Abstract
Andersen - Tawil syndrome (ATS) is an autosomal - dominant or sporadic disorder characterized by ventricular arrhythmias, periodic paralysis, and distinctive facial and skeletal dysmorphism. Mutations in KCNJ2, which encodes the α-subunit of the potassium channel Kir2.1, were identified in patients with ATS. This genotype has been designated as type-1 ATS (ATS1). KCNJ2 mutations are detectable in up to 60 % of patients with ATS. Cardiac manifestations of ATS include frequent premature ventricular contractions (PVC), Q-U interval prolongation, prominent U-waves, and a special type of polymorphic ventricular tachycardia (PMVT) called bidirectional ventricular tachycardia (BiVT). The presence of frequent PVCs at rest are helpful in distinguishing ATS from typical catecholaminergic polymorphic ventricular tachycardia (CPVT). In typical CPVT, rapid PMVT and BiVT usually manifest during or after exercising. Additionally, CPVT or torsade de pointes in LQTS are faster, very symptomatic causing syncope or often deteriorate into VF resulting in sudden cardiac death. PVCs at rest are quite frequent in ATS1 patients, however, in LQTS patients, PVCs and asymptomatic VT are uncommon which also contributes to differentiating them.
The article describes the new electrocardiographic criteria proposed for diagnosis of type-1 Andersen-Tawil syndrome. A differential diagnosis between Andersen-Tawil syndrome, the catecholamine polymorphic ventiruclar tachycardia and long QT syndrome is depicted. Special attention is paid on the repolarization abnormalities, QT interval and the pathologic U wave. In this article, we aim to provide five new electrocardiographic clues for the diagnosis of ATS1.
Keywords: Andersen-Tawil syndrome, QT interval, U-wave, LQTS, CPVT.
INTRODUCTION
Andersen - Tawil syndrome (ATS) is an autosomal - dominant or sporadic disorder characterized by ventricular arrhythmias, periodic paralysis, and distinctive facial and skeletal dysmorphism [1-4]. Mutations in KCNJ2, which encodes the α-subunit of the potassium channel Kir2.1, were identified in patients with ATS [5, 6]. This genotype has been designated as type-1 ATS (ATS1) [5-7]. KCNJ2 mutations are detectable in only 60 % of patients with ATS making genetic screening limited as a universal diagnostic tool [8]. Several ECG manifestations were previously reported, however, the diagnosis of ATS1 remains elusive and sometimes difficult to distinguish from other primary electrical disorders such as Long QT and catecholaminergic polymorphic ventricular tachycardia (CPVT). In this article, we aim to provide five new electrocardiographic clues for the diagnosis of ATS1 by analyzing a series of genetically confirmed cases.
CARDIAC MANIFESTATIONS
Cardiac manifestations of ATS include frequent premature ventricular contractions (PVC), Q-U interval prolongation,prominent U-waves, and a special type of polymorphic ventricular tachycardia (PMVT) called bidirectional ventricular tachycardia (BiVT) [4, 9, 10]. The burden of PVCs or non-sustained VT (NSVT) is very high, however, ATS patients may be asymptomatic or minimally symptomatic. ATS patients are at a higher risk of developing life-threatening arrhythmias such as PMVT or ventricular fibrillation (VF) albeit the risk is relatively uncommon than in other genetic arrhythmia syndromes [8, 10]. There are some reports of sudden cardiac deaths in ATS patients [11, 12]. The incidence of lethal arrhythmias in ATS KCNJ2 – positive mutation patients is estimated at about 3 % in the largest series reported to date [9].
DIFFERENTIAL DIAGNOSIS
ATS patients present with similar cardiac manifestations to other genetic, primary electrical disorders such as long QT syndrome (LQTS) or CPVT. Phenotypically, CPVT mimics closely type-1 LQTS, with nearly 30 % of CPVT cases having been misdiagnosed as LQTS with normal QT interval or concealed LQTS [13]. Tester et al. demonstrated that about 6 % of negative-genotype patients referred for LQTS consideration were diagnosed as CPVT 1 involving RyR 2 mutations [13]. The authors also found ATS1 in about 40% of females diagnosed initially as CPVT [13]. Eckhardt et al. found that none of these KCNJ2 mutations carriers displayed QT prolongation, periodic paralysis, or skeletal developmental abnormalities. Rather they only showed BiVT or polymorphic VT on exercise test which is typically associated with CPVT. It is noticeable that females with KCNJ2 mutations are more likely to develop BiVT than males with KCNJ2 mutations, and thus they are overrepresented in ATS1 patients misdiagnosed as CPVT.
THE VALUE OF THE SURFACE ELECTROCARDIOGRAM IN THE DIFFERENTIAL DIAGNOSIS
ATS1, LQTS and CPVT are three well-distinguished electrical diseases that may clinically manifest with some commonalities. Detailed ECG analysis may be helpful in establishing the proper diagnosis.
VENTRICULAR ARRHYTHMIA. POLYMORPHIC VENTRICULAR TACHYCARDIA (PMVT)
PMVT is a common life-threatening arrhythmia in ATS1, LQTS and CPVT but its subtype, BiVT, is the hallmark for ATS1 and CPVT. In ATS1, PMVT and/or BiVT are relatively slow, well-tolerated, usually asymptomatic with a heart rate of about 130-140 bpm. Frequent ventricular ectopy at rest is helpful in distinguishing ATS1 from typical CPVT. In typical CPVT, rapid PVMT and BiVT are usually associated with exercise. PMVT and torsades de pointes associated with LQTS are much faster arrhythmias and usually very symptomatic leading to syncope or deteriorating into VF and sudden cardiac death.
Asymptomatic premature ventricular contractions (PVCs) are frequently observed in ATS1 patients whereas in LQTS patients, PVCs and asymptomatic non-sustained VT are uncommon [9]. Zhang et al. [9] showed that frequent PVCs were present in 41% of ATS1 cases and ventricular bigeminy in 59 % of the cases. Non-sustained PMVT was detected in 23 % of the cases including 68% of the patients who presented with BiVT. Torsades de pointes was recorded (by Holter monitoring) in 3 % of this population [9]. In CPVT, ventricular arrhythmias, including PMVT, are usually induced by exercise, stress or isoproterenol/adrenaline infusion. In type-1 LQTS life-threatening arrhythmias are provoked by stress or exercise.
CONDUCTION ABNORMALITIES
In the study of Zhang et al. which included 96 patients with ATS1, [9] conduction disturbances were found in 23 % of the cases; first degree atrio-ventricular block in 7 %, right bundle branch block in 9 %, left bundle branch block in 2 %, and non-specific intraventricular conduction delay in 2 % [9].
QT INTERVAL
The classification of ATS as part of the congenital LQTS syndromes remains controversial. Some authors would classify ATS as Type-7 LQTS [15]. The first descriptions of ATS and its ECG manifestations included the U–wave as part of the QT interval thus overestimating the true QT interval. In this way, all ATS patients presented with abnormally prolonged QT intervals. Zhang et al. were the first to offer a solution for this dilemma: the real QT interval is normal in almost all ATS patients if the U-wave is excluded from the analysis [9]. The corrected QT interval in ATS1 patients was 440 ms vs 420 ms in matched normal subjects [9] and the distribution of the QTc interval in ATS1 patients overlapped with the normal subjects in 87 %, whilst only 17 % of ATS1 patients had a QTc > 460 ms [9]. The corrected Q-U interval was significantly longer in ATS1 patients than matched normal subjects at 655 ms vs 600 ms respectively.
U-WAVE AND “REPOLARIZATION COMPLEX” ABNORMALITIES
A manifest U-wave is present in about 30 % of patients with CPVT and in about 90 % of ATS1 patients [9, 16]. A prominent U-wave in ATS1 patients is best seen in leads V2-V4 and in the limb leads. Although U-waves can be observed in normal individuals at low heart rates, during parasympathetic stimulation or in hypokalemic states [17], the ones described in ATS1 patients occur at faster heart rates, suggesting that this may represent a manifestation of primary electrical disease rather than a normal variant [9]. In Type-4 LQTS, caused by an ankyrin B mutation, the surface ECG can also display a prominent U-wave, but it is usually associated with bradycardia or atrio-ventricular dissociation, and borderline QTc prolongation [18].
In ATS patients, Zhang et al. [9] described abnormal prominent U-wave and increased QT-U interval but not QT interval prolongation. They showed that abnormal T- U wave was present in 91 % of the patients with ATS-KCNJ2 mutations. These abnormalities included:
Prolonged terminal portion of the T-wave descending in about 70 % of the cases.
Wide T- U wave junction in 43 % of the cases.
Biphasic U-wave 16 % of the cases.
Large U-wave in 73 % of the cases.
These ECG abnormalities had a high predictive accuracy for KCNJ-2 mutation (ATS1) with sensitivity, specificity, positive and negative predictive value at 84%, 97%, 94% and 91 % respectively [9].
In ATS1, T-wave duration was found to be longer than normal matched subjects or non-KCNJ2-mutation patients; 220 ms vs 190 ms vs 200 ms, respectively. There was no difference in T-wave amplitude between the groups [9].
Similarly, the U-wave duration was longer in ATS1 pts compared to ATS-non-KCNJ 2 mutation or normal subjects; 210 ms vs 165 ms vs 170 ms, respectively. The U-wave amplitude was higher in ATS1 – 0. 15 mV than normal subjects - 0.08 mV (p < 0.0001) [9]. Finally the T peak – U peak interval was increased in ATS1 patients compared to ATS-non-KCNJ 2 mutation and normal subjects; 240 ms vs 175 ms vs 180 ms, respectively (p < 0.0001) [9].
NEW ELECTROCARDIOGRAPHIC CRITERIA FOR THE DIAGNOSIS OF ATS1
We collected information in 6 patients with genetic confirmation of ATS1. We analyzed surface ECGs and Holter recordings of 6 patients. All patients were females with a mean age 30.6 years [range 8 - 57 years] and 4 of them had facial and/or dental dysmorphic features. We analyzed several electrocardiograms available for each patient (average = 4.5 ± 2 per patient). All the measurements were performed using manual calipers by one person experienced in ECG analysis (P.K.). The presence of prominent U-waves in leads V2-V4 was seen in all patients, U wave in lead V5 in 87% and U wave in the inferior leads in 50 %. The mean QT interval were 370 ± 17 ms and the mean QU interval were 615 ms ± 33ms. Electrocardiographic characteristics can be seen in Table 1. Clinical characteristics can be seen in Table 2. The new 5 electrocardiographic clues for the diagnosis of ATS1 could be summarized as follows:
Table 1.
ECG characteristics of patients with ATS1.
| ECG Parameters | Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 | Pt 6 | Mean |
|---|---|---|---|---|---|---|---|
| QT interval lead V2 [ms] | 360 | 360 | 360 | 360 | 380 | 400 | 370±17 |
| QT + U interval V2 [ms] | 600 | 600 | 580 | 600 | 670 | 640 | 615±33 |
| Pseudo Tee-Pee sign | - | + | + | + | + | + | 5/6 |
| U on P sign | - | + | + | + | + | + | 5/6 |
| Post-PVC pseudo-LQTS pattern | - | + | + | + | + | + | 5/6 |
| U-wave inferior leads | - | + | + | + | + | + | 5/6 |
| U-wave duration [ms] | 200 | 240 | 180 | 180 | 230 | 200 | 205±25 |
| T-wave peak – U-wave peak [ms] | 320 | 240 | 200 | 240 | 240 | 220 | 243±40 |
| U-wave peak- U-wave end duration [ms] | 120 | 110 | 110 | 110 | 160 | 160 | 128±24 |
(-) – absent, (+) – present
Table 2.
Clinical characteristics.
| Clinical Data | N |
|---|---|
| Female | 6 |
| mean age [years old] | 27 [17-35] |
| KCNJ 2 mutation | 6 |
| first symptoms [years] | 9.2 ± 2.1 |
| dysmorphic features (facial, dental) | 4 |
| periodic paralysis | 4 |
| ECG abnormalities: short PQ interval RBBB Bi VT BiVT heart rate range [bpm] |
1 1 6 120 – 170 |
| U wave in precordial V2 - V3 leads | 6 |
| mean QT interval [ms] | 370 ± 17 |
| mean QT+U interval [ms] | 615 ± 33 |
| family history / SD/VT | 4 / 1 / 1 |
| ablation/ICD/pacemaker | 2 / 1 / 1 |
RBBB- right bundle branch block, SD- sudden death, Bi VT – bidirectional VT, VT –ventricular tachycardia, ICD – implanted defibrillator – cardioverter.
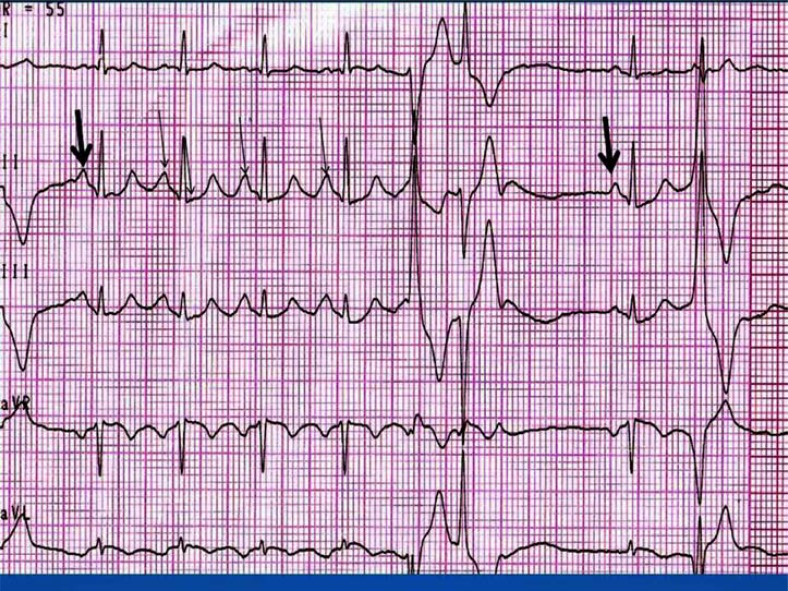
“U on P” sign (U-wave masquerading P-wave) (Fig. 1). The P-wave during sinus tachycardia is inscribed on the U-wave of the preceding beat. It resembles the morphology of the “P-pulmonale” pattern with P-wave amplitude > 3 mm. This finding was observed in 5/6 (83.3%) patients. In one case, the available ECGs did not allow us to perform this analysis.
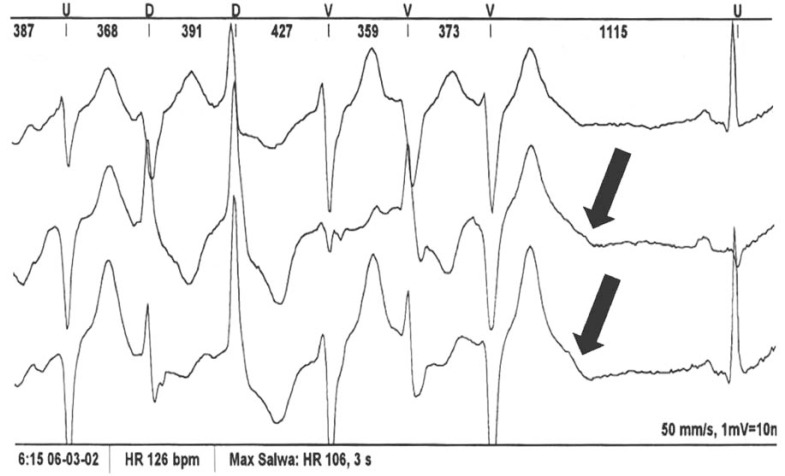
Pseudo “Tee-pee sign”. During a PVC, there is a prolongation of the descending limb of the T+U-wave (Fig. 2). The morphology resembles that described by Johri et al. as the “Tee-pee” sign observed in a patient with multiple electrolyte disorder (hyperkalemia, hypokalcemia and hypomagnesemia) [19]. In ATS1, the QT interval can appeared prolonged and difficult to quantify because of a prominent U-wave. In order to properly determine the QT interval, the tangent technique should be carefully applied [20, 21]. When the proper technique for QT measurement was applied (by excluding the U-wave), corrected QT was normal in all of our cases, confirming the observation by Zhang et al. [9]. In our series, this phenomenon was observed in 5/6 (83.3 %) patients.
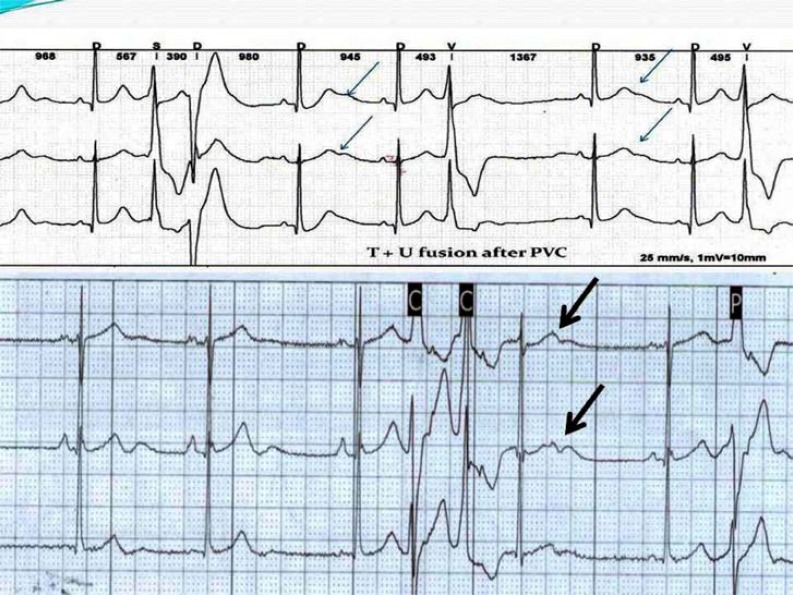
Post-extrasystolic “pseudo-LQTS-pattern”. This is detected in a sinus beat following a PVC. The fusion of T+U-waves can mimic LQTS (Fig. 3). This pattern is not observed in the subsequent sinus beats. The prevalence of this observation was 83.3 %.
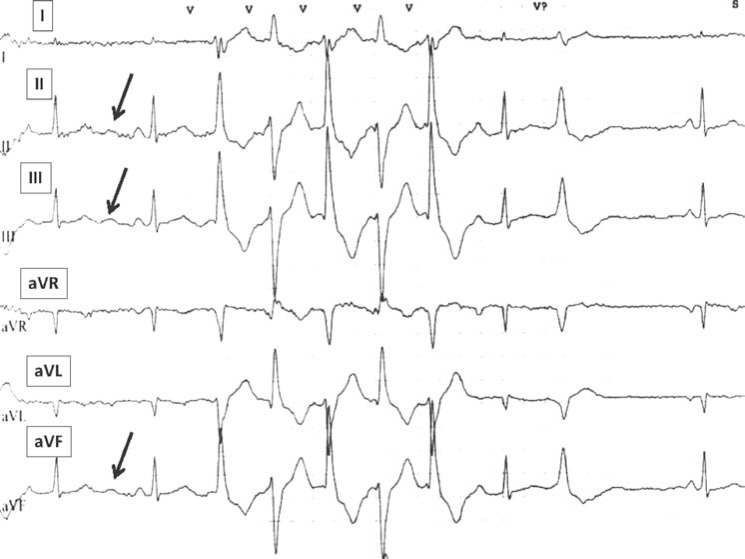
The presence of U-waves in the inferior leads and precordial leads V2-V3. (Fig. 4). In normal subjects, bradycardia increases the U-wave amplitude and tachycardia decreases or totally eliminates it. Conversely, in ATS1 patients, tachycardia increases the U-wave amplitude. In resting ECGs, a prominent U-wave can be seen in the inferior leads. This phenomenon was observed in 5/6 patients of our series.
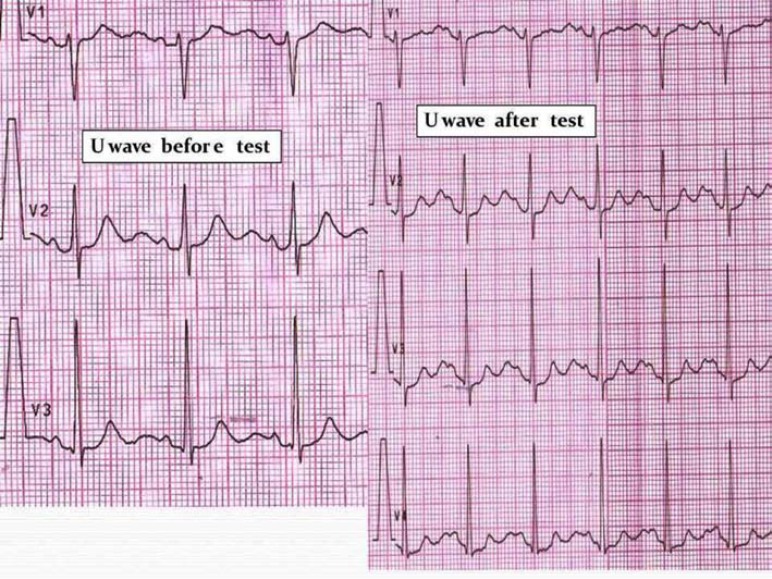
U-wave amplitude augmentation after adrenaline administration. (Fig. 5). We observed that in some patients with ATS1, adrenaline administration increases the U-wave amplitude. The ratio U-wave / T-wave amplitude becomes > 1 after the adrenaline administration when compared to the usual ratio < 1 before the administration of the inotropic drug.
Fig. (1).
“U on P” sign (black arrow). “U on P” sign (U-wave masquerading P-wave) ; the P-wave during sinus tachycardia is inscribed on the U-wave of the preceding beat.
Fig. (2).
Pseudo “Tee - Pee sign” (black arrow). Pseudo “Tee-pee sign” during a PVC, there is a prolongation of the descending limb of the T+U-wave. In ATS1, the QT interval can appeared prolonged and difficult to quantify because of a prominent U-wave. In order to properly determine the QT interval, the tangent technique should be carefully applied.
Fig. (3).
Post- extrasystolic “pseudo – LQTS pattern” (arrows). This is detected in a sinus beat following a PVC. The fusion of T+U-waves can mimic LQTS. This pattern is not observed in the subsequent sinus beats.
Fig. (4).
U-wave visible in the inferior limb leads (arrows).
Fig. (5).
Increased U-wave amplitude after “adrenaline test” in an ATS1 patient. Adrenaline administration increases the U-wave amplitude. The ratio U-wave / T-wave amplitude becomes > 1 after the adrenaline administration when compared to the usual ratio < 1 before the administration of the inotropic drug.
LIMITATIONS
These observations were performed in a small series of patients, however; the reproducibility in each subject was validated in several ECG recordings ( average of 4.5 ECGs per 1 patient). Concomitant factors that may influence the ECG recordings were not collected and this could have introduced some bias in the analysis. However, given the high prevalence of these five new ECG clues in the analyzed series, confirmation with a larger population of ATS1 patients is paramount.
ATS1 is a rare disease that involves the heart and specifically the electrical conduction system. Patients with this disease may suffer from life-threatening ventricular arrhythmias. There is an overlap with some other primary electrical diseases, so proper ECG identification is of paramount importance. Some specific ECG clues to the diagnosis of ATS1 were found in this small series.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Andersen E, Krasilnikoff PA, Overvad H. Intermittent muscular weakness. extrasystles.and multiple developmental anomalies new syndrome?. . Acta Pediatric Scan. 1971;60: 559–64. doi: 10.1111/j.1651-2227.1971.tb06990.x. [DOI] [PubMed] [Google Scholar]
- 2.Tawil R, Ptacek LJ, Pavlakis SG , et al. Andersen`s syndrome: potassium-sensitive periodic paralysis. ventricular ecopy.and dysmorphic features. Ann Neurol . 1994; 35:326–30. doi: 10.1002/ana.410350313. [DOI] [PubMed] [Google Scholar]
- 3.Donaldson MR, Yoon G, Fu YH, Ptacek LJ. Andersen-Tawil syndrome: a model of clinical variability. pleiotopy.and genetic heterogeneity. Ann Med . 2004; 36 (Suppl 1 ):92–7. doi: 10.1080/17431380410032490. [DOI] [PubMed] [Google Scholar]
- 4.Plaster NM, Tawil R, Tristani-Firouzi M , et al. Mutations in Kir..1 cause the developmental and episodic electrical phenotypes of Andresen`s syndrome. Cell. 2001;105:511–9. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 5.Hosaka Y, Hanawa H, Washizuka T , et al. Function. subcellular localization and assembly of a novel mutation of KCNJ2 in Andersen’s syndrome. J Mol Cell Cardiol. 2003;35:409–15. doi: 10.1016/s0022-2828(03)00046-4. [DOI] [PubMed] [Google Scholar]
- 6.Andelfinger G, Tapper AR, Welch RC etal. KCNJ2 mutation result in Andersen syndrome with sex-specific cardiac and skeletal muscle phenotypes. Am J Hum Genet. 2002;71:663–8. doi: 10.1086/342360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai T, Fujiwara Y, Tsuji Ketal. Novel KCNJ2 mutation in familial periodic paralysis with ventricular dysrhythmia. Circulation. 2002;105:2592– 4. doi: 10.1161/01.cir.0000019906.35135.a3. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson MR, Jensen JL, Tristani-Firouzi Metal. PIP2 binding residues of Kir 2. are common targets of mutations causing Andersen syndrome. Neurology. 2003;60:1811–6. doi: 10.1212/01.wnl.0000072261.14060.47. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Benson DW, Tristani-Firouzi Metal. Electrocardiographic features in Andersen-Tawil syndrome patients with KCNJ2 mutations characteristics T-U-wave patterns predict the KCNJ 2 genotype. Circulation. 2005;111:2720–6. doi: 10.1161/CIRCULATIONAHA.104.472498. [DOI] [PubMed] [Google Scholar]
- 10.Chun TU, Epstein MR, Dick Metal. polymorphic ventricular tachycardia and KCNJ2 mutations. Heart Rhythm. 2004;1:235–41. doi: 10.1016/j.hrthm.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Airey KJ, Etheridge SP, Tawil R, Tristani-Firouzi M. Resuscitated sudden cardiac death in Andersen-Tawil syndrome. Heart Rhythm. 2009;6(12):1814–7. doi: 10.1016/j.hrthm.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters S, Schulze-Bahr E, Etheridge SP, Tristani-Firouzi M. Sudden cardiac death in Andersen-Tawil syndrome. Europace. 2007;9(3):162–6. doi: 10.1093/europace/eul188. [DOI] [PubMed] [Google Scholar]
- 13.Tester DJ, Arya P, Will M , et al. Genotypic heterogeneity and phenotypic mimicry among unrelated patients referred for catecholaminergic polymorphic ventricular tachycardia genetic testing. Heart Rhythm. 2006;3:800–5. doi: 10.1016/j.hrthm.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Eckhardt LL, Farley AL, Rodriguez E , et al. KCNJ2 mutations in arrhythmia patients referred for LQT testing: a mutation T305A with novel effect on rectification properties. Heart Rhythm. 2007;4 (3):323–9. doi: 10.1016/j.hrthm.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tristani-Firouzi M, Jensen JL, Donaldson MR , et al. Functional and clinical characterization of KCNJ2 mutations associated with LQTS 7 (Andersen syndrome). J Clin Invest. 2002;110:381–8. doi: 10.1172/JCI15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aizawa Y, Komura S, Okada S , et al. Distinct U wave changes in patients with catechoalminergic polymorphic ventricular tachycardia. Int Heart J. 2006;47:381–9. doi: 10.1536/ihj.47.381. [DOI] [PubMed] [Google Scholar]
- 17.Surawicz B. U wave: facto. hypothses.misconceptions., and misnomers. J. Cardiovasc. Electrophysiol. 1998; 9:1117–28. doi: 10.1111/j.1540-8167.1998.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 18.Mohler PJ, Schott JJ, Gramolini AO , et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421(6923):634–9. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 19.Johri AM, Baranchuk A, Simpson CS, Abdollah H, Redfearn DP. ECG manifestations of multiple electrolyte imbalance: peaked T wave to P wave ("tee-pee sign"). Ann Noninvasive Electrocardiol. 2009;14(2):211–4. doi: 10.1111/j.1542-474X.2009.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou T, Knilans TK. Electrocardiography in clinical practice: Adult and Pediatric 4th edition. Philadelphia PA W B Saunders Compan. 1996 [Google Scholar]
- 21.Postema PG, De Jong JS, Van der Bilt IA, Wilde AA. Accurate electrocardiographic assessment of the QT interval: teach the tangent. Heart Rhythm. 2008;5(7):1015–8. doi: 10.1016/j.hrthm.2008.03.037. [DOI] [PubMed] [Google Scholar]