Abstract
Monoclonal gammopathy of undetermined significance is one of the most common pre-malignant disorders. IgG and IgA monoclonal gammopathy of undetermined significance are precursor conditions of multiple myeloma; light-chain monoclonal gammopathy of undetermined significance of light-chain multiple myeloma; and IgM monoclonal gammopathy of undetermined significance of Waldenström’s macroglobulinemia and other lymphoproliferative disorders. Clonal burden, as determined by bone marrow plasma cell percentage or M-protein level, as well as biological characteristics, including heavy chain isotype and light chain production, are helpful in predicting risk of progression of monoclonal gammopathy of undetermined significance to symptomatic disease. Furthermore, alterations in the bone marrow microenvironment of monoclonal gammopathy of undetermined significance patients result in an increased risk of venous and arterial thrombosis, infections, osteoporosis, and bone fractures. In addition, the small clone may occasionally be responsible for severe organ damage through the production of a monoclonal protein that has autoantibody activity or deposits in tissues. These disorders are rare and often require therapy directed at eradication of the underlying plasma cell or lymphoplasmacytic clone. In this review, we provide an overview of the clinical relevance of monoclonal gammopathy of undetermined significance. We also give general recommendations of how to diagnose and manage patients with monoclonal gammopathy of undetermined significance.
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is one of the most common pre-malignant disorders and affects approximately 3.5% of the population over 50 years of age.1–3 IgG and IgA MGUS are defined by a M-protein less than 30 g/L, bone marrow (BM) plasma cell percentage less than 10%, and absence of signs or symptoms related to multiple myeloma (MM) (hypercalcemia, renal insufficiency, anemia, or bone lesions) or other lymphoproliferative malignancies such as Waldenström’s macroglobulinemia (WM), immunoglobulin light-chain (AL) amyloidosis, chronic lymphocytic leukemia (CLL), or B-cell lymphoma.3,4 For IgM MGUS, there is some controversy concerning the diagnostic criteria. In the Second International Workshop on WM, a consensus panel defined IgM MGUS by the presence of an IgM M-protein (irrespective of IgM concentration) without bone marrow infiltration by lymphoplasmacytic lymphoma,5 whereas the Mayo Clinic criteria require less than 10% BM involvement and IgM M-protein less than 30 g/L6 (Online Supplementary Table S1).
There is an average risk of progression to MM or, to a lesser extent, other lymphoproliferative disorders of 1% per year. Typically, patients with IgG or IgA MGUS progress to MM, and patients with IgM MGUS progress to WM or other lymphoproliferative disorders7 (Figure 1). Light-chain MGUS is the precursor of light-chain MM,1,8,9 and is defined by an abnormal κ/λ free light-chain (FLC) ratio, increase in concentration of the involved light-chain, and absence of expression of a monoclonal peak of immunoglobulin heavy-chain in the serum on immunofixation.1 In contrast, in renal disease and in case of polyclonal B-cell activation there may be increased levels of κ and κ chains, but with a normal ratio. Light-chain MGUS has a prevalence of approximately 0.7–0.8% in persons aged 50 years and older.1,2 Smoldering (asymptomatic) MM (SMM) is a pre-malignant disorder with a higher tumor burden and higher risk of progression compared to MGUS (for a definition see Online Supplementary Table S1). Smoldering WM and idiopathic Bence Jones proteinuria are equivalents of SMM in IgM and light-chain gammopathies, respectively (Online Supplementary Table S1).
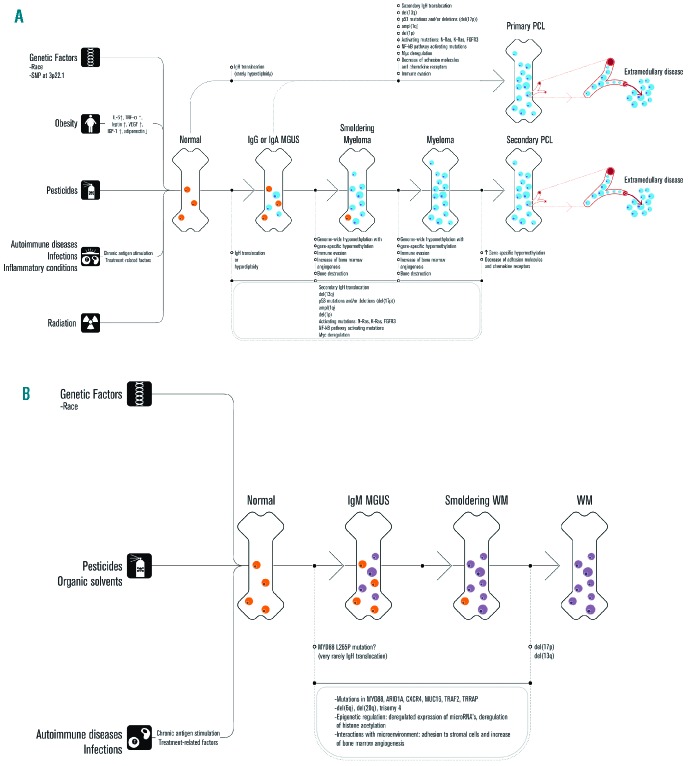
Figure 1.
Model for the mechanisms that contribute to the development and progression of MGUS. Obesity, exposure to pesticides, radiation exposure, and personal history of autoimmune diseases, inflammatory conditions and infections are associated with an increased risk of MGUS. In addition, there is a genetic predisposition to MGUS. (A) Primary immunoglobulin heavy chain (IgH) translocations with 5 recurrent chromosomal partners (4p16, 6p21, 11q13, 16q23, 20q11) and hyperdiploidy are early events and associated with the initiation of limited clonal plasma cell proliferation in non-IgM MGUS. Acquisition of secondary chromosomal abnormalities (such as deletions of (parts of) chromosomes or secondary chromosomal translocations) and mutations involving individual genes results in the stepwise progression from MGUS to newly diagnosed symptomatic MM, and finally aggressive forms of MM such as sPCL or extramedullary MM. During this process there is a progressive replacement of normal/polyclonal plasma cells (orange) by clonal plasma cells (blue). Progression of the plasma cell disorder is also accompanied by altered interactions of the tumor cells with various components of their microenvironment such as osteoclasts, endothelial cells, and cells of the immune system. Recent evidence suggests the presence of intraclonal heterogeneity in MGUS, adding a further level of genetic complexity to the initiation and progression of myeloma. (B) Progression from IgM MGUS to symptomatic Waldenström’s macroglobulinemia also involves a series of genetic changes (activating mutations, inactivating mutations, and chromosomal abnormalities) and altered interactions with the microenvironment. This results in a progressive decrease in normal B cells and normal plasma cells (orange) and increase in clonal B cells and clonal plasma cells (purple).
The importance of MGUS not only lies in the increased risk of developing a hematologic malignancy, but the small clone may also be responsible for severe organ damage through the production of a toxic M-protein which has autoantibody activity or deposits in tissues. In addition, recent studies show that pre-malignant clone-induced alterations in the BM microenvironment result in an increased risk of venous and arterial thrombosis, infections, and osteoporosis. Here, we will discuss the clinical relevance of MGUS, and provide recommendations for diagnosis and follow up.
Methodology
Clinical experts on plasma cell dyscrasias developed these recommendations based on evidence of published data through February 2014. Expert consensus was used to suggest recommendations in case of insufficient data. Grades of recommendations were assigned using the GRADE criteria for grade of recommendation (Online Supplementary Table S2). The recommendations were circulated among the panel members and also discussed in the 2013 EMN Trialist meeting. The manuscript subsequently underwent revision in two rounds until the EMN experts reached a consensus.
Clinical importance of MGUS
1. Regression
Disappearance of the M-protein occurs in approximately 2–5% of patients with MGUS.4,7,10 The majority of these patients have low initial concentrations of M-protein.4 Disappearance of M-protein is often observed after stopping immunosuppressive treatment or initiation of treatment for other disorders such as autoimmune diseases and infections.4,10,11
2. Progression
Analysis of pre-diagnostic serum samples has shown that MM is virtually always preceded by MGUS.8,9 It is unknown whether WM is preceded by IgM MGUS in the majority of the cases.
Progression of MGUS to MM or other related malignancies occurs at a rate of approximately 1% per year.4,10,12 There is no reduction of the risk of progression, even after 25 or 35 years of follow up. However, many MGUS patients are elderly and will die from unrelated diseases.
The risk of progression for light-chain MGUS is lower when compared to conventional MGUS. Only 3 out of 133 light-chain MGUS patients experienced progression to MM during 1100 patient-years of follow up (progression rate 0.27% per year). All 3 patients developed light-chain MM.1 Similarly, in a German study, none of 34 light-chain MGUS cases had progression during a median observation time of five years.2 It cannot be excluded that a small proportion of the patients with apparent light-chain MGUS, do not have a true clonal disorder but rather renal dysfunction or polyclonal activation.1
Predictors of malignant transformation in MGUS
Presenting features as well as the dynamics of the plasma cell clone during the first years of follow up are helpful in predicting risk of progression of MGUS to symptomatic disease (Table 1), and, therefore, in guiding follow up. Clonal burden as determined by BM plasma cell percentage10,12–16 and/or M-protein size4,10,12–15,17–23 is an important risk factor for malignant transformation of MGUS. Furthermore, Rosinol et al. showed that a progressive increase of the M-protein (evolving MGUS) is predictive of progression.14
Table 1.
Predicting factors of malignant transformation in MGUS.
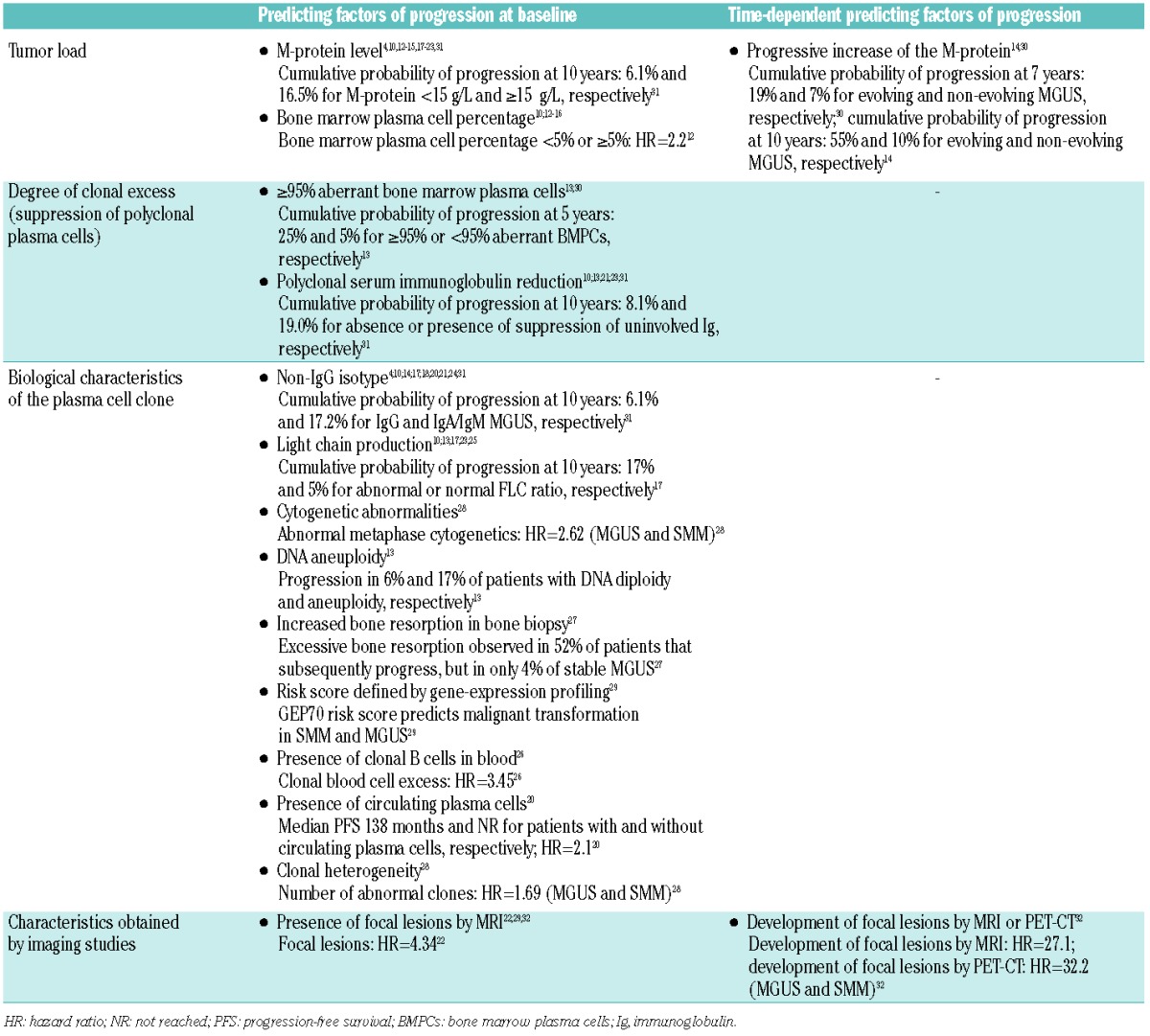
Biological characteristics of the clone also have predictive value in conventional MGUS and these include heavy chain isotype (IgA/IgM > IgG);4,10,14,17,18,20,21,24 light chain production as determined by abnormal serum FLC ratio17,25 or presence of Bence Jones proteinuria;10,13,23 detection of circulating clonal B cells26 or plasma cells;20 increased bone resorption in bone biopsy;27 clonal heterogeneity;28 DNA aneuploidy assessed by flow cytometry;13 and abnormal metaphase cytogenetics.28 Gene expression profiling of purified plasma cells has recently been demonstrated to have prognostic value.29 It is currently unknown whether specific chromosomal abnormalities, including del(17p) and t(4;14), are predictive of malignant progression in MGUS.
In addition, suppression of non-clonal BM plasma cells, based on multiparameter flow cytometric analysis, is a risk factor for progression13,30 (Figure 2). This explains the predictive value of reduction of polyclonal serum immunoglobulins.10,13,21,23,31 Also several imaging techniques may prove to be useful in predicting progression of MGUS. Detection of focal lesions by MRI at baseline22,29,32 or development of focal lesions by MRI or PET-CT predicted for progression to active MM.32 However, there is ongoing discussion of whether patients with focal lesions on MRI should be considered as having early myeloma requiring therapy.
Figure 2.
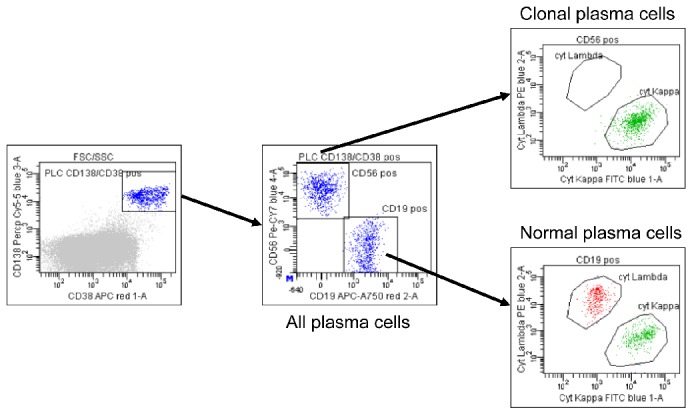
Immunophenotypic characterization of the clonal plasma cells in MGUS. In MGUS normal and malignant plasma cells coexist. The median percentage of clonal plasma cells is approx. 40–73% in MGUS, 97% in SMM, and >99% in MM. Representative dot plots from a MGUS patient show that the total percentage of CD38+ and CD138+ bone marrow plasma cells is 0.74%. Among all bone marrow plasma cells, 48% are polyclonal plasma cells (CD56− and CD19+) and 52% are clonal plasma cells (CD56+, CD19−, and cytoplasmic kappa+).
There are currently no data available on extrinsic factors that promote progression. For instance, patients with pre-transplant MGUS do not seem to be at an increased risk of progression because of chronic immunosuppression.33
When IgM MGUS is evaluated as a separate entity, the average risk for progression is approximately 1.5% per year.7 Serum M-protein and albumin levels were independent predictors of progression in one study,7 whereas in another study M-protein concentration, hemoglobin level, and male sex independently predicted for malignant evolution.34 IgM MGUS and IgM-related disorders (characterized by the presence of specific properties of the IgM M-protein without evidence of underlying lymphoma) have a similar probability of transformation into a lymphoid malignancy.35
So far no predictive factors for progression have been identified for light-chain MGUS. It is unknown whether higher levels of the involved light-chain result in a higher risk of transformation in light chain MGUS. However, in idiopathic Bence Jones proteinuria serum FLC ratio (<0.01 or >100) is associated with a higher risk of progression to MM or AL amyloidosis.36 Other prognostic factors in idiopathic Bence Jones proteinuria include size of urine M-protein, BM plasma cell percentage, serum creatinine, and reduction of all 3 uninvolved immunoglobulins.36
Prediction models
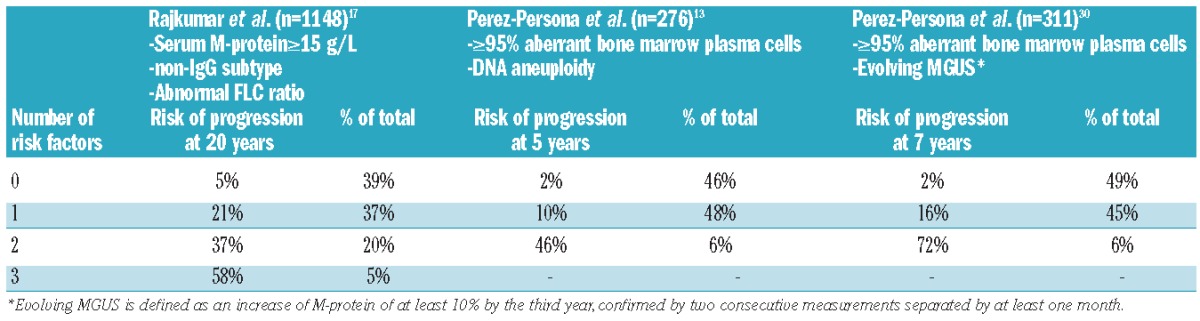
Rajkumar et al. constructed a model for predicting risk of progression based on the size of the M-protein (≥15 g/L), type of M-protein (non-IgG), and the presence of an abnormal FLC ratio.17 The absolute risk of progression at 20 years was 5% for patients without risk factors (low-risk), but the risk increased to 21%, 37%, and 58% for patients with 1 (low-intermediate risk), 2 (high-intermediate risk), or 3 (high risk) of these risk factors, respectively (Table 2). Another prognostic stratification system was proposed by Perez-Persona et al. which is based on the percentage of aberrant plasma cells and DNA aneuploidy, both assessed by flow cytometry.13 Also combination of percentage of aberrant plasma cells with presence of an evolving M-protein identifies 3 different risk groups30 (Table 2).
Table 2.
Models that predict risk of progression of MGUS.
3. Secondary MGUS
During the course of MM, new monoclonal gammopathies of an isotype distinct from the original MM can emerge. This entity has been called secondary MGUS and occurs in 10–73% of MM patients after autologous stem cell transplantion (autoSCT),37–44 and in 1.6–33% of non-transplant patients.37,45 Clinicians should be aware that secondary MGUS does not represent disease recurrence or development of a new malignancy to avoid unnecessary treatment. Oligoclonal reconstitution of the immune system after myeloablative conditioning and impaired T-cell regulation of B-cell proliferation in the BM after autoSCT are likely implicated in the pathogenesis of secondary MGUS.
Interestingly, the quality of response is better among patients with secondary MGUS.40,42,44,45 This may explain the superior overall survival in non-transplant patients with secondary MGUS when compared to MM patients who did not develop secondary MGUS.37 After autoSCT this survival advantage was observed in some studies40–42,44 but not in others.37–39,43
Also after allogeneic (allo)-SCT development of secondary MGUS has been described. Myeloablative conditioning, cytomegalovirus (CMV) infection, and graft-versus-host disease (GVHD) are associated with a higher incidence of monoclonal or oligoclonal bands.46
4. MGUS-associated conditions without direct relationship to the M-protein
MGUS is often diagnosed in the workup of another disease. Since MGUS has a relatively high prevalence and serum immune electrophoresis is performed in patients with certain clinical symptoms, it is often difficult to differentiate true pathogenetic associations from coincidental relationships.47
In this section, we describe disorders that probably have a causal relationship to MGUS through clone-related alterations in the BM microenvironment, such as suppression of normal plasma cells and osteoclast activation. However, it cannot be fully excluded that part of the increased risk for these conditions from MGUS is due to other underlying conditions that are prevalent in persons who are tested for monoclonal gammopathies.
Infections
MGUS patients have an approximately 2-fold increased risk of developing bacterial and viral infections compared to controls.48,49 This can be explained by underlying immunodeficiency including reduced levels of uninvolved immunoglobulins in approximately 25% of the MGUS patients.3,4,10,13,18,21,50,51 In addition, MGUS patients have significantly lower absolute numbers of both CD4+ and CD8+ T cells compared to healthy controls.26
Osteoporosis
MGUS patients have an increased risk of osteoporosis and fractures (axial >distal).47,52–56 Conversely, the prevalence of MGUS is 3.6% in patients with osteoporosis, compared to 2% in patients without osteoporosis.57 The prevalence of MGUS is even higher in patients presenting with acute osteoporotic vertebral fractures (15%).58 There was no difference in risk of malignant transformation in patients with or without fracture.55 Importantly, patients with osteoporosis or fractures should be carefully evaluated to exclude the presence of MM (see section on diagnostic considerations).
Interestingly, MGUS patients have already an altered bone microstructure when compared to healthy controls.53 In addition, some studies show that biochemical markers of bone formation are reduced in MGUS, whereas markers of bone resorption are increased compared to healthy controls.59–61 This may be related to elevated levels of the Wnt pathway inhibitor DKK1 resulting in reduced bone formation,53 combined with increased MIP-1α levels and RANKL/OPG ratio leading to increased bone resorption.53,59
Malignancies
There is an approximately 2–8-fold increased risk of developing myeloid malignancies including myelodysplastic syndrome (MDS), acute myeloid leukemia, and polycythemia vera in MGUS.62,63 Risk factors include IgG/IgA isotype and M-protein greater than 15 g/L.62,63 Host- and MGUS-related factors that contribute to development of myeloid malignancies remain to be defined.62 However, the altered BM microenvironment may play an important role.
Furthermore, MGUS patients have an approximately 1.5-fold increased risk of developing a non-hematologic malignancy.62,64 Apart from a biological association, the higher incidence can also be a reflection of increased surveillance of MGUS patients leading to the detection of earlier cases with cancer. In addition, part of the association can be explained by widespread use of serum electrophoresis as a diagnostic tool in patients suspected of having malignant disease.62,64 Interestingly, first-degree relatives of MGUS65 and MM66 patients also have a small (approx. 10%) but significantly increased risk of developing solid tumors.
Thrombosis
Several studies report an approximately 2–3-fold increased risk of developing deep venous thrombosis or pulmonary embolism in MGUS patients.67–70 Also superficial venous thrombosis seems to be associated with MGUS.47 In addition, a population-based study shows that risk of arterial thrombosis, including coronary artery disease and cerebrovascular disease, is increased in MGUS.68 On the other hand, 2 studies fail to find an association between MGUS and venous or arterial thrombosis.71,72
Prothrombotic changes, such as increases in FVIII and von Willebrand factor in MGUS to similar levels as observed in newly diagnosed MM, may be causally related to the increased thrombosis risk.73 The increased risk of venous and arterial thrombosis was only observed in IgG and IgA MGUS, but not in IgM MGUS.68 Risk of thrombosis did not vary by M-protein level in one study,68 whereas in 2 studies risk of venous thrombosis was increased in patients with higher M-protein levels.72,74
Survival
MGUS patients have a poorer survival than the general population.4,18,48,51 For example, in a large cohort of MGUS patients, median survival was 8.1 years, whereas it was 11.8 years for age- and sex-matched Minnesota residents.4 Excess mortality is not only caused by development of MM and other lymphoproliferative diseases, but there is also an increased risk of dying of myeloid malignancies, non-hematologic cancers, bacterial infections, heart disorders, liver diseases, and renal disorders.18,48,51 This may be explained by a combination of pathogenic mechanisms related to MGUS as well as factors related to co-existing unrelated diseases which lead to medical workup eventually leading to detection of MGUS.48 This indicates that after exclusion of MM and other lymphoproliferative disorders, attention should also be paid to the presence of coexisting diseases that may contribute to the increased mortality in MGUS. In addition, it cannot be ruled out that the increased risk of dying due to infections, renal diseases, and heart disorders is a reflection of early MM, WM, amyloidosis, or another lymphoproliferative malignancy.48
5. M-protein related disorders: presence or development of symptoms related to the M-protein
Sometimes the small clone in MGUS is responsible for severe organ damage through the production of an M-protein which has autoantibody activity or deposits in tissues (AL amyloidosis, monoclonal immunoglobulin deposition disease, and type I cryoglobulinemia).75 Pathogenesis of some of these associated disorders is not well understood and may be related to both M-protein and growth factors produced by the underlying clone, such as in POEMS syndrome.
M-protein related disorders with systemic manifestations
Some of the M-protein related disorders have multi-organ involvement. In AL amyloidosis there is production of misfolded immunoglobulin light-chains that deposit in tissues as amyloid fibrils leading to organ dysfunction. Major clinical manifestations include heart failure, hepatosplenomegaly, nephrotic syndrome, macroglossia, carpal tunnel syndrome, diarrhea, sensorimotor neuropathy, and autonomic neuropathy.
Cryoglobulins are immunoglobulins that precipitate when cooled and re-dissolve when heated, resulting in temperature-dependency of clinical manifestations such as Raynaud phenomenon, acrocyanosis, and cold urticaria. Other cryoglobulinemic symptoms include peripheral neuropathy, renal failure, and skin vasculitis. MGUS may be accompanied by type I or type II cryoglobulinemia.76 Type I consists of monoclonal immunoglobulins (typically IgM or IgG), while in type II cryoglobulinemia, which is often associated with HCV infection, there is a monoclonal autoantibody (mainly IgM) which binds to the Fc portion of polyclonal antibodies.76
The main features of POEMS syndrome include polyneuropathy, organomegaly, endocrinopathy, M-protein (mainly IgG-76 and IgA-λ), sclerotic bone changes, and skin changes. VEGF levels are often markedly elevated and correlate with disease activity.75
Neurological diseases
IgM MGUS is associated with (demyelinating) peripheral neuropathy. Half of the patients with IgM MGUS and peripheral neuropathy have anti-myelin-associated glyco-protein (MAG) antibodies. However, the IgM M-protein can also be directed to other neural antigens including GD1b ganglioside, sulphatide, and chondroitin sulphate, thus leading to immune-mediated nerve damage.77 Rituximab is effective in part of the patients with IgM MGUS-associated neuropathy.77,78 The association between IgA and IgG MGUS and neuropathy is less clear, and MGUS may be an incidental finding without relationship to the neuropathy,77 when AL amyloidosis and POEMS syndrome have been excluded. Especially, in case of neuropathy associated with monoclonal gammopathy with a rapidly progressive disease course an underlying hematologic malignancy has to excluded.79
Blood diseases
Cold agglutinin disease (CAD) is frequently associated with MGUS (mainly IgM-κ). The monoclonal immunoglobulin binds to erythrocyte carbohydrate antigens resulting in hemolytic anemia, Raynaud phenomenon, and acrocyanosis after cold exposure.80 Warm antibody autoimmune hemolytic anemia in MGUS is a very rare phenomenon.
Immune thrombocytopenic purpura (ITP) is more frequent in MGUS patients than in the general population.81 The M-protein may act as an anti-platelet autoantibody.81 Acquired von Willebrand disease is an infrequent complication of MGUS caused by the M-protein that interferes with platelet or collagen binding or accelerates VWF clearance from the circulation.82 Autoantibody-mediated acquired deficiency of FVIII in association with MGUS is very rare.83
Skin
The M-protein seems to play a role in the development of several skin disorders including plane xanthoma and Schnitzler syndrome. However, the contributing role of cytokines released by the plasma cell clone cannot be excluded. In plane xanthomas with monoclonal gammopathy (mainly IgG), the M-protein has antilipoprotein activity in some patients. This results in enhanced lipid accumulation by macrophages and complement activation.84,85 In Schnitzler syndrome there is deposition of para-protein (IgM-κ is most common) at the dermoepidermal junction and in capillary walls which probably contributes to development of chronic urticaria, intermittent fever, arthralgia, bone pain, and lymphadenopathy.86 Treatment with anakinra can be very effective.86 Acquired C1 esterase inhibitor deficiency leading to angio-edema may also be related to the presence of an M-protein with C1 esterase inhibitor-binding activity.87
The contributing role of the M-protein and/or growth factors secreted by the clonal cells is even less clear for other skin diseases that are associated with MGUS including scleromyxedema (mainly IgG-λ), scleredema (mainly IgG-κ), necrobiotic xanthogranuloma (mainly IgG-κ), Sweet syndrome, and pyoderma gangrenosum.88
Metabolic disturbances
Hyperlipidemia is a rare condition in MGUS (mainly IgA), and may be accompanied by xanthomas, hyperviscosity syndrome, and/or atherosclerosis. Binding of the M-protein to lipoproteins, LDL receptor, or lipoprotein lipases results in reduced lipid degradation.89
Renal diseases
Various kidney disorders are the result of toxic M-proteins produced by the MGUS clone, including monoclonal immunoglobulin deposition disease (MIDD, which includes light-chain deposition disease (LCDD), heavy-chain deposition disease (HCDD), and light- and heavy-chain deposition disease (LHCDD)), light-chain proximal tubulopathy (with or without Fanconi syndrome), immunotactoid glomerulopathy, proliferative glomeru-lonephritis with monoclonal Ig deposits (PGNMID), and type 1 and type 2 cryoglobulinemic glomerulonephritis.90–92 In these conditions, the M-protein is the direct cause of the kidney disease and these disorders are characterized by the presence of monoclonal deposits in the kidney. Importantly, M-protein-associated kidney diseases have a high recurrence rate after kidney transplantation. Recently, the term monoclonal gammopathy of renal significance (MGRS) has been proposed for these pathological conditions, to discriminate them from asymptomatic MGUS.90 Importantly, although light-chain MGUS has a low rate of malignant progression, 23% of the patients have or will develop renal disease.1
Treatment of M-protein related disorders
Treatment of M-protein-related disorders is dependent on severity. Institution of supportive care alone may be sufficient in cases with only mild symptoms. However, in general, the most effective treatment of M-protein-related disorders is directed to the underlying clone. Since this approach is potentially toxic, clone-directed therapy should only be considered in cases of aggressive and disabling disease. In addition, therapy directed at eradication of the MGUS clone is only justified when there is a clear causal relationship between MGUS and the associated disorder.
Rituximab monotherapy is recommended in cases of IgM-related disease, such as anti-MAG polyneuropathy. Addition of chemotherapy to rituximab can be considered in cases with severe symptoms and the need for rapid tumor reduction. Since tumor burden is low in IgM-related disease, the duration of immunochemotherapy is generally shorter compared to patients with symptomatic WM.
In non-IgM MGUS-related disorders therapy should rely on antimyeloma agents. In younger patients (≤65–70 years) high-dose melphalan with autoSCT to induce a long-term remission can be considered if the symptoms are severe, progressive and/or disabling, like in POEMS syndrome. Induction therapy preceding autoSCT is probably not needed in case of a small clone, but it may be advantageous for patients with a poor performance status due to the MGUS-associated disorder, and in case with a significant plasma cell clone (M-protein ≥10 g/L). A lenalidomide-based regimen is the first choice in patients with neuropathy, while bortezomib has the highest efficacy in M-protein-associated renal disorders, since it rapidly reduces tumor load and toxic M-proteins. In addition, bortezomib clearance is independent of renal function.92
Diagnostic considerations
Patients with symptoms or laboratory abnormalities, which may be attributable to the underlying MGUS clone
MGUS patients are frequently identified when serum protein electrophoresis is requested as part of a diagnostic assessment for various symptoms including fatigue, recurrent infections and back pain, but also in cases with laboratory abnormalities such as anemia, hypercalcemia, elevated total protein, or renal failure, as well as osteolytic bone lesions. In these settings, we recommend excluding the presence of MM, WM, AL amyloidosis, or CLL by laboratory tests (complete blood count with differential, blood chemistry (including calcium, albumin, and creatinine), serum and urine protein electrophoresis with immunofixation, and measurement of FLCs), BM biopsy and aspiration, and imaging studies (grade of recommendation 1C). These investigations are also recommended in patients with a monoclonal gammopathy and osteoporosis, especially when this combination is present in males and pre-menopausal women.
Asymptomatic patients
An Italian study with 1217 patients showed that the risk of finding a plasma cell infiltration of 10% or over in patients without bone pain with an M-protein of 15 g/L or less or of 10 g/L or less is very low (7.3% and 5.0%, respectively). However, this risk is dependent on IgH isotype (4.7% and 3.5% for IgG isotype; 20.5% and 14.0% for IgA).93 These data are in agreement with a Dutch study that showed that the presence of M-protein concentration 10–15 g/L or more is an important parameter in discriminating between MM and non-myeloma patients.94 No separate analysis was reported for IgG and IgA M-proteins. The Italian study also showed that the probability of finding bone lesions at skeletal survey is very low for both IgG (1.7% and 2%) and IgA isotypes (6.4% and 0.0% for M-protein ≤15 and ≤10 g/L, respectively).93 There are currently no data available to verify whether the FLC assay is of additional value in discriminating between MM/SMM and MGUS.
Based on these data, most experts do not routinely recommend BM examination in asymptomatic patients with apparent IgG MGUS if the serum M-protein is 15 g/L or less and there is no end-organ damage, until there is evidence of progression to symptomatic disease (grade of recommendation 2C). BM examination should be part of the diagnostic workup for all IgA and IgM M-proteins. Imaging is not routinely recommended in patients with a serum IgG M-protein of 15 g/L or less or IgA M-protein of 10 g/L or less without bone pain (grade of recommendation 2C). For all other patients with apparent conventional MGUS imaging should be considered (skeletal survey for non-IgM M-protein; CT scan of chest, abdomen, and pelvis for IgM MGUS) (grade of recommendation 2C). In addition, it can be justified to exclude bone marrow investigation and imaging from the diagnostic workup when there is limited life expectancy because of advanced age or comorbidities.
Although conventional X-rays are still considered the gold standard for diagnosing MM bone disease,95 low-dose whole-body computed tomography (CT), which is faster and more comfortable for the patient, may be a good alternative in MGUS as previously suggested by the IMWG consensus panel on imaging techniques95 (grade of recommendation 2C). Importantly, CT imaging has superior sensitivity and reveals more lesions compared to conventional radiography, which may, therefore, lead to earlier institution of therapy. The value of earlier treatment is currently unknown. At this moment, we do not recommend whole body magnetic resonance imaging (MRI) or positron emission tomography (PET)/CT except in the clinical trial setting.
There are currently no data available to guide diagnostic workup in light-chain MGUS. We do not recommend as routine BM examination and imaging in asymptomatic patients with apparent light-chain MGUS. However, BM evaluation and imaging should be considered in patients with high levels of the involved light-chain (e.g. FLC ratio >10 or <0.10).
Altogether these recommendations will reduce invasive procedures during diagnostic workup in asymptomatic patients with low-risk monoclonal gammopathy.
Other considerations
At diagnosis, not only presence of MM, WM, or other malignant disorders has to be excluded, but also other MGUS-related disorders. Fat, BM, or rectum biopsy with Congo red staining should be performed when AL amyloidosis is suspected. In cases of significant proteinuria or renal insufficiency kidney biopsy is often indicated. Immunofluorescence and electron microscopic studies are essential to demonstrate monoclonal deposits and their pattern of organization.90,92 Other tests may be useful under certain circumstances (Table 3).
Table 3.
Diagnostic evaluation for MGUS.

Furthermore, since there is an increased risk of fractures and osteoporosis in MGUS, it may be appropriate to evaluate patients for excessive bone loss by using dual-energy X-ray absorptiometry (DXA), especially when other risk factors for osteoporosis are present. Bisphosphonates (alendronate or zoledronic acid) have been shown to improve bone mineral density in MGUS patients with osteopenia/osteoporosis or osteoporotic fractures (grade of recommendation 1B).96,97 Patients with reduced bone mineral density or prevalent fractures should receive treatment with bisphosphonates,54 and also calcium and vitamin D supplementation if dietary intake is insufficient.
Although the risk of venous thromboembolism in MGUS is increased, the absolute risk is low. Therefore, there is no indication for standard thrombosis prophylaxis.
Follow up
Since there is no decline in risk of progression, lifelong follow up is generally advised for the majority of MGUS patients to diagnose malignant transformation before the onset of serious complications in order to avoid hospitalizations and costs and to preserve quality of life.98 However, follow up can be optimized based on the patient’s risk of progression and life expectancy. We recommend using the Mayo Clinic risk stratification model to predict progression17 since the three prognostic factors in this model can be easily determined in all MGUS patients (grade of recommendation 1B) (Table 2). Follow up consists of a careful history, physical examination, and laboratory studies (quantification of M-protein, complete blood count, creatinine, and calcium). Therapy should be initiated only when symptomatic disease develops.
There are currently no prospective data available regarding the efficacy of monitoring and optimal frequency of follow up in MGUS patients. However, a retrospective SEER database analysis showed that complication rates of fractures, acute kidney injury, cord compression, or hyper-calcemia were lower in patients with follow up for MGUS and subsequently developed MM or WM (n=1037) compared to those patients who developed MM or WM without preceding follow up (n=16392) (any complication: 20.8% vs. 32.6%) (Online Supplementary Table S3).99 In contrast, a smaller retrospective analysis from the Mayo Clinic of symptomatic myeloma patients with preceding MGUS (n=116) showed that optimal follow up (at least every 3 years) did not result in reduced hospitalizations or decreased myeloma-related complications, compared to suboptimal follow up.98 Overall survival from the time of myeloma diagnosis was similar in both groups.98 Progression between screening visits may contribute to the inadequacy of follow up in this study.98
Patients with intermediate risk (risk of progression at 20 years: 21–37% according to Mayo Clinic risk stratification model17) or high-risk MGUS (risk of progression at 20 years: 58%) should be monitored more closely (at 6 months, and annually thereafter) than patients with low-risk MGUS (risk of progression at 20 years: 5%) for whom less frequent follow up can be justified (at 6 months, and every 1–2 years thereafter) (grade of recommendation 2C) (Table 4). Many patients can receive appropriate follow up of MGUS in primary care. Alternatively, low-risk MGUS patients may not need annual follow up, but only laboratory investigations or BM analysis when symptoms suggestive of MM or related diseases develop. No follow up can also be considered in elderly patients or in patients with significant comorbidity with a short life expectancy. Because of competing causes of death, these patients will probably die before progression of MGUS.
Table 4.
Follow up according to risk of progression and life expectancy.
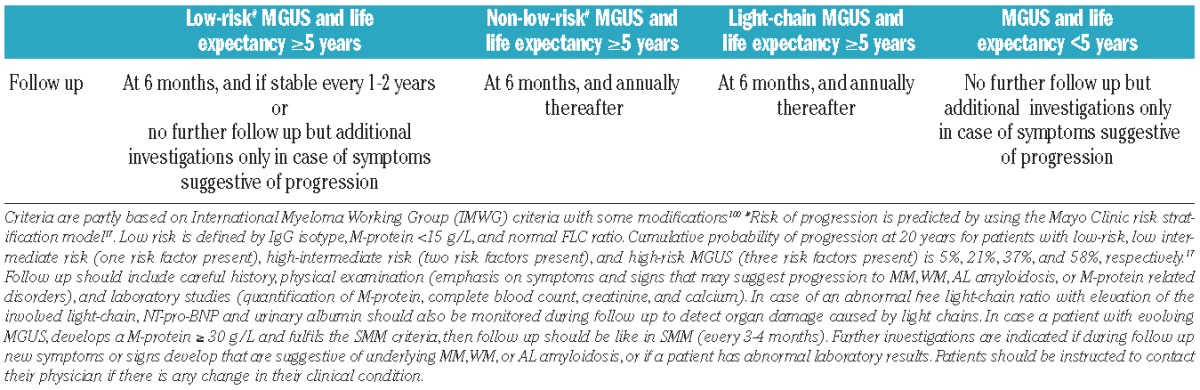
Although the progression rate in light-chain MGUS is low (approx. 0.3% per year) there is a considerable risk of developing renal disease.1 We, therefore, recommend that patients with light-chain MGUS should receive follow up at six months, and every year thereafter (grade of recommendation 2C).1 MGUS patients with elevated free light-chains should also be monitored for development of amyloidosis or LCDD by measuring NT-pro-BNP and urine albumin during follow up. In patients with abnormal findings, additional investigations may include 24-h urine for total protein, echocardiography, and ultrasound for organomegaly.
These recommendations (Table 4) are partly based on the 2010 IMWG guidelines100 with incorporation of life expectancy.
Preventive strategies
There are currently no interventions to prevent or delay progression of MGUS. Intervention approaches should only be performed in the setting of a clinical trial. However, even in studies with high-risk MGUS, extensive follow up and large numbers of patients will be required to demonstrate a meaningful impact on survival and quality of life, in the absence of long-term adverse effects.
Population screening
Screening of the general population is not recommended outside of studies. It is also unknown whether early detection of a monoclonal gammopathy is beneficial among relatives of MGUS, MM, or WM patients. Since there are currently no intervention strategies available, together with the low absolute risk of detecting MGUS or MM in relatives, we recommend screening only as part of a research protocol.
Conclusions and future prospects
MGUS patients have an average risk of progression to MM or, to a lesser extent, other lymphoproliferative disorders of 1% per year. However, at the time of diagnosis and during follow up attention should also be paid to the presence of disorders related to autoantibody activity of the M-protein or resulting from deposition of M-protein in tissues. These M-protein-related disorders are rare but may cause significant morbidity, thereby justifying clone-directed therapy. In addition, there is emerging evidence that the underlying plasma cell or lymphoplasmacytic clone may also contribute to the development of rather common disorders such as osteoporosis and venous thrombosis through induction of alterations in the BM microenvironment (Figure 3). Altogether this clearly demonstrates that the significance of MGUS, for both clinicians and patients, has increased considerably over the last years. We have incorporated these new data in recommendations for diagnostic evaluation and follow up. These recommendations are personalized and based on both life expectancy and risk of progression. We expect that in the nearby future increased knowledge of mechanisms underlying the progression of MGUS to MM or WM results in a further improvement in the identification of patients at high-risk of progression, which will hopefully lead to an even more tailored follow up with start of therapy before serious complications develop.
Figure 3.
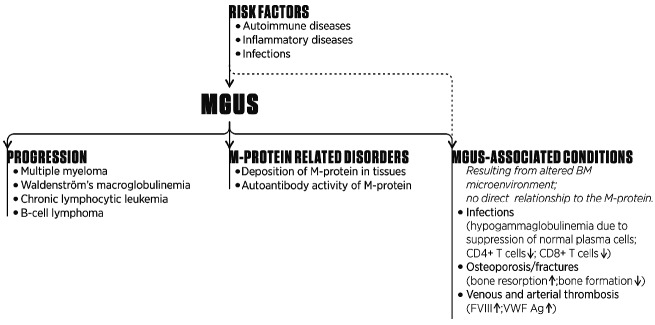
Associations between MGUS and other disorders. MGUS patients have a life-long risk of progression to MM or, to a lesser extent, other lymphoproliferative disorders. In addition, MGUS is also associated with several conditions that may partly result from an altered BM microenvironment due to the underlying plasma cell or lymphoplasmacytic clone. It cannot be fully excluded that part of the increased risk for these conditions from MGUS is due to other underlying conditions that are prevalent in persons who are tested for monoclonal gam-mopathies. Furhermore, several disorders are present or develop in MGUS patients due to deposition of the M-protein in tissues or because of autoantibody activity of the M-protein.
Acknowledgments
The authors thank Victor Muñoz Sanz (Sanz Serif Design Agency) for creating Figure 1 and 3; and Jeroen van Velzen and Andries C. Bloem (Department of Immunology, University Medical Center Utrecht, Utrecht, The Netherlands) for help with the selection of morphological and flow cytometric images; and all participants and discussants of the 6th Trialist Forum, European Myeloma Network, Baveno, 15–16 September, 2013.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
Reference
- 1.Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Melton LJ, III, Colby CL, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet 2010; 375(9727):1721–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisele L, Durig J, Huttmann A, Duhrsen U, Assert R, Bokhof B, et al. Prevalence and progression of monoclonal gammopathy of undetermined significance and light-chain MGUS in Germany. Ann Hematol 2012; 91(2):243–8 [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 2006;354(13):1362–9 [DOI] [PubMed] [Google Scholar]
- 4.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med 2002;346(8):564–9 [DOI] [PubMed] [Google Scholar]
- 5.Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol 2003;30(2):110–5 [DOI] [PubMed] [Google Scholar]
- 6.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2009;23(1):3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyle RA, Therneau TM, Rajkumar SV, Remstein ED, Offord JR, Larson DR, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood 2003;102(10):3759–64 [DOI] [PubMed] [Google Scholar]
- 8.Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood 2009;113(22):5412–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009;113(22):5418–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesana C, Klersy C, Barbarano L, Nosari AM, Crugnola M, Pungolino E, et al. Prognostic factors for malignant transformation in monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. J Clin Oncol 2002;20(6):1625–34 [DOI] [PubMed] [Google Scholar]
- 11.Murray DL, Seningen JL, Dispenzieri A, Snyder MR, Kyle RA, Rajkumar SV, et al. Laboratory persistence and clinical progression of small monoclonal abnormalities. Am J Clin Pathol 2012;138(4):609–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montoto S, Blade J, Montserrat E. Monoclonal gammopathy of undetermined significance. N Engl J Med 2002;346(26): 2087–8 [PubMed] [Google Scholar]
- 13.Perez-Persona E, Vidriales MB, Mateo G, Garcia-Sanz R, Mateos MV, de Coca AG, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 2007;110(7):2586–92 [DOI] [PubMed] [Google Scholar]
- 14.Rosinol L, Cibeira MT, Montoto S, Rozman M, Esteve J, Filella X, et al. Monoclonal gammopathy of undetermined significance: predictors of malignant transformation and recognition of an evolving type characterized by a progressive increase in M protein size. Mayo Clin Proc 2007;82(4):428–34 [DOI] [PubMed] [Google Scholar]
- 15.Van De Donk N, de Weerdt O, Eurelings M, Bloem A, Lokhorst H. Malignant transformation of monoclonal gammopathy of undetermined significance: cumulative incidence and prognostic factors. Leuk Lymphoma 2001;42(4):609–18 [DOI] [PubMed] [Google Scholar]
- 16.Mian M, Franz I, Wasle I, Herold M, Griesmacher A, Prokop W, et al. “Idiopathic Bence-Jones proteinuria”: a new characterization of an old entity. Ann Hematol 2013; 92(9):1263–70 [DOI] [PubMed] [Google Scholar]
- 17.Rajkumar SV, Kyle RA, Therneau TM, Melton LJ, III, Bradwell AR, Clark RJ, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005;106(3):812–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaar CG, le Cessie S, Snijder S, Franck PF, Wijermans PW, Ong C, et al. Long-term follow-up of a population based cohort with monoclonal proteinaemia. Br J Haematol 2009;144(2):176–84 [DOI] [PubMed] [Google Scholar]
- 19.Kyle RA, Rajkumar SV, Therneau TM, Larson DR, Plevak MF, Melton LJ., III Prognostic factors and predictors of outcome of immunoglobulin M monoclonal gammopathy of undetermined significance. Clin Lymphoma 2005;5(4):257–60 [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Rajkumar SV, Kyle RA, Lacy MQ, Dispenzieri A, Fonseca R, et al. Prognostic value of circulating plasma cells in monoclonal gammopathy of undetermined significance. J Clin Oncol 2005;23(24):5668–74 [DOI] [PubMed] [Google Scholar]
- 21.Gregersen H, Mellemkjaer L, Ibsen JS, Dahlerup JF, Thomassen L, Sorensen HT. The impact of M-component type and immunoglobulin concentration on the risk of malignant transformation in patients with monoclonal gammopathy of undetermined significance. Haematologica 2001;86(11): 1172–9 [PubMed] [Google Scholar]
- 22.Hillengass J, Weber MA, Kilk K, Listl K, Wagner-Gund B, Hillengass M, et al. Prognostic significance of whole-body MRI in patients with monoclonal gammopathy of undetermined significance. Leukemia 2014;28(1):174–8 [DOI] [PubMed] [Google Scholar]
- 23.Rossi F, Petrucci MT, Guffanti A, Marcheselli L, Rossi D, Callea V, et al. Proposal and validation of prognostic scoring systems for IgG and IgA monoclonal gammopathies of undetermined significance. Clin Cancer Res 2009;15(13):4439–45 [DOI] [PubMed] [Google Scholar]
- 24.Blade J, Lopez-Guillermo A, Rozman C, Cervantes F, Salgado C, Aguilar JL, et al. Malignant transformation and life expectancy in monoclonal gammopathy of undetermined significance. Br J Haematol 1992; 81(3):391–4 [DOI] [PubMed] [Google Scholar]
- 25.Rajkumar SV, Kyle RA, Therneau TM, Clark RJ, Bradwell AR, Melton LJ, III, et al. Presence of monoclonal free light chains in the serum predicts risk of progression in monoclonal gammopathy of undetermined significance. Br J Haematol 2004;127(3): 308–10 [DOI] [PubMed] [Google Scholar]
- 26.Isaksson E, Bjorkholm M, Holm G, Johansson B, Nilsson B, Mellstedt H, et al. Blood clonal B-cell excess in patients with monoclonal gammopathy of undetermined significance (MGUS): association with malignant transformation. Br J Haematol 1996;92(1):71–6 [DOI] [PubMed] [Google Scholar]
- 27.Bataille R, Chappard D, Basle MF. Quantifiable excess of bone resorption in monoclonal gammopathy is an early symptom of malignancy: a prospective study of 87 bone biopsies. Blood 1996;87(11):4762–9 [PubMed] [Google Scholar]
- 28.Papanikolaou X, Waheed S, Dhodapkar MV, Usmani SZ, Heuck C, van Rhee F, et al. DNA Flow Cytometry and Metaphase Cytogenetics Can Predict Progression of Asymptomatic Monoclonal Gammopathies (AMG) to Symptomatic Multiple Myeloma (MM). ASH Annual Meeting Abstracts 2012; 120(21):2915 [Google Scholar]
- 29.Dhodapkar MV, Sexton R, Waheed S, Usmani S, Papanikolaou X, Nair B, et al. Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120). Blood 2014;123(1):78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Persona E, Mateo G, Garcia-Sanz R, Mateos MV, de Las HN, de Coca AG, et al. Risk of progression in smouldering myeloma and monoclonal gammopathies of unknown significance: comparative analysis of the evolution of monoclonal component and multiparameter flow cytometry of bone marrow plasma cells. Br J Haematol 2010; 148(1):110–4 [DOI] [PubMed] [Google Scholar]
- 31.Katzmann JA, Clark R, Kyle RA, Larson DR, Therneau TM, Melton LJ, III, et al. Suppression of uninvolved immunoglobulins defined by heavy/light chain pair suppression is a risk factor for progression of MGUS. Leukemia 2013;27(1):208–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heuck C, Sexton R, Dhodapkar M, Zhang Q, Usmani S, Nair B, et al. SWOG S0120 Observational Trial for MGUS and Asymptomatic Multiple Myeloma (AMM): Imaging Predictors of Progression for Patients Treated At UAMS. ASH Annual Meeting Abstracts 2011;118(21):3955 [Google Scholar]
- 33.Jimenez-Zepeda VH, Heilman RL, Engel RA, Carey EJ, Freeman C, Rakela J, et al. Monoclonal gammopathy of undetermined significance does not affect outcomes in patients undergoing solid organ transplants. Transplantation 2011;92(5):570–4 [DOI] [PubMed] [Google Scholar]
- 34.Baldini L, Goldaniga M, Guffanti A, Broglia C, Cortelazzo S, Rossi A, et al. Immunoglobulin M monoclonal gammopathies of undetermined significance and indolent Waldenstrom’s macroglobulinemia recognize the same determinants of evolution into symptomatic lymphoid disorders: proposal for a common prognostic scoring system. J Clin Oncol 2005;23(21):4662–8 [DOI] [PubMed] [Google Scholar]
- 35.Morra E, Cesana C, Klersy C, Barbarano L, Varettoni M, Cavanna L, et al. Clinical characteristics and factors predicting evolution of asymptomatic IgM monoclonal gammopathies and IgM-related disorders. Leukemia 2004;18(9):1512–7 [DOI] [PubMed] [Google Scholar]
- 36.Kyle RA, Larson D, Therneau TM, Dispenzieri A, Benson JT, Melton LJ, III, et al. Idiopathic Bence Jones Proteinuria (Smoldering Monoclonal Light-Chain Proteinuria): Clinical Course and Prognosis. ASH Annual Meeting Abstracts 2012; 120(21):1861 [Google Scholar]
- 37.Wadhera RK, Kyle RA, Larson DR, Dispenzieri A, Kumar S, Lazarus HM, et al. Incidence, clinical course, and prognosis of secondary monoclonal gammopathy of undetermined significance in patients with multiple myeloma. Blood 2011;118(11): 2985–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manson GV, Campagnaro E, Balog A, Kaplan D, Sommers SR, Fu P, et al. Secondary MGUS after autologous hematopoietic progenitor cell transplantation in plasma cell myeloma: a matter of undetermined significance. Bone Marrow Transplant 2012;47(9):1212–6 [DOI] [PubMed] [Google Scholar]
- 39.Hovenga S, de Wolf JT, Guikema JE, Klip H, Smit JW, Smit Sibinga CT, et al. Autologous stem cell transplantation in multiple myeloma after VAD and EDAP courses: a high incidence of oligoclonal serum Igs post transplantation. Bone Marrow Transplant 2000;25(7):723–8 [DOI] [PubMed] [Google Scholar]
- 40.Maisnar V, Tichy M, Smolej L, Zak P, Radocha J, Palicka V, et al. Isotype class switching after transplantation in multiple myeloma. Neoplasma 2007;54(3):225–8 [PubMed] [Google Scholar]
- 41.Alejandre ME, Madalena LB, Pavlovsky MA, Facio ML, Corrado C, Milone G, et al. Oligoclonal bands and immunoglobulin isotype switch during monitoring of patients with multiple myeloma and autologous hematopoietic cell transplantation: a 16-year experience. Clin Chem Lab Med 2010; 48(5):727–31 [DOI] [PubMed] [Google Scholar]
- 42.Zent CS, Wilson CS, Tricot G, Jagannath S, Siegel D, Desikan KR, et al. Oligoclonal protein bands and Ig isotype switching in multiple myeloma treated with high-dose therapy and hematopoietic cell transplantation. Blood 1998;91(9):3518–23 [PubMed] [Google Scholar]
- 43.Sucak G, Suyani E, Ozkurt ZN, Yegin ZA, Aki Z, Yagci M. Abnormal protein bands in patients with multiple myeloma after haematopoietic stem cell transplantation: does it have a prognostic significance? Hematol Oncol. 2010;28(4):180–4 [DOI] [PubMed] [Google Scholar]
- 44.Jimenez-Zepeda VH, Franke N, Winter A, Sr, Trudel S, Chen CI, Tiedemann RE, et al. Monoclonal and Oligoclonal Bands After Single Autologous Stem Cell Transplantation in Patients with Multiple Myeloma: Impact On Overall Survival and Progression-Free Survival. ASH Annual Meeting Abstracts 2012;120(21):595 [Google Scholar]
- 45.Mark T, Jayabalan D, Coleman M, Pearse RN, Wang YL, Lent R, et al. Atypical serum immunofixation patterns frequently emerge in immunomodulatory therapy and are associated with a high degree of response in multiple myeloma. Br J Haematol 2008;143(5):654–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hebart H, Einsele H, Klein R, Fischer I, Buhler S, Dietz K, et al. CMV infection after allogeneic bone marrow transplantation is associated with the occurrence of various autoantibodies and monoclonal gammopathies. Br J Haematol 1996;95(1):138–44 [DOI] [PubMed] [Google Scholar]
- 47.Bida JP, Kyle RA, Therneau TM, Melton LJ, III, Plevak MF, Larson DR, et al. Disease associations with monoclonal gammopathy of undetermined significance: a population-based study of 17,398 patients. Mayo Clin Proc 2009;84(8):685–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kristinsson SY, Bjorkholm M, Andersson TM, Eloranta S, Dickman PW, Goldin LR, et al. Patterns of survival and causes of death following a diagnosis of monoclonal gammopathy of undetermined significance: a population-based study. Haematologica 2009;94(12):1714–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gregersen H, Madsen KM, Sorensen HT, Schonheyder HC, Ibsen JS, Dahlerup JF. The risk of bacteremia in patients with monoclonal gammopathy of undetermined significance. Eur J Haematol 1998;61(2):140–4 [DOI] [PubMed] [Google Scholar]
- 50.Vuckovic J, Ilic A, Knezevic N, Marinkovic M, Zemunik T, Dubravcic M. Prognosis in monoclonal gammopathy of undetermined significance. Br J Haematol 1997;97(3):649–51 [DOI] [PubMed] [Google Scholar]
- 51.Gregersen H, Ibsen J, Mellemkjoer L, Dahlerup J, Olsen J, Sorensen HT. Mortality and causes of death in patients with monoclonal gammopathy of undetermined significance. Br J Haematol 2001;112(2):353–7 [DOI] [PubMed] [Google Scholar]
- 52.Melton LJ, III, Rajkumar SV, Khosla S, Achenbach SJ, Oberg AL, Kyle RA. Fracture risk in monoclonal gammopathy of undetermined significance. J Bone Miner Res 2004; 19(1):25–30 [DOI] [PubMed] [Google Scholar]
- 53.Ng AC, Khosla S, Charatcharoenwitthaya N, Kumar SK, Achenbach SJ, Holets MF, et al. Bone microstructural changes revealed by high-resolution peripheral quantitative computed tomography imaging and elevated DKK1 and MIP-1alpha levels in patients with MGUS. Blood 2011;118(25):6529–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pepe J, Petrucci MT, Nofroni I, Fassino V, Diacinti D, Romagnoli E, et al. Lumbar bone mineral density as the major factor determining increased prevalence of vertebral fractures in monoclonal gammopathy of undetermined significance. Br J Haematol 2006;134(5):485–90 [DOI] [PubMed] [Google Scholar]
- 55.Kristinsson SY, Tang M, Pfeiffer RM, Bjorkholm M, Blimark C, Mellqvist UH, et al. Monoclonal gammopathy of undetermined significance and risk of skeletal fractures: a population-based study. Blood 2010;116(15):2651–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gregersen H, Jensen P, Gislum M, Jorgensen B, Sorensen HT, Norgaard M. Fracture risk in patients with monoclonal gammopathy of undetermined significance. Br J Haematol 2006;135(1):62–7 [DOI] [PubMed] [Google Scholar]
- 57.Abrahamsen B, Andersen I, Christensen SS, Skov MJ, Brixen K. Utility of testing for monoclonal bands in serum of patients with suspected osteoporosis: retrospective, cross sectional study. BMJ 2005;330(7495):818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golombick T, Diamond T. Prevalence of monoclonal gammopathy of undetermined significance/myeloma in patients with acute osteoporotic vertebral fractures. Acta Haematol 2008;120(2):87–90 [DOI] [PubMed] [Google Scholar]
- 59.Politou M, Terpos E, Anagnostopoulos A, Szydlo R, Laffan M, Layton M, et al. Role of receptor activator of nuclear factor-kappa B ligand (RANKL), osteoprotegerin and macrophage protein 1-alpha (MIP-1a) in monoclonal gammopathy of undetermined significance (MGUS). Br J Haematol 2004; 126(5):686–9 [DOI] [PubMed] [Google Scholar]
- 60.Woitge HW, Horn E, Keck AV, Auler B, Seibel MJ, Pecherstorfer M. Biochemical markers of bone formation in patients with plasma cell dyscrasias and benign osteoporosis. Clin Chem 2001;47(4):686–93 [PubMed] [Google Scholar]
- 61.Pecherstorfer M, Seibel MJ, Woitge HW, Horn E, Schuster J, Neuda J, et al. Bone resorption in multiple myeloma and in monoclonal gammopathy of undetermined significance: quantification by urinary pyridinium cross-links of collagen. Blood 1997; 90(9):3743–50 [PubMed] [Google Scholar]
- 62.Mailankody S, Pfeiffer RM, Kristinsson SY, Korde N, Bjorkholm M, Goldin LR, et al. Risk of acute myeloid leukemia and myelodysplastic syndromes after multiple myeloma and its precursor disease (MGUS). Blood 2011;118(15):4086–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roeker LE, Larson DR, Kyle RA, Kumar S, Dispenzieri A, Rajkumar SV. Risk of acute leukemia and myelodysplastic syndromes in patients with monoclonal gammopathy of undetermined significance (MGUS): a population-based study of 17 315 patients. Leukemia 2013;27(6):1391–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gregersen H, Mellemkjaer L, Salling IJ, Sorensen HT, Olsen JH, Pedersen JO, et al. Cancer risk in patients with monoclonal gammopathy of undetermined significance. Am J Hematol 2000;63(1):1–6 [DOI] [PubMed] [Google Scholar]
- 65.Kristinsson SY, Goldin LR, Bjorkholm M, Turesson I, Landgren O. Risk of solid tumors and myeloid hematological malignancies among first-degree relatives of patients with monoclonal gammopathy of undetermined significance. Haematologica 2009;94(8): 1179–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kristinsson SY, Bjorkholm M, Goldin LR, Blimark C, Mellqvist UH, Wahlin A, et al. Patterns of hematologic malignancies and solid tumors among 37,838 first-degree relatives of 13,896 patients with multiple myeloma in Sweden. Int J Cancer 2009; 125(9):2147–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kristinsson SY, Fears TR, Gridley G, Turesson I, Mellqvist UH, Bjorkholm M, et al. Deep vein thrombosis after monoclonal gammopathy of undetermined significance and multiple myeloma. Blood 2008;112(9): 3582–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kristinsson SY, Pfeiffer RM, Bjorkholm M, Goldin LR, Schulman S, Blimark C, et al. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: a population-based study. Blood 2010;115(24):4991–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Srkalovic G, Cameron MG, Rybicki L, Deitcher SR, Kattke-Marchant K, Hussein MA. Monoclonal gammopathy of undetermined significance and multiple myeloma are associated with an increased incidence of venothromboembolic disease. Cancer 2004; 101(3):558–66 [DOI] [PubMed] [Google Scholar]
- 70.Gregersen H, Norgaard M, Severinsen MT, Engebjerg MC, Jensen P, Sorensen HT. Monoclonal gammopathy of undetermined significance and risk of venous thromboembolism. Eur J Haematol 2011. 86(2):129–34 [DOI] [PubMed] [Google Scholar]
- 71.Cohen AL, Sarid R. The relationship between monoclonal gammopathy of undetermined significance and venous thromboembolic disease. Thromb Res 2010; 125(3):216–9 [DOI] [PubMed] [Google Scholar]
- 72.Za T, De SV, Rossi E, Petrucci MT, Andriani A, Annino L, et al. Arterial and venous thrombosis in patients with monoclonal gammopathy of undetermined significance: incidence and risk factors in a cohort of 1491 patients. Br J Haematol 2013;160(5):673–9 [DOI] [PubMed] [Google Scholar]
- 73.Auwerda JJ, Sonneveld P, de Maat MP, Leebeek FW. Prothrombotic coagulation abnormalities in patients with paraprotein-producing B-cell disorders. Clin Lymphoma Myeloma 2007;7(7):462–6 [DOI] [PubMed] [Google Scholar]
- 74.Sallah S, Husain A, Wan J, Vos P, Nguyen NP. The risk of venous thromboembolic disease in patients with monoclonal gammopathy of undetermined significance. Ann Oncol 2004;15(10):1490–4 [DOI] [PubMed] [Google Scholar]
- 75.Merlini G, Stone MJ. Dangerous small B-cell clones. Blood 2006; 108(8):2520–30 [DOI] [PubMed] [Google Scholar]
- 76.Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet 2012; 379(9813):348–60 [DOI] [PubMed] [Google Scholar]
- 77.Ramchandren S, Lewis RA. An update on monoclonal gammopathy and neuropathy. Curr Neurol Neurosci Rep 2012;12(1):102–10 [DOI] [PubMed] [Google Scholar]
- 78.Dalakas MC, Rakocevic G, Salajegheh M, Dambrosia JM, Hahn AF, Raju R, et al. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein antibody demyelinating neuropathy. Ann Neurol 2009;65(3):286–93 [DOI] [PubMed] [Google Scholar]
- 79.Eurelings M, Notermans NC, van de Donk NW, Lokhorst HM. Risk factors for hematological malignancy in polyneuropathy associated with monoclonal gammopathy. Muscle Nerve 2001;24(10):1295–302 [DOI] [PubMed] [Google Scholar]
- 80.Berentsen S, Ulvestad E, Langholm R, Beiske K, Hjorth-Hansen H, Ghanima W, et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica 2006;91(4):460–6 [PubMed] [Google Scholar]
- 81.Rossi D, De Paoli L, Franceschetti S, Capello D, Vendramin C, Lunghi M, et al. Prevalence and clinical characteristics of immune thrombocytopenic purpura in a cohort of monoclonal gammopathy of uncertain significance. Br J Haematol 2007;138(2):249–52 [DOI] [PubMed] [Google Scholar]
- 82.Ojeda-Uribe M, Caron C, Itzhar-Baikian N, Debliquis A. Bortezomib effectiveness in one patient with acquired von Willebrand syndrome associated to monoclonal gammopathy of undetermined significance. Am J Hematol 2010;85(5):396. [DOI] [PubMed] [Google Scholar]
- 83.Taher A, Abiad R, Uthman I. Coexistence of lupus anticoagulant and acquired haemophilia in a patient with monoclonal gammopathy of unknown significance. Lupus 2003;12(11):854–6 [DOI] [PubMed] [Google Scholar]
- 84.Loo DS, Kang S. Diffuse normolipidemic plane xanthomas with monoclonal gammopathy presenting as urticarial plaques. J Am Acad Dermatol 1996;35(5 Pt 2):829–32 [DOI] [PubMed] [Google Scholar]
- 85.Szalat R, Arnulf B, Karlin L, Rybojad M, Asli B, Malphettes M, et al. Pathogenesis and treatment of xanthomatosis associated with monoclonal gammopathy. Blood 2011; 118(14):3777–84 [DOI] [PubMed] [Google Scholar]
- 86.Jain T, Offord CP, Kyle R, Dingli D. Schnitzler syndrome - an under diagnosed clinical entity. Haematologica 2013; 98(10):1581–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cicardi M, Beretta A, Colombo M, Gioffre D, Cugno M, Agostoni A. Relevance of lymphoproliferative disorders and of anti-C1 inhibitor autoantibodies in acquired angio-oedema. Clin Exp Immunol 1996;106(3): 475–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Daoud MS, Lust JA, Kyle RA, Pittelkow MR. Monoclonal gammopathies and associated skin disorders. J Am Acad Dermatol 1999; 40(4):507–35 [DOI] [PubMed] [Google Scholar]
- 89.Misselwitz B, Goede JS, Pestalozzi BC, Schanz U, Seebach JD. Hyperlipidemic myeloma: review of 53 cases. Ann Hematol 2010;89(6):569–77 [DOI] [PubMed] [Google Scholar]
- 90.Leung N, Bridoux F, Hutchison CA, Nasr SH, Cockwell P, Fermand JP, et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood 2012;120(22):4292–5 [DOI] [PubMed] [Google Scholar]
- 91.Gertz M, Buadi FK. Case vignettes and other brain teasers of monoclonal gammopathies. Hematology Am Soc Hematol Educ Program. 2012:582–5 [DOI] [PubMed] [Google Scholar]
- 92.Fermand JP, Bridoux F, Kyle RA, Kastritis E, Weiss BM, Cook MA, et al. How I treat monoclonal gammopathy of renal significance (MGRS). Blood 2013;122(22):3583–90 [DOI] [PubMed] [Google Scholar]
- 93.Mangiacavalli S, Cocito F, Pochintesta L, Pascutto C, Ferretti V, Varettoni M, et al. Monoclonal gammopathy of undetermined significance: a new proposal of workup. Eur J Haematol 2013;91(4):356–60 [DOI] [PubMed] [Google Scholar]
- 94.Ong F, Hermans J, Noordijk EM, De Keviet W, Seelen PJ, Wijermans PW, et al. Development of a “Myeloma Risk Score” using a population-based registry on para-proteinemia and myeloma. Leuk Lymphoma 1997;27(5–6):495–501 [DOI] [PubMed] [Google Scholar]
- 95.Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M, Sezer O, et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia 2009; 23(9):1545–56 [DOI] [PubMed] [Google Scholar]
- 96.Pepe J, Petrucci MT, Mascia ML, Piemonte S, Fassino V, Romagnoli E, et al. The effects of alendronate treatment in osteoporotic patients affected by monoclonal gammopathy of undetermined significance. Calcif Tissue Int 2008;82(6):418–26 [DOI] [PubMed] [Google Scholar]
- 97.Berenson JR, Yellin O, Boccia RV, Flam M, Wong SF, Batuman O, et al. Zoledronic acid markedly improves bone mineral density for patients with monoclonal gammopathy of undetermined significance and bone loss. Clin Cancer Res 2008;14(19):6289–95 [DOI] [PubMed] [Google Scholar]
- 98.Bianchi G, Kyle RA, Colby CL, Larson DR, Kumar S, Katzmann JA, et al. Impact of optimal follow-up of monoclonal gammopathy of undetermined significance on early diagnosis and prevention of myeloma-related complications. Blood 2010;116(12):2019–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gundrum JD, Neuner JM, Go RS. Cancer Complications At the Time of Multiple Myeloma Diagnosis and the Impact of Monoclonal Gammopathy of Undetermined Significance (MGUS) Follow-up: A Population-Based Study. ASH Annual Meeting Abstracts 2012;120(21):3996 [Google Scholar]
- 100.Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 2010;24(6):1121–7 [DOI] [PMC free article] [PubMed] [Google Scholar]