Abstract
Percutaneous pericardial access for epicardial mapping and ablation of ventricular arrhythmias has expanded considerably in recent years. After its description in patients with Chagas disease, the technique has provided relevant in-formation on the arrhythmia substrate in other cardiomyopathies and has improved the results of ablation procedures in various clinical settings. Electrocardiographic criteria proposed for the recognition of the epicardial origin of ventricular tachycardias are mainly based on analysis of the first QRS components. Ventricular activation at the epicardium has a slow initial component reflecting the transmural activation and influenced by the absence of Purkinje system in the epicardium. Various parameters (pseudodelta wave, intrinsicoid deflection and shortest RS interval) of these initial intervals predict an epicardial origin in patients with scar-related ventricular tachycardias with right bundle branch block morphology. Using the same concept, the maximum deflection index was defined for the location of idiopathic epicardial tachycardias remote from the aortic root. Electrocardiogram criteria based on the morphology of the first component of the QRS (q wave in lead I) have been proposed in patients with nonischemic cardiomyopathy. All these criteria seem to be substrate-specific and have several limitations. Other information, including type of underlying heart disease, previous failed endocardial ablation, and evidence of epicardial scar on magnetic resonance imaging, can help to plan the ablation procedure and decide on an epicardial approach.
Keywords: Arrhythmia, catheter ablation, electrocardiogram, epicardium, ventricles, ventricular tachycardia.
INTRODUCTION
Ventricular tachycardia (VT) mapping and ablation has increased significantly in recent years. Nowadays, VT ablation is an essential option to control recurrent VTs [1]. In the case of idiopathic VTs, radiofrequency (RF) catheter ablation is curative in a large proportion of patients. In structural heart disease, catheter ablation is usually adjunctive therapy to antiarrhythmic drugs and/or an implantable defibrillator. In these patients, RF catheter ablation was initially relegated to a last-resort tachycardia therapy for VT recurrence despite antiarrhythmic drug therapies. In experienced centers, catheter ablation now can be considered early in the management of patients with heart disease and symptomatic monomorphic VT [2].
In the majority of cases, scar-related VTs can be treated endocardially, particularly in post-myocardial infarction patients. Most of the circuits observed in infarct scars are located subendocardially and most idiopathic VTs have an endocardial focus. However, earlier studies on VT mapping observed subepicardial target sites to be involved in catheter ablation failure [3, 4].
Since the initial description by Sosa et al. in a patient group with Chagas disease, [5] a percutaneous approach to catheter ablation treatment of VT has expanded considerably. Although surgery was the only option for recurrent scar-related VT refractory to endocardial ablation and antiarrhythmic drugs for many years, [6] the usefulness of the percutaneous epicardial approach has now been proven in various substrates [7, 8]. In two recent multicenter studies, epicardial mapping or ablation was involved in 13% to 17% of VT ablation procedures [9, 10]. However, the use of the technique is still restricted to rather high-volume centers due to the high skill level required and the potential risk of severe complications.
One of the main issues when planning a VT ablation procedure is deciding when to go to the epicardium. The present article aims to provide an overview of data from the clinical history, imaging studies and electrocardiogram and the characteristics of the underlying heart disease that can provide useful information and an indication to suspect epicardial origin of tachycardia [11].
Electrocardiographic methods for the diagnosis of epicardial VT are mainly based on the concept that when ventricular activation starts at the epicardial level, the initial part of the wavefront progresses slowly through the myocardial wall until reaching the Purkinje system at the subendocardium. This slow transmural activation is reflected as slow onset of the QRS on the surface electrocardiogram [12].
The incidence of epicardial VTs varies dramatically between different arrhythmogenic substrates. In a recent multicenter report on epicardial VT ablation, epicardial mapping was more frequently required in some arrhythmogenic substrates than others. Pericardial access was performed in 16% of patients with coronary artery disease (n=58), 35% of patients with idiopathic dilated cardiomyopathy (n=39), 41% of patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) (n=14), 18% of patients with other cardiomyopathies (n=13), and 6% of patients without structural heart disease (n=17).
We focus this review on electrocardiographic methods developed for identifying epicardial VTs. A variety of electrocardiographic tools have been developed to recognize the epicardial origin of VTs in different clinical situations. These are summarized in Table 1. We discuss these according to the arrhythmogenic substrate.
Table 1.
Electrocardiographic criteria proposed for the identification of epicardial VTs.
| Reference | Underlying heart disease | Limitations | Technique | ECG criteria |
|---|---|---|---|---|
| Berruezo et al. [12] | CAD 72% IDCM 28% |
RBBB VT | Pace mapping and clinical VT | Pseudodelta wave ≥34 ms Intrinsicoid deflection V2 ≥85 ms Shortest RS complex ≥121 ms |
| Daniels et al. [22] | No SHD | Described for LVOT VT | Clinical VT | Precordial maximum deflection index ≥0.55 |
| Bazan et al. [52] VallÈs et al. [53] |
NICM | Absence of Q wave in sinus rhythm | Pace mapping and clinical VT | Q wave in lead I for anterolateral epi VT Q wave in inferior lead for inferior epi VT |
| Bazan et al. [66] | CAD: 2, IDCM: 4, ARVC: 2, No SHD: 5 | No tested in ARVC VTs. Absence of Q wave in sinus rhythm | Pace mapping in RV | Q wave in lead I / QS in lead V2 for anterior epi RV VT Q wave in leads II, III, and aVF is inferior epi RV VT |
ARVC, Arrhythmogenic right ventricular cardiomyopathy; CAD, Coronary artery disease; IDCM, Idiopathic dilated cardiomyopathy; SHD, Structural heart disease; VT: ventricular tachycardia.
IDIOPATHIC EPICARDIAL VENTRICULAR TACHYCARDIA
Idiopathic VTs are a small proportion of all monomorphic VTs, which are mostly related to myocardial scars in patients with structural heart disease. Idiopathic VTs have good prognosis and can be effectively treated with radiofrequency ablation. Classically, idiopathic VTs have been divided into outflow VTs, the most frequent site of origin, and fascicular VTs. Ventricular arrhythmias arising from mitral and tricuspid annulus have similar mechanisms but are less common. Around 15% of idiopathic VTs have an epicardial origin [13].
Triggered activity is the most widely accepted arrhythmogenic mechanism for the outflow tract tachycardias. The appearance of delayed afterdepolarizations is related to intracellular calcium overload. Drugs such as adenosine, beta blockers or verapamil that reduce the inward calcium current inhibit the occurrence of VTs.
In most cases, the site of origin of the tachycardia is located in the right ventricular outflow tract (RVOT), just below the pulmonary cusps. Left ventricular outflow tract (LVOT) origin occurs in 15% to 30% of outflow VTs. Three locations have been described in LVOT VTs: LVOT endocardium, sinuses of Valsalva, and the LVOT epicardium. Although VTs arising from the coronary cusp can be ablated endocardially via retroaortic approach, these origins also can be considered epicardial.
The typical electrocardiographic pattern of outflow tract ventricular arrhythmias shows left bundle branch block (LBBB) pattern and inferior axis. The presence of very early precordial transition (≤ V2) indicates LVOT origin [14]. The late transition (≥ V4) predicts RVOT origin. Ventricular arrhythmias with intermediate transition (V3 transition) may have an origin in either outflow tract.
Presence of myocardial extensions into the aortic root, beyond the annulus, is relatively common. In an autopsy series, 21 of 95 human hearts showed ventricular myocardial extensions beyond the ventriculo-arterial junction. Ventricular extensions into the aortic root (7% of cases) were located within the adventitia or on the epicardial surface [15]. However, the exclusive presence of these muscle fibers does not seem to be enough to justify the arrhythmia. On the other hand, the semilunar shape of the sinuses of Valsalva allows three triangles of myocardium to exceed the ventriculo-arterial junction. Left coronary cusp is the most frequent origin of idiopathic ventricular arrhythmias from the aortic root. In a group of 44 idiopathic ventricular arrhythmias from the aortic root, the site of origin was the left coronary cusp in 24 patients, the right coronary cusp in 14, the junction between left and right cusps in 5, and the non-coronary cusp in 1 patient [16]. The non-coronary cusp is intimately related to the anterior aspect of the interatrial septum and rarely is involved with ventricular arrhythmias.
Several different electrocardiographic features have been proposed for the diagnosis of ventricular arrhythmias originating from the sinuses of Valsalva. Ouyang et al. studied a group of 15 patients with VT or premature ventricular contractions (PVCs) with the earliest ventricular activation in the septal RVOT (8 patients) or aortic cusp (7 patients). The R wave duration and the R/S wave amplitude in V1 and V2 were higher in arrhythmias coming from the aortic root. Concretely, R-wave duration index ≥50% (longer R wave duration V1 or V2 / QRS duration) and R/S-wave amplitude index ≥30% (greater R wave amplitude / S wave amplitude ratio in V1 or V2) showed a sensitivity of 87.5% and a specificity of 100% [17]. Fig. (1) shows two electrocardiograms of PVCs with site of origin in the RVOT and in the right coronary cusp. More recent studies report that pacing in the left coronary cusp produces a polyphasic QRS morphology in V1 (with “W” or “M” shape) with a precordial transition at V2 or before. Pacemapping from the right sinus of Valsalva results in a LBBB pattern with precordial transition in V3 [18]. Yamada et al. thoroughly studied the characteristics of ventricular arrhythmias of the aortic root. The maximum amplitude of the R-wave in the inferior leads was larger in ventricular arrhythmias originating from the left coronary cusp, compared to the right coronary cusp. Notably, 6 of 24 VTs from the left coronary cusp showed the RBBB pattern. In this series, a ratio between amplitude at lead III and lead II (III/II) greater than 0.9 predicts a left coronary cusp origin with a sensitivity of 100% and specificity of 64% [16]. The presence of a qrS pattern in leads V1-V3 has been specifically associated with an origin in the junction of the left and right coronary cusps [19].
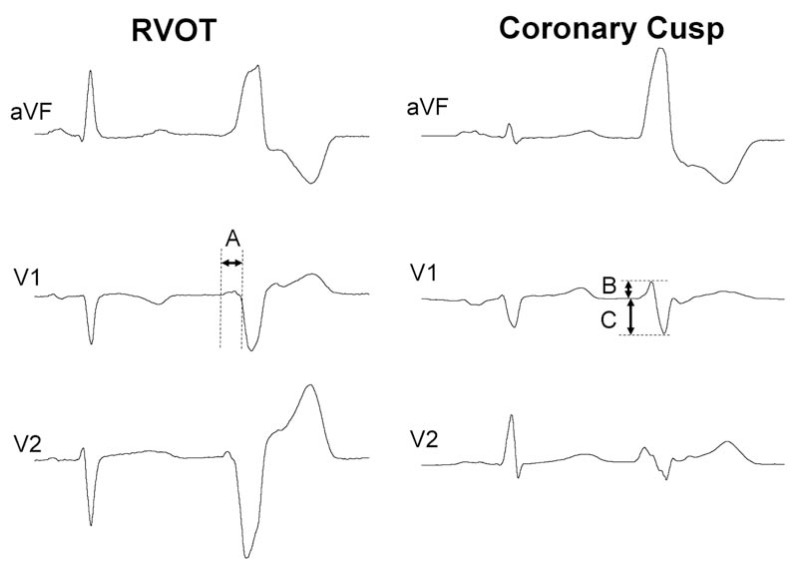
Fig. (1).
Electrocardiogram of two patients with frequent premature ventricular contractions (PVCs) and normal heart referred for radiofrequency ablation, one of them ablated (left) in the septal right ventricular outflow tract, under the pulmonary valve. The other was successfully ablated in the right coronary cusp. The R-wave duration index is obtained by dividing the longer R wave (A) in V1-V2 into the total duration of the QRS. R-wave duration index ≥50‰ predicts origin in the aortic root. The R-wave duration index is 31‰ in the RVOT PVC and 64‰ in the coronary cusp PVC. The R/S-wave amplitude index is calculated from the greater percentage of the R/S-wave amplitude ratio (B divided by C, expressed as a percentage) in V1 or V2. The cut-off point proposed is 30%. In these examples, the R/S-wave amplitude index is 9‰ in the RVOT PVC and 49‰ in the left-sided PVC.
Idiopathic epicardial VTs with an origin remote from the sinuses of Valsalva are usually perivascular. Transvenous and transpericardial approaches can be used. In a series of 48 patients with previous failed endocardial VT ablation procedure, Schweikert et al. performed endo and epicardial mapping. They found that 17 of 20 idiopathic VTs had the earliest activation at the epicardium. Eight of them were ablated in the aortic root, 9 from in the epicardial surface, and 1 failed. Most of the VTs successfully ablated with epicardial RF were located close to major epicardial vessels in the atrioventricular and interventricular grooves [20]. These VTs appear to showed predilection for locating in the distal portion of the great cardiac vein [21].
Slow initial QRS activation allows differentiation of idiopathic epicardial VTs (usually perivascular) from RVOT VTs, endocardial LVOT VTs, and ventricular arrhythmias coming from sinuses of Valsalva. An electrocardiographic marker has been proposed to quantify the prolongation of the initial part of the QRS [22]. The maximum deflection index (MDI) is calculated as follows: earliest time to maximum deflection in any precordial lead divided by the total QRS duration (Table 1) [22]. An MDI larger than 0.55 was related to epicardial origin remote from the sinus of Valsalva compared to other sites of origin with a sensitivity of 100% and specificity of 98.7% [22]. However, two later studies showed that the MDI had limited value for the differentiation between VTs arising from the epicardium and from the aortic cusps (Fig. 2) [13, 23]. The presence of preferential conduction within the aortic root has been proposed to explain the variability in the electrocardiographic features of aortic cusp arrhythmias and the limited reliability of electrocardiographic algorithms to determine the site of origin [24]. Baman et al. report that an R wave in V1 broader than 75 ms can help to differentiate idiopathic epicardial VTs from the coronary venous system [13]. In this series, most of the epicardial PVCs (74%) were successfully ablated by RF ablation within the coronary venous system. Ventricular arrhythmias with a site of origin in the proximal part of the great cardiac vein showed RBBB morphology and those with a distal origin had LBBB morphology [13].
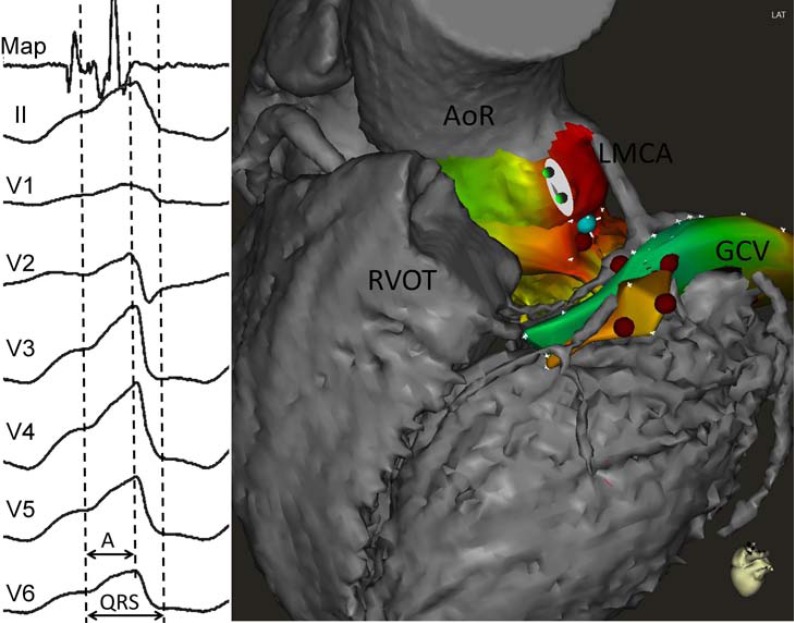
Fig. (2).
Example of idiopathic ventricular tachycardia (VT). Electrocardiogram (left panel) shows a maximum deflection index (MDI) of 0.71 (interval A divided by QRS duration). However, radiofrequency application (right panel, red dots) in the distal great cardiac vein (GCV) failed to eliminate VT. In this case MDI was not useful to predict an epicardial origin of the VT. Mapping in the aortic root (AoR) showed the earliest bipolar electrogram ("Map" in the left panel, blue dot in the right panel) in the left cusp. LMCA, left main coronary artery; RVOT, right ventricular outflow tract.
Crux cordis also have been defined as a potential source of idiopathic epicardial VTs. In a series of 340 patients referred for idiopathic VT ablation, 4 of them revealed earliest activation in the middle cardiac vein or proximal coronary sinus at the crux cordis [25]. All electrocardiograms showed left superior axis, MDI >0.55, pseudo-delta >34 ms and intrinsicoid deflection >85 ms in lead V2 [12, 22]. Beyond the epicardial electrocardiogram criteria, deeply negative deltoid waves in the inferior leads and prominent R wave in lead V2 can lead to suspicion of a crux cordis origin [25].
In our center, we follow a stepwise mapping strategy for outflow tract VTs with a V3 transition, in which the ECG has a low discriminative capacity to differentiate between RVOT and LVOT. Mapping starts at the RV and the highest density of points is taken on the septal RVOT. Right ventricle mapping is used for merging with computed tomography or cardiac magnetic resonance images. The spatial displacement between point position acquired in sinus rhythm and PVC must be taken into account. This is particularly relevant for fusion [26]. Afterwards, the coronary sinus is mapped up to the distal great cardiac vein and the first portion of the anterior interventricular vein. In the case of absence of appropriate parameters for ablation, the LVOT (endocardial LVOT and aortic sinuses of Valsalva) is mapped via retrograde aortic access [27]. After comparing all the points mapped in the different chambers, the point with the highest precocity electrogram during PVC with respect to the QRS complex is targeted for RF ablation. Finally, if endocardial access attempts fail to abolish PVC/VT, epicardial access is performed [11].
VENTRICULAR TACHYCARDIA AFTER MYOCARDIAL INFARCTION
Earlier studies with endocardial and epicardial mapping during surgical treatment of post-myocardial infarction VTs showed that 20% of VTs have earliest epicardial activation rather than endocardial activation [28]. The presence of an epicardial exit does not exclude successful VT ablation from the endocardium. The VT isthmuses can be long and complex with a 3-dimensional pathway that can involve subendocardial and subepicardial layers. In other series during this same era of VT treatment, around 15% of circuits were complete subepicardial circuits [4]. In histology studies of healed infarctions, viable myocardial fiber tracts, recognized as the substrate of reentry, have a complex architecture and can be displayed through different levels of the wall thickness [29, 30]. The infarct location and transmural extent influence the incidence of epicardial VTs. They are more common in inferior infarction compared to other locations [7, 8, 31]. The presence of epicardial circuits has been recognized as a cause of recurrence after endocardial VT ablation [32]. Initial reports showed the usefulness of percutaneous epicardial VT ablation in patients with healed infarction and/or incessant VT [7, 8].
Di Biase et al. have studied extensive endo- and epicardial substrate ablation in patients with healed infarction and electrical storm [33]. They treated 98 patients by limited endocardial substrate ablation or by endocardial and epicardial abolition of abnormal electrogram (scar homogenization). In the first group, epicardial ablation was necessary in 8% of patients because of VT inducibility after endocardial ablation. Epicardial mapping was obtained in all patients (n=49), but epicardial ablation was required in only 33% of these patients. Endo- and epicardial homogenization was related to a reduction in the recurrence of ventricular arrhythmia during follow-up (19% vs 47% of recurrence during a mean follow-up of 25 months). However, this result is probably more related to ablation technique (scar homogenization vs limited ablation) than to the epicardial access. In fact, there was no difference in outcome between patients undergoing epicardial ablation and those who only received endocardial homogenization. Remarkably, only a third of patients who systematically received epicardial mapping had any substrate at that level to be treated [33]. Although data are lacking, patients with subendocardial infarctions appear unlikely to benefit from an epicardial approach. The absence of scar at subepicardial layers makes the presence of VT isthmuses at that level very unlikely.
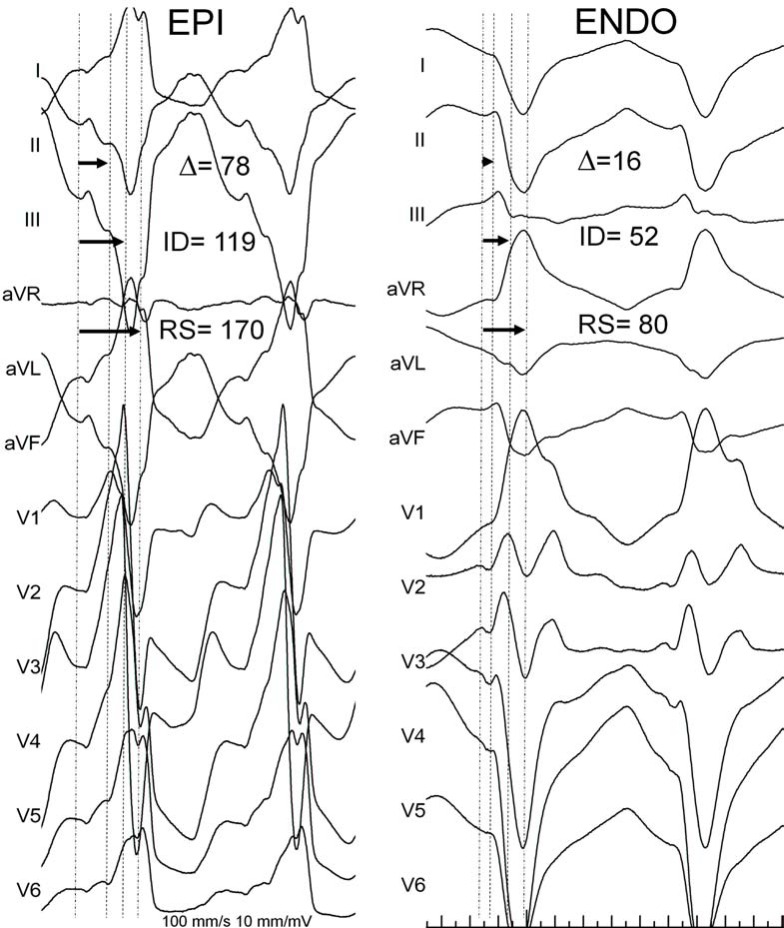
Electrocardiography can be useful for determining an epicardial VT exit in ischemic patients. In 2004, our group analyzed the electrocardiographic features during epicardial and endocardial left ventricular pacing in 9 patients to verify the hypothesis that the epicardial origin of the ventricular activation widens the initial part of the QRS complex. We also analyzed the electrocardiographic characteristics of 69 VTs in patients with ischemic and dilated cardiomyopathy. A number of electrocardiographic parameters were defined. Pseudo delta wave was measured from the earliest ventricular activation to the earliest fast deflection in any precordial lead. The intrinsicoid deflection time was defined as the interval measured from the earliest ventricular activation to the peak of the R wave in V2. The shortest RS complex was defined as the interval from the earliest ventricular activation to the nadir of the first S wave in any precordial lead (Table 1). The duration of this pseudodelta wave coincided with the duration of the transmural activation time.
Epicardial pacing was significantly related to prolongation of the shortest RS interval, intrinsicoid deflection, pseudodelta wave, and QRS duration. Patients with VTs were divided into 3 groups according to the site of successful VT ablation: epicardial, endocardial, and failed endocardial. Electrocardiographic parameters described were longer in patients with epicardial ablation and failed endocardial ablation. Numerous cut-off points were tested, and the best sensitivity and specificity obtained for the identification of an epicardial origin on the VT were as follows: pseudodelta wave ≥34 ms, 83% and 95%; intrinsicoid deflection time ≥85 ms 87% and 90%; and RS complex duration ≥121 ms, 76% and 85%, respectively (Fig. 3) [12]. The main limitation of these criteria is that they are only applicable to VTs with RBBB pattern. Ventricular arrhythmias in patients with structural heart disease and LBBB morphology were not included in this study. These VTs commonly arise from the interventricular septum and may be inappropriate for epicardial ablation.
Fig. (3).
Electrocardiographic criteria for the recognition of the epicardial origin in patients with coronary artery disease (CAD). Left panel shows a 12-lead electrocardiogram during ventricular tachycardia (VT) from a patient with healed inferior myocardial infarction. Epicardial VT exit was confirmed after pericardial percutaneous access Right panel shows the electrocardiogram from a patient with subendocardial myocardial infarction and VT. Endocardial radiofrequency ablation successfully terminated VT.
The reliability of various electrocardiographic criteria was recently tested in a series of patients with ischemic cardiomyopathy [34]. The QRS characteristics of 24 VTs successfully ablated from the epicardium in 14 patients and 39 VTs ablated from the endocardium in 25 patients were retrospectively analyzed. Electrocardiographic criteria (pseudodelta, intrinsicoid deflection time, shortest RS complex, Q wave in lead I, and MDI) failed to discriminate between VTs ablated from endocardium and VTs requiring epicardial ablation [35]. The presence of Q waves, the myocardial scar extension and slow conduction within the scar have been proposed to explain the failure of the electrocardiographic criteria in post-myocardial infarction patients. It must also be taken into account that the electrocardiogram pattern only indicates the exit of the VT circuit. The VT isthmus can be complex, and most of them have an endocardial and epicardial trajectory permitting successful ablation from endocardium in a VT with an epicardial exit. Moreover, irrigated tip ablation catheters cause deep lesions that may achieve profound circuits by RF application at the endocardium [36]. This is of particular significance in patients with transmural infarction and thin left ventricular walls.
In our center, patients with myocardial infarction and VT referred for RF ablation are initially approached endocardially. We perform a substrate-guided ablation strategy, attempting to eliminate all the abnormal electrograms in the scar area with RF application al the slow conducting zones entrance (scar dechanneling technique) [37, 38]. After substrate ablation, VT inducibility is tested. Residual VTs are targeted by entrainment and pacemapping techniques. In the case of monomorphic VTs still inducible after an endocardial ablation attempt, percutaneous epicardial mapping and ablation is performed. The transmurality of the myocardial infarction (scar reaching the epicardium), analyzed by means of echocardiogram or preferably magnetic resonance imaging, is taken into account before deciding on an epicardial approach.
NON-ISCHEMIC CARDIOMYOPATHY
Myocardial diseases are categorized as dilated cardiomyopathy, hypertrophic cardiomyopathy, restrictive cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy (ARVC), and unclassified [39]. The incidence and type of ventricular arrhythmias varies between pathologies, and these are not the only cause of sudden death in this context. In advanced stages of the disease, bradycardia, thromboembolism, and electromechanical dissociation are a frequent cause of death [40]. Sustained monomorphic VT is only part of the spectrum of ventricular arrhythmias in patients with cardiomyopathy [41]. The occurrence of clusters of VT episodes tend to be recurrent and impair survival in patients with idiopathic dilated cardiomyopathy, despite the use of antiarrhythmic drugs [42].
Most of the available knowledge about VT pathophysiology is based on ischemic models. Myocardial scar reentry causes the vast majority of VTs in post-infarction patients. The mechanism of ventricular arrhythmias in other cardiac diseases is less established. Experience of VT ablation in patients with non-ischemic cardiomyopathy (NICM) is less well known, but the outcomes after ablation seem poorer [43]. The arrhythmogenic mechanisms in non-ischemic heart disease are: bundle-branch reentry, focal activity, and scar-related reentry. As with ischemic heart disease, reentry is most frequently the mechanism involved [44, 45].
The success rate of endocardial ablation is lower in patients with NICM than in ischemic patients [1]. Reentry circuits deep in the endocardium and epicardium appear to be a likely explanation. Cardiac magnetic resonance imaging with delayed enhancement demonstrates that scars can be located intramurally and subepicardially in NICM [46, 47]. Epicardial mapping after a failed endocardial approach usually shows more extensive substrate at epicardium and improves the success rate [48]. Certain etiologies, such as Chagas disease or sarcoidosis, frequently show epicardial substrate [49, 50].
Soejima et al. published a series of 28 consecutive patients with dilated cardiomyopathy refereed for VT ablation. Only 6 (27%) were successfully ablated from the endocardium; ablation was not attempted in 2 patients (9%) and in 14 patients (64%) endocardial ablation failed. Epicardial mapping and ablation was performed in 7 patients and succeeded in 6 of them. Most low-voltage areas identified in the electroanatomic maps are close to the atrioventricular annulus, being larger at the epicardial surface. This predilection for the basal segments of the left ventricle was also found in another study and remains unexplained [44]. In a consecutive series of 22 consecutive patients with NICM and VTs with endo- and epicardial mapping, confluent low-voltage areas were present in 82% of patients. Moreover, epicardial scar areas were significantly greater than endocardial ones [51]. The predominant location of the scar in the subepicardium may explain the low success rate of endocardial catheter ablation in NICM.
Bazan et al. analyzed electrocardiogram features during endocardial and epicardial pace mapping in a cohort of patients with VT not related to myocardial infarction, most of them with dilated cardiomyopathy [52]. They proposed different morphological criteria depending on the site of origin of the VT determined by the polarity of the initial vector of depolarization (Table 1). The presence of a Q wave in inferior leads for basal inferior and apical inferior VTs reflects the initial epi to endo activation in the inferior wall and therefore suggests an epicardial origin. In the same way, a Q wave in lead I for basal superior and apical superior VTs and the absence of a Q wave in any of the inferior leads for basal superior VTs were associated with an epicardial origin [52]. The sensitivity and specificity of the previously reported criteria may differ depending on the myocardial wall of origin of the VT [12, 52]. More recently, the same group reported the data from 14 patients with NICM undergoing endo- and epicardial VT ablation. They focused the analysis on the anterior-basal and lateral-basal segments of the left ventricle (Josephson sites 8, 10, 12). Using pace mapping they tested the proposed interval criteria (pseudo-delta wave, intrinsicoid deflection time, shortest RS complex, and MDI) and morphology criteria (q wave in lead I and no q waves). The presence of q waves in inferior leads, pseudo-delta wave ≥75 ms, MDI ≥0.59, and q wave in lead I had 95% specificity and 20% sensitivity in identifying epicardial and endocardial origin for pace maps in the mentioned regions (see Fig. 4) [53]. A four-step algorithm based on these criteria was proposed and showed 93% sensitivity and 86% specificity for an epicardial origin in a validation cohort of 11 patients [53]. The main restriction of this algorithm is that it can be applied only to patients with NICM and myocardial scars in the anterolateral basal region of the LV. Although a predominance of perimitral zones has been observed, scars in the NICM may appear at any myocardial segment [54].
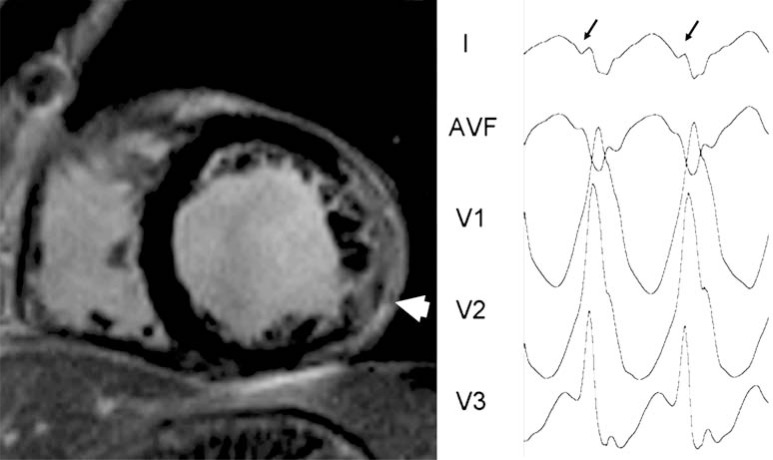
Fig. (4).
Epicardial VT in nonischemic cardiomyopathy. Left panel shows short axis view of contrast-enhanced cardiac magnetic resonance from a patient with non-ischemic cardiomyopathy. An extensive area of hyperenhancement is observed in the lateral wall of the left ventricle (white arrow). Right panel shows the electrocardiogram of the clinical ventricular tachycardia. Q waves (lead I, black arrows) represent the initial forces of the ventricular activation going from epicardium to endocardium.
ARRHYTHMOGENIC RIGHT VENTRICULAR CARDIOMYOPATHY
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a myocardial disease characterized by fibrofatty replacement and ventricular arrhythmias. This replacement involves predominantly the right ventricle, starting at the epicardium and extending towards the endocardium [55]. Left ventricular involvement is present in more than half of all cases and a left dominant phenotype have been described [56, 57]. Heart failure, ventricular arrhythmias, and sudden cardiac death (SCD) are the most severe clinical manifestations of ARVC. The diagnosis of ARVC can be arduous. Task Force criteria proposed for ARVC diagnosis includes electrocardiographic, structural (cardiac imaging and histology), clinical, and familial variables [58].
Radiofrequency ablation has been restricted to patients with incessant VTs or electrical storm refractory to antiarrhythmic drugs. Endocardial RF ablation has proven inadequate in both acute and long-term results [59-62]. Substrate-guided mapping techniques may improve these results [63]. The high recurrence rate published in earlier series has been attributed to the progressive nature of the disease. However, the epicardial nature of the disease also may have a role in the failure of the endocardial ablation approach.
Recent studies in substrate-guided ablation have shown that ARVC exhibits a predominantly epicardial arrhythmogenic substrate. Garcia et al. performed endocardial and epicardial mapping in 13 patients with ARVC after failed endocardial ablation [64]. The area of low voltage (<1 mV and<1.5 mV at epicardium and endocardium, respectively) were larger at epicardium in each patient. During a follow-up of 18±13 months, 77% of patients remained free of VT recurrence. Recently, our group reported the results of a combined endocardial and epicardial substrate-guided ablation (using the scar dechanneling technique) as a first-line approach for patients with ARVC and VT referred for VT ablation [37]. These patients also had a larger epicardial than endocardial arrhythmogenic substrate. Remarkably, 86% of the induced VTs required an epicardial ablation. The scar dechanneling technique aims to eliminate every slow conducting zone and minimize the extension of RF application, which is especially desirable at the epicardium [37, 38]. After a follow-up of 11 months (6-24 months) only one patient (9%) had a VT recurrence. Bai et al. compared two groups of patients (23 vs 26 patients) with ARVC who received endocardial or combined endo- and epicardial substrate-based mapping and ablation. After a 3-year follow-up, 52% of patients in the endocardial group and 85% of patients in the endo-epicardial group were free of VT recurrence [65].
Bazan et al. studied the usefulness of electrocardiogram to predict an epicardial origin in VT arising from the right ventricle (Table 1). Pacemapping was used in multiple endocardial and epicardial sites in a group of patients who underwent combined endo- and epicardial mapping. Two patients had coronary artery disease, 4 had dilated cardiomyopathy, 2 had ARVC and 5 had normal hearts. Interval criteria appeared not to be applicable with pace mapping in the right ventricle. The presence of Q wave in inferior leads was more frequent when pacing in inferior epicardial sites and the presence of Q waves in lead I and QS in V2 when pacing in anterior epicardial sites. No distinctive electrocardiogram feature for epicardial pacing at the RVOT was identified [66]. These criteria have not been validated with clinical VTs in patients with ARVC. The described interval criteria (pseudodelta wave, intrinsicoid deflection time, shortest RS complex) are probably inapplicable in this disease, primarily because most of the VTs in ARVC patients have LBBB morphology and these criteria have been validated for VTs with RBBB morphology.
Considering the data reported about the epicardial arrhythmogenic substrate and the results of endo- and epicardial ablation, in our center a combined endocardial and epicardial ablation is the first-line approach in all ARVC patients referred for ablation.
WHEN TO GO TO THE EPICARDIUM? DATA BEYOND THE ELECTROCARDIOGRAM.
In addition to electrocardiographic criteria detailed here, other clinical parameters from cardiac imaging may be useful.
As we have emphasized, the presence of epicardial substrate varies greatly depending on the underlying heart disease. A first-line percutaneous pericardial approach may be reasonable in entities with proven extensive epicardial substrate. This is the case of ARVC, Chagas disease, myocarditis or NICM. In the latter case, it would be preferable to select cases for epicardial approach using additional criteria because some patients may have only endocardial or midmyocardial substrate [67].
On the other hand, it is generally established that a failed endocardial ablation procedure is sufficient reason to attempt an epicardial ablation. In our experience, epicardial ablation in patients with incessant VT and prior failed endocardial ablation offers good results in terms of acute success and follow-up [8].
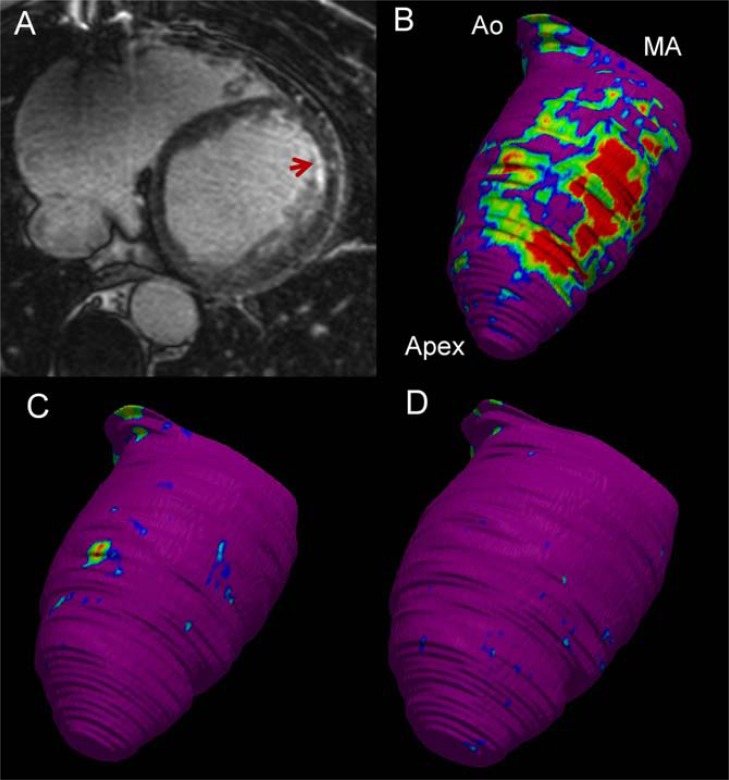
Most VTs in patients with structural heart disease are scar-related myocardial reentries. Identification of scar tissue by contrast-enhanced cardiac magnetic resonance matches the histology of the myocardial infarction, is related to VT inducibility and spontaneous VT occurrence, has prognostic value for long-term total mortality, and is correlated with 3D voltage maps [68-73]. Cardiac magnetic resonance allows characterization of the scar in core and border zone and can identify VT isthmuses as border zone corridors [70, 74-76]. Scar identification and characterization could help to restrict mapping to potentially arrhythmogenic areas, estimate scar extent, and examine the endocardial/epicardial involvement of the ventricular wall. In a series of 29 patients with NICM, Bogun et al. showed that the distribution of the scar at the myocardial wall can be useful in the preparation of the ablation strategy. Five patients showed endocardial scar and ablation was effective from endocardium in all of them. Two patients had both endo- and epicardial scar, and both ablations were only partially effective. Finally, two patients had scars confined to epicardium and epicardial ablation was successful in both [77]. In selecting an epicardial approach for reentry VTs (the vast majority of non-idiopathic VTs), the principle of "no scar no VT" should be kept in mind: it is very unlikely that a VT has an epicardial origin in a patient with evidence of an exclusively subendocardial scar, and vice versa (Fig. 5).
Fig. (5).
Contrast-enhanced cardiac magnetic resonance (CeCMR) from a patient with subendocardial anterior myocardial infarction and ventricular tachycardia (12-lead electrocardiogram is shown in the right panel of Fig. (3)). Panel A shows axial-axis view of CeCMR showing subendocardial hyperenhancement in the anterior wall of the left ventricle. Panels B to D show signal intensity maps obtained from CeCMR projected over 3D color-coded shells at subendocardium (panel B), midmyocardium (panel C) and subepicardium (panel D). Normal myocardium is coded in purple, core of the infarct in red and border zone in blue-green-yellow. Identification by CeCMR of scar confined to subendocardium indicates that an endocardial approach will be adequate to abolish the arrhythmia. Apex left ventricular apex; Ao, Aortic root; MA, mitral annulus.
In the case of patients with coronary artery disease (CAD), the evidence of transmural infarction can lead us to suspect the presence of epicardial circuits. However, it is well known that most infarct-related VTs can be successfully ablated from the endocardium. The presence of complex isthmus with endo- and epicardial paths and wall thinning in the infarct area may allow appropriate ablation results from the endocardium in transmural infarcts. Therefore, we think it is reasonable to be more conservative in the indication of epicardial mapping in patients with CAD.
In patients with a normal heart, electrocardiogram criteria appear to fail in the differentiation of VTs arising from the aortic root and the epicardial surface [23]. Moreover, epicardial transvenous ablation can eliminate most of the epicardial foci remote from the aortic cusps [13]. Finally, epicardial ablation from the pericardial sac can be limited in idiopathic VTs because the atrioventricular and interventricular grooves (the most frequent origins) are often covered with abundant fat. Therefore, we perform pericardial access for idiopathic VT ablation in selected patients only after mapping the RVOT, endocardial LVOT, sinuses of Valsalva, and coronary venous system and ablation failure.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Aliot EM, Stevenson WG, Almendral-Garrote JM , et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA). a Registered Branch of the European Society of Cardiology (ESC) and the Heart Rhythm Society HRS) in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm . 2009; 6(6):886–933. doi: 10.1016/j.hrthm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 2.Wissner E, Stevenson WG, Kuck KH. Catheter ablation of ventricular tachycardia in ischaemic and non-ischaemic cardiomyopathy: where are we today?. A clinical review. Eur Heart J. 2012;33(12):1440–50. doi: 10.1093/eurheartj/ehs007. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard SM, Walcott GP, Wharton JM , et al. Why is catheter ablation less successful than surgery for treating ventricular tachycardia that results from coronary artery disease?. PACE. 1994;17(12 Pt 1):2315–35. doi: 10.1111/j.1540-8159.1994.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaltenbrunner W, Cardinal R, Dubuc M , et al. Epicardial and endocardial mapping of ventricular tachycardia in patients with myocardial infarction.Is the origin of the tachycardia always subendocardially localized?. Circulation. 1991;84(3):1058–71. doi: 10.1161/01.cir.84.3.1058. [DOI] [PubMed] [Google Scholar]
- 5.Sosa E, Scanavacca M, D'Avila A , et al. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7(6):531–6. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 6.Guiraudon G, Fontaine G, Frank R , et al. Encircling Endocardial Ventriculotomy: A New Surgical Treatment for Life-Threatening Ventricular Tachycardias Resistant to Medical Treatment Following Myocardial Infarction. Ann Thorac Surg. 1978;26(5):438–44. doi: 10.1016/s0003-4975(10)62923-2. [DOI] [PubMed] [Google Scholar]
- 7.Sosa E, Scanavacca M, d'Avila A, Oliveira F, Ramires JAF. Nonsurgical transthoracic epicardial catheter ablation to treat recurrent ventricular tachycardia occurring late after myocardial infarction. J Am Coll Cardiol. 2000;35(6):1442–9. doi: 10.1016/s0735-1097(00)00606-9. [DOI] [PubMed] [Google Scholar]
- 8.Brugada J, Berruezo A, Cuesta Aetal. Nonsurgical transthoracic epicardial radiofrequency ablation An alternative in incessant ventricular tachycardia. J Am Coll Cardiol. 2003;41(11):2036–43. doi: 10.1016/s0735-1097(03)00398-x. [DOI] [PubMed] [Google Scholar]
- 9.Sacher F, Roberts-Thomson K, Maury P , et al. Epicardial ventricular tachycardia ablation a multicenter safety study. J Am Coll Cardiol. 2010;55(21):2366–72. doi: 10.1016/j.jacc.2009.10.084. [DOI] [PubMed] [Google Scholar]
- 10.Della Bella P, Brugada J, Zeppenfeld K , et al. Epicardial ablation for ventricular tachycardia a European multicenter study. Circ Arrhythm Electrophysiol. 2011;4(5):653–9. doi: 10.1161/CIRCEP.111.962217. [DOI] [PubMed] [Google Scholar]
- 11.Berruezo A. Complex ventricular arrhythmias a therapeutic nightmare. Heart. 2010;96(9):723–8. doi: 10.1136/hrt.2008.163337. [DOI] [PubMed] [Google Scholar]
- 12.Berruezo A, Mont L, Nava Setal. Electrocardiographic recognition of the epicardial origin of ventricular tachycardias. Circulation. 2004;109(15):1842–7. doi: 10.1161/01.CIR.0000125525.04081.4B. [DOI] [PubMed] [Google Scholar]
- 13.Baman TS, Ilg KJ, Gupta SKetal. Mapping and ablation of epicardial idiopathic ventricular arrhythmias from within the coronary venous system. Circ Arrhythm Electrophysiol. 2010;3(3):274–9. doi: 10.1161/CIRCEP.109.910802. [DOI] [PubMed] [Google Scholar]
- 14.Callans DJ, Menz MDV, Schwartzman DS , et al. Repetitive Monomorphic Tachycardia From the Left Ventricular Outflow Tract Electrocardiographic Patterns Consistent With a Left Ventricular Site of Origin. J Am Coll Cardiol. 1997;29(5):1023–7. doi: 10.1016/s0735-1097(97)00004-1. [DOI] [PubMed] [Google Scholar]
- 15.Hasdemir CAN, Ulucan CEM, Yavuzgil O , et al. Tachycardia-induced cardiomyopathy in patients with idiopathic ventricular arrhythmias: the incidence. clinical and electrophysiologic characterisics.and the predictors. . J Cardiovasc Electrophysiol. 2011;22(6):663–8. doi: 10.1111/j.1540-8167.2010.01986.x. [DOI] [PubMed] [Google Scholar]
- 16.Yamada T, McElderry HT, Doppalapudi H , et al. Idiopathic ventricular arrhythmias originating from the aortic root prevalence. electrocardiographic and electrophysiologic characterisics.and results of radiofrequency catheter ablation. . J Am Coll Cardiol . 2008; 52(2):139–47. doi: 10.1016/j.jacc.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang F, Fotuhi P, Ho SY , et al. repetitive monomorphic ventricular tachycardia originating from the aortic sinus cusp electrocardiographic characterization for guiding catheter ablation. J Am Coll Cardiol. 2002;39(2):500–8. doi: 10.1016/s0735-1097(01)01767-3. [DOI] [PubMed] [Google Scholar]
- 18.Lin D, Ilkhanoff L, Gerstenfeld E , et al. Twelve-lead electrocardiographic characteristics of the aortic cusp region guided by intracardiac echocardiography and electroanatomic mapping. Heart Rhythm. 2008;5(5):663–9. doi: 10.1016/j.hrthm.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Yamada T, Yoshida N, Murakami Y , et al. Electrocardiographic characteristics of ventricular arrhythmias originating from the junction of the left and right coronary sinuses of Valsalva in the aorta: the activation pattern as a rationale for the electrocardiographic characteristics. Heart Rhythm. 2008;5(2):184–92. doi: 10.1016/j.hrthm.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 20.Schweikert RA, Saliba WI, Tomassoni G , et al. Percutaneous pericardial instrumentation for endo-epicardial mapping of previously failed ablations. Circulation. 2003;108(11):1329–35. doi: 10.1161/01.CIR.0000087407.53326.31. [DOI] [PubMed] [Google Scholar]
- 21.Obel OA, d'Avila A, Neuzil P , et al. Ablation of left ventricular epicardial outflow tract tachycardia from the distal great cardiac vein. J Am Coll Cardiol. 2006;48(9):1813–7. doi: 10.1016/j.jacc.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Daniels DV, Lu YY, Morton JB , et al. Idiopathic epicardial left ventricular tachycardia originating remote from the sinus of Valsalva: electrophysiological characteristics. catheter ablaion.and identification from the 12-lead electrocardiogram. Circulation . 2006; 113(13):1659–66. doi: 10.1161/CIRCULATIONAHA.105.611640. [DOI] [PubMed] [Google Scholar]
- 23.Yamada T, McElderry HT, Okada T , et al. Idiopathic left ventricular arrhythmias originating adjacent to the left aortic sinus of valsalva: electrophysiological rationale for the surface electrocardiogram. J Cardiovasc Electrophysiol. 2010;21(2):170–6. doi: 10.1111/j.1540-8167.2009.01608.x. [DOI] [PubMed] [Google Scholar]
- 24.Yamada T, Yoshida Y, Inden Y , et al. Idiopathic premature ventricular contractions exhibiting preferential conduction within the aortic root. PACE. 2010;33(1):e10–3. doi: 10.1111/j.1540-8159.2009.02571.x. [DOI] [PubMed] [Google Scholar]
- 25.Doppalapudi H, Yamada T, Ramaswamy K , et al. Idiopathic focal epicardial ventricular tachycardia originating from the crux of the heart. Heart Rhythm. 2009;6(1):44–50. doi: 10.1016/j.hrthm.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 26.Andreu D, Berruezo A, Fernandez-Armenta J , et al. Displacement of the target ablation site and ventricles during premature ventricular contractions: Relevance for radiofrequency catheter ablation. Heart Rhythm. 2012;9(7):1050–7. doi: 10.1016/j.hrthm.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 27.Herczku C, Berruezo A, Andreu D , et al. Mapping data predictors of a left ventricular outflow tract origin of idiopathic ventricular tachycardia with V3 transition and septal earliest activation. Circ Arrhythm Electrophysiol. 2012;5(3):484–91. doi: 10.1161/CIRCEP.111.969592. [DOI] [PubMed] [Google Scholar]
- 28.Pagé PL, Cardinal R, Shenasa M , et al. Surgical treatment of ventricular tachycardia.Regional cryoablation guided by computerized epicardial and endocardial mapping. Circulation. 1989;80(3 Pt 1):I124–34. [PubMed] [Google Scholar]
- 29.de Bakker JM, van Capelle FJ, Janse MJ , et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988;77(3):589–606. doi: 10.1161/01.cir.77.3.589. [DOI] [PubMed] [Google Scholar]
- 30.de Bakker JM, Coronel R, Tasseron S , et al. Ventricular tachycardia in the infarcted. Langendorff-perfused human heart: role of the arrangement of surviving cardiac fibers. J Am Coll Cardiol. 1990;15(7):1594–607. doi: 10.1016/0735-1097(90)92832-m. [DOI] [PubMed] [Google Scholar]
- 31.Lacroix D, Klug D, Grandmougin D , et al. Ventricular tachycardia originating from the posteroseptal process of the left ventricle with inferior wall healed myocardial infarction. Am J Cardiol. 1999;84(2):181–6. doi: 10.1016/s0002-9149(99)00231-3. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt B, Chun KR, Baensch D , et al. Catheter ablation for ventricular tachycardia after failed endocardial ablation: epicardial substrate or inappropriate endocardial ablation?. Heart Rhythm. 2010;7(12):1746–52. doi: 10.1016/j.hrthm.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Di Biase L, Santangeli P, Burkhardt DJ , et al. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2012;60(2):132–41. doi: 10.1016/j.jacc.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 34.Page SP, Duncan ER, Thomas G , et al. Epicardial catheter ablation for ventricular tachycardia in heparinized patients. Europace. 2013;15(2):284–9. doi: 10.1093/europace/eus258. [DOI] [PubMed] [Google Scholar]
- 35.Martinek M, Stevenson WG, Inada K , et al. QRS characteristics fail to reliably identify ventricular tachycardias that require epicardial ablation in ischemic heart disease. J Cardiovasc Electrophysiol. 2012;23(2):188–93. doi: 10.1111/j.1540-8167.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 36.Pilcher TA, Sanford AL, Saul JP , et al. convective cooling effect on cooled-tip catheter compared to large-tip catheter radiofrequency ablation. Pacing Clin Electrophysiol. 2006;29(12):1368–74. doi: 10.1111/j.1540-8159.2006.00549.x. [DOI] [PubMed] [Google Scholar]
- 37.Berruezo A, Fernández-Armenta J, Mont L , et al. Combined endocardial and epicardial catheter ablation in arrhythmogenic right ventricular dysplasia incorporating scar dechanneling technique. Circ Arrhythm Electrophysiol. 2012;5(1):111–21. doi: 10.1161/CIRCEP.110.960740. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-Armenta J, Berruezo A, Ortiz-Perez JT , et al. Improving safety of epicardial ventricular tachycardia ablation using the scar dechanneling technique and the integration of anatomy. scar components and coronary arteries into the navigation system. Circulation. 2012;125(11):e466–8. doi: 10.1161/CIRCULATIONAHA.111.087858. [DOI] [PubMed] [Google Scholar]
- 39.Elliott P, Andersson B, Arbustini E , et al. Classification of the cardiomyopathies: a position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2008;29(2):270–6. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 40.Luu M, Stevenson WG, Stevenson LW , et al. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation. 1989;80(6):1675–80. doi: 10.1161/01.cir.80.6.1675. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson WG, Stevenson LW, Middlekauff HR , et al. Sudden death prevention in patients with advanced ventricular dysfunction. Circulation. 1993;88(6):2953–61. doi: 10.1161/01.cir.88.6.2953. [DOI] [PubMed] [Google Scholar]
- 42.Bänsch D, Böcker D, Brunn J , et al. Clusters of ventricular tachycardias signify impaired survival in patients with idiopathic dilated cardiomyopathy and implantable cardioverter defibrillators. J Am Coll Cardiol. 2000;36(2):566–73. doi: 10.1016/s0735-1097(00)00726-9. [DOI] [PubMed] [Google Scholar]
- 43.Kottkamp H, Hindricks G, Chen X , et al. Radiofrequency catheter ablation of sustained ventricular tachycardia in idiopathic dilated cardiomyopathy. Circulation. 1995;92(5):1159–68. doi: 10.1161/01.cir.92.5.1159. [DOI] [PubMed] [Google Scholar]
- 44.Hsia HH, Callans DJ, Marchlinski FE. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation. 2003;108(6):704–10. doi: 10.1161/01.CIR.0000083725.72693.EA. [DOI] [PubMed] [Google Scholar]
- 45.Delacretaz E, Stevenson W, Ellison K , et al. Mapping and radiofrequency catheter ablation of the three types of sustained monomorphic ventricular tachycardia in nonischemic heart disease. J Cardiovasc Electrophysiol. 2000;11(1):11–7. doi: 10.1111/j.1540-8167.2000.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 46.Mahrholdt H, Wagner A, Judd RM , et al. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26(15):1461–74. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 47.Nazarian S, Bluemke DA, Lardo AC , et al. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112(18):2821–5. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soejima K, Stevenson WG, Sapp JL , et al. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: The importance of low-voltage scars. J Am Coll Cardiol. 2004;43(10):1834–42. doi: 10.1016/j.jacc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 49.Sosa E, Scanavacca M, D'Avila A , et al. Endocardial and epicardial ablation guided by nonsurgical transthoracic epicardial mapping to treat recurrent ventricular tachycardia. J Cardiovasc Electrophysiol. 1998;9(3):229–39. doi: 10.1111/j.1540-8167.1998.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 50.Hsia HH, Marchlinski FE. Electrophysiology studies in patients with dilated cardiomyopathies. Cardiac Electrophysiol Rev. 2002;6(4):472–81. doi: 10.1023/a:1021109130276. [DOI] [PubMed] [Google Scholar]
- 51.Cano O, Hutchinson M, Lin D , et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009;54(9):799–808. doi: 10.1016/j.jacc.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 52.Bazan V, Gerstenfeld EP, Garcia FC , et al. Site-specific twelve-lead ECG features to identify an epicardial origin for left ventricular tachycardia in the absence of myocardial infarction. Heart Rhythm. 2007;4(11):1403–10. doi: 10.1016/j.hrthm.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Valles E, Bazan V, Marchlinski FE. ECG Criteria to Identify Epicardial Ventricular Tachycardia in Nonischemic Cardiomyopathy. Circ Arrhythm Electrophysiol. 2010;3(1):63–71. doi: 10.1161/CIRCEP.109.859942. [DOI] [PubMed] [Google Scholar]
- 54.McCrohon JA, Moon JC, Prasad SK , et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108(1):54–9. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 55.Basso C, Corrado D, Marcus FI , et al. Arrhythmogenic right ventricular cardiomyopathy. The Lancet. 2009;373(9671):1289–300. doi: 10.1016/S0140-6736(09)60256-7. [DOI] [PubMed] [Google Scholar]
- 56.Thiene G, Basso C. Arrhythmogenic right ventricular cardiomyopathy: An update. Cardiovascular pathology. 2001;10(3):109–17. doi: 10.1016/s1054-8807(01)00067-9. [DOI] [PubMed] [Google Scholar]
- 57.Sen-Chowdhry S, Syrris P, Prasad SK , et al. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol. 2008;52(25):2175–87. doi: 10.1016/j.jacc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 58.Marcus FI, McKenna WJ, Sherrill D , et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121(13):1533–41. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arbelo E, Josephson ME. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol. 2010;21(4):473–86. doi: 10.1111/j.1540-8167.2009.01694.x. [DOI] [PubMed] [Google Scholar]
- 60.Arruda M, Armaganijan L, Fahmy T , et al. Catheter ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia. J Interv Card Electrophysiol. 2009;25(2):129–33. doi: 10.1007/s10840-008-9317-z. [DOI] [PubMed] [Google Scholar]
- 61.Dalal D, Jain R, Tandri H , et al. Long-term efficacy of catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2007;50(5):432–40. doi: 10.1016/j.jacc.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 62.Miljoen H, State S, de Chillou C , et al. Electroanatomic mapping characteristics of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Europace. 2005;7(6):516–24. doi: 10.1016/j.eupc.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Verma A, Kilicaslan F, Schweikert RA , et al. Short- and long-term success of substrate-based mapping and ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia. Circulation. 2005;111(24):3209–16. doi: 10.1161/CIRCULATIONAHA.104.510503. [DOI] [PubMed] [Google Scholar]
- 64.Garcia FC, Bazan V, Zado ES , et al. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009;120(5):366–75. doi: 10.1161/CIRCULATIONAHA.108.834903. [DOI] [PubMed] [Google Scholar]
- 65.Bai R, Di Biase L , et al. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy: arrhythmia-free survival after endo-epicardial substrate based mapping and ablation. Circ Arrhythm Electrophysiol. 2011;4(4):478–85. doi: 10.1161/CIRCEP.111.963066. [DOI] [PubMed] [Google Scholar]
- 66.Bazan V, Bala R, Garcia FC , et al. Twelve-lead ECG features to identify ventricular tachycardia arising from the epicardial right ventricle. Heart Rhythm. 2006;3(10):1132–9. doi: 10.1016/j.hrthm.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 67.Haqqani HM, Tschabrunn CM, Tzou WS , et al. Isolated septal substrate for ventricular tachycardia in nonischemic dilated cardiomyopathy: Incidence characterization. and implications. . Heart Rhythm. 2011;8(8):1169–76. doi: 10.1016/j.hrthm.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 68.Roes SD, Borleffs CJ, van der Geest RJ , et al. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging. 2009;2(3):183–90. doi: 10.1161/CIRCIMAGING.108.826529. [DOI] [PubMed] [Google Scholar]
- 69.Amado LC, Gerber BL, Gupta SN , et al. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol. 2004;44(12):2383–9. doi: 10.1016/j.jacc.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 70.Ashikaga H, Sasano T, Dong J , et al. Magnetic resonance-based anatomical analysis of scar-related ventricular tachycardia: implications for catheter ablation. Circ Res. 2007;101(9):939–47. doi: 10.1161/CIRCRESAHA.107.158980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Desjardins B, Crawford T, Good E , et al. Infarct architecture and characteristics on delayed enhanced magnetic resonance imaging and electroanatomic mapping in patients with postinfarction ventricular arrhythmia. Heart Rhythm. 2009;6(5):644–51. doi: 10.1016/j.hrthm.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wijnmaalen AP, van der Geest RJ, van Huls van Taxis CF , et al. Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: real-time image integration and reversed registration. Eur Heart J. 2011;32(1):104–14. doi: 10.1093/eurheartj/ehq345. [DOI] [PubMed] [Google Scholar]
- 73.Fernández-Armenta J, Berruezo A, Mont L , et al. Use of myocardial scar characterization to predict ventricular arrhythmia in cardiac resynchronization therapy. Europace. 2012;14(11):1578–86. doi: 10.1093/europace/eus104. [DOI] [PubMed] [Google Scholar]
- 74.Estner HL, Zviman MM, Herzka D , et al. The critical isthmus sites of ischemic ventricular tachycardia are in zones of tissue heterogeneity. visualized by magnetic resonance imaging. Heart Rhythm. 2011;8(12):1942–9. doi: 10.1016/j.hrthm.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 75.Perez-David E, Arenal A, Rubio-Guivernau JL , et al. Noninvasive identification of ventricular tachycardia-related conducting channels using contrast-enhanced magnetic resonance imaging in patients with chronic myocardial infarction: comparison of signal intensity scar mapping and endocardial voltage mapping. J Am Coll Cardiol. 2011;57(2):184–94. doi: 10.1016/j.jacc.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 76.Andreu D, Berruezo A, Ortiz-Perez JT , et al. Integration of 3D electroanatomic maps and magnetic resonance scar characterization into the navigation system to guide ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2011;4(5):674–83. doi: 10.1161/CIRCEP.111.961946. [DOI] [PubMed] [Google Scholar]
- 77.Bogun FM, Desjardins B, Good E , et al. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy: utility for identifying the ventricular arrhythmia substrate. J Am Coll Cardiol. 2009;53(13):1138–45. doi: 10.1016/j.jacc.2008.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]