Abstract
Various noninvasive tests for risk stratification of sudden cardiac death (SCD) were studied, mostly in the context of structural heart disease such as coronary artery disease (CAD), cardiomyopathy and heart failure but have low positive predictive value for SCD. Fragmented QRS complexes (fQRS) on a 12-lead ECG is a marker of depolarization abnormality. fQRS include presence of various morphologies of the QRS wave with or without a Q wave and includes the presence of an additional R wave (R’) or notching in the nadir of the R’ (fragmentation) in two contiguous leads, corresponding to a major coronary artery territory. fQRS represents conduction delay from inhomogeneous activation of the ventricles due to myocardial scar. It has a high predictive value for myocardial scar and mortality in patients CAD. fQRS also predicts arrhythmic events and mortality in patients with implantable cardioverter defibrillator. It also signifies poor prognosis in patients with nonischemic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and Brugada syndrome. However, fQRS is a nonspecific finding and its diagnostic prognostic should only be interpreted in the presence of pertinent clinical evidence and type of myocardial involvement (structural vs. structurally normal heart).
Keywords: Fragmented QRS, coronary artery disease, cardiomyopathy.
INTRODUCTION
Presently available invasive and noninvasive tests for risk stratification of sudden cardiac death (SCD) were studied, mostly in the context of structural heart disease such as coronary artery disease (CAD), cardiomyopathy and heart failure [1]. One of the recent noninvasive tests, microwave T-wave alternans (MTWA) and signal-averaged electrocardiogram (SAECG) have high negative predictive values but have a low positive predictive value in patients with history of myocardial infarction (MI) or cardiomyopathy [1]. A low left ventricular ejection fraction (LVEF), in patients with structural heart disease is used routinely in clinical practice for risk stratification of SCD., the major limitation of these tests is their lack of desirable positive predictive value for SCD.
Malignant ventricular arrhythmias which include ventricular tachycardia (VT) and ventricular fibrillation (VF) are responsible for two thirds of SCD [2]. The initiation and maintenance of sustained reentrant ventricular arrhythmias depend on a trigger such as a premature ventricular complex and a vulnerable myocardial substrate. Focally initiated ventricular arrhythmias are also perpetuated by barriers which may be functional or anatomical. For reentry to occur, an abnormal repolarization of the tissues and/or disorder of depolarization resulting in abnormal impulse conduction are required. These repolarization and depolarization abnormalities of the heart can be detected by various invasive and noninvasive tests. Major depolarization repolarization abnormalities include the MTWA and QT prolongation and dispersion are predictors of SCD. The late potentials (LP) recorded on SAECG are the major depolarization abnormality that predicts SCD.
We have described the presence of fragmented QRS complexes (fQRS) on a routine 12-lead ECG as another marker of depolarization abnormality [3]. The data suggested that the fQRS represents conduction delay from inhomogeneous activation of the ventricles due to myocardial scar. However, fQRS is not specific for CAD and is also encountered in other myocardial diseases such as cardiomyopathy and congenital heart disease. fQRS has also been described in arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) [4] and Brugada syndrome [5].
DEFINITION OF FRAGMENTED QRS
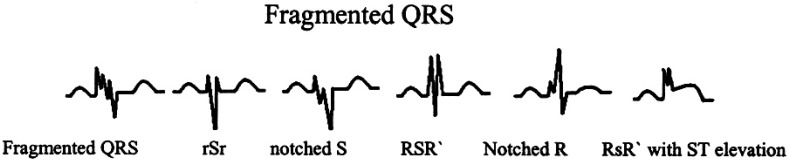
Fragmented QRS (fQRS) is defined on the routine 12 lead ECG (filter range 0.15-100 Hz, AC filter 60 Hz, 25mm/s, 10mm/mV), with the presence of various morphologies of the QRS wave with or without a Q wave and includes the presence of an additional R wave (R’) or notching in the nadir of the R’ (fragmentation) in two contiguous leads, corresponding to a major coronary artery territory [3]. (Fig. 1) In the presence of wide complex QRS (≥ 120 ms) such as a bundle branch block (BBB), premature ventricular complexes (PVC) and paced QRS (pQRS) complexes, it is defined to be present if fragmentation (greater than two notches in the R or S wave ) was recorded in ≥ 2 contiguous anterior leads (V1 to V5) or in ≥ 2 lateral (I, aVL and V6) or in ≥ 2 inferior leads (II, III and aVF). (Fig. 2) [6] fQRS in a premature ventricular complex (PVC) is defined to be present when there are 2 notches in the R waves, > 40 ms apart and present in 2 contiguous leads. Morita et al. defined presence of fQRS in right bundle branch (RBBB) as 1) ≥4 spikes in one or 2) ≥8 spikes in all of the leads V1, V2 and V3 [5].
Fig. (1).
Different morphologies of an fQRS on a 12-lead ECG [3].
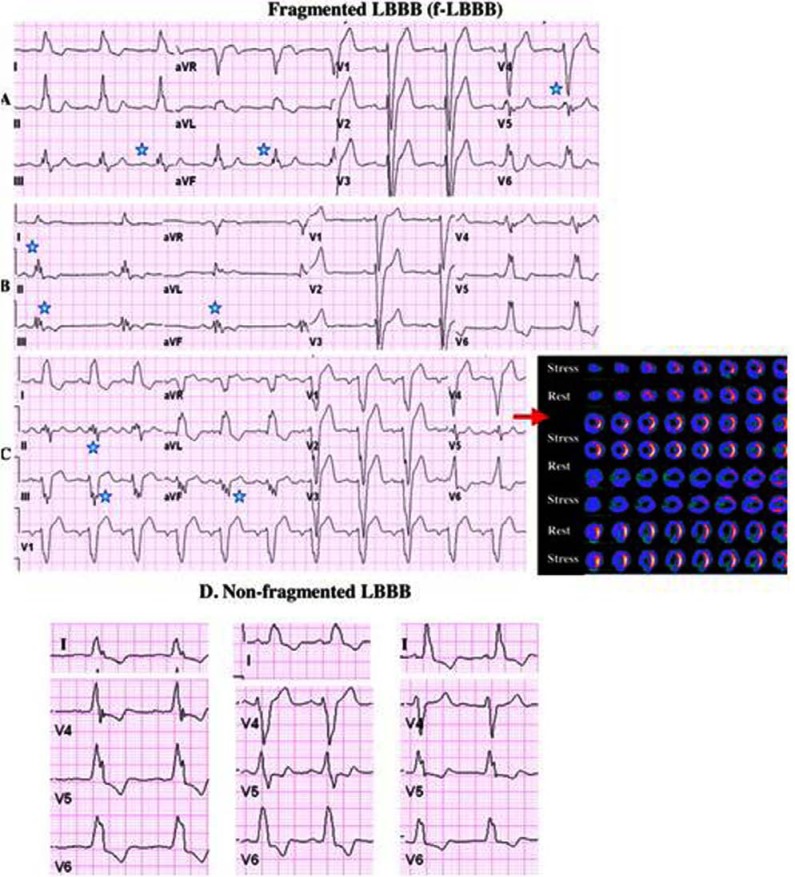
Fig. (2).
Examples of fragmented LBBB (f-LBBB) of 3 different patients are shown in panels A, B, and C. The corresponding myocardial SPECT imaging (upper panels show stress images and the lower panels show the corresponding rest images) of the patient in panel C demonstrates myocardial scar in the LAD territory. Asterisks denote fragmented QRS complexes. Examples of nonfragmented LBBB is shown in panel D. Das M K et al. [6].
SIGNIFICANCE OF FRAGMENTED QRS IN CORONARY ARTERY DISEASE
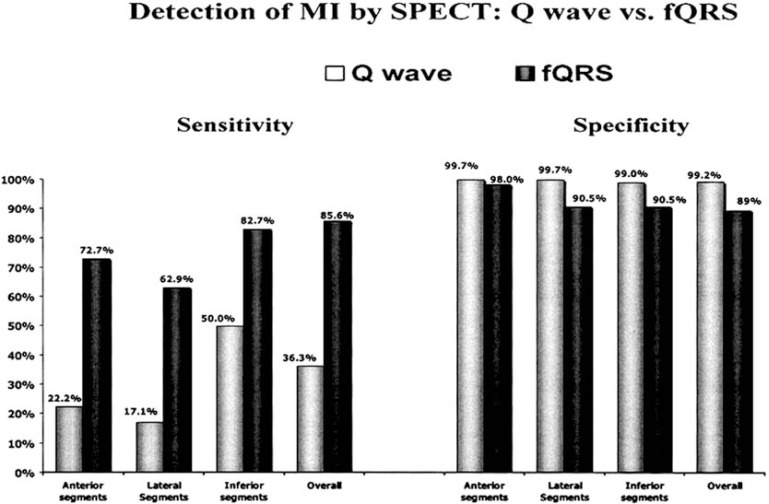
We demonstrated in a cohort of 479 consecutive patients (mean ± SD age, 58 ± 13 years; 283 males) with QRS duration < 120ms who were evaluate for CAD, that fQRS is more sensitive, specific and has a higher negative predictive (91.4%, 89% and 94.2% versus 36.3%, 99.2%, and 70.8%) value than Q wave for myocardial scar (Figs. 3-4) [3]. We extended the concept of fQRS in wide QRS complexes and in patients (n= 879)with wide QRS complex, we showed that fragmented wide QRS (f-wQRS) also represents myocardial scar [6]. The sensitivity, specificity, positive predictive value and negative predictive values of f-QRS for myocardial scar were 87, 93, 92 and 88%, respectively.
Fig. (3).
Sensitivity and specificity of the Q wave and fQRS are shown for each myocardial segment as well as any segment (overall) [3].
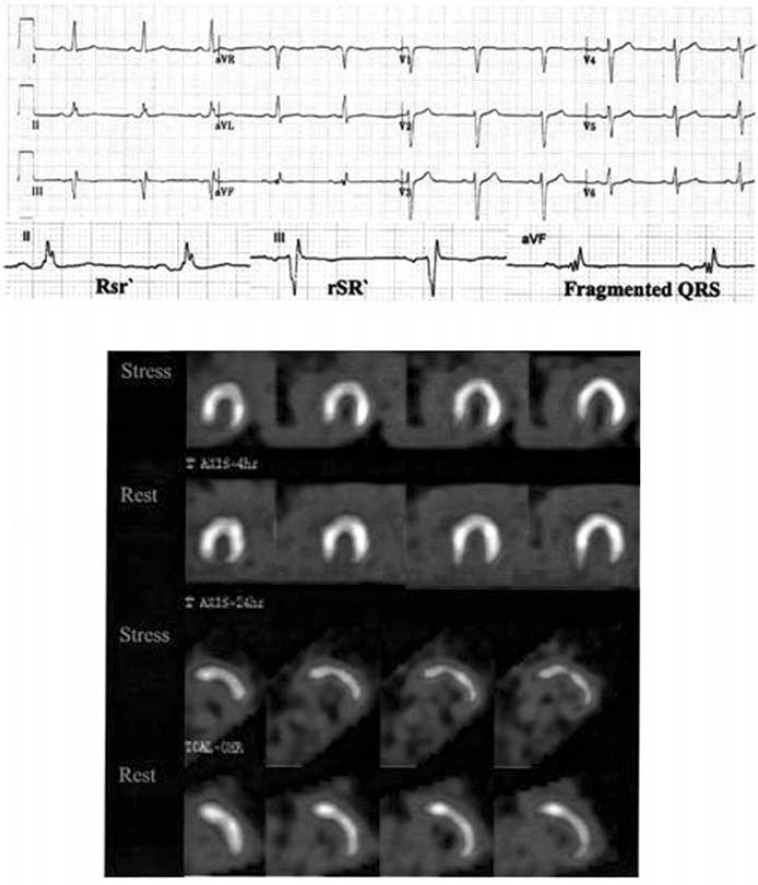
Fig. (4).
Twelve-lead ECG, showing an fQRS (various RSR patterns; QRS duration <120 ms) in inferior leads that is correlated with an inferior wall MI on a myocardial perfusion study (QRS complexes are enlarged in the lower row). The fQRS (a variant of the RSR pattern) is present in lead aVF. There is no Q wave. Nuclear imaging revealed a fixed inferior defect [3].
These above studies raised the question about the time of appearance of fQRS after MI. Therefore, we studied serial ECGs of 896 patients presenting with acute coronary syndrome (ACS) who underwent cardiac catheterization [7]. Serial ECGs were obtained every 6-8 h during the first 24 h of diagnosis of myocardial infarction (MI) and the next day (within 48 h). fQRS developed within 48 h of presentation in 51% of patients who had MI as compared with only 3.7% of patients who had unstable angina (these patients were ruled out for MI). The sensitivity of fQRS for ST elevation MI (STEMI) and non-ST elevation MI (NSTEMI) was 55% and 50%, respectively. The specificity of fQRS was 96% for diagnosing an acute MI in patients with acute coronary syndrome.
SIGNIFICANCE OF fQRS IN PATIENTS WITH CAD AND RELATIVELY PRESERVED LV FUNCTION
Yan et al., evaluated 176 patients ([68 ± 9 years, 145 (82%]) for left ventricular (LV) myocardial strain in patients with CAD with narrow QRS and preserved LV ejection fraction (>45%) [8]. Fifty-five patients (31%) had fQRS complex. Global, middle, and apical LV longitudinal, radial, and circumferential strains and strain rates were significantly lower in the fQRS groups than in the non-fQRS group (all P < 0.05). Multivariate analysis revealed that the fQRS complex was associated with decreased global circumferential strain (odds ratio 1.19; 95% confidence interval 1.06-1.33; P = 0.03) and multi-vessel disease (odds ratio 3.69; 95% confidence interval 1.35-10.08; P =0.011). Kaplan Meier analysis showed that event –free survival for cardiac events was significantly lower in the fQRS group than in the non-fQRS group (P = 0.036).
fQRS AND MYOCARDIAL REPERFUSION IN ACUTE STEMI
In the setting of acute STEMI, it has been shown that fQRS has a significant negative correlation with successful reperfusion after thrombolytic therapy or percutaneous coronary intervention (PCI) [9]. In 100 patients who presented with their first STEMI, myocardial reperfusion was found to be adequate in 34 patients and inadequate in 65 patients. fQRS was detected in 57% in the group having inadequate myocardial reperfusion while only 24% of those having adequate myocardial reperfusion had fQRS(p=0.002). In a smaller study, fQRS after 48 hours from the onset of acute MI was a predictor for cardiac events (death, acute MI, revascularization) in patients with STEMI who had undergone primary PCI [10].
f-QRS AS A PREDICTOR OF ARRHYTHMIC EVENTS AND MORTALITY IN CORONARY ARTERY DISEASE
In 896 patients with ACS with a mean follow up period of 34 ± 16 months the mortality was significantly higher in the fQRS group than the non-fQRS group (56[23%] vs. 81 [12%], p<0.001) [7]. Kaplan-Meier survival analysis revealed significantly decreased survival for the fQRS group compared to the non-fQRS group. After controlling for age, diabetes, hypertension, hypercholesterolemia, history of smoking, and family history of CAD, fQRS, ST segments depression, and T-wave inversion were significant predictors of mortality whereas Q waves and QRS duration did not predict mortality.
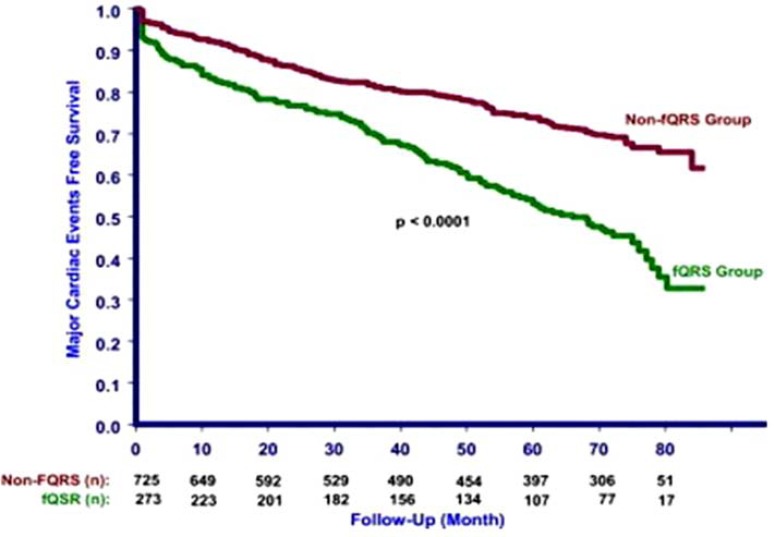
We studied the cardiac events (MI, need for revascularization, or cardiac death) and all-cause mortality in 998 patients (mean age 65.5 +/- 11.9 years, male 967) who underwent nuclear stress test [11]. During a mean follow-up of 57 +/- 23 months, all-cause mortality (93 [34.1%] vs. 188 [25.9%]) and cardiac event rate (135 [49.5%] vs. 200 [27.6%]) were higher in the fQRS group compared with the non-fQRS group. A Kaplan-Meier survival analysis revealed significantly lower event-free survival for cardiac events (P <0.001) and all-cause mortality (P = 0.02). (Fig. 5) Multivariate Cox regression analysis revealed that fQRS was an independent significant predictor for cardiac events but not for all-cause mortality. The Kaplan-Meier survival analysis showed no significant difference between fQRS and Q waves groups for cardiac events (P = 0.48) and all-cause mortality (P = 0.08) [11].
Fig. (5).
Kaplan-Meier analysis showing cardiac events in patients with fragmented QRS (fQRS group) and without fragmented QRS (non-fQRS group). Das MK, et al. [11].
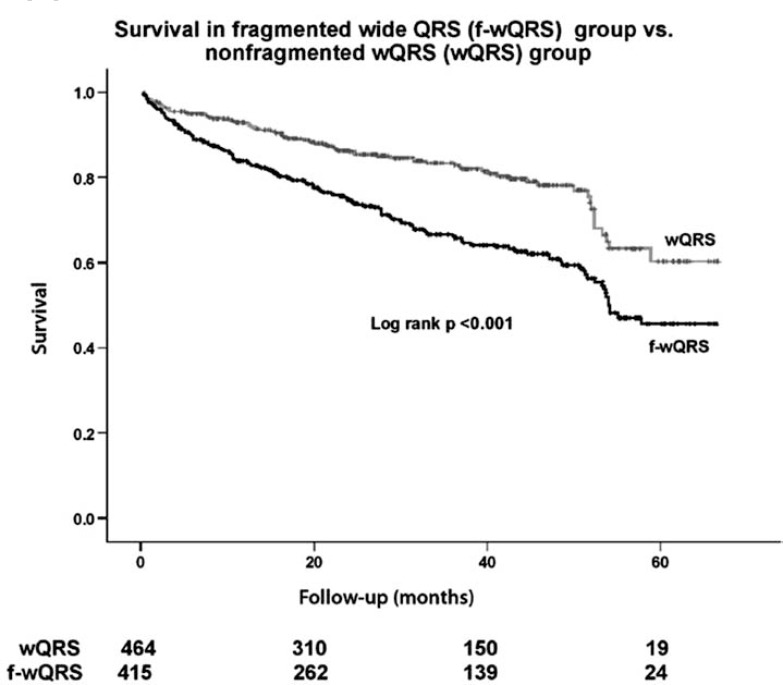
In the study involving wide QRS patients, we showed that in patients with f-wQRS had a significantly higher mortality when compared patients without f-wQRS (P < 0.001) during a mean follow up of 29+18 months (Fig. 6) [12].
Fig. (6).
Kaplan-Meier analysis shows the all-cause mortality in patients with fragmented wide QRS (f-wQRS) group and nonfragmented f-wQRS group. Number of patients at risk during follow-up is shown below the abscissa [12].
Pietrasik et al., studied the predictive value of fQRS in patients with Q wave MI. In this population, fQRS predicted over two fold higher risk (adjusted hazard ratio 2.68, P =0.004) of recurrent cardiac events (cardiac death and non fatal MI) compared with those without fQRS and persistent Q waves [13].
Brenyo et al., in their study of 1,040 ECG’s review of MADIT II enrollment of ischemic cardiomyopathy patients showed presence of fQRS in 33% of patients in any leads with a normal QRS [14]. Inferior location of fQRS was found to be predictive of SCD/ICD shock (hazard ratio 1.46, P=0.032), SCD (HR 2.05, P= 0.007), and total mortality (HR 1.44, P=0.036).
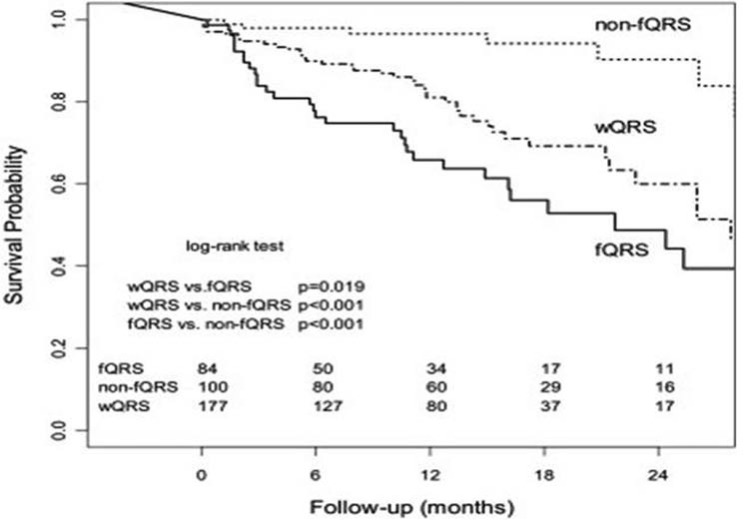
Our study showed that arrhythmic events and mortality in 361 patients (91% male, age 63.3 ± 11.4 years, mean follow-up 16.6 ± 10.2 months) with CAD and NICM who received an ICD for primary or secondary prophylaxis [15]. fQRS was present in 84 (23%) patients (fQRS group) and absent in 100 (28%) patients (non-fQRS group). Wide QRS (wQRS; QRS duration ≥120 ms) was present in 177 (49%) patients. Kaplan-Meier analysis revealed that event-free survival for an arrhythmic event (ICD shock or antitachycardia pacing) was significantly lower in the fQRS group than in the non-fQRS and wQRS groups (P <0.001 and P <.019, respectively). (Fig. 7) fQRS was an independent predictor of an arrhythmic event but not of death.
Fig. (7).
Kaplan-Meier survival analysis for an arrhythmic event in wide QRS (wQRS), fragmented QRS (fQRS,) and nonfragmented QRS (non-fQRS) groups. The wide QRS group included patients with right and left bundle branch block as well as intraventricular conduction delay (QRS ≥120 ms). Numbers of subjects at risk are shown at the bottom of the graph [22].
fQRS AND NONISCHEMIC CARDIOMYOPATHY (NICM)
The diagnostic and prognostic value of QRS fragmentation has not been well studied in patients with NICM; however, it is a common finding on a 12-lead ECG in patients who receive an ICD for primary and secondary prophylaxis. In patients with NICM, QRS fragmentation has been recorded during wavelet ECG analysis recorded from 6 unipolar left precordial leads, using a high precision amplifier [16]. When compared with the control subjects with no heart disease, these wavelets predicted frequent PVCs and SCD.
Sha J et al., studied the prognostic significance of fQRS in 108 patients with NICM and left ventricular dysfunction (ejection fraction, EF </= 40%) [17]. According to QRS duration and the existence of fQRS, the study populations were divided into three groups: (1) the fQRS group (QRS<120 ms and with fragmented QRS, n = 51), (2) the wide QRS (wQRS) group (QRS ≥ 120 ms, n = 48), and (3) the nonfragmented QRS (non-fQRS) group (QRS < 120 ms and without f - QRS, n = 29). The combined end point of all-cause mortality and ventricular tachyarrhythmias was significantly higher in the fQRS and wide QRS groups than the non-fQRS group (23.5%, 25%, and 3.4%, respectively; P < 0.05 for both) during a mean follow-up of 14+/-5 months.
Basaran et al., studied 20 patients with NICM and fQRS by cardiac MRI. Of these 19 patients had significant systolic dyssynchrony with echocardiography. Among 19 patients with significant dyssynchrony, 14 (74%) patients had fQRS complexes in the most delayed contracting segment or one of the dyssynchronous segments, whereas five patients (26%) had fQRS complexes in a lead which is discordant with the dyssynchronous segment on echocardiography [18].
We studied arrhythmic events and all-cause mortality in 105 patients (age 58.7 ± 15.5 years; male, 70) with nonischemic cardiomyopathy who received an ICD for primary and secondary prophylaxis. The study showed that fQRS independently predicts ventricular arrhythmic events (ICD shock or antitachycardia pacing) in patients with CAD or NICM (HR=7.62) who had received an ICD for primary or secondary prophylaxis, but it did not predict mortality [15]. fQRS was present in 54 (51%) patients. During a mean follow-up of 21.6 ± 21.9 months, 29 (53.4%) patients in the fQRS group received ICD therapy (antitachycardia therapy and/or ICD shock) as compared with only 5 (10%) patients in the non-fQRS group (P < .001). The study concluded that combined endpoint of ICD therapy and mortality was also significantly higher in the fQRS group as compared with the non-fQRS group (70% vs. 17.6%, P < .001). Mortality was 24% in the fQRS group and 14% in the non-fQRS group (P = 0.18). Arrhythmic events treated by ICD shocks are generally interpreted as an event of aborted SCD. In fact, appropriate ICD shocks occur more frequently than SCD in patients with nonischemic cardiomyopathy. The data analysis of recent ICD trials have that nearly half of these episodes of nonsustained VT frequently terminate spontaneously in such patients.
However, fragmented QRS is not predictive of arrhythmic events in cardiac resynchronization therapy (CRT) patients and non-fragmented QRS is associated with an improvement of echocardiographic parameters in CRT patients, thus it could be a good marker in identifying responders. Instead, the persistence of paced fragmented QRS is associated with lack of response to CRT making this subgroup of patients less likely to benefit from CRT. Rickard et al. evaluated the effect of fQRS on the efficiency of cardiac resynchronization therapy (CRT) in the cohort of 233 patients with LVEF <= 40% and NYHA class II–VI undergoing implantation of CRT device. Presence of fQRS was assessed on 12-lead standard ECG before the implantation. The end-points of the study were LVEF, LVEDV and LVESV as well as all cause mortality. During mean follow-up of 4.4 ± 1.9 years, there was no difference in all-cause mortality between patients with and without fQRS (44% vs. 36.8%; p = 0.31). Therefore, this study showed no significant association between fQRS and response to CRT, and no effect of fQRS complex on the all-cause mortality among the patients on CRT.
FRAGMENTED QRS: A MARKER FOR ARRHYTHMOGENIC RIGHT VENTRICULAR DYSPLASIA/ CARDIOMYOPATHY (ARVD/C)
Peters et al. reported the role of fQRS in a standard 12 lead ECG in 360 patients with ARVD/C (176 men, mean age: 47.3 ± 13.7 years) and compared its presence with the detection of the epsilon wave in highly amplified right precordial and modified limb leads in a subgroup of 207 patients [4]. In that study, 52 phenotypically and genotypically nonaffected subjects from systematic family screening in 10 families with known plakophilin-2 and desmoplakin mutations served as a controls. fQRS was found in 85% of patients and 4% of controls, whereas epsilon waves in highly amplified right precordial and modified limb leads could be found in 77% of the patients. The number of leads with fQRS in patients with ARVC was associated with a severe form of the disease including the LV involvement. Other ECG signs of ARVD/C include QRS prolongation, prolonged S-wave upstroke, terminal activation delay, and epsilon potentials. Most of these signs are incorporated in typical fQRS.
FRAGMENTED QRS AND CARDIAC SARCOIDOSIS
We studied the usefulness of fQRS in sarcoidosis in 17 patients with biopsy-proven extracardiac sarcoidosis who underwent cardiac magnetic resonance (CMR) with Gadolinium-delayed enhancement (GDE) to evaluate for cardiac sarcoidosis. fQRS and Q wave were present in 9(53%) and 1(6%) patient [19]. The sensitivity and specificity of fQRS for detecting abnormal GDE was 100% and 80%, respectively. Sensitivity and specificity of Q waves were 11% and 100% respectively. In another study of 46 patients with pulmonary sarcoidosis, 75% of patients with cardiac sarcoidosis had fQRS, whereas only 34% of patients without cardiac sarcoidosis had fQRS [20].
FRAGMENTED QRS AND OTHER DEPOLARIZATION ABNORMALITIES IN BRUGADA SYNDROME
Morita et al., showed a 43% incidence of fQRS in 115 patients with Brugada syndrome resuscitated from VF, 28 with syncope, and 74 asymptomatic [5]. The incidence of fQRS was significantly higher in the VF group as compared with the syncope or asymptomatic groups (incidence of fQRS: VF 85%, syncope 50%, and asymptomatic 34%, P< 0.01). Interestingly, the SCN5A mutation in Brugada syndrome ocurred more often in presence of fQRS (33%) than in without fQRS (5%). In patients with syncope or VF, only 6% of patients without fQRS experienced VF during a 43-month follow-up, whereas 58% of patients with fQRS had recurrent syncope caused by VF (P < .0004). Kaplan-Meier survival analysis showed a significantly decreased event (VF)–free survival in the fQRS group as compared with the non-fQRS group. However, the existence of the LP, the mutation of SCN5A and VF induced by programmed electrical stimulation, did not predict the recurrence of syncope. Furthermore, the PRELUDE study has shown that fQRS is useful in determining patients who will benefit from prophylactic ICD placement in the case of Brugada syndrome (HR=4.902), as well as those with spontaneous type1 ECG, history of syncope and short ventricular refractory periods [21].
fQRS AND ACQUIRED LONG QT SYNDROME
In their study, Morita et al. found that fQRS was present in 81% of acquired long QTS patients with syncope/torsades de pointes [22]. Although the onset of torsades de pointes is triggered by early after-depolarization, myocardial scar represented by fQRS is what causes its propagation in the form of reentrant arrhythmia.
J-WAVE AND fQRS
We believe that J wave is a part of depolarization abnormalities and qualifies for our definition of fQRS. Hassaguerre et al., have shown that J wave (early repolarization abnormalities) is associated with idiopathic ventricular fibrillation (IVF) [23]. He compared the incidence of fQRS in 206 who were resuscitated after cardiac arrest due to IVF and assessed the prevalence of electrocardiographic early repolarization as compared to the age, sex, race and level of physical activity matched control group (n= 412). The incidence of J wave was significantly higher in IVF group vs. control (31% vs. 5%, p<0.001). During a mean follow-up of 61±50 months, there was a higher incidence of recurrent ventricular fibrillation in case subjects with a repolarization abnormality than in those without such an abnormality (hazard ratio, 2.1; 95% confidence interval, 1.2 to 3.5; P=0.008). Wang et al., in a smaller population (n-21) of patients, who were resuscitated after cardiac arrest due to Idiopathic Ventricular Fibrillation (IVF), showed that the incidence of events such as syncope, survived cardiac arrest and VF episodes recorded in ICD or pacemakers was the most frequent in the group that showed both fQRS as well as J waves [HR= 3.2 (95%CI, 1.1–7.9; P = 0.01)] [24].
fQRS IN CONGENITAL HEART DISEASE
fQRS is prevalent in patients with congenital heart diseases. In a study involving right ventricle and in adult patients with repaired tetralogy of Fallot, fQRS predicted ventricular fibrosis and was associated with operative scar of the RV, and patients with fQRS had larger right ventricular (RV) volume and lower RV EF [25]. fQRS was closely associated with more extensive RV fibrosis and dysfunction. Delayed and prolonged depolarization of the RV is common in patients with Ebstein anomaly. In a study of 63 patients, the QRS duration was a marker of RV enlargement and dysfunction. QRS fractionation is associated with a greater atrialized RV volume [25]. A preserved surface ECG identifies a subset of patients with Ebstein anomaly with mild morphological and functional abnormalities and better clinical profile [26]. Ventricular arrhythmia events have been reported to occur more frequently in fQRS group than in non-fQRS group of adult patients with Ebstein`s anomaly. Ning et al., have shown that in left ventricular noncompaction cardiomyopathy (n=64) f-fQRS was present in 38% patients while f-wQRS was found in 11% and during follow-up, independent predictor of all-cause mortality (HR: 5.33; P = 0.045) [27].
OTHER ASSOCIATIONS OF fQRS
fQRS has been reported in isolated case studies to be associated with mitral stenosis, delayed Gadolinium enhancement in Hypertrophic Cardiomyopathy (HCM) and with left ventricular non-compaction. However, there are no large enough studies for any significant conclusions. In patients with mitral stenosis caused by rheumatic fever, fQRS was associated with low EF, pulmonary hypertension, poor NYHA functional class, and decreased mitral valve area. A case study of HCM, aborted SCD and implanted with ICD who developed progressive fragmentation of her surface 12 lead EKG raised the question of an association between HCM and fQRS. In their poster presentation, Sheikh et al. showed that the presence of fQRS on 12-lead ECG correlates with DGE (delayed Gadolinium enhancement) on cardiac MRI in patients with HCM, with a specificity of 85.7% and positive predictive value of 85.7%. Electrocardiographic territories containing fragmentation also correlate with myocardial segments of DGE on cardiac MRI thus proving valuable for predicting scar or fibrosis in patients with HCM.
The prevalence of fQRS was significantly higher in patients (n=111) with cardiac AL amyloidosis (28.5% vs. 11.7%; p <0.001) and during a median follow-up of 561days, Kaplan-Meier survival analysis revealed a significantly higher mortality in the fQRS group when compared with the "normal" QRS group (p<0.001) [28]. The prevalence of fQRS has been reported to be higher in patients with rheumatic arthritis and ankylosing spondylitis (37% and 32% respectively) as compared to 6% and 7% in age and sex matched controls.
MECHANISMS OF fQRS IN ARRHYTHMOGENESIS
The mechanism of fragmentation in the QRS complex on the surface 12-lead ECG has been explained by inhomogeneous impulse conduction of the ventricles because of myocardial scar and/or ischemia. Prior studies have defined notching of the QRS wave after an MI as peri-infarction conduction block, which also can be defined as fQRS [29-31]. Flower et al., have shown that the extent of LPs on SAECG in patients with the peri-infarction exceeded those found in patients without peri-infarction block (P < .0001) [31]. There was a significantly higher incidence of sustained ventricular arrhythmias and SCD in patients with peri-infarction block on the surface ECG. Fragmentation of QRS complexes has also been shown to be associated with a remote myocardial scar in smaller studies and in computer models [32, 33]. This concept was also supported by studies with spectral analysis of high frequency electrograms that revealed increased notches and or “slurring” in the electrograms after myocardial injury [34]. The possible mechanism of fragmentation were documented by autopsies of patients with MI and left ventricular aneurysm that confirmed the presence of significant myocardial necrosis, with “islands” of viable myocardial tissue interspersed in abundant fibrous tissue [35, 36]. The islands of chronically ischemic myocardium displayed slow activation as a result of the partially depolarized and depressed action potential upstroke velocities and is probably responsible for inhomogeneous activation of the left ventricle and zigzag conduction around the scarred myocardium causing multiple spikes within the QRS complexes.
The amount, distribution, and pattern of scar depends on the disease states. For example, scar in CAD is usually subendocardial or transmural in the distribution of an occluded coronary artery, whereas scars in patients with nonischemic cardiomyopathy are patchy and mid myocardial or subepicardial. Therefore, different morphologies of fQRS are caused by shifting of the QRS vector during depolarization in and around the areas of scarred or ischemic myocardium, depending on their extent and location in the ventricles. Altered conduction pattern results in slow conduction in the myocardial scar border zones, which may promote reentry and malignant ventricular arrhythmias. The increased arrhythmic events and mortality associated with fQRS reflects significant underlying myocardial disease with an arrhythmic substrate.
Morita et al., showed that fQRS was a marker of conduction abnormality in the absence of structural heart using an isolated canine right ventricular tissue model of Brugada syndrome [5]. In this model, the activation delay in the epicardium reproduced similar fQRS in the transmural ECG. With optical mapping of multisite action potentials, ST-segment elevation resulted from a large phase 1 notch of the action potential in the epicardium and local epicardial activation delay reproduced fQRS in the transmural ECG. A reduced Na+ current in Brugada syndrome not only deepens the phase 1 notch of the AP, but also slows the conduction velocity and reduces the safety factor of conduction, resulting in conduction abnormalities (with regional delay or block of conduction). Although local conduction slowing may be caused by myocardial fibrosis in Brugada syndrome, dynamic and spontaneous changes in the fQRS wave suggest that functional modulation of conduction rather than a fixed scarred myocardium, such as caused by autonomic nerve activity, aging, temperature, or heart rate, can also cause such fragmentation.
LIMITATIONS OF fQRS
Accurate recording of fQRS on a 12-lead ECG requires an optimal low-pass filter setting (100 or 150 Hz). Fragmentation may be missed with lower filter settings such as 40 or 60 Hz. Apart from notching, slurring of fQRS may also represent myocardial scar; however, the present definition of fQRS being qualitative, it cannot be incorporated. A quantitative definition of fQRS with development of specific software is underway. It is to be emphasized that fQRS is a nonspecific finding and should only be interpreted in the presence of pertinent clinical evidence of myocardial scar as in CAD or primary electrical abnormalities of depolarization.
CONCLUSIONS
fQRS represents myocardial scar or inhomogenous myocardial conduction. It is a marker of poor prognosis in patients with acute MI. It is a predictor of ventricular arrhythmia events in patients with ischemic and nonischemic cardiomyopathy. It is also a predictor of mortality in patients with ischemic cardiomyopathy. It is also a marker of poor prognosis and/or risk for arrhythmic events in various cardiomyopathies including nonischemic cardiomyopathy sarcoidosis, and certain congenital heart disease. Above mentioned studies show that fQRS is a nonspecific finding and its diagnostic prognostic should only be interpreted in the presence of pertinent clinical evidence and type of myocardial involvement (structural vs. strutuarlly normal heart). The mechanism of SCD needs to be explored further and will require coordinated translational and clinical studies. The study should focus upon the pathological vs. physiological abnormalities of conduction represented by fQRS. . We, as well as other researchers, have demonstrated the potential role of fQRS in arrhythmogenic mechanisms and as a predictor of mortality and arrhythmic events. fQRS may be of value in determining the risk for SCD and guiding selection for device therapy in patients with structural heart disease and Brugada syndrome. The predictive value of fQRS (a marker of depolarization abnormality) for SCD can be enhanced further by combining a marker of repolarization abnormality such as MTWA or a newer test.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Zipes DP, Camm AJ, Borggrefe Metal. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114(10):e385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 2.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345(20):1473–82. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 3.Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113(21):2495–501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 4.Peters S, Trummel M, Koehler B. QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia-cardiomyopathy. Heart Rhythm. 2008;5(10):1417–21. doi: 10.1016/j.hrthm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Morita H, Kusano KF, Miura Detal. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118(17):1697–704. doi: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 6.Das MK, Suradi H, Maskoun Wetal. Fragmented Wide QRS on a 12-Lead ECG A Sign of Myocardial Scar and Poor Prognosis. Circ Arrhythmia Electrophysiol. 2008;1(4):258–68. doi: 10.1161/CIRCEP.107.763284. [DOI] [PubMed] [Google Scholar]
- 7.Das MK, Michael MA, Suradi H. Usefulness of fragmented QRS on a 12-lead electrocardiogram in acute coronary syndrome for predicting mortality. Am J Cardiol. 2009;104(12):1631–7. doi: 10.1016/j.amjcard.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 8.Yan GH, Wang M, Yiu KHetal. Subclinical left ventricular dysfunction revealed by circumferential 2D strain imaging in patients with coronary artery disease and fragmented QRS complex. Heart Rhythm. 2012;9(6):928–35. doi: 10.1016/j.hrthm.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Erdem FH, Tavil Y, Yazici H, Aygül N, Abaci A, Boyaci B. Association of fragmented QRS complex with myocardial reperfusion in acute ST-elevated myocardial infarction. Ann Noninvasive Electrocardiol. 2013;18(1):69–74. doi: 10.1111/anec.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ari H, Cetinkaya S, Ari S, Koca V, Bozat T. The prognostic significance of a fragmented QRS complex after primary percutaneous coronary intervention. Heart Vessels. 2012;27(1):20–8. doi: 10.1007/s00380-011-0121-9. [DOI] [PubMed] [Google Scholar]
- 11.Das MK, Saha C, El Masry Hetal. Fragmented QRS on a 12-lead ECG a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4(11):1385–92. doi: 10.1016/j.hrthm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Das MK, Suradi H, Maskoun W , et al. Fragmented wide QRS on a 12-lead ECG a sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008;1(4):258–68. doi: 10.1161/CIRCEP.107.763284. [DOI] [PubMed] [Google Scholar]
- 13.Pietrasik G, Goldenberg I, Zdzienicka J, Moss AJ, Zareba W. Prognostic significance of fragmented QRS complex for predicting the risk of recurrent cardiac events in patients with Q-wave myocardial infarction. Am J Cardiol. 2007;100(4):583–6. doi: 10.1016/j.amjcard.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 14.Brenyo A, Pietrasik G, Barsheshet A , et al. QRS fragmentation and the risk of sudden cardiac death in MADIT II. J Cardiovasc Electrophysiol. 2012;23(12):1343–8. doi: 10.1111/j.1540-8167.2012.02390.x. [DOI] [PubMed] [Google Scholar]
- 15.Das MK, Maskoun W, Shen C , et al. Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm. 2010;7(1):74–80. doi: 10.1016/j.hrthm.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 16.Maehara K, Kokubun T, Awano N , et al. Detection of abnormal high-frequency components in the QRS complex by the wavelet transform in patients with idiopathic dilated cardiomyopathy. Jpn Circ J. 1999;63(1):25–32. doi: 10.1253/jcj.63.25. [DOI] [PubMed] [Google Scholar]
- 17.Sha J, Zhang S, Tang M, Chen K, Zhao X, Wang F. Fragmented QRS is associated with all-cause mortality and ventricular arrhythmias in patient with idiopathic dilated cardiomyopathy. Ann Noninvasive Electrocardiol. 2011;16(3):270–5. doi: 10.1111/j.1542-474X.2011.00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tigen K, Karaahmet T, Gurel E , et al. The utility of fragmented QRS complexes to predict significant intraventricular dyssynchrony in nonischemic dilated cardiomyopathy patients with a narrow QRS interval. Can J Cardiol. 2009;25(9):517–22. doi: 10.1016/s0828-282x(09)70137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Homsi M, Alsayed L, Safadi B, Mahenthiran J, Das MK. Fragmented QRS complexes on 12-lead ECG: a marker of cardiac sarcoidosis as detected by gadolinium cardiac magnetic resonance imaging. Ann Noninvasive Electrocardiol. 2009;14(4):319–26. doi: 10.1111/j.1542-474X.2009.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuller JL, Olson MD, Zipse MM , et al. Electrocardiographic characteristics in patients with pulmonary sarcoidosis indicating cardiac involvement. J Cardiovasc Electrophysiol. 2011;22(11):1243–8. doi: 10.1111/j.1540-8167.2011.02099.x. [DOI] [PubMed] [Google Scholar]
- 21.Priori SG, Gasparini M, Napolitano C , et al. Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J Am Coll Cardiol. 2012;59(1):37–45. doi: 10.1016/j.jacc.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 22.Haraoka K, Morita H, Saito Y , et al. Fragmented QRS is associated with torsades de pointes in patients with acquired long QT syndrome. Heart Rhythm. 2010;7(12):1808–14. doi: 10.1016/j.hrthm.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Haïssaguerre M, Derval N, Sacher F , et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358(19):2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Tang M, Mao KX , et al. Idiopathic ventricular fibrillation with fragmented QRS complex and J wave in resting electrocardiogram. J Geriatr Cardiol. 2012;9(2):143–7. doi: 10.3724/SP.J.1263.2011.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamanari H, Nakayama K, Morita H , et al. Effects of cardiac sympathetic innervation on regional wall motion abnormality in patients with long QT syndrome. Heart. 2000;83(3):295–300. doi: 10.1136/heart.83.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egidy Assenza G, Valente AM, Geva T , et al. QRS duration and QRS fractionation on surface electrocardiogram are markers of right ventricular dysfunction and atrialization in patients with Ebstein anomaly. Eur Heart J. 2013;34(3):191–200. doi: 10.1093/eurheartj/ehs362. [DOI] [PubMed] [Google Scholar]
- 27.Ning XH, Tang M, Chen KP , et al. The prognostic significance of fragmented QRS in patients with left ventricular noncompaction cardiomyopathy. Can J Cardiol. 2012;28(4):508–14. doi: 10.1016/j.cjca.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Perlini S, Salinaro F, Cappelli F , et al. Prognostic value of fragmented QRS in cardiac AL amyloidosis. Int J Cardiol. 2013;167(5):2156–61. doi: 10.1016/j.ijcard.2012.05.097. [DOI] [PubMed] [Google Scholar]
- 29.Flowers NC, Horan LG, Thomas JR, Tolleson WJ. The anatomic basis for high-frequency components in the electrocardiogram. Circulation. 1969;39(4):531–9. doi: 10.1161/01.cir.39.4.531. [DOI] [PubMed] [Google Scholar]
- 30.Flowers NC, Horan LG, Tolleson WJ, Thomas JR. Localization of the site of myocardial scarring in man by high-frequency components. Circulation. 1969;40(6):927–34. [PubMed] [Google Scholar]
- 31.Flowers NC, Horan LG, Wylds AC , et al. Relation of peri-infarction block to ventricular late potentials in patients with inferior wall myocardial infarction. Am J Cardiol. 1990;66(5):568–74. doi: 10.1016/0002-9149(90)90483-h. [DOI] [PubMed] [Google Scholar]
- 32.el-Sherif N. The rsR' pattern in left surface leads in ventricular aneurysm. Br Heart J. 1970;32(4):440–8. doi: 10.1136/hrt.32.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varriale P, Chryssos BE. The RSR' complex not related to right bundle branch block: diagnostic value as a sign of myocardial infarction scar. Am Heart J. 1992;123(2):369–76. doi: 10.1016/0002-8703(92)90648-f. [DOI] [PubMed] [Google Scholar]
- 34.Schick TD, Powers SR Jr. Spectral analysis of the high-frequency electrocardiogram in contusive myocardial injury. Ann Biomed Eng. 1978;6(2):154–60. doi: 10.1007/BF02584541. [DOI] [PubMed] [Google Scholar]
- 35.Gardner PI, Ursell PC, Fenoglio JJ Jr, Wit AL. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation . 1985;72(3):596–611. doi: 10.1161/01.cir.72.3.596. [DOI] [PubMed] [Google Scholar]
- 36.Friedman BM, Dunn MI. Postinfarction ventricular aneurysms. Clin Cardiol. 1995;18(9):505–11. doi: 10.1002/clc.4960180905. [DOI] [PubMed] [Google Scholar]