Abstract
The ECG is abnormal in most patients with arrhythmogenic right ventricular dysplasia (ARVD). Right ventricular parietal block, reduced QRS amplitude, epsilon wave, T wave inversion in V1-3 and ventricular tachycardia in the morphology of left bundle branch block are the characteristic changes that reflect the underlying genetic predetermined pathology and pathoelectrophysiology. Recognizing the characteristic ECG changes in ARVD will be of help in making a correct diagnosis of this rare disease.
Keywords: ECG, ARVD, desmosomal genes, ventricular tachyarrhythmia, SCD.
INTRODUCTION
The electrocardiogram (ECG) is the most commonly used test in cardiology. In this era of high technology and evidence-based medicine of the 21st century, the fundamentals of ECG applications in the diagnosis of arrhythmogenic right ventricular dysplasia (ARVD) still remain unshakable.
Since the beginning recognition of ARVD, [1-4] it is now appreciated as a genetic arrhythmogenic cardiomyopathy associated with an increased risk of sudden cardiac death (SCD). The pathology is characterized by the gradual loss of myocardium with fatty or fibrofatty tissue replacement, predominantly affecting the right ventricle and resulting in regional and global myocardial dysfunction. Areas of slow conduction and disparate refractoriness set the stage for reentrant ventricular tachyarrhythmias. ARVD development is mostly a concealed process in its early stages. Thus, SCD can be the first symptom occurring in young, apparently healthy individuals and endurance athletes. High risk individuals often experience the so-called 'hot phase' of the disease [5] featuring recurrent malignant ventricular tachyarrhythmias, whereas low risk individuals may have an uneventful clinical course. Because of the disease progression, congestive biventricular failure is the main cause of death and heart transplant may be required at the end stage of the disease [6, 7].
GENETIC BASIS AND THE PATHOGENESIS OF ARVD
Like many other familial disorders, there is a high degree of genetic heterogeneity in ARVD. To date, mutations of over 12 genes have been identified that can cause ARVD, or result in an ARVD phenotype, although some of these geneare also responsible for other diseases. Genes can be sub-grouped into desmosomal and non-desmosomal types. Desmosomal genes are JUP encoding plakoglobin, [8-11] DSP for desmoplakin [12], PKP2, plakophilin-2 [13-15], PKP4, plakophilin-4 [16], DSG, desmoglein-2 [17, 18], and DSC2 for desmocollin-2 [19, 20]. Non-desmosomal genes are: TMEM43 [21] containing a response element for peroxisome proliferator-activated receptor gamma (PPAR-gamma), which is an adipogenic transcription factor regulating adipogenic pathway; CTNNA3, a gene encoding adherens junctional protein alpha-T-catenin [22] that binds plakophilins; and a list of genes that can result in “overlap syndromes” such as DES encoding desmin, a major intermediate filament protein interacting with the desmosome. DES mutations can cause skeletal myopathies, conduction disorders and an ARVD phenotype [23, 24]. TTN, a gene encoding titin expressed in both cardiac and skeletal muscle, is the largest protein in mammals. TTN mutations can cause hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), tibial muscular dystrophy and an ARVD phenotype [25]. PLN, encoding phospholamban, a Ca2+-ATPase regulator involved in assembly and disassembly of the desmosome. PLN mutations are associated with both DCM and ARVD [26]. LMNA, encoding Lamin A/C the nuclear matrix proteins and mutations of this gene can cause a variable of phenotypes so called ‘laminopathies’ characterized by alterations in adipose localization and replacement of skeletal myocytes by fibrofatty tissue. With over 100 mutations identified in DCM, LMNA is also responsible for ARVD [27]. RYR2, the cardiac ryanodine receptor gene mainly accounting for catecholaminergic polymorphic ventricular tachycardia, is linked to ARVD as well [28, 29].
Radical mutations have a high probability of being disease-causing in ARVD [30]. Compound or double heterozygous desmosomal mutations are not rare, and the extent of the disease is high compared to that in single-mutation carriers [13, 30-32]. Among genotyped individuals, a PKP2 mutation is the most common according to multicenter/multinational registry studies [33-39]. In ARVD, the proband often exhibits a severe phenotype whereas reduced penetrance is prevalent among gene carriers [33-39].
The exact mechanism of the causative relationship between mutations in genes encoding both desmosomal and non-desmosomal proteins and the gradual loss of ventricular cardiomyocytes and fatty/fibrofatty replacement predominant in the right ventricle has not been elucidated [40-44]. The reduced desmosomal protein expression shown in endocardial biopsy samples [45] and fat droplet formation within the cardiomyotypes of the diseased hearts observed from histological studies [46, 47] have been replicated recently in PKP2 mutant induced pluripotent stem cells (iPSCs)-derived cardiomyocytes [48]. The findings from patient specific iPSC-derived cardiomyocytes indicate that the PPAR-gamma pathway and cell metabolic derangement may account for apoptosis and lipogenesis [48]. Moreover, the PKP2 knockout epicardial progenitor cells have shown adipocyte enlargement and migration, a plausible explanation for fat infiltration predominantly occurred in the right ventricle [43]. The vast majority of genotyped ARVD patients present with autosomal dominant inheritance, although an autosomal recessive pattern has also reported [8-4, 17, 18, 21, 49-51].
Establishing a clinical diagnosis of ARVD is based on a combination of characteristic abnormalities using an updated taskforce scoring system [52] that includes family history, ECG, cardiac imaging, endomyocardial biopsy and the identification of an ARVD-causing gene that can confirm the diagnosis. Since up to 50% of ARVD patients have no mutations in known genes, clinical assessment is essential for a correct diagnosis. A timely diagnosis can facilitate tailored treatment for arrhythmia control [53-55] and for prevention of SCD in high risk individuals using an implantable cardioverter-defibrillators (ICD) [56-60].
Among diagnostic modalities, the ECG is the first test. The vast majority of patients who meet ARVD Task force criteria present with ECG abnormalities, and many studies stress that ECG abnormalities are important elements in ARVD assessment and for family screening. The purpose of this article, therefore, is to describe the common ECG abnormalities associated with ARVD that have been observed by the authors over the years, and by literature review. Recognizing the characteristic ECG changes can increase diagnostic accuracy, and help identify affected members at an early and hopefully pre-symptomatic stage, in a timely and cost effective manner.
I. ECG IN SINUS RHYTHM
As with any ECG interpretations, the sequence of ECG assessment in ARVD should start from heart rate, rhythm, P-QRS-ST-T morphology, amplitude, duration and axis. Visual inspection may take a few minutes. It is important to observe ECG patterns in all 12 leads. Patient’s age, gender, medication and/or device therapy should be taken into consideration during ECG assessment.
1. Heart Rate
Based on our observations, the average heart rate in ARVD adults (n= 120, age 39±13 yrs) is within the lower normal range (63±13 bpm). Sinus bradycardia seems more common (37% vs. 20%, p=ns), though the difference did not reach statistical significance when compared with age and gender matched controls. There are case reports of ARVD patients presenting with sick sinus syndrome [61, 62]. Those observations may have been influenced by several factors: 1) sino-atrial involvement during disease progression; 2) some of the ARVD patients were athletes; and 3) use of sotalol, a drug with both beta-blocker and Class III antiarrhythmic effects, commonly prescribed to patients with frequent ventricular tachyarrhythmias [53]. The lower heart rate in ARVD may be of use in differentiating it from acute myocarditis, pericarditis and pulmonary embolism (PE). Although right precordial T wave inversion can be seen in those conditions, they are more likely associated with inappropriate sinus tachycardia. Moreover, the presence of SIQIIITIII, defined as a deep or broad S wave in lead I, Q wave and T wave inversion in lead III, is a signature change of acute PE [63-65].
2. P Wave
P wave abnormalities are common in ARVD [66]. Notched, widened (P wave duration >110 ms), flat or small peaked P wave are seen in up to 50% of ARVD patients in our study cohort consisting of 120 adults and 20 children. Nevertheless, P wave morphology variations are also found in 37% of age and gender comparable control subjects. Thus presence of P wave abnormalities in ARVD patients may reflect atrial involvement, but they may not necessarily add power to an ARVD diagnosis.
3. P-R Interval
A-V conduction delay is common in ARVD [67, 68]. Based on our observations the P-R interval is significantly prolonged in ARVD compared to control subjects (170±32 ms vs 154±21 ms, p <0.0001). First degree A-V block (AVB) (PR interval >200 msec) is seen in 16% of ARVD patients. In our ARVD cohort first degree AVB is seen in 15% of ARVD children, indicating that the conduction system may be involved at the early stage of the disease process. Second and third degree AVB are also reported in ARVD patients [67].
We do need to keep in mind that AVB is also common in patients with right ventricular cardiac sarcoidosis (CS) [69, 70]. The latter is a multisystem granulomatous disorder of unknown etiology. CS can mimic ARVD by ECG presentations, EP study results and even with desmosomal gene expression [71]. Unfortunately, applying the taskforce criteria cannot reliably separate CS from ARVD [72, 75].
4. QRS Complex
The regional conduction delay and the loss of myocardium due to ARVD are well reflected in the QRS complex, including changes in duration, morphology and amplitude.
4.1. Regional Prolongation of QRS Duration
Since the first description of right ventricular parietal block [76], localized QRS prolongation in the right precordial leads, such as QRS duration (QRSD) >110 ms, and the terminal S wave prolongation (≥ 55 ms) in V1-3 have been much appreciated as non-invasive indicators of activation delay occurred in the diseased region of the right ventricle [77-82]. Those ECG markers have been factored in the taskforce criteria for ARVD diagnosis [52, 83].
4.2. QRS Fragmentation
A fragmented QRS (fQRS) refers to the ‘slurs or notches’ appeared on the R or S wave or if the total QRS complex had ≥ 4 spikes. fQRS can be a normal variant if it appeared randomly in just a few leads. fQRS presenting in multiple leads is more likely pathologic. The underlying cause is the regional delay in propagation of ventricular depolarization [84]. fQRS is highly prevalent in ARVD patients when applied to amplified and modified ECG recording techniques, including the use of the Fontaine Leads System [85-87]. In real world practice, nevertheless, most ECGs available from ARVD patients and family members were obtained by using standard ECG recording technique. Based on our observations, fQRS is easily recognizable from standard ECGs and they are much more common in ARVD patients (n=140) when compared with 100 control subjects (61% vs 37%, p < 0.001). Among them a notch before the end of R or S wave (Fig. 1) is characteristic, seen in 51% of ARVD vs 26% in controls, p <0.0001. In ARVD, fQRS is often seen in multiple leads. Such changes, however, are common in CS as well. In the latter, the QRS complex is wider [72]. Since fQRS is also prevalent in other types of cardiomyopathies (both ischemic and non-ischemic) [88, 89], Brugada syndrome [84] and in normal subjects, its use in ARVD diagnosis is limited.
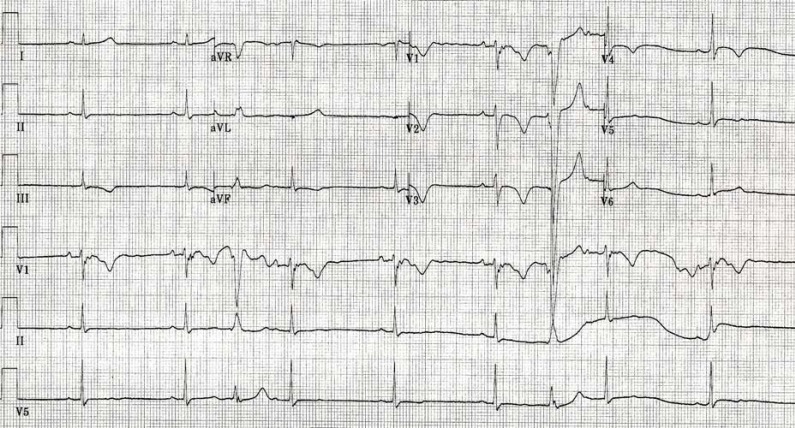
Fig. (1).
An ECG from a 46-y-old male Caucasian ARVD patient showing 1) sinus bradycardia; 2) interpolated PVCs in LBBB morphology; 3) IRBBB; 4) though there are some artifacts, the terminal S wave prolongation (≥55 ms) with a slur, and an epsilon wave that is best appreciated in Lead V1; 4) T wave inversion inV1-4.
4.3. Reduced QRS Amplitude
We have found that reduced QRS amplitude in ≥ 6 leads is seen in 66% of 140 ARVD patients in our study cohort. This feature may be of help in differentiating ARVD from athlete heart. The loss of heart muscle is a major feature in ARVD, and the likely cause of reduced QRS amplitude seen in ARVD patients, especially those in the advanced or late stage of the disease [78, 80]. Since reduced QRS amplitude can be a normal variant or found in many other diseases, its lone use in ARVD diagnosis is limited.
4.4. Poor R Wave Progression (PRWP)
The most prevalent ECG manifestation in ARVD, caused by p.S358L mutation in TMEM43 gene, is PRWP. Affected males are twice as likely to develop PRWP as affected females [90]. PRWP is also common in other ARVD patients with severe RV dilatation [80]. The most likely cause of PRWP is clockwise rotation caused by RV enlargement.
4.5. QRS Axis
Unlike other types of RV enlargement, left axis deviation is common in ARVD, seen in 22% in our study cohort. Left axis deviation, when associated with left anterior fascicular block, is more common in patients with LV involvement or in the later stages of ARVD [80].
4.6. Right Bundle Branch Block (RBBB)
Complete or incomplete RBBB is common in ARVD [76, 79, 91], indicating that the right heart Purkinje network of the conduction system is impaired. In advanced or later stages, CRBBB is associated with a poor prognosis [92]. CRBBB in ARVD has the following features (Fig. 2): 1) a lower magnitude of both R wave and QRS complex in V1-2; 2) a smaller ratio of R’/S in V1-2; and 3) T wave inversion in V1-3 or beyond is much more common (67% vs. 33%, p <0.0001). The rather different looking RBBB morphology seen in ARVD, based on the findings from epicardial mapping and histological data, is likely attributable not only to the impaired bundle branch but also to distal block in the right ventricular wall due to the irregular and delayed propagation of activation in the zones of dysplasia [76]. Therefore, we call it a more than complete (or incomplete) RBBB.
Fig. (2).
ECG from a 41-y-old male ARVD patient showing 1) sinus rhythm and frequent multifoci PVCs and non-sustained VT; 2) Notched P waves in 12 leads; 3) a more than complete RBBB; 4) Characteristic QRS pragmentation including Epsilon waves in both sinus and ectopic beats in 12 leads; 5) Inverted T waves in V1-6.
Fig. (2). ECG from a 41-y-old male ARVD patient showing 1) sinus rhythm and frequent multifoci PVCs and non-sustained VT; 2) Notched P waves in 12 leads; 3) a more than complete RBBB; 4) Characteristic QRS pragmentation including Epsilon waves in both sinus and ectopic beats in 12 leads; 5) Inverted T waves in V1-6.
5. Epsilon Wave
First reported in 1977 [1], the Epsilon wave is a reflection of ‘mega’ late potentials on the surface ECG that can be seen in patients with advanced or late stage of ARVD [68, 79, 80, 93, 94]. In the diseased region of the right ventricle, the islands of the surviving cardiomyocytes bordered by or embedded in interstitial fibrosis and/or fat causes delayed activation in those areas which results in delayed (late) potentials. Depending on the severity of myocardial damage, the delayed and distort activation may occur before the end of the QRS complex or after the ST segment [1]. Nevertheless, the Epsilon wave is best appreciated in the beginning of the ST segment as small wiggles or a tiny protrusion [1, 87]. Moreover, Epsilon waves are most prominent in the right precordial leads though they can be seen in the limb leads or in all 12 leads (Figs. 2-3). Since the magnitude of the Epsilon wave is generally small, applying Fontaine leads system [87], a very simple method, can amplify Epsilon waves and thereby increase the detection sensitivity significantly [86, 95]. Presence of Epsilon wave is considered as a major diagnostic criterion in ARVD [52, 83] but it is also found in other diseases if late potentials are manifest on the surface ECG [96-101]. For example, Epsilon waves in CS are much more coarse due to extensive formation of fibrosis in the RV [98].
Fig. (3).
An endurance athlete first diagnosed with an ‘athlete heart’ for the “unexplained T wave inversion in V1-3” at age 25, and remained asymptomatic until reached age 45, he had an viral infection that initiated the ‘hot phase’ sustained VTs and become very symptomatic. His right heart was significantly dilated by the time of ARVD diagnosis. His arrhythmia was under control by using sotalol, amiodarone and subsequently an ICD. This ECG tracing was taken at age 49, showing 1) marked sinus bradycardia (48 bpm); 2) bizarre QRS complex with evidence of significant conduction distortion and delay in the right ventricle by epsilon waves in all 12 leads; 3) reduced QRS amplitude; 4) Inverted T waves in V1-3.
6. ST Segment
ST elevation is not uncommon in ARVD [102]. In our study cohort, 37% of ARVD patients had a ST elevation. Among these, 42% showed a small notch (Fig. 1) in the first half of the ST segment and such findings are more frequently seen in patients in the presence of Epsilon waves.
7. T Wave Inversion in the Right Precordial Leads
Based on a number of multicenter/multinational studies [3, 78-80, 103, 104], T wave inversion in V1-3 is one of the most common ECG abnormalities in ARVD. Therefore it has been upgraded to a major taskforce diagnostic criterion [52]. In ARVD the regional conduction delay and RV dilatation [80, 105] may be the causes of T wave inversion in V1-3 or beyond. In other words, it is a secondary rather than a primary repolarization abnormality. T wave inversion beyond V3 is more common in ARVD patients in the advanced stage of the disease with severe RV dilatation and LV involvement [80, 106]. Thus it has been considered a risk factor102 and perhaps an indication of a poor prognosis. T wave inversion in the right precordial leads can also be seen in many other conditions such as acute PE, athlete heart [107-109], Brugada syndrome, long QT syndrome caused by KCNH2 mutations or compound mutations, and occasionally in normal adult female.
II. ARVD-RELATED ARRHYTHMIA
1. Atrial Arrhythmia
Atrial extrasystoles, flutter and atrial fibrillation are not uncommon in ARVD [110] especially in those with moderate/severe tricuspid regurgitation and markedly enlarged right ventricles [5, 111, 112]. They can occur prior to or during manifest ventricular arrhythmia [111]. Atrial involvement is a likely cause of these arrhythmias [61, 62].
2. Ventricular Arrhythmia
The increased susceptibility to ventricular tachyarrhythmia and SCD is a main feature of ARVD that led to its first recognition in 1977 [1]. Symptoms such as palpitations, dizziness, presyncope and syncope are caused by frequent premature contractions (PVCs), sustained, or non-sustained VT. Since ectopic beats from the ‘triangle of dysplasia’ [3] right ventricular origin is most common, these VTs have a left bundle branch block (LBBB) morphology with superior, inferior or indeterminate axis [52]. VT and PVCs (>500 per 24 hours) in LBBB morphology are considered a minor diagnostic criteria [52]. In many cases VT comes in ‘hot phases’ thus viral infection has been suspected as a prerequisite [113] or a superimposed factor onto ARVD. Drug therapy is empiric. Sotalol or amiodarone in combination with beta-blockers is preferred but their effectiveness is limited due to the nature of the disease [53, 60]. The slow conduction in the diseased region first recorded during epicardial mapping confirmed re-entry as the mechanism of resistant ventricular tachycardia (VT). This led the pioneer investigators to use aggressive therapeutic approaches such as ventriculotomy [1] and multi-session fulgurations with high energy DC ablation [114, 119]. In recent years, the combined endocardial and epicardial VT ablation incorporating scar de-channeling seems promising in achieving short- and mid-term success [54].
Since ARVD is often a silent cardiomyopathy among affected young people or endurance athletes, and VT degenerating into ventricular fibrillation (VF) is the cause of SCD, ICD therapy is highly effective in cases of aborted SCD and unstable VT, and is recommended to high risk individuals for primary and secondary SCD prevention purposes [56-60].
The differential diagnosis for individuals with recurrent VT with a LBBB morphology is idiopathic right ventricular outflow tract VT (RVOT). The prognosis for these patients is usually benign. Unlike ARVD triggered automatic activity accounts for the vast majority of RVOT VTs [120]. A number of studies have reported the differences in VT morphology between ARVD and ROVT with regard to the duration, fragmentation, frontal axis and the origin of QRS complex [121, 122]. Nevertheless, there is a considerable overlap in VT morphology between these two entities. Sometimes at fast rates, separating the oscillating-like R wave from S wave or the wide QRS complex from T wave is nearly impossible, especially in emergency situations. A careful ECG evaluation in VT and in sinus rhythm is necessary [52, 123, 124].
III. LATE POTENTIALS
Late potentials refer to the low-amplitude, high-frequency, and altered frequency components in the terminal QRS complex. They are too small to be visible on the surface ECG tracing. This limitation however was solved by high resolution signal-averaged ECG (SAECG), a summation averaging technique first proposed in 1977 [1] and refined thoroughly over time [125, 126]. The underlying mechanism is all the same regarding late potentials, Epsilon waves, delayed or fragmented electrograms recorded during endomyocardial mapping and the presence of low-voltage areas detected by electroanatomic voltage mapping in the right ventricle of ARVD patients. Presence of late potentials is a minor task force criterion [52]. Utilizing SAECG has shown an improved diagnostic accuracy [127]. In ARVD the prevalence of late potentials is much higher (69%) than that of Epsilon waves in individuals met the taskforce criteria [127]. The prevalence is also higher in ARVD family members (16%), therefore, it can be used for family screenings [128]. In contrast, late potential is mostly negative in RVOT individuals with no apparent structural heart abnormalities [129].
CONCLUSIONS
In summary, ARVD is a genetic arrhythmogenic cardiomyopathy associated with an increased risk of SCD. Mutations in both desmosomal and non-desmosomal genes can lead to a gradual loss of myocardium and fatty or fibrofatty replacement predominantly affecting the right ventricle. ARVD development is a concealed process at the early stage. Upon diagnosis, most patients present with ECG abnormalities, indicating the ECG changes may have occurred long before the development of malignant ventricular arrhythmias. ECG abnormalities are characteristic in the majority of patients who meet the task force criteria. Recognizing the common manifestations of ARVD on ECG may help improve diagnostic accuracy. Moreover it can be useful in identifying affected family members. ECG, nevertheless, cannot replace or substitute other diagnostic modalities towards ARVD diagnosis.
ACKNOWLEDGEMENTS
Grants: AHA SDG0735474N, SSRF 2011-2012 and W.W. Smith Charitable Trust.
Organizations and individuals: North American ARVD Registry, Johns Hopkins ARVD Registry, Chinese ARVD Registry, Mrs. Micheline (Tink) Long and the International ARVD Family Support Network, as well as colleagues from France, Italy and all over the world who contributed to the ARVD investigations since 1977.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Fontaine G, Guiraudon G, Frank R. Stimulation studies and epicardial mapping in ventricular tachycardia A study of mechanisms and selection for surgery HE Kulbertus (Ed). Reentrant Arrhythmias Lancaster England. 1977:334–50. [Google Scholar]
- 2.Fontaine G, Guiraudon G, Frank R , et al. Arrhythmogenic right ventricular dysplasia and uhl's disease. Arch Mal Coeur Vaiss. 1982;75:361–71. [PubMed] [Google Scholar]
- 3.Marcus FI, Fontaine GH, Guiraudon G , et al. Right ventricular dysplasia: A report of 24 adult cases. Circulation. 1982;65:384–98. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 4.Dungan WT, Garson A Jr, Gillette PC. Arrhythmogenic right ventricular dysplasia: A cause of ventricular tachycardia in children with apparently normal hearts. Am Heart J. 1981;102:745–50. doi: 10.1016/0002-8703(81)90101-0. [DOI] [PubMed] [Google Scholar]
- 5.Peters S. Long-term follow-up and risk assessment of arrhythmogenic right ventricular dysplasia/cardiomyopathy: Personal experience from different primary and tertiary centres. J Cardiovasc Med (Hagerstown) 2007;8:521–6. doi: 10.2459/01.JCM.0000278450.35107.b3. [DOI] [PubMed] [Google Scholar]
- 6.Nemec J, Edwards BS, Osborn MJ, Edwards WD. Arrhythmogenic right ventricular dysplasia masquerading as dilated cardiomyopathy. Am J Cardiol. 1999;84:237–9. doi: 10.1016/s0002-9149(99)00244-1. [DOI] [PubMed] [Google Scholar]
- 7.Hulot JS, Jouven X, Empana JP, Frank R, Fontaine G. Natural history and risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2004;110:1879–84. doi: 10.1161/01.CIR.0000143375.93288.82. [DOI] [PubMed] [Google Scholar]
- 8.McKoy G, Protonotarios N, Crosby A , et al. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (naxos disease). Lancet. 2000;355:2119–24. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- 9.Protonotarios N, Tsatsopoulou A, Anastasakis A , et al. Genotype-phenotype assessment in autosomal recessive arrhythmogenic right ventricular cardiomyopathy (naxos disease) caused by a deletion in plakoglobin. J Am Coll Cardiol. 2001;38:1477–84. doi: 10.1016/s0735-1097(01)01568-6. [DOI] [PubMed] [Google Scholar]
- 10.Asimaki A, Syrris P, Wichter T, Matthias P, Saffitz JE, McKenna WJ. A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2007;81:964–73. doi: 10.1086/521633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coonar AS, Protonotarios N, Tsatsopoulou A , et al. Gene for arrhythmogenic right ventricular cardiomyopathy with diffuse nonepidermolytic palmoplantar keratoderma and woolly hair (naxos disease) maps to 17q21. Circulation. 1998;97:2049–58. doi: 10.1161/01.cir.97.20.2049. [DOI] [PubMed] [Google Scholar]
- 12.Rampazzo A, Nava A, Malacrida S , et al. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2002;71:1200–6. doi: 10.1086/344208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerull B, Heuser A, Wichter T , et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–4. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 14.Dalal D, Molin LH, Piccini J , et al. Clinical features of arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in plakophilin-2. Circulation. 2006;113:1641–9. doi: 10.1161/CIRCULATIONAHA.105.568642. [DOI] [PubMed] [Google Scholar]
- 15.van der Zwaag PA, Cox MG, van der Werf C , et al. Recurrent and founder mutations in the netherlands : Plakophilin-2 p.rg79x mutation causing arrhythmogenic right ventricular cardiomyopathy/dysplasia. . Neth Heart J. 2010;18:583–91. doi: 10.1007/s12471-010-0839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu T, Yang Z, Vatta M , et al. Multidisciplinary Study of Right Ventricular Dysplasia I.Compound and digenic heterozygosity contributes to arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2010;55:587–97. doi: 10.1016/j.jacc.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pilichou K, Nava A, Basso C , et al. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:1171–9. doi: 10.1161/CIRCULATIONAHA.105.583674. [DOI] [PubMed] [Google Scholar]
- 18.Awad MM, Dalal D, Cho E , et al. Dsg2 mutations contribute to arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Hum Genet. 2006;79:136–42. doi: 10.1086/504393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heuser A, Plovie ER, Ellinor PT , et al. Mutant desmocollin-2 causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2006;79:1081–8. doi: 10.1086/509044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beffagna G, De Bortoli M, Nava A , et al. Missense mutations in desmocollin-2 n-terminus. associated with arrhythmogenic right ventricular cardiomyopthy.affect intracellular localization of desmocollin-2 in vitro. BMC Med Genet. 2007; 8:65. doi: 10.1186/1471-2350-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merner ND, Hodgkinson KA, Haywood AF , et al. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant. lethal arrhythmic disorder caused by a missense mutation in the tmem43 gene. . Am J Hum Genet. 2008;82:809–21. doi: 10.1016/j.ajhg.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Hengel J, Calore M, Bauce B , et al. Mutations in the area composita protein alphat-catenin are associated with arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2013;34:201–10. doi: 10.1093/eurheartj/ehs373. [DOI] [PubMed] [Google Scholar]
- 23.van Spaendonck-Zwarts KY, van der Kooi AJ, van den Berg MP , et al. Recurrent and founder mutations in the netherlands: The cardiac phenotype of des founder mutations p.13f and p.N342d. Neth Heart J. 2012;20:219–28. doi: 10.1007/s12471-011-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Tintelen JP, Van Gelder IC, Asimaki A , et al. Severe cardiac phenotype with right ventricular predominance in a large cohort of patients with a single missense mutation in the des gene. Heart Rhythm. 2009;6:1574–83. doi: 10.1016/j.hrthm.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 25.Taylor M, Graw S, Sinagra G , et al. Genetic variation in titin in arrhythmogenic right ventricular cardiomyopathy-overlap syndromes. Circulation. 2011;124:876–85. doi: 10.1161/CIRCULATIONAHA.110.005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Zwaag PA, van Rijsingen IA, Asimaki A, Jongbloed JD, van Veldhuisen DJ, Wiesfeld AC, Cox MG, van Lochem LT, de Boer RA , et al. Phospholamban r14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: Evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail. 2012;14:1199–207. doi: 10.1093/eurjhf/hfs119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quarta G, Syrris P, Ashworth M , et al. Mutations in the lamin a/c gene mimic arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2012;33:1128–36. doi: 10.1093/eurheartj/ehr451. [DOI] [PubMed] [Google Scholar]
- 28.Tiso N, Stephan DA, Nava A , et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (arvd2). Hum Mol Genet. 2001;10:189–94. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- 29.Pamuru PR, Maithili DV, Mohiuddin K, Calambur N, Nallari P. Novel mutations in arrhythmogenic right ventricular cardiomyopathy from indian population. Indian J Hum Genet. 2011;17:70–6. doi: 10.4103/0971-6866.86182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapplinger JD, Landstrom AP, Salisbury BA , et al. Distinguishing arrhythmogenic right ventricular cardiomyopathy/dysplasia-associated mutations from background genetic noise. J Am Coll Cardiol. 2011;57:2317–27. doi: 10.1016/j.jacc.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen AH, Benn M, Bundgaard H, Tybjaerg-Hansen A, Haunso S, Svendsen JH. Wide spectrum of desmosomal mutations in danish patients with arrhythmogenic right ventricular cardiomyopathy. J Med Genet. 2010;47:736–44. doi: 10.1136/jmg.2010.077891. [DOI] [PubMed] [Google Scholar]
- 32.Bauce B, Nava A, Beffagna G , et al. Multiple mutations in desmosomal proteins encoding genes in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2010;7:22–9. doi: 10.1016/j.hrthm.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 33.Dalal D, James C, Devanagondi R , et al. Penetrance of mutations in plakophilin-2 among families with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2006;48:1416–24. doi: 10.1016/j.jacc.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 34.van der Smagt JJ, van der Zwaag PA, van Tintelen JP , et al. Clinical and genetic characterization of patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy caused by a plakophilin-2 splice mutation. Cardiology. 2012;123:181–9. doi: 10.1159/000342717. [DOI] [PubMed] [Google Scholar]
- 35.van Tintelen JP, Entius MM, Bhuiyan ZA , et al. Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2006;113:1650–8. doi: 10.1161/CIRCULATIONAHA.105.609719. [DOI] [PubMed] [Google Scholar]
- 36.den Haan AD, Tan BY, Zikusoka MN , et al. Comprehensive desmosome mutation analysis in north americans with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Cardiovasc Genet. 2009;2:428–35. doi: 10.1161/CIRCGENETICS.109.858217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu X, Liu W, Hu D , et al. Mutations of plakophilin-2 in chinese with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol. 2009;103:1439–44. doi: 10.1016/j.amjcard.2009.01.356. [DOI] [PubMed] [Google Scholar]
- 38.Fressart V, Duthoit G, Donal E , et al. Desmosomal gene analysis in arrhythmogenic right ventricular dysplasia/cardiomyopathy: Spectrum of mutations and clinical impact in practice. Europace. 2010;12:861–8. doi: 10.1093/europace/euq104. [DOI] [PubMed] [Google Scholar]
- 39.Ohno S, Nagaoka I, Fukuyama M , et al. Age-dependent clinical and genetic characteristics in japanese patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ J. 2013;77(6):1534–42. doi: 10.1253/circj.cj-12-1446. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Gras E, Lombardi R, Giocondo MJ , et al. Suppression of canonical wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116:2012–21. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delmar M. Desmosome-ion channel interactions and their possible role in arrhythmogenic cardiomyopathy. Pediatr Cardiol. 2012;33:975–9. doi: 10.1007/s00246-012-0257-0. [DOI] [PubMed] [Google Scholar]
- 42.Joshi-Mukherjee R, Coombs W, Musa H, Oxford E, Taffet S, Delmar M. Characterization of the molecular phenotype of two arrhythmogenic right ventricular cardiomyopathy (arvc)-related plakophilin-2 (pkp2) mutations. Heart Rhythm. 2008;5:1715–23. doi: 10.1016/j.hrthm.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthes SA, Taffet S, Delmar M. Plakophilin-2 and the migration. differentiation and transformation of cells derived from the epicardium of neonatal rat hearts. . Cell Commun Adhes. 2011;18:73–84. doi: 10.3109/15419061.2011.621561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oxford EM, Everitt M, Coombs Wetal. Molecular composition of the intercalated disc in a spontaneous canine animal model of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Heart Rhythm. 2007;4:1196–205. doi: 10.1016/j.hrthm.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asimaki A, Tandri H, Huang Hetal. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075–84. doi: 10.1056/NEJMoa0808138. [DOI] [PubMed] [Google Scholar]
- 46.d'Amati G, di Gioia CR, Giordano C, Gallo P. Myocyte transdifferentiation A possible pathogenetic mechanism for arrhythmogenic right ventricular cardiomyopathy. Arch Pathol Lab Med. 2000;124:287–90. doi: 10.5858/2000-124-0287-MT. [DOI] [PubMed] [Google Scholar]
- 47.d'Amati G, Leone O, di Gioia CRetal. Arrhythmogenic right ventricular cardiomyopathy Clinicopathologic correlation based on a revised definition of pathologic patterns. Hum Pathol. 2001;32:1078–86. doi: 10.1053/hupa.2001.28232. [DOI] [PubMed] [Google Scholar]
- 48.Kim C, Wong J, Wen Jetal. Studying arrhythmogenic right ventricular dysplasia with patient-specific ipscs. Nature. 2013;494:105–10. doi: 10.1038/nature11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simpson MA, Mansour S, Ahnood Detal. Homozygous mutation of desmocollin-2 in arrhythmogenic right ventricular cardiomyopathy with mild palmoplantar keratoderma and woolly hair. Cardiology. 2009;113:28–34. doi: 10.1159/000165696. [DOI] [PubMed] [Google Scholar]
- 50.Awad MM, Dalal D, Tichnell Cetal. Recessive arrhythmogenic right ventricular dysplasia due to novel cryptic splice mutation in pkp2. Hum Mutat. 2006;27:1157. doi: 10.1002/humu.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alcalai R, Metzger S, Rosenheck S, Meiner V, Chajek-Shaul T. A recessive mutation in desmoplakin causes arrhythmogenic right ventricular dysplasia. skin disoder.and woolly hair. J Am Coll Cardiol . 2003; 42:319–27. doi: 10.1016/s0735-1097(03)00628-4. [DOI] [PubMed] [Google Scholar]
- 52.Marcus FI, McKenna WJ, Sherrill Detal. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia Proposed modification of the task force criteria. Eur Heart J. 2010;31:806–14. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcus GM, Glidden DV, Polonsky B , et al. Multidisciplinary Study of Right Ventricular Dysplasia I.Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy A report from the north american arvc registry. J Am Coll Cardiol. 2009;54:609–15. doi: 10.1016/j.jacc.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berruezo A, Fernandez-Armenta J, Mont Letal. Combined endocardial and epicardial catheter ablation in arrhythmogenic right ventricular dysplasia incorporating scar dechanneling technique. Circ Arrhythm Electrophysiol. 2012;5:111–21. doi: 10.1161/CIRCEP.110.960740. [DOI] [PubMed] [Google Scholar]
- 55.Pokushalov E, Romanov A, Turov A, Artyomenko S, Shirokova N, Karaskov A. Percutaneous epicardial ablation of ventricular tachycardia after failure of endocardial approach in the pediatric population with arrhythmogenic right ventricular dysplasia. Heart Rhythm. 2010;7:1406–10. doi: 10.1016/j.hrthm.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 56.Corrado D, Leoni L, Link MSetal. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108:3084–91. doi: 10.1161/01.CIR.0000103130.33451.D2. [DOI] [PubMed] [Google Scholar]
- 57.Bhonsale A, James CA, Tichnell C. Incidence and predictors of implantable cardioverter-defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy undergoing implantable cardioverter-defibrillator implantation for primary prevention. J Am Coll Cardiol. 2011;58:1485–96. doi: 10.1016/j.jacc.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 58.Dalal D, Nasir K, Bomma Cetal. Arrhythmogenic right ventricular dysplasia A united states experience. Circulation. 2005;112:3823–32. doi: 10.1161/CIRCULATIONAHA.105.542266. [DOI] [PubMed] [Google Scholar]
- 59.Li CH, Lin YJ, Huang JLetal. Long-term follow-up in patients with arrhythmogenic right ventricular cardiomyopathy. J Cardiovasc Electrophysiol. 2012;23:750–6. doi: 10.1111/j.1540-8167.2011.02288.x. [DOI] [PubMed] [Google Scholar]
- 60.Wichter T, Paul TM, Eckardt Letal. Arrhythmogenic right ventricular cardiomyopathy. Antiarrhythmic drugs catheter ablation or icd?. Herz. 2005;30:91–101. doi: 10.1007/s00059-005-2677-6. [DOI] [PubMed] [Google Scholar]
- 61.Takemura N, Kono K, Tadokoro K , et al. Right atrial abnormalities in a patient with arrhythmogenic right ventricular cardiomyopathy without ventricular tachycardia. J Cardiol. 2008;51:205–9. doi: 10.1016/j.jjcc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Balderramo DC, Caeiro AA. Arrhythmogenic right ventricular dysplasia and sick sinus syndrome. Medicina (B Aires) 2004;64:439–441. [PubMed] [Google Scholar]
- 63.Ryu HM, Lee JH, Kwon YS , et al. Electrocardiography patterns and the role of the electrocardiography score for risk stratification in acute pulmonary embolism. Korean Circ J. 2010;40:499–506. doi: 10.4070/kcj.2010.40.10.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sinha N, Yalamanchili K, Sukhija R , et al. Role of the 12-lead electrocardiogram in diagnosing pulmonary embolism. Cardiol Rev. 2005;13:46–9. doi: 10.1097/01.crd.0000134647.55135.4a. [DOI] [PubMed] [Google Scholar]
- 65.Serra W, De Iaco G, Reverberi C, Gherli T. Pulmonary embolism and patent foramen ovale thrombosis: The key role of tee. Cardiovasc Ultrasound. 2007;5:26. doi: 10.1186/1476-7120-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Platonov PG, Christensen AH, Holmqvist F, Carlson J, Haunso S, Svendsen JH. Abnormal atrial activation is common in patients with arrhythmogenic right ventricular cardiomyopathy. J Electrocardiol. 2011;44:237–41. doi: 10.1016/j.jelectrocard.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Peters S, Trummel M, Koehler B, Westermann KU. Mechanisms of syncopes in arrhythmogenic right ventricular dysplasia-cardiomyopathy beyond monomorphic ventricular tachycardia. Int J Cardiol. 2006;106:52–4. doi: 10.1016/j.ijcard.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 68.Pu JL, Wang HT, Liu TK , et al. Clinical and ecg features of arrhythmogenic right ventricular cardiomyopathy: A retrospective analysis of 31 cases. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:24–7. [PubMed] [Google Scholar]
- 69.Yodogawa K, Seino Y, Ohara T , et al. Non-invasive detection of latent cardiac conduction abnormalities in patients with pulmonary sarcoidosis. Circ J. 2007;71:540–5. doi: 10.1253/circj.71.540. [DOI] [PubMed] [Google Scholar]
- 70.Banba K, Kusano KF, Nakamura K , et al. Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis. Heart Rhythm. 2007;4:1292–9. doi: 10.1016/j.hrthm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Asimaki A, Tandri H, Duffy ER , et al. Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:743–52. doi: 10.1161/CIRCEP.111.964890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dechering DG, Kochhauser S, Wasmer K , et al. Electrophysiological characteristics of ventricular tachyarrhythmias in cardiac sarcoidosis versus arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2013;10:158–64. doi: 10.1016/j.hrthm.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 73.Ladyjanskaia GA, Basso C, Hobbelink MG , et al. Sarcoid myocarditis with ventricular tachycardia mimicking arvd/c. J Cardiovasc Electrophysiol. 2010;21:94–8. doi: 10.1111/j.1540-8167.2009.01479.x. [DOI] [PubMed] [Google Scholar]
- 74.Mohsen A, Panday M, Wetherold S, Jimenez A. Cardiac sarcoidosis mimicking arrhythmogenic right ventricular dysplasia with high defibrillation threshold requiring subcutaneous shocking coil implantation. Heart Lung Circ. 2012;21:46–9. doi: 10.1016/j.hlc.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 75.Ott P, Marcus FI, Sobonya RE, Morady F, Knight BP, Fuenzalida CE. Cardiac sarcoidosis masquerading as right ventricular dysplasia. Pacing Clin Electrophysiol. 2003;26:1498–503. doi: 10.1046/j.1460-9592.2003.t01-1-00217.x. [DOI] [PubMed] [Google Scholar]
- 76.Fontaine G, Frank R, Guiraudon G , et al. Significance of intraventricular conduction disorders observed in arrhythmogenic right ventricular dysplasia. Arch Mal Coeur Vaiss. 1984;77:872–9. [PubMed] [Google Scholar]
- 77.Fontaine G, Umemura J, Di Donna P, Tsezana R, Cannat JJ, Frank R. Duration of qrs complexes in arrhythmogenic right ventricular dysplasia.A new non-invasive diagnostic marker. Ann Cardiol Angeiol (Paris) 1993;42:399–405. [PubMed] [Google Scholar]
- 78.Peters S, Trummel M. Diagnosis of arrhythmogenic right ventricular dysplasia-cardiomyopathy: Value of standard ecg revisited. Ann Noninvasive Electrocardiol. 2003;8:238–45. doi: 10.1046/j.1542-474X.2003.08312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jain R, Dalal D, Daly A , et al. Electrocardiographic features of arrhythmogenic right ventricular dysplasia. Circulation. 2009;120:477–87. doi: 10.1161/CIRCULATIONAHA.108.838821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steriotis AK, Bauce B, Daliento L , et al. Electrocardiographic pattern in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2009;103:1302–8. doi: 10.1016/j.amjcard.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 81.Cox MG, Nelen MR, Wilde AA , et al. Activation delay and vt parameters in arrhythmogenic right ventricular dysplasia/ cardiomyopathy: Toward improvement of diagnostic ecg criteria. J Cardiovasc Electrophysiol. 2008;19:775–81. doi: 10.1111/j.1540-8167.2008.01140.x. [DOI] [PubMed] [Google Scholar]
- 82.Cox MG, van der Smagt JJ, Wilde AA , et al. New ecg criteria in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol. 2009;2:524–30. doi: 10.1161/CIRCEP.108.832519. [DOI] [PubMed] [Google Scholar]
- 83.McKenna WJ, Thiene G, Nava A , et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy.Task force of the working group myocardial and pericardial disease of the european society of cardiology and of the scientific council on cardiomyopathies of the international society and federation of cardiology. Br Heart J. 1994;71:215–8. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morita H, Kusano KF, Miura D , et al. Fragmented qrs as a marker of conduction abnormality and a predictor of prognosis of brugada syndrome. Circulation. 2008;118:1697–704. doi: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 85.Peters S, Trummel M, Koehler B. Qrs fragmentation in standard ecg as a diagnostic marker of arrhythmogenic right ventricular dysplasia-cardiomyopathy. Heart Rhythm. 2008;5:1417–21. doi: 10.1016/j.hrthm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 86.Kukla P, Jastrzebski M, Kurdzielewicz W. Higher right precordial leads and fontaine leads: The better detection of qrs fragmentation and epsilon wave in arrhythmogenic right ventricular dysplasia-cardiomyopathy. Kardiol Pol. 2012;70:958–9. [PubMed] [Google Scholar]
- 87.Hurst JW. Naming of the waves in the ecg. with a brief account of their genesis. Circulation. 1998;98:1937–42. doi: 10.1161/01.cir.98.18.1937. [DOI] [PubMed] [Google Scholar]
- 88.Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented qrs complex versus a q wave in patients with coronary artery disease. Circulation. 2006;113:2495–501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 89.Das MK, Maskoun W, Shen C , et al. Fragmented qrs on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm. 2010;7:74–80. doi: 10.1016/j.hrthm.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 90.Hodgkinson K, Connors S, Merner N , et al. The natural history of a genetic subtype of arrhythmogenic right ventricular cardiomyopathy caused by a p.358l mutation in tmem43. . Clin Genet. 2013;83(4):321–31. doi: 10.1111/j.1399-0004.2012.01919.x. [DOI] [PubMed] [Google Scholar]
- 91.Corrado D, Basso C, Buja G, Nava A, Rossi L, Thiene G. Right bundle branch block. right precordial st-segment elevaion.and sudden death in young people. . Circulation. 2001; 103:710–7. doi: 10.1161/01.cir.103.5.710. [DOI] [PubMed] [Google Scholar]
- 92.Peters S, Trummel M, Koehler B. Special features of right bundle branch block in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Int J Cardiol. 2012;157:102–3. doi: 10.1016/j.ijcard.2011.09.070. [DOI] [PubMed] [Google Scholar]
- 93.Obata H, Mitsuoka T, Kikuchi Y , et al. Twenty-seven-year follow-up of arrhythmogenic right ventricular dysplasia. Pacing Clin Electrophysiol. 2001;24:510–1. doi: 10.1046/j.1460-9592.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- 94.Wu S, Wang P, Hou Y, Yang P, Xiao Y, Zhan X. Epsilon wave in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Pacing Clin Electrophysiol. 2009;32:59–63. doi: 10.1111/j.1540-8159.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 95.Wang J, Yang B, Chen H , et al. Epsilon waves detected by various electrocardiographic recording methods: In patients with arrhythmogenic right ventricular cardiomyopathy. Tex Heart Inst J. 2010;37:405–11. [PMC free article] [PubMed] [Google Scholar]
- 96.Aldakar M, Perchet H, Coutte R, Dauptain J, Lefort JF, Charon P. Association of an epsilon wave and syncope. Presse Med. 1998;27:1893–6. [PubMed] [Google Scholar]
- 97.Zorio E, Arnau MA, Rueda J , et al. The presence of epsilon waves in a patient with acute right ventricular infarction. Pacing Clin Electrophysiol. 2005;28:245–7. doi: 10.1111/j.1540-8159.2005.40021.x. [DOI] [PubMed] [Google Scholar]
- 98.Santucci PA, Morton JB, Picken MM, Wilber DJ. Electroanatomic mapping of the right ventricle in a patient with a giant epsilon wave. ventricular tachycadia.and cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2004;15:1091–4. doi: 10.1046/j.1540-8167.2004.03708.x. [DOI] [PubMed] [Google Scholar]
- 99.Mivelaz Y, Di Bernardo S, Pruvot E, Meijboom EJ, Sekarski N. Brugada syndrome in childhood: A potential fatal arrhythmia not always recognised by paediatricians.A case report and review of the literature. Eur J Pediatr. 2006;165:507–11. doi: 10.1007/s00431-006-0150-z. [DOI] [PubMed] [Google Scholar]
- 100.Valdivia CR, Medeiros-Domingo A, Ye B , et al. Loss-of-function mutation of the scn3b-encoded sodium channel {beta}3 subunit associated with a case of idiopathic ventricular fibrillation. Cardiovasc Res. 2010;86:392–400. doi: 10.1093/cvr/cvp417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Frigo G, Rampazzo A, Bauce B , et al. Homozygous scn5a mutation in brugada syndrome with monomorphic ventricular tachycardia and structural heart abnormalities. Europace. 2007;9:391–7. doi: 10.1093/europace/eum053. [DOI] [PubMed] [Google Scholar]
- 102.Peters S, Peters H, Thierfelder L. Risk stratification of sudden cardiac death and malignant ventricular arrhythmias in right ventricular dysplasia-cardiomyopathy. Int J Cardiol. 1999;71:243–50. doi: 10.1016/s0167-5273(99)00142-4. [DOI] [PubMed] [Google Scholar]
- 103.Ma KJ, Li N, Wang HT , et al. Clinical study of 39 chinese patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Chin Med J (Engl) 2009;122:1133–8. [PubMed] [Google Scholar]
- 104.Nava A, Bauce B, Basso C , et al. Clinical profile and long-term follow-up of 37 families with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2000;36:2226–33. doi: 10.1016/s0735-1097(00)00997-9. [DOI] [PubMed] [Google Scholar]
- 105.Nava A, Canciani B, Buja G , et al. Electrovectorcardiographic study of negative t waves on precordial leads in arrhythmogenic right ventricular dysplasia: Relationship with right ventricular volumes. J Electrocardiol. 1988;21:239–45. doi: 10.1016/0022-0736(88)90098-2. [DOI] [PubMed] [Google Scholar]
- 106.Sen-Chowdhry S, Syrris P, Ward D, Asimaki A, Sevdalis E, McKenna WJ. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation. 2007;115:1710–20. doi: 10.1161/CIRCULATIONAHA.106.660241. [DOI] [PubMed] [Google Scholar]
- 107.Wilson MG, Sharma S, Carre F , et al. Significance of deep t-wave inversions in asymptomatic athletes with normal cardiovascular examinations: Practical solutions for managing the diagnostic conundrum. Br J Sports Med. 2012;46(Suppl 1 ):i51–8. doi: 10.1136/bjsports-2011-090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Magalski A, Maron BJ, Main ML , et al. Relation of race to electrocardiographic patterns in elite american football players. J Am Coll Cardiol. 2008;51:2250–5. doi: 10.1016/j.jacc.2008.01.065. [DOI] [PubMed] [Google Scholar]
- 109.Magalski A, McCoy M, Zabel M , et al. Cardiovascular screening with electrocardiography and echocardiography in collegiate athletes. Am J Med. 2011;124:511–8. doi: 10.1016/j.amjmed.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 110.Tonet JL, Castro-Miranda R, Iwa T, Poulain F, Frank R, Fontaine GH. Frequency of supraventricular tachyarrhythmias in arrhythmogenic right ventricular dysplasia. Am J Cardiol. 1991;67:1153. doi: 10.1016/0002-9149(91)90886-p. [DOI] [PubMed] [Google Scholar]
- 111.Chu AF, Zado E, Marchlinski FE. Atrial arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and ventricular tachycardia. Am J Cardiol. 2010;106:720–2. doi: 10.1016/j.amjcard.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 112.Jinnouchi J, Nakane H, Kitayama J, Fukahori M, Ibayashi S, Iida M. A case of cardioembolic brain infarction in arrhythmogenic right ventricular dysplasia. Rinsho Shinkeigaku. 2005;45:744–7. [PubMed] [Google Scholar]
- 113.Sabel KG, Blomstrom-Lundqvist C, Olsson SB, Enestrom S. Arrhythmogenic right ventricular dysplasia in brother and sister: Is it related to myocarditis?. Pediatr Cardiol. 1990;11:113–6. doi: 10.1007/BF02239576. [DOI] [PubMed] [Google Scholar]
- 114.Fontaine G, Cansell A, Tonet JL , et al. Techniques and methods for catheter endocardial fulguration. Pacing Clin Electrophysiol. 1988;11:592–602. doi: 10.1111/j.1540-8159.1988.tb04555.x. [DOI] [PubMed] [Google Scholar]
- 115.Fontaine G, Frank R, Gallais Y , et al. Radiofrequency catheter ablation of ventricular tachycardia. Arch Mal Coeur Vaiss. 1996;89 Spec No 1:99–107. [PubMed] [Google Scholar]
- 116.Fontaine G, Frank R, Gallais Y , et al. Fulguration and radiofrequency in ventricular tachycardia. Arch Mal Coeur Vaiss. 1994;87:1589–607. [PubMed] [Google Scholar]
- 117.Fontaine G, Tonet J, Gallais Y , et al. Ventricular tachycardia catheter ablation in arrhythmogenic right ventricular dysplasia: A 16-year experience. Curr Cardiol Rep. 2000;2:498–506. doi: 10.1007/s11886-000-0034-1. [DOI] [PubMed] [Google Scholar]
- 118.Frank R, Tonet J, Rougier I, Gallais Y, Fontaine G, Grosgogeat Y. Endocardial fulguration for the treatment of ventricular tachycardia.Experience with 69 cases. Arch Inst Cardiol Mex. 1990;60:491–7. [PubMed] [Google Scholar]
- 119.Leclercq JF, Coumel P. Characteristics. prognosis and treatment of the ventricular arrhythmias of right ventricular dysplasia. Eur Heart J. 1989;10(Suppl D ):61–7. doi: 10.1093/eurheartj/10.suppl_d.61. [DOI] [PubMed] [Google Scholar]
- 120.O'Donnell D, Cox D, Bourke J, Mitchell L, Furniss S. Clinical and electrophysiological differences between patients with arrhythmogenic right ventricular dysplasia and right ventricular outflow tract tachycardia. Eur Heart J. 2003;24:801–10. doi: 10.1016/s0195-668x(02)00654-1. [DOI] [PubMed] [Google Scholar]
- 121.Ainsworth CD, Skanes AC, Klein GJ, Gula LJ, Yee R, Krahn AD. Differentiating arrhythmogenic right ventricular cardiomyopathy from right ventricular outflow tract ventricular tachycardia using multilead qrs duration and axis. Heart Rhythm. 2006;3:416–23. doi: 10.1016/j.hrthm.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 122.Hoffmayer KS, Machado ON, Marcus GM , et al. Electrocardiographic comparison of ventricular arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy and right ventricular outflow tract tachycardia. J Am Coll Cardiol. 2011;58:831–8. doi: 10.1016/j.jacc.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 123.Morin DP, Mauer AC, Gear K , et al. Usefulness of precordial t-wave inversion to distinguish arrhythmogenic right ventricular cardiomyopathy from idiopathic ventricular tachycardia arising from the right ventricular outflow tract. Am J Cardiol. 2010;105:1821–4. doi: 10.1016/j.amjcard.2010.01.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoffmayer KS, Bhave PD, Marcus GM , et al. An electrocardiographic scoring system for distinguishing right ventricular outflow tract arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy from idiopathic ventricular tachycardia. Heart Rhythm. 2013;10:477–82. doi: 10.1016/j.hrthm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 125.Kinoshita O, Fontaine G, Rosas F , et al. Optimal high-pass filter settings of the signal-averaged electrocardiogram in patients with arrhythmogenic right ventricular dysplasia. Am J Cardiol. 1994;74:1074–5. doi: 10.1016/0002-9149(94)90866-4. [DOI] [PubMed] [Google Scholar]
- 126.Kinoshita O, Fontaine G, Rosas F , et al. Time- and frequency-domain analyses of the signal-averaged ecg in patients with arrhythmogenic right ventricular dysplasia. Circulation. 1995;91:715–21. doi: 10.1161/01.cir.91.3.715. [DOI] [PubMed] [Google Scholar]
- 127.Kamath GS, Zareba W, Delaney J , et al. Value of the signal-averaged electrocardiogram in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011;8:256–62. doi: 10.1016/j.hrthm.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hermida JS, Minassian A, Jarry G , et al. Familial incidence of late ventricular potentials and electrocardiographic abnormalities in arrhythmogenic right ventricular dysplasia. Am J Cardiol. 1997;79:1375–80. doi: 10.1016/s0002-9149(97)00143-4. [DOI] [PubMed] [Google Scholar]
- 129.Grimm W, List-Hellwig E, Hoffmann J , et al. Magnetic resonance imaging and signal-averaged electrocardiography in patients with repetitive monomorphic ventricular tachycardia and otherwise normal electrocardiogram. Pacing Clin Electrophysiol. 1997;20:1826–33. doi: 10.1111/j.1540-8159.1997.tb03573.x. [DOI] [PubMed] [Google Scholar]