the T wave. Circulation. 1987;75:379–86. doi: 10.1161/01.cir.75.2.379. [DOI] [PubMed] [Google Scholar]
- 13.Toyoshima H, Burgess MJ. Electrotonic interaction during canine ventricular repolarization. Circ Res. 1978;43:348–56. doi: 10.1161/01.res.43.3.348. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman BR. Electrotonic modulation of the T wave. Am J Cardiol. 1982;50:361–2. doi: 10.1016/0002-9149(82)90189-8. [DOI] [PubMed] [Google Scholar]
- 15.Costard-Jäckle A, Goetsch B, Antz M, Franz MR. Slow and long-lasting modulation of myocardial repolarization produced by ectopic activation in isolated rabbit hearts. Evidence for cardiac “memory”. Circulation. 1989;80:1412–20. doi: 10.1161/01.cir.80.5.1412. [DOI] [PubMed] [Google Scholar]
- 16.Shvilkin A, Danilo P Jr, Wang J , et al. Evolution and resolution of long-term cardiac memory. Circulation. 1998;97:1810–7. doi: 10.1161/01.cir.97.18.1810. [DOI] [PubMed] [Google Scholar]
- 17.Wecke L, Rubulis D, Lundahl G, Rosen M, Bergfeldt L. Right ventricular pacing–induced electrophysiological remodeling in the human heart and its relationship to cardiac memory. Heart Rhythm. 2007;4:1477–86. doi: 10.1016/j.hrthm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Ozgen N, Rosen MR. Cardiac memory: A work in progress. Heart Rhythm. 2009;6:564–71. doi: 10.1016/j.hrthm.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Rosen MR, Cohen IS. Cardiac memory…new insights into molecular mechanisms. J Physiol. 2006;570:209–18. doi: 10.1113/jphysiol.2005.097873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricard P, Danilo P Jr, Cohen IS, Burkoff D, Rosen MR. A role for the renin-angiotensin system in the evolution of cardiac memory. J Cardiovasc Electrophysiol. 1999;10:545–51. doi: 10.1111/j.1540-8167.1999.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 21.Sosunov E, Anyukhovsky EP, Rosen MR. Altered ventricular stretch contributes to initiation of cardiac memory. Heart Rhythm. 2009;5:106–13. doi: 10.1016/j.hrthm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, McKinnon D, Dixon JE , et al. Transient outward current.to1.is altered in cardiac memory. . Circulation . 1999; 99:1898–905. doi: 10.1161/01.cir.99.14.1898. [DOI] [PubMed] [Google Scholar]
- 23.Plotnikov AN, Yu H, Geller JC , et al. Role of L type calcium channels in pacing-induced short-term and long-term cardiac memory in canine heart. Circulation. 2003;107:2844–9. doi: 10.1161/01.CIR.0000068376.88600.41. [DOI] [PubMed] [Google Scholar]
- 24.Patberg KW, Plotnikov AN, Quamina A , et al. Cardiac memory is associated with decreased levels of the transcriptional factor CREB modulated by angiotensin II and calcium. Circ Res. 2003;93:472–8. doi: 10.1161/01.RES.0000088785.24381.2F. [DOI] [PubMed] [Google Scholar]
- 25.Janse MJ, Sosunov EA, Coronel R , et al. Repolarization gradients in the canine left ventricle after induction of short term cardiac memory. Circulation. 2005;112:1711–8. doi: 10.1161/CIRCULATIONAHA.104.516583. [DOI] [PubMed] [Google Scholar]