Natural killer (NK) cells are involved in immune surveillance of various malignancies, including multiple myeloma (MM).1 IPH2101 is a fully human monoclonal antibody that blocks HLA-C binding KIR2D receptors (KIR2DL/DS-1, -2 -3) expressed on the surface of NK-cells, enhancing their cytotoxicity against HLA class I mediated tumor targets by preventing inhibitory KIR signaling. In vitro studies confirm that IPH2101 enhances NK-cell killing of MM-cell lines,2 while data from KIR ligand-mismatched allogeneic stem cell transplants (SCT)3 and infusions of KIR ligand-mismatched allogeneic NK cells prior to autologous SCT4 suggest a potential therapeutic benefit. Since deteriorated NK-cell function tends to occur in later clinical stages of MM,5 we hypothesized that smoldering multiple myeloma (SMM) may be a unique time point for NK-cell based interventions prior to evasion of host immune system by tumor cells.
We conducted a 2-stage phase II clinical trial in SMM patients (Table 1)6–9 by administering IPH2101 at 1 mg/kg intravenously every other month for 6 cycles. A total of 9 SMM patients were enrolled onto the first stage of the study at the National Institutes of Health (NIH) Clinical Center between December 2010 and May 2011. After completion of the first stage, the study was terminated due to lack of patients meeting the defined primary objective (50% decline in M-protein). Clinical response rates during the first 6 cycles of IPH2101 yielded 1 of 9 (11%) patients with minimal response (MR: >25% and <50% decrease in M-protein), 6 of 9 (66%) patients with stable disease (SD), 1 of 9 (11%) with biochemical progression (BP), and 1 of 9 (11%) patients with clinical progression to symptomatic MM. During the follow-up period (median follow up 32 months, range 8–37), 2 additional patients (ns. #5 and #9) progressed to symptomatic MM within 3–6 months after IPH2101 infusions had stopped. IPH2101 infusions were well tolerated with no grade 3 or 4 toxicities reported.
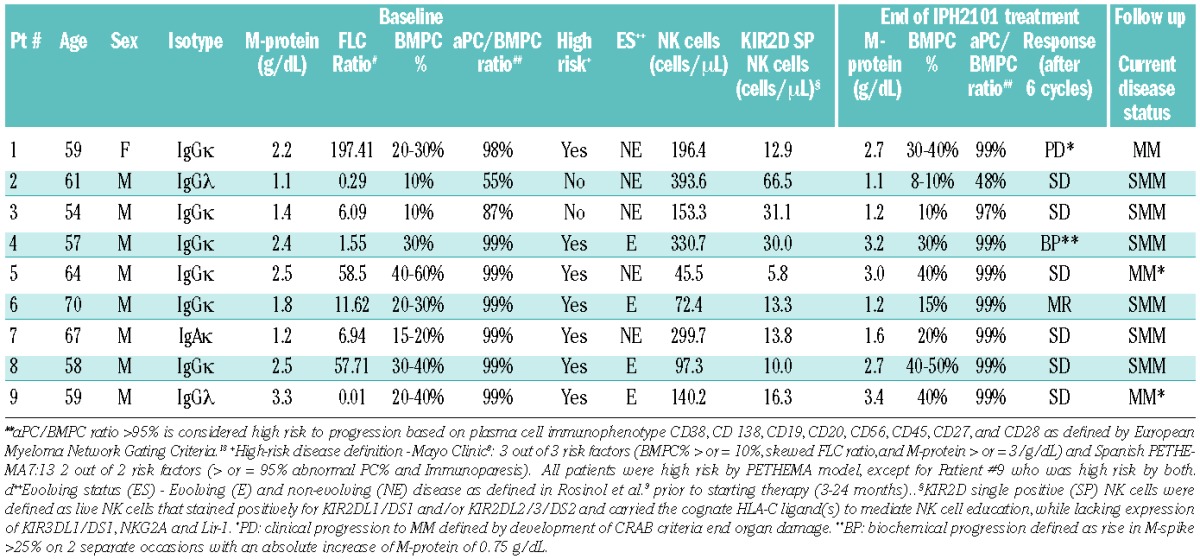
Table 1.
Patients’ characteristics and results. This phase II clinical trial was open for SMM patients (serum M-protein ≥ 3 g/dL and/or bone marrow plasma cells ≥ 10% and absence of end organ damage). The study was planned as a single arm Simon 2-stage design where the first 9 patients were enrolled and monthly responses evaluated after receiving 6 cycles of IPH2101. A cycle was defined as being completed 2 months after the last IPH2101 infusion. If 3 or more patients achieved a 50% reduction in M-protein, the study was designed to go into a second stage to enroll a total of 21 patients. After completion of the first stage interim analysis, the study was terminated due to the lack of patients meeting the defined primary objective (50% decline in M-protein). Current disease status reported after median follow up of 32 months (range 8–37). Clinical progressive disease to MM was based on the IMW criteria for MM.6 In addition to standard criteria for progressive disease, patients were monitored for biochemical progression (asymptomatic, ≥25% M-protein increase from baseline and an absolute increase of M-protein of 0.75 g/dL demonstrated on two separate occasions). Patients #2, 3, 4, 6, 7, and 8 remain asymptomatic with SMM. Patient #6 (MR) demonstrated a 33% decline of base-line M-protein and a 50% decline in CD138+ plasma cells compared to baseline. Of note, the patient suffered an asthma flare requiring a brief course of systemic steroids (50 mg of prednisone for 14 days during cycle 4). Given the sustained response for 6 months and objective decline in M-protein and CD138+ bone marrow plasma cells, the patient received an additional 6 cycles of IPH2101. This patient continues to have no evidence of clinical symptomatic MM, however his subsequent treatment with IPH2101 was again confounded by another short course of steroids for arthritis. Patient #4 with biochemical progression remains asymptomatic with SMM. Patient #1 had clinical progression and was treated for newly diagnosed MM. Patients 5 and 9 had clinical progression during the follow up period and were treated for newly diagnosed MM. The median baseline (pre-treatment) absolute KIR2D SP NK cell counts were compared in patients who had stable disease (SD) or a minimal response (MR) (13.8 cells/mL) versus those who had progressive disease (PD) or biochemical progression (BP) (14.6 cells/mL) during the trial or at follow up with no difference found between the two groups using a Mann-Whitney test (P=0.56) (Figure not shown).
The most common toxicities included: constitutional, i.e. chills, fever (11% grade 1, 22% grade 2); gastrointestinal (GI)-related, i.e. diarrhea, nausea, abdominal discomfort (22% grade 1); liver function test elevation, i.e. increases in ALT, AST (11% grade 2); fatigue (11% grade 1); and creatinine increase (11% grade 1).
To exclude the possibility that the lack of clinical responses observed in this study was related to dysfunctional patient NK cells, we assessed their ability to recognize the gold standard NK-cell target K562. As shown in Figure 1A, degranulation by patient NK cells versus K562 targets was similar to that of healthy subjects. To evaluate whether clinical outcome was related to other NK-cell properties, we measured the absolute number of all NK cells and the number of NK that express KIR2D as their only HLA class I-binding inhibitory receptor (KIR2D single positive (SP) NK cells) (Table 1). It would be predicted that the latter NK-cell subset would be fully augmented against MM cells following KIR blockade with IPH2101. However, neither of these two parameters was found to be associated with disease outcome in our cohort (data not shown).
Figure 1.
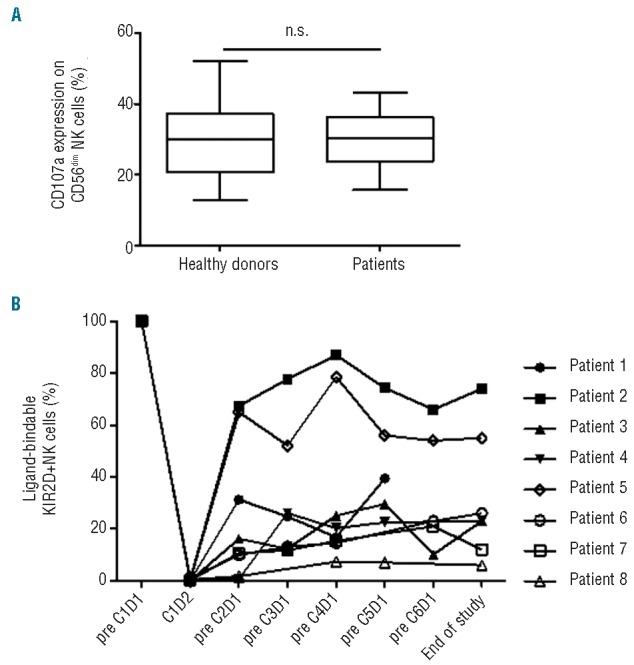
Evaluation of baseline NK cell function and proportion of ligand-bindable KIR2D+ NK-cells. (A) Evaluation of base-line NK cell function. Degranulation, as measured by CD107a expression, by peripheral blood NK cells isolated from patients with smoldering multiple myeloma or from healthy donors was assessed after co-culture with or without K562 cells. CD107a expression on CD56dim NK cells isolated from healthy donors (n=18) versus SMM patients (n=9) following co-culture with K562 cells. Error bars; max and min. Box; quartiles and median. (B) Proportion of ligand-bindable KIR2D+ NK cells per patient. Methodology of assay is described elsewhere.10,11 The proportion of ligand-bindable KIR2D+ NK cells was measured in peripheral blood prior to first infusion (pre-C1D1), one day after first infusion (C1D2) and then before each infusion of IPH2101 (n=8). Six (6) out of eight (8) patients show less than 50% ligand-bindable KIR2D+ NK-cells in peripheral blood throughout the trial. The following patients had missing data point assessment: patient #4 (pre-C6D1), patients #6 and #7 (pre-C5D1), and patient #8 (pre-C3D1 and -C6D1). No pre-treatment sample was obtained for patient #9.
Another factor that potentially impacts the efficacy of this antibody is how efficiently IPH2101 blocks KIR2D on NK cells. We, therefore, measured the proportion of free ligand-bindable KIR2D receptors throughout the trial using previously described methods.10,11 Ligand-bindable KIR2D expression was found to decrease to 0% in all 9 subjects 24 h after first infusion (Figure 1B). In 6 out of 8 patients, this level was maintained at less than 50% compared to baseline when measured prior to each subsequent IPH2101 infusion, suggesting that IPH2101 treatment was able to significantly reduce ligand-bindable KIR2D levels for up to 60 days following each infusion in most patients. Of the 2 patients with 50% or more ligand-bindable KIR2D, Patient #2 had SD and remains asymptomatic with SMM and Patient #5 had clinical progression during the follow-up period. Interestingly, Patient #1 who had clinical progression of disease while being treated with IPH2101 demonstrated 50% or more ligand-bindable KIR2D levels at all time points assessed while on study. These findings suggest base-line numbers of KIR2D expressing NK cells and the efficiency of KIR blockade does not predict clinical outcome in SMM following treatment with IPH2101, despite their having normal base-line NK-cell function.
Recent evidence from a randomized phase III study shows that early treatment with lenalidomide and dexamethasone improves time to progression and overall survival in high-risk SMM patients when compared to observation.12 Several studies are currently exploring platforms of therapy in SMM patients.13 We assessed the potential impact of treating ‘early myeloma’ using an immune-based strategy with IPH2101 in a patient cohort (n=9) that presumably had preserved NK-cell function. Our results show no patients (0 of 9) obtained a 50% reduction in M-protein concentration and the study did not continue to the second stage of enrollment due to lack of efficacy as defined by our criteria. One possible explanation for lack of clinical response may be that recognition of patient MM cells by NK cells is poor. However, we consider this explanation unlikely since NK-cell function was preserved in our cohort (Figure 1A) and previous reports have demonstrated that MM cells from patients with early myeloma14 have sufficient expression of ligands to activate NK-cell receptors. The observation that NK cells can kill MM cells following HLA class I blockade15 further supports the notion that NK cells are capable of recognizing MM cells. Using the previously described indirect method to measure ‘KIR occupancy’10,11 our data show IPH2101 infusions effectively decreased free KIR2D receptor levels on NK cells for up to eight weeks (Figure 1B). However, such methodology assumes that KIR2D surface levels on NK cells are maintained at a constant steady state before and after IPH2101 infusion. Recognizing this limitation, we proposed the term “ligand-bindable KIR2D expression” rather than the “KIR occupancy” used in previous publications. This terminology avoids the assumption that all reductions in NK-cell KIR2D expression occur as a consequence of IPH2101 antibody ‘occupying’ KIR2D, but rather could potentially be attributed to decreased expression of KIR2D molecules on NK cells (decreased production or loss of existing KIR2D receptors) or elimination of KIR2D expressing NK cells.
Although our study showed no clinical response to single agent IPH2101, we cannot exclude the possibility that KIR blockade given in combination with other agents (e.g. lenalidomide) to bolster NK-cell cytotoxicity or in the setting of adoptive transfer of allogeneic or ex vivo expanded autologous NK cells may generate clinically significant responses in future trials.
Acknowledgments
The authors would like to thank all of the patients who contributed to this study. The study was an investigator initiated clinical trial. Study drug was donated by Innate-Pharma under a Clinical Trial Agreement (CTA) with the NCI and NHLBI Division of Intramural Research.
Footnotes
clinicaltrials.gov identifier: NCT01248455
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Godfrey J, Benson DM., Jr The role of natural killer cells in immunity against multiple myeloma. Leuk Lymphoma 2012;53(9):1666–76 [DOI] [PubMed] [Google Scholar]
- 2.Benson DM, Bakan CE, Zhang S, Collins SM, Liang J, Srivastava S, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood 2011;118(24):6387–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroger N, Shaw B, Iacobelli S, Zabelina T, Peggs K, Shimoni A, et al. Comparison between antithymocyte globulin and alemtuzumab and the possible impact of KIR-ligand mismatch after dose-reduced conditioning and unrelated stem cell transplantation in patients with multiple myeloma. Br J Haematol 2005;129(5):631–43 [DOI] [PubMed] [Google Scholar]
- 4.Shi J, Tricot G, Szmania S, Rosen N, Garg TK, Malaviarachchi PA, et al. Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br J Haematol 2008; 143(5):641–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richter J, Neparidze N, Zhang L, Nair S, Monesmith T, Sundaram R, et al. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood 2013;121(3):423–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 2011;117(18):4691–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Persona E, Vidriales MB, Mateo G, Garcia-Sanz R, Mateos MV, de Coca AG, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 2007;110(7):2586–92 [DOI] [PubMed] [Google Scholar]
- 8.Dispenzieri A, Kyle RA, Katzmann JA, Therneau TM, Larson D, Benson J, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood 2008;111(2):785–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosinol L, Blade J, Esteve J, Aymerich M, Rozman M, Montoto S, et al. Smoldering multiple myeloma: natural history and recognition of an evolving type. Br J Haematol 2003;123(4):631–6 [DOI] [PubMed] [Google Scholar]
- 10.Benson DM, Jr, Hofmeister CC, Padmanabhan S, Suvannasankha A, Jagannath S, Abonour R, et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood 2012;120(22):4324–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood 2012;120(22):4317–23 [DOI] [PubMed] [Google Scholar]
- 12.Mateos MV, Hernandez MT, Giraldo P, de la Rubia J, de Arriba F, Corral LL, et al. Lenalidomide plus Dexamethasone for High-Risk Smoldering Multiple Myeloma. N Engl J Med 2013;369(5):438–47 [DOI] [PubMed] [Google Scholar]
- 13.Landgren O, Mailankody S, Kwok M, Manasanch E, Bhutani M, Tageja N, et al. Carfilzomib, lenalidomide, and dexamethasone in high-risk smoldering multiple myeloma. 14th International Myeloma Workshop; 2013; Kyoto, Japan [Google Scholar]
- 14.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, et al. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood 2009;113(15):3503–11 [DOI] [PubMed] [Google Scholar]
- 15.Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood 2005;105(1):251–8 [DOI] [PubMed] [Google Scholar]


