Abstract
The differential diagnosis of a regular, monomorphic wide QRS complex tachycardia (WCT) mechanism represents a great diagnostic dilemma commonly encountered by the practicing physician, which has important implications for acute arrhythmia management, further work-up, prognosis and chronic management as well. This comprehensive review discusses the causes and differential diagnosis of WCT, and since the ECG remains the cornerstone of WCT differential diagnosis, focuses on the application and diagnostic value of different ECG criteria and algorithms in this setting and also provides a practical clinical approach to patients with WCTs.
Keywords: Supraventricular tachycardia, ventricular tachycardia, wide QRS complex tachycardia.
INTRODUCTION
A relatively common frustrating and anxiety-provoking situation in medical practice is the confrontation of a physician with a wide QRS complex tachycardia (WCT) ECG tracing. The elucidation of the mechanism of WCT is vital not only for acute arrhythmia management, but also for the further work-up, prognosis and chronic management. Despite the published numerous ECG algorithms and criteria, the accurate, rapid diagnosis in patients with WCT remains a significant clinical problem, because many of these ECG criteria are complicated, not applicable in a large proportion of cases and difficult to recall in an urgent setting. This review will focus on sustained, regular, monomorphic WCTs. A WCT is defined as a rhythm with a rate >100/min with a QRS duration >120 ms.
CAUSES OF WCT
The most common cause of WCT is ventricular tachycardia (VT), which accounts for up to 80% of cases [1-5] (see Table 1). Supraventricular tachycardia (SVT) with abnormal intraventricular conduction is the second commonest cause of WCTs, accounting for 15-25% of WCT cases. The majority of SVTs with abnormal intraventricular conduction is due to SVT with aberrant conduction (SVT-A), which means that the widening of the QRS complex results from conduction delay or block or both along the bundle branches or fascicles. SVT-A may be manifested as pre-existent bundle branch block (BBB), when the BBB is fixed (i.e. present at all heart rates) or as SVT with functional BBB, when the BBB is cycle length- or rate-dependent (usually tachycardia-dependent) and accounts for 15-20% of WCT cases. Another cause of abnormal intraventricular conduction might be when the site of conduction delay is not in the His-Purkinje system, butintramyocardial, due to slowed muscle-to-muscle conduction, which might occur in ventricular hypertrophy and dilation, cardiomyopathy and congenital heart disease [1, 2, 6, 7]. Preexcited SVT (SVT with anterograde conduction over an accessory pathway) is another cause of SVT with abnormal intraventricular conduction and may be an antidromic AV reentrant tachycardia (AVRT) with anterograde conduction over a typical or atypical (such as atriofascicular, nodoventricular, nodofascicular) bypass tract, which is participating in the reentrant circuit or may be due to AV nodal reentrant tachycardia (AVNRT), atrial tachycardia or atrial flutter with anterograde conduction over an accessory pathway functioning as a bystander. Ventricular paced rhythm should also be considered in the WCT differential diagnosis, because contemporary pacing systems are commonly associated with small and almost imperceptible stimulus artifact on the ECG [1, 2]. Because the vast majority (95%) of WCTs are either VT or SVT-A, the main differential diagnosis for WCT is to distinguish VT from SVT-A. The remaining underlying causes, such as preexcited SVTs, drug- (class IA, IC antiarrhythmic drugs and amiodarone) or electrolyte disorder (hyperkalemia)-induced WCTs, ventricular paced rhythm altogether account for only 1-5% of WCTs [1, 2, 7].
Table 1.
Causes of regular, monomorphic WCT.
 |
WCT= wide QRS complex tachycardia, BBB=bundle branch block, AVRT=atrioventricular reentrant tachycardia, AVNRT=atrioventricular nodal reentrant tachycardia.
PATIENT HISTORY AND PHYSICAL EXAMINATION IN WCT DIFFERENTIAL DIAGNOSIS
Patient History
A history of structural heart disease-prior myocardial infarction, angina or congestive heart failure-carry a positive predictive value (PPV) for VT of >95% [1-4, 8]. However, about 10% of patients with VT (idiopathic VT) have no structural heart disease [9, 10]. Patients with SVT may or may not have structural heart disease. Patients with VT tend to be older than those with SVT. Patients >35 years of age carry a PPV of 85% and a sensitivity of 92% for VT diagnosis. Patients less than 35 years of age have a PPV of 70% for SVT diagnosis [1, 2, 8]. If the tachycardia recurred over a period of more than 3 years SVT is more likely, conversely the first occurrence of the tachycardia after a myocardial infarction strongly suggests VT [2, 3, 11, 12]. Although VT is more likely than SVT to cause hypotension and hemodynamic instability, the hemodynamic stability of a patient does not distinguish VT from SVT, because a significant proportion of patients with VT and most patients with SVT are hemodynamically stable [1-4, 8, 13].
Several medications also might result in WCT. Class I agents (sodium channel blocking agents) or amiodarone, which also has class I antiarrhythmic activity can cause nonspecific widening of the QRS complex. Class IC drugs (flecainide, propafenone), -by causing use-dependent sodium channel block-, may provoke sustained, monomorphic, incessant VT at fast heart rate during exercise even in young, healthy persons without structural heart disease or history of ventricular arrhythmia. Class IC drugs may also cause WCT by slowing atrial rate during atrial flutter until 1:1 AV conduction occurs with a high ventricular rate and wide QRS complex (partly may be due to drug effect, partly to aberrant conduction). Digoxin may cause monomorphic VT as well [1, 2 14, 15].
Detection of AV Dissociation
Physical findings that indicate the presence of AV dissociation suggest VT with a very high likelihood. These findings include variable intensity of the first heart sound, variation in systolic arterial blood pressure unrelated to respiration and the presence of “cannon” A waves that may be observed on examination of the jugular pulsation on the neck reflecting occasional simultaneous atrial and ventricular contraction during AV dissociation. “Cannon” A waves should be distinguished from frog sign, which occurs not only occasionally but during every beat due to simultaneous atrial and ventricular contraction that is usually seen in AVNRT [1-4, 8, 13]. Interestingly the frog sign is usually visible only in typical slow-fast AVNRT, where the RP interval is the shortest, and not in VT with retrograde 1:1 VA conduction or orthodromic AVRT, where the RP interval is somewhat longer [4].
Valsalva maneuver, carotid sinus massage or adenosine administration may facilitate the elucidation of WCT mechanism. The termination of tachycardia strongly suggests SVT (AVNRT or AVRT will either terminate or remain unaffected). However, VT due to triggered mechanism such as idiopathic outflow tract VT may be terminated as well with these maneuvers. Even if the arrhythmia itself remains unaffected, these maneuvers may clarify the mechanism of WCT by exposing VA dissociation in the case of VT or by slowing down the sinus or atrial rate directly during sinus or automatic atrial tachycardia or the ventricular rate during atrial tachycardia and flutter, because of increased AV nodal blocking effect [2, 3].
There are several electrocardiographic and echocardiographic methods that may facilitate the detection of AV dissociation. The use of Lewis leads may improve the detection of P waves on the ECG. Lewis lead is a special bipolar chest lead with the right arm electrode applied to the right side of the sternum at the 2nd intercostal space and the left arm electrode applied to the right 4th intercostal space adjacent to the sternum. The recording of the tracing can be seen in lead I. Calibration should be adjusted to 1 mV=20 mm (see Fig. 1) [4, 16]. A new electrocardiographic method of AF toolbox dveloped to improve visualization of atrial activity by suppression of electrical activity by the ventricles (QRST suppression) during atrial fibrillation and atrial flutter by providing a separate view of ventricular and atrial activity might also be used to detect AV dissociation in the future [4, 17]. Echocardiographic verification of AV dissociation is also possible by 2D guided M-mode or color tissue doppler M-mode recordings in the parasternal long axis view, in which one can simultaneously visualize the right ventricular and left atrial contraction [4].
Fig. (1).
Recognition of AV dissociation using the Lewis leads. The upper panel shows a WCT due to VT in a patient with recurrent monomorphic VTs. Although the suspicion of AV dissociation might emerge looking at the standard 12 lead ECG, the P waves cannot be discerned with certainty. The lower panel shows a somewhat later recorded rhythm strip with the Lewis lead, which should be interpreted in lead I, while the patient was on amiodarone treatment. The vertical black arrows indicate dissociated P waves (courtesy of András Simon MD). For further explanation see text.
Electrocardiographic Criteria
Fundamentally all ECG criteria or algorithms developed for the differential diagnosis of WCT are based on a few simple principles.
If the morphology of the WCT QRS complex is compatible with any combination of typical BBB or fascicular block, the WCT is caused by SVT-A. If there is no combination of BBB or fascicular blocks that could result in the particular QRS morphology, the WCT is caused by VT or preexcited SVT [1, 2] (all morphological criteria).
Most VTs (myocardial VTs) are associated with slow initial ventricular activation close to the site of origin due to slow muscle-to-muscle conduction, which results in a more significantly prolonged QRS duration or time to the intrinsicoid deflection, when the mechanism of WCT is VT vs. SVT (classic QRS duration and Kindwall criteria, 2nd Brugada RS>100 ms criterion, lead II R-wave peak time criterion).
The relative fastness of initial and terminal ventricular activation is different during SVT-A and VT. During SVT-A the initial activation is always fast, because it occurs via the normal His-Purkinje system, and the conduction delay causing the wide QRS occurs in the mid to terminal part of the QRS. During VT the initial ventricular activation is usually slower due to initial muscle-to-muscle conduction than the later or terminal ventricular activation [18, 19] (vi/vt criterion).
During SVT-A both the initial rapid septal activation (which can be either left-to-right or right-to-left) and the later main ventricular activation wavefront proceed in a direction away from lead aVR yielding a negative or predominantly negative QRS complex in lead aVR, therefore an initial R or Rs wave cannot be present in lead aVR or a northwest QRS axis (between ±180 degrees and -90 degrees) cannot be present during SVT-A. Another consequence of this direction of impulse propagation is that it should give rise an R wave at least in one or several precordial leads during SVT, therefore the absence of RS complex in the precordial leads strongly suggests VT (presence of initial R or Rs wave in lead aVR criterion, classic northwest axis criterion, 1st Brugada criterion: absence of RS complex).
The direction of initial septal activation and that of the main ventricular activation wavefront during sinus rhythm or SVT is different and give rise both predominantly positive and negative QRS complexes in different precordial leads, therefore concordance of the QRS complexes in the precordial leads strongly suggests VT.
The presence of AV dissociation or VA block suggests VT with a close to 100% specificity.
Traditional Criteria and the Brugada Algorithm
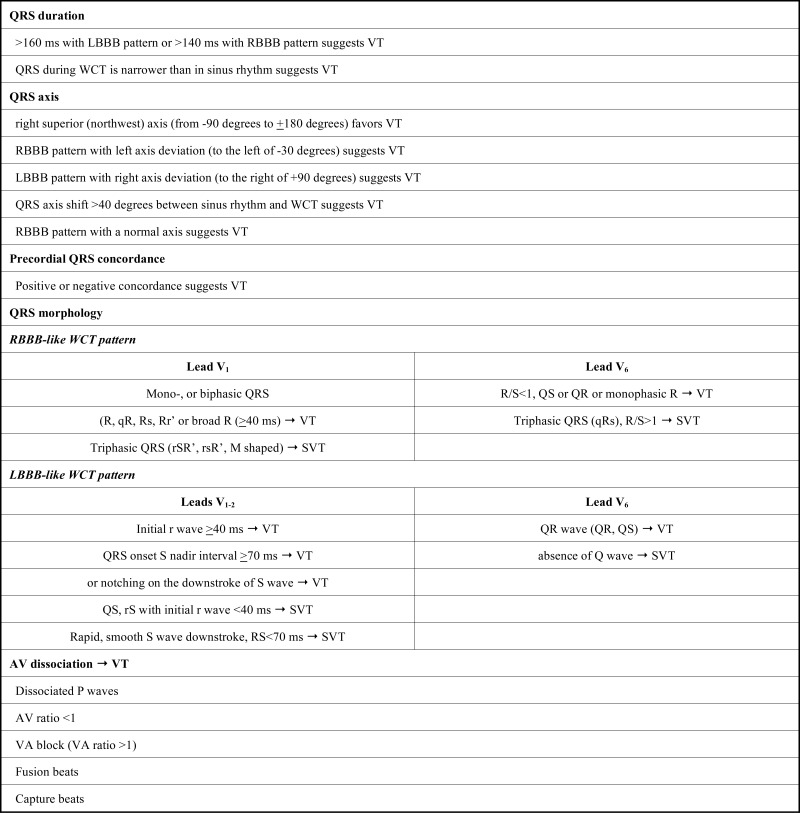
The most important contributions to the development of the traditional criteria were made by Sandler, Swanick, Marriott in 1965, 1966 and 1972 [20-22], Wellens et al. in 1978 [23], Coumel et al. in 1984 [24] and Kindwall et al. in 1988 [25]. The traditional criteria are essentially based on principles 1), 2), 4), 5), 6). Table 2 demonstrates the traditional criteria. For application of the traditional criteria one should determine that the WCT has a right bundle branch block (RBBB)-like or a left bundle branch block (LBBB)-like pattern. When in leads V1 and V2 the QRS polarity is predominantly positive the WCT has a RBBB-like, when the QRS polarity is predominantly negative, the WCT has a LBBB-like pattern.
Table 2.
Traditional ECG criteria for the differentiation of WCTs.
 |
QRS Duration
A QRS duration >160 ms might occur in SVT with pre-existent BBB or in patients taking drugs that capable of slowing intraventricular conduction (class IA and IC drugs, amiodarone). Of note, the QRS duration may be ≤120 ms in 2-5% of VTs (e.g. verapamil sensitive fascicular VT, VT that originates in the interventricular septum close to the His-Purkinje system) [2, 8, 26, 27].
A QRS complex that is narrower during WCT than during sinus rhythm suggests VT, but this is rare occurring in less than 1% of VTs [2].
Precordial QRS Concordance
Although positive concordance (QRS complexes are positive in all precordial leads) strongly suggests VT, this pattern may also be caused by prexcited SVT using a left posterior accessory pathway [2, 4, 27]. Negative concordance, when strictly defined (QRS complexes are negative i. e. QS complexes are present in all precordial leads), nearly always caused by VT. However, rarely patients with heart failure, cardiomyopathy and very dilated hearts with LBBB pattern or patients with an abnormal anatomical position of the heart, such as in patients with pectus excavatum, where the right ventricle lies completely under the anterolateral precordial area, may manifest with negative concordance during an SVT [4, 6, 28, 29]. Concordance is a very specific (>90% specificity) but insensitive criterion for VT, because only about 20% of all VTs have a concordant pattern, which is approximately evenly split between positive and negative concordance [6].
QRS Morphology
When there is a double-peaked R wave in lead V1 (its two peaks are called “rabbit ears’) only when the “left ear” is taller than the “right ear” suggests strongly VT, and it is a misconception that when a “right ear” is taller than the “left ear” it favors SVT, because this configuration is equally likely in VT [30]. An R wave in lead V1 during a WCT taller than during sinus rhythm favors VT [2].
Contralateral BBB during WCT and sinus rhythm strongly favors VT. The rationale behind this criterion is that when during sinus rhythm one type of BBB is present, when a contralateral BBB occurs during an SVT-A a complete heart block should occur, if complete AV block is not present, then the contralateral BBB-like pattern is due to a VT. However, rarely when both type of BBB are peripheral due to intramyocardial delay, contralateral BBB may occur during SVT-A [1].
Subtle non-rate related variations in QRS morphology or multiple QRS morphologies suggest reentrant, scar-related VT, whereas SVTs (unless the tachycardia rate changes) or focal idiopathic VTs present with uniform QRS morphology [2, 5]
Presence of QR Complexes
The presence of QR (but not QS, which not necessarily imply structural damage, but rather an elecrtrical impulse moving away from the recording site) complex in any leads except lead aVR during WCT usually in the same leads as in sinus rhythm indicates scar in the myocardium usually caused by a remote myocardial infarction suggesting VT. QR complexes during VT are present in approximately 40% of postinfarction VT [1, 5, 24, 27].
AV Dissociation
AV dissociation is a hallmark of VT with a specificity approaching 100%, however its sensitivity is low and only present in 20-50% of all VTs. An AV ratio<1 and VA ratio >1 are equally diagnostic of VT, the latter occurs in an additional 15-20% of VTs [1, 3, 5]. A 1:1 AV association doesn’t indicate SVT, because approximately 30% of VTs have 1:1 retrograde VA conduction. In patients with slow VT capture or fusion beats may be present resulting from complete or partial activation of the ventricles from the atria during WCT, which implies the presence of AV dissociation and therefore diagnostic of VT. The capture beat is a sinus (or supraventricular) usually narrow QRS complex conducted beat occurring earlier than the next expected WCT beat, that gain momentary control of (i. e. captures) the ventricle. If the WCT were due to SVT-A an earlier beat than the next expected WCT beat cannot have a markedly different morphology and be narrower than the WCT beats (but should have the same or very similar morphology with greater degree of aberration), thus a capture beat with markedly different morphology and/or narrow QRS complex indicates VT [5, 27]. Very rarely a WCT with AV dissociation can be due to a SVT, such as junctional tachycardia with aberrant conduction and AV dissociation. Another caveat when AV dissociation is looked for, when atrial fibrillation, atrial flutter or tachycardia are simultaneously present together with a VT, evidently dissociated P waves cannot be visualized. A ventricular premature beat ipsilateral to the “blocked” ventricle occurring simultaneously with the SVT QRS complex may mimick a fusion beat and could be erroneously interpreted as evidence of AV dissociation and VT [2, 3].
WCT with BBB-like or Typical BBB Pattern
It is important to know whether a WCT has only a BBB-like pattern or a typical BBB pattern, because the presence of a typical BBB pattern significantly restricts the potential underlying causes compared with a BBB-like pattern. For example the differential diagnosis of WCT with typical LBBB pattern is limited to 5 entities: 1) SVT with fixed LBBB, 2) SVT with functional aberrancy, 3) preexcited SVT using an atriofascicular or nodofascicular accessory pathway as anterograde limb of the circuit, the retrograde limb is usually the normal His-Purkinje system, but may be a second accessory pathway, 4) SVT with a bystander atriofascicular or nodofascicular pathway, 5) bundle branch reentrant VT [31].
If the WCT presents with a typical RBBB pattern the potential possibilities are: 1) SVT with fixed RBBB, 2) SVT with functional aberrancy, 3) verapamil sensitive “fascicular” VT, 4) Rare type of bundle branch reentry VT, 5) very rarely interfascicular reentry tachycardia, which can be associated either with RBBB+left anterior fascicular block or RBBB+left posterior fascicular block patterns.
Brugada Algorithm
In 1991 Brugada and coworkers [32] published a stepwise, decision-tree like algorithm in which 4 criteria for VT are sequentially considered (see Fig. 2). The first two criteria in their 4-step algorithm were new, in the 3rd and 4th step the algorithm used the old traditional criteria of AV dissociation and morphological criteria in leads V1-2 and V6. The Brugada algorithm is the most widely known and commonly used algorithm. In the first step precordial leads are assesssed for the absence of an RS complex (only the presence or absence of an RS complex is valuable for the diagnosis, QR, QRS, QS, monophasic R or rSR complexes are not considered RS complexes), which would indicate VT with a specificity of 100% and a sensitivity of 21% for VT diagnosis. In the next step, when an RS complex is present in one or more precordial leads, the longest RS interval in any precordial lead is measured (between the onset of the R wave and the nadir of the S wave). If the longest RS interval >100 ms VT is diagnosed with a reported specificity of 98% and sensitivity of 66% for VT diagnosis. If the longest RS interval is<100 ms, in the 3rd step when AV dissociation is present VT diagnosis can be made with 21% sensitivity and 100% specificity. Fourth, if the RS interval<100 ms and AV dissociation cannot be detected, the traditional QRS morphology criteria in leads V1-2 and V6 are considered. When QRS morphology criteria are present both in leads V1-2 and V6 VT is diagnosed, if either the V1-2 and V6 criteria are not consistent, or none are consistent with VT, SVT-A is diagnosed by exclusion. The sensitivity and specificity of the 4th step wasn’t reported. The authors prospectively analyzed 554 WCTs and reported a very high sensitivity and specificity of the 4 consecutive steps of 98.7% and 96.5% respectively. The two new (first two) criteria of the Brugada algorithm correspond to the basic principles 2), 4), 5). Limitations of the Brugada algorithm are that it was tested in patients without antiarrhythmic drug treatment and the authors didn’t state in the article whether they studied patients with pre-existent BBB, idiopathic VT, preexcited SVT or not.
Fig. (2).
The Brugada algorithm. For further explanation see text.
Newer Algorithms and Criteria
Vereckei Algorithms
In 2007 and 2008 Vereckei et al. [18, 19] published two new, 4-step algorithms with the incorporation of two new criteria:
The vi/vt criterion based on the estimation of initial (vi) and terminal (vt) ventricular activation velocity ratio (vi/vt) by measuring the vertical excursion (in millivolts) recorded on the ECG during the initial (vi) and terminal 40 ms (vt) of the QRS complex.
The presence of an initial R wave in lead aVR criterion.
The rationale behind the vi/vt criterion is that during WCT due to SVT the initial activation of the septum (occurring either left-to-right or right-to-left) should be invariably rapid over the normal His-Purkinje system and the intraventicular conduction delay causing the wide QRS complex occurs in the mid to terminal part of the QRS, thus the vi/vt >1 during SVT. During WCT due to VT, however, an initial slower muscle-to-muscle spread of activation occurs until the impulse reaches the His-Purkinje system, after which the rest of the ventricular muscle is more rapidly activated, thus, the vi/vt ≤1 during VT. This assumption should hold true regardless of the mechanism of VT or presence or absence of structural heart disease. We used another assumption while devising the vi/vt criterion, that the steepness of the QRS (which was measured by voltage in mV the impulse traveled in vertical direction during a given time period) is directly proportional with the conduction velocity of the propagating impulse in the ventricle.
The presence of an initial R wave in lead aVR is similar to the traditional northwest axis criterion, but not the same, because the range of the resultant QRS vector that yields an initial R wave in lead aVR is between -60 degrees and +120 degrees. The initial ventricular activation wavefront during SVT and sinus rhythm should go away from lead aVR yielding a negative QRS (QS) complex (an rS complex in lead aVR may be present as a normal variant or in patients with inferior myocardial infarction due to loss of initial inferiorly directed forces, but with an R/S ratio<1). Thus, an initial dominant R wave (such as R or Rs complex) in lead aVR should not be present in SVT-A and suggests VT. Figure 3 shows that the presence of an initial R wave in lead aVR criterion is different from the traditional northwest axis criterion not only in the minimal difference in QRS axis that is needed to yield an R wave in lead aVR vs. a right superior quadrant axis, but also in the fact that our aVR criterion suggests VT only in the presence of an initial R or Rs wave and not in the presence of a predominantly positive QRS complex, which might have a small, initial negative deflection.
Fig. (3).
An example of a WCT due to SVT demonstrating why the presence of an initial R wave in lead aVR criterion might be superior to the traditional northwest axis criterion. The QRS axis is -160o, northwest axis suggesting the misdiagnosis of VT. Although the QRS complex is predominantly positive in lead aVR, there is no initial R wave, because the QRS complex starts with a q wave, and the vi/vt in lead aVR >1, suggesting correctly SVT. Reproduced from Ref. 18. with permission.
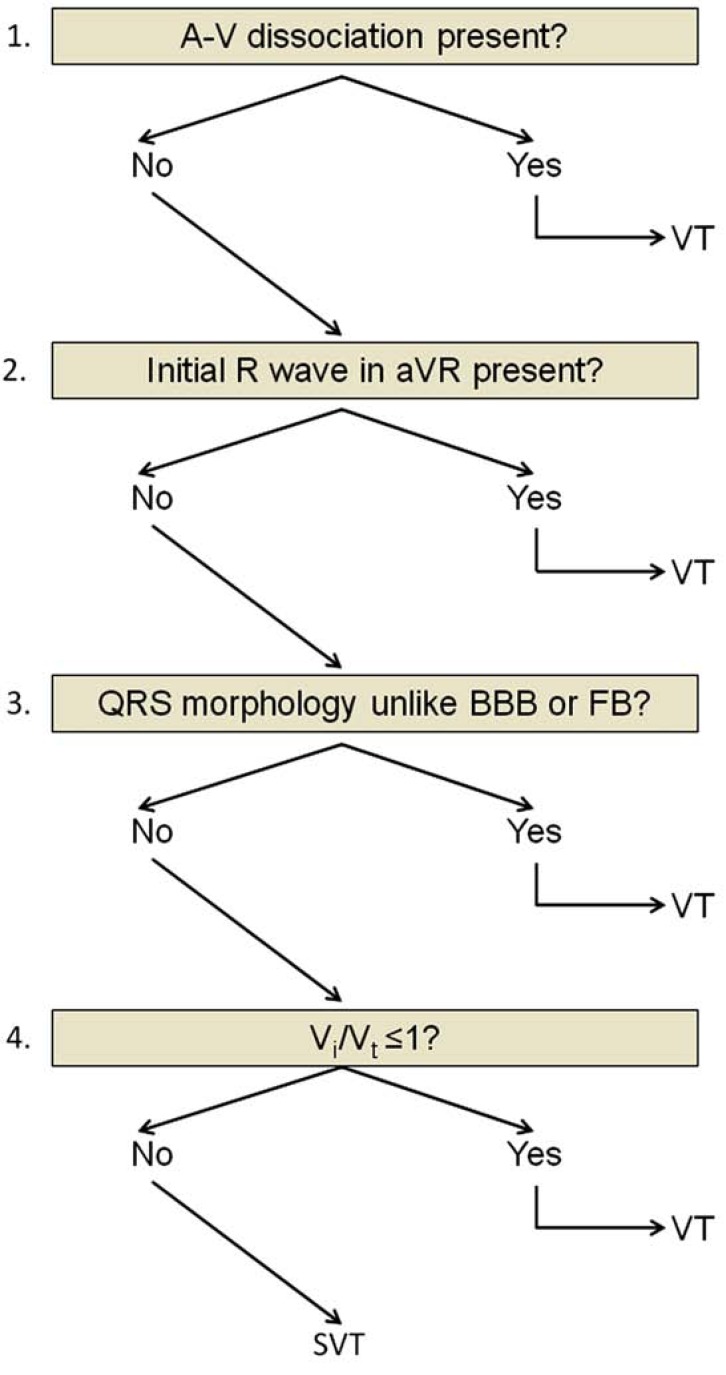
First Vereckei Algorithm
The four criteria of this newer algorithm [18] were organized in a stepwise, decision-tree format similar to the Brugada algorithm (see Fig. 4). The algorithm was tested in 453 monomorphic WCT-ECG tracings recorded from 287 patients. The four steps were used in the following sequence: 1) If AV dissociation was present the diagnosis of VT was made and the analysis was stopped. 2) If an initial R wave was present in lead aVR the diagnosis of VT was made and the analysis was stopped. 3) If the morphology of WCT did not correspond to BBB or fascicular block the diagnosis of VT was made and the analysis was stopped. 4) In the last step when the vi/vt was ≤1 the diagnosis of VT, if the vi/vt was >1 the diagnosis of SVT was made. This algorithm, as well as the traditional criteria and the Brugada algorithm, are unable to reliably differentiate VTs from preexcited SVTs in most WCT cases [with the exception of the presence of A-V dissociation and possibly that of an initial R wave in lead aVR along with other criteria suggested by Antunes et al [18, 33] that are infrequently present] thus, the final diagnosis of VT in the 3rd and 4th step of the algorithm included also preexcited SVTs [18]. This algorithm had a superior overall test accuracy to that of the Brugada algorithm (90.3% vs. 84.8% respectively). The superiority of this algorithm to the Brugada algorithm was mostly due to the significantly better overall test accuracy of the vi/vt criterion in the 4th step than that of the 4th Brugada criterion (82.2% vs. 68% respectively). The significantly and borderline better overall test accuracy of the first Vereckei algorithm in the pre-existent BBB and idiopathic VT patient subgroups to that of the Brugada algorithm (92.2% vs 85%, p<0.05 for the pre-existent BBB; 86.5% vs 67.6%, p=0.06 for the idiopathic VT subgroups) also contributed to the superiority of the first Vereckei algorithm.
Fig. (4).
The first Vereckei algorithm. FB=fascicular block. For further explanation see text.
The vi/vt criterion has several advantages and limitations. A potential advantage that antiarrhythmic drugs that impair conduction in the His-Purkinje system and/or ventricular myocardium (such as class I drugs and amiodarone) and decrease the diagnostic accuracy of the 2nd Brugada criterion, would be expected to decrease the vi and vt approximately to the same degree, therefore the vi/vt ratio will not change significantly. A possible limitation of the vi/vt criterion that disorders involving the myocardium locally can alter the vi or vt, and may lead to misdiagnosis. For example a decreased vi with unchanged vt may be present in the case of an SVT occurring in the presence of an anteroseptal myocardial infarction leading to the misdiagnosis of VT. Or a scar situated at a late activated ventricular site may result in a decreased vt in the presence of VT leading to the misdiagnosis of SVT. Other possible limitations that in the case of a fascicular VT, the vi is not slower than the vt; and if the exit site of the VT reentry circuit is very close to the His-Purkinje system, it might result in a VT with a relatively narrow QRS complex and the slowing of the vi may last for such a short time that it cannot be detected by the surface ECG.
A limitation of the initial R wave in lead aVR criterion that wasn’t described in the original manuscript might be, that very rarely in patients with chronic cor pulmonale or severe emphysema associated with right ventricular hypertrophy an R wave might be present in lead aVR during sinus rhythm.
The aVR Vereckei Algorithm
Despite the superiority of the first Vereckei algorithm to the Brugada algorithm its application required more time in most cases than that of the Brugada algorithm. Therefore another simplified algorithm was devised by the total omission of complicated morphological criteria and limiting the leads to be studied to lead aVR [19]. Figure 5 shows the aVR Vereckei algorithm. The following criteria were analyzed in lead aVR: 1) The presence of an initial R wave? 2) Presence of an initial r or q wave of >40 ms width? 3) Notching on the descending limb of a negative onset, predominantly negative QRS complex? 4) vi/vt ratio? When any of the first three criteria of the algorithm was met, a diagnosis of VT was made and the analysis was stopped at that step. In the 4th step a vi/vt ≤1 diagnosed VT, if vi/vt was >1 a diagnosis of SVT was made.
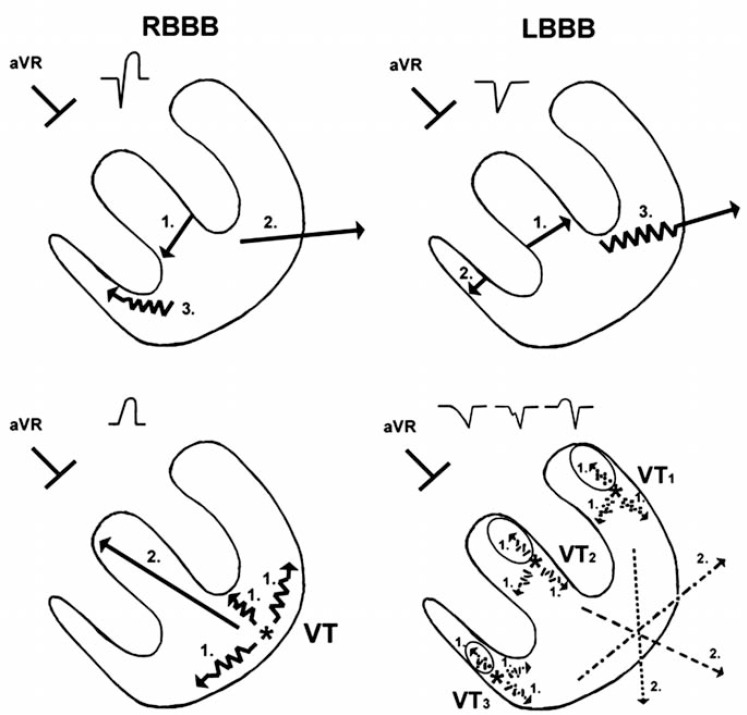
The aVR Vereckei algorithm is based solely on the principle of differences in the direction and velocity of the initial and terminal ventricular activation during WCT due to VT and SVT. Although the aVR Vereckei algorithm does not contain any fundamentally new criteria compared with the first Vereckei algorithm, it is based on three novel concepts: 1) Selection of lead aVR exclusively for the differential diagnosis of WCTs; 2) classification of VTs into two main groups: a) VTs arising from the inferior or apical region of the ventricles yielding an initial R wave in lead aVR, b) VTs arising from other regions and lacking an initial R wave in aVR, but with slowing of the initial part of the predominantly negative QRS complex (in contrast to SVTs that show more rapid initial QRS forces); and 3) elimination of the complex morphological criteria (and the AV dissociation criterion) used by all prior algorithms and traditional criteria.
Fig. (6) demonstrates the explanation of the rationale of the aVR Vereckei criteria. The left lower panel shows that the presence of an initial R wave in lead aVR suggests VT, typically arising from the inferior or apical region of the ventricles. The right lower panel shows that VTs originating from sites other than the inferior or apical wall of the ventricles, not showing an initial R wave in lead aVR should yield a slow, initial upward vector component of variable size pointing toward lead aVR (which is absent in SVT), even if the main vector in these VTs points downward yielding a totally or predominantly negative QRS in lead aVR. Thus, in VT without an initial R wave in lead aVR, the initial part of the QRS in lead aVR should be less steep (“slow”) due to the slower initial ventricular activation having an initial upward vector component, which may be manifested as an initial r or q wave >40 ms width, a notch on the downstroke of the QRS, or a slower ventricular activation during the initial 40 ms than during the terminal 40 ms of the QRS (vi/vt ≤1) in lead aVR. In contrast to that, in SVT-A the initial part of the QRS in lead aVR is steeper (“fast”), due to the invariably rapid septal activation going away from lead aVR, resulting in a narrow (≤40 ms) initial r or q wave and a vi/vt >1 as shown in the upper two panels of (Fig. 6). Fig. (7) shows how closely similar ECG patterns to those predicted by the schematic (Fig. 6) could be observed in lead aVR of real WCT-ECG tracing examples. The aVR Vereckei algorithm was tested in 483 WCT tracings recorded from 313 patients. The overall test accuracy of the aVR Vereckei algorithm was similar to that of the first Vereckei algorithm and superior to that of the Brugada algorithm (91.5% vs. 90.7% and 85.5% respectively). A limitation of the aVR Vereckei algorithm similar to that of the first Vereckei and Brugada algorithms was its inability to differentiate VTs from preexcited SVTs with the possible exception of the presence of an initial R wave criterion. In fact none of the 20 preexcited SVTs that were analyzed during the study had an initial R wave in lead aVR.
Fig. (6).
Schematic explanation of the rationale behind the aVR Vereckei algorithm criteria. The vectors marked with serrated lines and number 1 in the two lower panels are representing the slow initial upward vector components pointing toward lead aVR, which are present in all VTs regardless of the site of origin. The vectors marked with number 2 in the two lower panels represent the resultant QRS vectors of ventricular activation. For further explanation see text.
Fig. (7).
Representative examples of the most common lead aVR ECG patterns taken from real tracings recorded from patients with WCTs superimposed on a grid (small box=40 ms). For further explanation see text. Reproduced from Ref. 19. with permission.
Lead II R-wave Peak Time (RWPT) Criterion
Pava et al. [34] recently published a new criterion that is essentially consistent with principle 2. They proposed that the time to the intrinsicoid deflection measured in lead II as an interval from the QRS onset to the peak of the first positive or negative wave, when >50 ms suggested VT, when<50 ms suggested SVT diagnosis. The RWPT criterion was analyzed in 218 WCTs. The authors reported a very high sensitivity (93.2%), specificity (99.3%), PPV (98.2%) and negative predictive values (NPV) (93.3%) for VT diagnosis. The authors didn’t specify how many of their patients had pre-existent BBB, were on antiarrhythmic medication and it was also not clear how many idiopathic, septal myocardial and bundle branch reentry VTs were among the WCTs, which can have shorter RWPT [2].
Other Criteria
Several other approaches [35-38] were published for the differentiation of WCTs, which I don’t discuss here in detail, because they either not contain new criteria or very complicated and therefore difficult to use in an urgent setting.
The Differentiation of VT from Preexcited SVT
It is very difficult to distinguish VT from preexcited SVT because in both case ventricular activation begins outside the normal intraventricular conduction system. Preexcited SVT using a typical AV bypass tract behaves as a VT originating from the base of the ventricles. With the exception of an algorithm devised by the Brugada working group in 1994 [33], none of the traditional criteria or all other algorithms are able to differentiate VT from preexcited SVT unless AV dissociation is present, which rules out preexcited SVT. The proposed algorithm consists of three steps (see Fig. 8) and was tested in 149 VTs and 118 preexcited SVTs proven by electrophysiological study. Criteria favoring VT were: 1st step: Presence of a predominantly negative QRS complex in the precordial leads V4 to V6?,. 2nd step: Presence of a QR complex in one or more of the precordial leads V2 to V6?, 3rd step: AV relation different from 1:1 (more QRS complexes than P waves) ? When any of the first 3 criteria were met a VT diagnosis was made and the analysis was stopped. When none of the 3 criteria were present preexcited SVT was diagnosed. The reported sensitivity and specificity of the three consecutive steps for VT diagnosis were 75% and 100% respectively. The rationale behind the first criterion was based on the fact that activation of the ventricles over a typical AV bypass tract proceeds from the base toward the apex of the heart, which should yield a predominantly positive QRS complex in leads V4 to V6. Predominantly negative QRS complexes in these leads favor VT. The second criterion is based on the fact that in the absence of structural heart disease (which is usually the case in preexcited SVT) QR complexes should not be observed in one or more of the precordial leads V2 to V6. Thus, the presence of QR complexes in these leads favors VT The above described rationale behind the first criterion implying a ventricular activation going away from lead aVR also explains why possibly an initial R wave in lead aVR might rule out preexcited SVT. In fact in our study [19] none of the included preexcited SVTs had an initial R wave in lead aVR. However this assumption should be verified in further studies analyzing greater number of preexcited SVTs.
Fig. (8).
The algorithm of Brugada working group devised for distinguishing VT from PXT. For further explanation see text.
When ECG in sinus rhythm is available it might be helpful showing signs of WPW syndrome. In the presence of minimal preexcitation (presenting as slurring of the QRS upstroke with a QRS duration<120 ms and a PR interval >120 ms, which occurs especially in patients with left free wall pathway and slowly conducting accessory pathways, a normally occurring small q wave should not be present, this sign helps to distinguish minimal preexcitation from normal ventricular activation [39, 40]. An rS pattern in lead III associated with the absence of Q wave in lead I is considered very specific for atriofascicular bypass tract [41]. In the medical history younger age, the absence of structural heart disease favor preexcited SVT.
Evaluation of ECG Methods for WCT Diagnosis by Independent Authors
A common finding in all studies carried out by independent authors was that they failed to reproduce the very high overall test accuracy, sensitivity, specificity values reported by the original authors about the particular ECG method. Grimm et al. [42] compared the diagnostic value of the classical criteria published by Wellens in 1978 and the Brugada algorithm analyzing 240 WCTs, and found a sensitivity for VT diagnosis >90% for both criteria and a specificity of 70% and 72% for RBBB-like and 87% each for LBBB-like WCTs and found that the combined use of both criteria did not increase the sensitivity and specificity. Alberca et al. [43] demonstrated that the presence of pre-existing BBB and the use of class I antiarrhythmic drugs or amiodarone result in a low specificity (63%) of the 2nd Brugada criterion (i.e. in many of these patients with WCT due to SVT the longest RS interval in the precordial leads will be >100 ms suggesting a misdiagnosis of VT). The consecutive specificity of the 1st and 2nd Brugada criteria was only 57%. When the Brugada algorithm was applied by two board-certified emergency physicians and two board-certified cardiologists the sensitivity and specificity for cardiologist 1 and 2 and emergency physician 1 and 2 were 85% and 60%, 91% and 55%; 83% and 43%, 79% and 70% respectively [44). A head-to-head comparison of 5 ECG methods (RWPT criterion, Brugada, Griffith, Bayesian and aVR Vereckei algorithms) for WCT differential diagnosis were made by two board certified cardiologists [45] and they found that all five ECG methods had a rather moderate diagnostic accuracy (68.8 to 77.5%) but didn’t find significant difference between the overall test accuracy of the ECG methods with the exception that the RWPT criterion had a significantly lower overall test accuracy than the Brugada algorithm (p=0.04, 68.8% vs. 77.5%). The RWPT criterion had lower sensitivity (60%) than the Brugada (89%) aVR Vereckei (87.1%), Griffith (94.2%) and Bayesian (89%) algorithms (p<0.001). The Griffith algorithm showed lower specificity (39.8%) than the RWPT criterion (82.7%), Brugada (59.2%) and Bayesian (52%) algorithms (p<0.005). However, in contrast to our approach and the recommended approach by Brugada working group [38] and Fernando Pava (personal communication), when the mechanism of WCT was preexcited SVT and the final diagnosis by the applied aVR Vereckei, Brugada algorithms and RWPT criterion was VT, they considered it as an incorrect diagnosis and the SVT diagnosis as a correct one. This important discrepancy renders difficult the comparison of the results, however the potential misclassification of their 23 preexcited SVTs out of 260 WCT tracings might have not changed significantly their conclusions. In a recent study [46] four emergency resident reviewers analyzed WCT-ECG tracings using the aVR Vereckei algorithm. When 2 of the 4 reviewers who left disproportionately high number of ECGs misclassified at the final step of the algorithm were eliminated from the analysis, the 2 remaining reviewers achieved an overall test accuracy of 70% and 74%. In pediatric population lower overall test accuracy for both Brugada and aVR Vereckei algorithm (69% vs. 66% respectively) has been reported with no significant difference in overall test accuracy between the two algorithms [47]. Very recently we compared the diagnostic value of aVR Vereckei algorithm vs. RWPT criterion by the participation of 7 reviewers (2 board-certified cardiologists, two board-certified emergency physicians, 1 internal medicine, 1 cardiology and 1 emergency medicine residents) (the author’s and coworkers’ yet unpublished observations, submitted for publication). The mean overall test accuracy of the aVR Vereckei algorithm by the 7 reviewers was superior to that of the RWPT criterion (p=0.003, 84.3% vs. 79% respectively). The mean sensitivity for VT diagnosis of the 7 reviewers for the aVR Vereckei algorithm was superior to that of the RWPT criterion (92.4% vs. 79.1%, p<0.0001 respectively), the mean specificity for the RWPT criterion was superior to that of the aVR Vereckei algorithm (80.9% vs. 64.7%, p<0.0001 respectively). The incorrect diagnosis using the aVR Vereckei algorithm was mainly due to misdiagnosis of SVT as VT (in 65.7%), which is the less dangerous misclassification, and those by the RWPT criterion were mostly due to misdiagnosis of VT as SVT (in 72.5%), which is a more dangerous error.
Practical Approach to a Patient with Hemodynamically Stable WCT
Figure 9 shows the ACC/AHA/ESC recommendation for the differential diagnosis of WCTs 48). If the WCT is irregular the likely possibilities are atrial fibrillation or atrial flutter or tachycardia (with variable AV conduction) with fixed or functional aberration or bystander anterograde conduction over an accessory pathway. A regular WCT can be due to VT or other type of SVTs with BBB or preexcited SVTs. However, irregularity of the WCT doesn’t rule out VT. VT can be irregular, particularly in the first 30 seconds of onset and in patients treated with antiarrhythmic drugs and with focal idiopathic VT [2, 8].
Fig. (9).
The algorithm proposed by the ACC/AHA/ESC guideline for WCT differential diagnosis.
When confronted with a hemodynamically stable WCT, a useful practical approach is to look for clinical signs of AV dissociation (e.g. “cannon” A waves), consider the patient’s clinical characteristics ( a history of prior myocardial infarction, congestive heart failure, angina strongly suggests VT, while age<35 years, recurrent tachycardia over a period of >3 years, absence of structural heart disease favor SVT) [2]. Then one should evaluate the 12-lead WCT-ECG for the most specific criteria in a sequence of decreasing specificity such as the presence of AV dissociation, northwest axis, initial R or Rs wave in lead aVR, absence of RS complex, concordant pattern in the precordial leads, and when confirmed by other investigators lead II RWPT >50 ms. All the above criteria when present strongly suggest VT. If the morphology of the WCT corresponds to any combination of a typical bundle branch block or fascicular block SVT diagnosis is highly likely, a vi/vt>1 also strongly suggests SVT. If the morphology of WCT definitely doesn’t correspond to any combination of typical BBB or fascicular block VT or PXT diagnosis is very likely, a vi/vt ≤1 strongly suggest VT or preexcited SVT as well [1]. When a sinus rhythm ECG is available and the QRS morphology in the sinus rhythm ECG is identical to that of the WCT-ECG, SVT diagnosis (or rarely antidromic AV reentrant tachycardia) is highly likely [1, 48]. However, bundle branch reentry VT, fascicular VT and high midseptal VT may be associated with identical QRS morphology during WCT to that during sinus rhythm as well, and this way may masquerade as SVT in patients with fixed intraventricular conduction disturbance [49]. Vagal maneuvers (Valsalva maneuver, carotid sinus massage) or adenosine administration may help in the elucidation of WCT mechanism. The termination of tachycardia strongly suggests SVT, however, idiopathic outflow tract VT may be terminated as well by these maneuvers [3]. Even if the tachycardia itself remains unaffected, these maneuvers may unmask the WCT mechanism by inducing AV or VA block or slowing down the sinus or atrial rate [2]. When all these measures fail to establish a certain diagnosis of SVT, then the WCT should be treated as a VT, because it is far better to be wrong with a few cases of SVT treated as VT than the reverse situation, since treating a VT as SVT may result in potentially disastrous consequences (e. g. iv. verapamil injection may cause severe hypotension and/or VT acceleration and ventricular fibrillation) [1, 50, 51] and iv. adenosine should also be used with great caution, because it can cause coronary steal producing ischemia in ischemic VT and in patients with antidromic AVRT it might precipitate atrial fibrillation with high ventricular rate and may result, if the accessory pathway has a short anterograde refractory period, in ventricular fibrillation [52].
CONCLUSIONS
After a period of relative stagnation, recently new concepts, ECG criteria and algorithms emerged in the exciting field of WCT differential diagnosis raising the hope that further improvement can be achieved in the accurate identification of WCT mechanism. ECG methods can diagnose the mechanism of WCT with certainty in the majority (approximately 90%) of cases. However, current ECG criteria and algorithms still misdiagnose up to 10% of WCTs, therefore physicians should be cautioned against overreliance in these ECG methods. If there remains any doubt in the mechanism of a WCT, it should be treated acutely as VT and later an electrophysiological study should be performed to clarify the WCT mechanism.
Fig. (5).
The aVR Vereckei algorithm. For further explanation see text.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
REFERENCES
- 1.Miller JM, Das MK, editors. In: Cardiac electrophysiology. From cell to bedside. 5th Edition. Eds. Elsevier.: Zipe DPJalife J. Saunders.; 2009. Differential diagnosis for wide QRS complex tachycardia. pp. 823–30. [Google Scholar]
- 2.Issa F, Miller JM, Zipes DP, editors. Approach to wide QRS complex tachycardias. 2nd Edition. Elsevier Saunders: 2012. Clinical arrhythmology and electrophysiology. pp. 499–511. [Google Scholar]
- 3.Pellegrini CN, Scheinman MM. Clinical management of ventricular tachycardia. Curr Probl Cardiol. 2010;35:453–504. doi: 10.1016/j.cpcardiol.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Alzand BSN, Crijns HJGM. Diagnostic criteria of broad QRS complex tachycardia: decades of evolution. Europace. 2011;13:465–72. doi: 10.1093/europace/euq430. [DOI] [PubMed] [Google Scholar]
- 5.Benito B, Josephson ME. Ventricular tachycardia in coronary artery disease. Revista Espanola de Cardiologia. 2012;65:939–55. doi: 10.1016/j.recesp.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Miller JM, Bhakta D, Scherschel JA, Yadav AV, editors. New York., London. Hong Kong Sidney Tokyo: Lippincott Williams and Wilkins Philadelphia Baltimore.Buenos Aires.; 2010. Approach to the patient with wide complex tachycardia.In: Electrophysiology. The basics. A companion guide for the cardiology fellow during the EP rotation. Wolters Kl wer. pp. 186–94. [Google Scholar]
- 7.Strauss DG. Differentiation between left bundle branch block and left ventricular hypertrophy: Implications for cardiac resynchronization therapy. J Eelectrocardiol. 2012;45:635–9. doi: 10.1016/j.jelectrocard.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Roberts-Thomson KC, Lau DH, Sanders P. The diagnosis and management of ventricular arrhythmias. Nat Rev Cardiol. 2011;8:311–21. doi: 10.1038/nrcardio.2011.15. [DOI] [PubMed] [Google Scholar]
- 9.Wilber DJ, Joshi S, Wilber DJ, Packer DL, editors. In: Catheter ablation of cardiac arrhythmias . 3rd Edition. Stevenson WG Blackwell Futura: 2008. Ablation of idiopathic right ventricular tachycardia. pp. 279–97. [Google Scholar]
- 10.Lerman BB, Zipes DP, Jalife J, editors. In: Cardiac electrophysiology. From cell to bedside. 5th Edition. Eds. Saunders Elsevier: 2009. Ventricular tachycardia in patients with structurally normal hearts. pp. 657–68. [Google Scholar]
- 11.Gupta AK, Thakur RK. Wide QRS complex tachycardias. Med Clin North Am. 2001;85:245–66. doi: 10.1016/s0025-7125(05)70315-1. [DOI] [PubMed] [Google Scholar]
- 12.Waxman MB, Wald RW, Finley JP, Bonet JF, Downar E, Sharma AD. Valsalva termination of ventricular tachycardia. Circulation. 1980;62:843–51. doi: 10.1161/01.cir.62.4.843. [DOI] [PubMed] [Google Scholar]
- 13.Morady F, Baerman JM, DiCarlo LA Jr, de Buitleir M, Krol RB, Wehr DW. A prevalent misconception regarding wide-complex tachycardias. JAMA. 1985;254:2790–2. [PubMed] [Google Scholar]
- 14.Eckardt L, Brethardt G, Zipes DP, Jalife J, editors. In: Cardiac electrophysiology. From cell to bedside. 5th Edition. Eds. Sauners.Elsevier: 2009. Drug-induced ventricular tachycardia. pp. 769–77. [Google Scholar]
- 15.Darbar D, Zipes DP, Jalife J, editors. In: Cardiac electrophysiology. From cell to bedside Saunders. 5th Edition. Eds. Elsevier: 2009. Standard antiarrhythmic drugs. pp. 959–73. [Google Scholar]
- 16.Bakker ALM, Nijkerk G, Groenemeijer BE , et al. The Lewis lead.Making recognition of P waves easy during wide QRS complex tachycardia. . Circulation. 2009;119:e592–3. doi: 10.1161/CIRCULATIONAHA.109.852053. [DOI] [PubMed] [Google Scholar]
- 17.Abacherli R, Leber R, Lemay Metal. Development of a toolbox for electrocardiogram-based interpretation of atrial fibrillation. J Electrocardiol. 2009;42:517–21. doi: 10.1016/j.jelectrocard.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Vereckei A, Duray G, Szénási G, Altemose GT, Miller JM. Application of a new algorithm in the differential diagnosis of wide QRS complex tachycardia. European Heart Journal. 2007;28:589–600. doi: 10.1093/eurheartj/ehl473. [DOI] [PubMed] [Google Scholar]
- 19.Vereckei A, Duray G, Szénási G, Altemose GT, Miller JM. A new algorithm using only lead aVR for the differential diagnosis of wide QRS complex tachycardia. Heart Rhythm. 2008;5:89–98. doi: 10.1016/j.hrthm.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Marriott HJL, Sandler IA. Criteria old and new for differentiating between ectopic ventricular beat and aberrant ventricular conduction in the presence of atrial fibrillation. Prog Cardiovasc Dis. 1966;9:18–28. [Google Scholar]
- 21.Sandler IA, Marriott HJL. The differential morphology of anomalous ventricular complexes of RBBB-type in lead V1.Ventricular ectopy versus aberration. . Circulation. 1965;31:551–6. doi: 10.1161/01.cir.31.4.551. [DOI] [PubMed] [Google Scholar]
- 22.Swanick EJ, LaCamera F, Marriott HJL. Morphologic features of right ventricular ectopic beats. Am J Cardiol. 1972;30:888–91. doi: 10.1016/0002-9149(72)90015-x. [DOI] [PubMed] [Google Scholar]
- 23.Wellens HJJ, Bar FW, Lie KL. The value of the electrocardiograms in the differential diagnosis of a tachycardia with a widened QRS complex. Am J Med. 1978;64:27–33. doi: 10.1016/0002-9343(78)90176-6. [DOI] [PubMed] [Google Scholar]
- 24.Coumel P, Leclercq JF, Attuel P, Maisonblanche P. The QRS morphology in post myocardial infarction ventricular tachycardia.A study of 100 tracings compared with 70 cases of idiopathic ventricular tachycardia. Eur Heart J. 1984;5:792–805. doi: 10.1093/oxfordjournals.eurheartj.a061568. [DOI] [PubMed] [Google Scholar]
- 25.Kindwall KE, Brown J, Josephson ME. Eelectrocardiographic criteria for ventricular tachycardia in wide complex left bundle branch block morphology tachycardias. Am J Cardiol. 1988;61:1279–83. doi: 10.1016/0002-9149(88)91169-1. [DOI] [PubMed] [Google Scholar]
- 26.Zimetbaum PJ, Josephson ME. Practical Clinical Eelectrophysiology Ventricular tachycardia Wolters Kluwer Health/Lippincott Williams and Wilkins Philadelphia Baltimore New York London. Buenos Aires Hong Kong Sidney Tokyo. 2009:137–61. [Google Scholar]
- 27.Wellens HJJ. Electrophysiology.Ventricular tachycardia: diagnosis of broad complex tachycardia. . Heart. 2001;86:579–85. doi: 10.1136/heart.86.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barold SS, Stroobandt RX, Herweg B. Limitations of the negative concordance pattern in the diagnosis of broad QRS tachycardia. J Electrocardiol. 2012;45:733–5. doi: 10.1016/j.jelectrocard.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Volders PG, Timmermans C, Rodriguez LM, van Pol PF, Wellens HJJ. Wide QRS complex tachycardia with negative precordial concordance: always a ventricular origin?. J Cardiovasc Electrophysiol. 2003;14:109–11. doi: 10.1046/j.1540-8167.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- 30.Marriott HJL, editor. 7th Edition. Baltimore/London: Williams and Wilins; 1983. Practical electrocardiography. pp. 190–210. [Google Scholar]
- 31.Neiger JS, Trohman RG. Differential diagnosis of tachycardia with typical left bundle branch morphology. Word J Cardiol. 2011;3:127–34. doi: 10.4330/wjc.v3.i5.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brugada P, Brugada J, Mont L, Smeets J, Andries EW. A new approach to the differential diagnosis of a regular tachycardia with a wide QRS complex. Circulation. 1991;83:1649–59. doi: 10.1161/01.cir.83.5.1649. [DOI] [PubMed] [Google Scholar]
- 33.Antunes E, Brugada J, Steurer G, Andries E, Brugada P. The differential diagnosis of a regular tachycardia with a wide QRS complex on the 12-lead ECG: ventricular tachycardia. supraventricular tachycardia with aberrant conduction and supraventricular tachycardia with anterograde conduction over an accessory pathway. PACE. 1994;17:1515–24. doi: 10.1111/j.1540-8159.1994.tb01517.x. [DOI] [PubMed] [Google Scholar]
- 34.Pava LF, Parafán P, Badiel M , et al. R-wave peak time at DII: a new criterion to differentiate between wide complex QRS tachycardias. Heart Rhythm. 2010;7:922–6. doi: 10.1016/j.hrthm.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Griffith M, de Belder MA, Linker NJ, Ward DE, Camm AJ. Multivariate analysis to simplify the differential diagnosis of broad complex tachycardia. Br Heart J. 1991;66:166–74. doi: 10.1136/hrt.66.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffith M, de Belder MA, Linker NJ, Ward DE, Camm AJ. Difficulties in the use of electrocardiographic criteria for the differential diagnosis of left bundle branch block pattern tachycardia in patients with structurally normal heart. Eur Heart J. 1992;13:478–83. doi: 10.1093/oxfordjournals.eurheartj.a060200. [DOI] [PubMed] [Google Scholar]
- 37.Griffith MJ, Garratt CJ, Mounsey P. Ventricular tachycardia as default diagnosis in broad complex tachycardia. Lancet. 1994;343:386–8. doi: 10.1016/s0140-6736(94)91223-8. [DOI] [PubMed] [Google Scholar]
- 38.Lau EW, Pathamanathan RK, NG GA, Cooper J, Skehan JD, Griffith MJ. The Bayesian approach improves the electrocardiographic diagnosis of broad complex tachycardia. Pacing Clin Eelectrophysiol. 2000;23:1519–26. doi: 10.1046/j.1460-9592.2000.01519.x. [DOI] [PubMed] [Google Scholar]
- 39.Chugh A, Bogun F, Morady F, Wilber DJ, Packer DL, Stevenson WG, editors. In: Catheter ablation of cardiac arrhythmias. 3rd Edition. Eds. Blackwell Futura: 2008. Catheter ablation of accessory pathways. pp. 149–72. [Google Scholar]
- 40.Chugh A, Morady F, Zipes DP, Jalife J, editors. In: Cardiac electrophysiology. From cell to bedside. 5th Edition Eds. Sauners.Elsevier : 2009. Atriovantricular reentry and variants. pp. 605–14. [Google Scholar]
- 41.Sternick EB, Timmermans C, Sosa E , et al. The elctrocardiogram in sinus rhythm and during tachycardia in patients with anterograde conduction over Mahaim fibers: the role of the “rS” pattern in lead III. J Am Coll Cardiol. 2004;44:1626–35. doi: 10.1016/j.jacc.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 42.Grimm W, Menz V, Hoffmann J, Maisch B. Value of old and new electrocardiographic criteria for differential diagnosis between ventricular tachycardia and supraventricular tachycardia with bundle branch block. Z Kardiol. 1996;85:932–42. [PubMed] [Google Scholar]
- 43.Alberca T, Almendral J, Sanz P, Almazan A, Cantalapiedra JL, Delcán JL. Evaluation of the specificity of morphological electrocardiographic criteria for the differential diagnosis of wide QRS complex tachycardia in patients with intraventricular conduction defects. Circulation. 1997;96:3527–33. doi: 10.1161/01.cir.96.10.3527. [DOI] [PubMed] [Google Scholar]
- 44.Isenhour JL, Craig S, Gibbs M, Littmann L, Rose G, Risch R. Wide-complex tachycardia: continued evaluation of diagnostic criteria. Acad Emerg Med. 2000;7:769–73. doi: 10.1111/j.1553-2712.2000.tb02267.x. [DOI] [PubMed] [Google Scholar]
- 45.Jastrzebski M, Kukla P, Czarnecka D, Kawacka-Jaszcz K. Comparison of five electrocardiographic methods for differentiation of wide QRS-complex tachycardias. Europace. 2012;14:1165–71. doi: 10.1093/europace/eus015. [DOI] [PubMed] [Google Scholar]
- 46.Baxi RP, Hart KW, Vereckei A , et al. Vereckei criteria as a diagnostic tool amongst emergency medicine residents to distinguish between ventricular tachycardia and supraventricular tachycardia with aberrancy. J Cardiol. 2012;59:307–12. doi: 10.1016/j.jjcc.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ceresnak SR, Liberman L, Avasarala K, Tanel R, Motonaga KS, Dubin AM. Are wide complex tachycardia algorithms applicable in children and patients with congenital heart disease?. J Electrocardiol. 2010;43:694–700. doi: 10.1016/j.jelectrocard.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Blomström-Lundqvist C, Scheinman MM, Aliot EM , et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular tachycardia: executive summary. Eur Heart J. 2003;24:1857–97. [Google Scholar]
- 49.Littmann L, McCall MM. Ventricular tachycardia may masquerade as supraventricular tachycardia in patients with preexisting bundle-branch block. Ann Emerg Med. 1995;26:98–101. doi: 10.1016/s0196-0644(95)70245-8. [DOI] [PubMed] [Google Scholar]
- 50.Dancy M, Camm AJ, Ward D. Misdiagnosis of chronic recurrent ventricular tachycardia. Lancet. 1985;2:320–3. doi: 10.1016/s0140-6736(85)90363-0. [DOI] [PubMed] [Google Scholar]
- 51.Buxton AE, Marchlinski FE, Doherty JU, Flores B, Josephson ME. Hazards of intravenous verapamil for sustained ventricular tachycardia. Am J Cardiol. 1987;59:1107–10. doi: 10.1016/0002-9149(87)90857-5. [DOI] [PubMed] [Google Scholar]
- 52.Lee KW, Badhwar N, Scheinman MM. Supraventricular tachycardia-Part I. Curr Probl Cardiol. 2008;33:467–546. doi: 10.1016/j.cpcardiol.2008.06.002. [DOI] [PubMed] [Google Scholar]