Abstract
Vascular permeability plays a key role in a wide array of life-threatening and sight-threatening diseases. Vascular endothelial growth factor can increase vascular permeability. Using a model system for nonproliferative diabetic retinopathy, we found that pigment epithelium-derived factor (PEDF) effectively abated vascular endothelial growth factor-induced vascular permeability. A 44-amino acid region of PEDF was sufficient to confer the antivasopermeability activity. Additionally, we identified four amino acids (glutamate-101, isoleucine-103, leucine-112, and serine-115) critical for this activity. PEDF, or a derivative, could potentially abate or restore vision loss from diabetic macular edema. Furthermore, PEDF may represent a superior therapeutic approach to sepsis-associated hypotension, nephrotic syndrome, and other sight-threatening and life-threatening diseases resulting from excessive vascular permeability.
Vascular permeability and its regulatory control are central to homeostasis. Increases in vascular permeability play a key role in the development of sepsis-associated hypotension, acute respiratory distress syndrome, nephrotic syndrome, diabetic nephropathy, and diabetic retinopathy. Although the physiologic importance of maintaining the normal vascular integrity is well appreciated, an understanding of how vascular integrity is maintained, and whether vascular permeability can be down-regulated, remains elusive.
The activity of vascular endothelial growth factor (VEGF) in promoting vascular permeability is well established (1). In addition to promoting vascular permeability in guinea pig skin (1), VEGF is an important mediator of angiogenesis in vivo (2, 3) and has neurotrophic/neuroprotective activity (4-6). VEGF exerts its effects on endothelial cells by means of two tyrosine kinase receptors, the fms-like tyrosine kinase-1 (Flt-1; VEGFR-1) and fetal liver kinase-1 (Flk-1/KDR; VEGFR-2) (7). VEGFR-2 is the dominant signaling receptor for many of VEGF's biological activities, including vascular permeability (8, 9).
Pigment epithelium-derived factor (PEDF), a 418-aa 50-kDa glycoprotein, is a member of the serine protease inhibitor (serpin) family (10, 11). Although PEDF has a putative protease-sensitive loop, unlike classical serpins such as α1-antichymotrypsin (ACT), PEDF lacks protease inhibitory activity. Among serpins, this absence of antiprotease activity is not unique to PEDF; heat shock protein 47 (HSP47), a collagen-specific chaperone protein from the serpin family, also lacks antiprotease activity (12). PEDF was originally identified as an extracellular component of the retinal interphotoreceptor matrix (13, 14). PEDF functions in promoting neurite outgrowth in Y79 retinoblastoma cells (15, 16). More recently, PEDF has been found to be a potent antiangiogenic factor (17), effectively inhibiting neovascularization in a murine model of ischemia-induced retinopathy (18).
The biological activities of VEGF and PEDF are similar in some cases but antagonistic in other cases. Both VEGF and PEDF are active in angiogenesis and motoneuron survival. In the vascular endothelial cell system, VEGF and PEDF have counterbalancing proangiogenic and antiangiogenic activities, respectively (17, 19-23). In motoneurons, both PEDF and VEGF function in concert as neurotrophic/neuroprotective agents (24-27). Although the relationships between PEDF and VEGF in angiogenesis and motoneuron survival have been established, it is unknown what effect PEDF has on VEGF's activity in vascular permeability.
The eye is a good model system for studying vascular permeability because the retinal vasculature is highly accessible, both for treatment and for observing subsequent effects. Furthermore, the eye is a relevant model system to study vascular permeability because this process plays a key role in the development of nonproliferative diabetic retinopathy. In this form of retinopathy, increased vascular permeability causes retinal edema and consequent loss of visual acuity. In this study, we delivered VEGF alone or VEGF in combination with PEDF or other agents, by injection into the vitreous of the eye, to assess their effect on vascular permeability. PEDF effectively abated VEGF-induced vascular permeability. We identified four amino acid residues in PEDF important for this activity.
Methods
Preparation of PEDF. Recombinant human PEDF was produced in human embryonic kidney carcinoma 293 (HEK293) cells. Human PEDF cDNA (image clone 235156, Research Genetics, Huntsville, AL) was inserted downstream from the cytomegalovirus promoter into the pRK5-KS plasmid at EcoRI and NotI sites. The human PEDF expression plasmid and pRSV-neomycin were cotransfected by electroporation into cells. Stable transfectants were selected with Geneticin (750 μg/ml, Invitrogen). From among 192 clones, the one secreting the highest level of PEDF was selected by Western blot analysis of conditioned medium (19). PEDF protein was purified from the conditioned medium of this clone according to previously described procedures (28). Instead of Sepharose S ion-exchange chromatography, we used Mono S FPLC, eluting with a linear NaCl gradient (20 mM NaH2PO4, pH 6.2, 0-500 mM NaCl, 10% glycerol). Purified PEDF was a broad 50-kDa band on Coomassie blue-stained SDS/PAGE (data not shown). Aliquots were stored in elution buffer at -80°C.
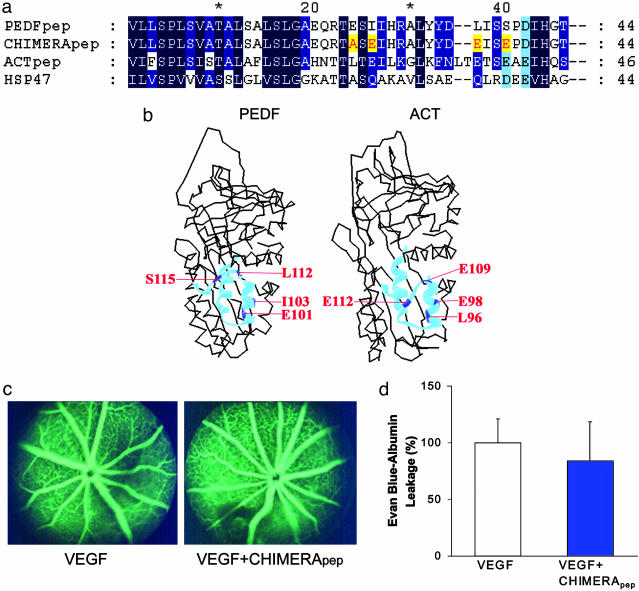
Preparation of Synthetic Peptides. Three peptides (see Fig. 4a) were synthesized by the Howard Hughes Medical Institute Biopolymer Laboratory at Johns Hopkins University School of Medicine. The PEDF peptide (PEDFpep) corresponded to amino acid residues 78-121 of the protein (GenBank accession no. P36955). The ACT peptide (ACTpep) corresponded to residues 73-118 of the protein (GenBank accession no. P01011). A chimeric peptide (CHIMERApep) was 44 amino acids in length, with 40 amino acid residues from PEDF plus four amino acid residues from ACT or HSP47 (GenBank accession no. P29043). The peptides were purified, and their molecular masses were verified by mass spectrometry.
Fig. 4.
Replacement of four amino acid residues on PEDFpep by corresponding residues from ACT or HSP47 abolishes modulation of vascular permeability. Four amino acid residues were substituted in PEDFpep to give CHIMERApep. Corresponding sequences of PEDFpep, CHIMERApep, ACTpep, and HSP47 are aligned (a). Identical and similar amino acid residues are shaded in dark and light blue, respectively. Amino acid substitutions in PEDFpep substituted to give CHIMERApep are highlighted in yellow. The crystallographic structures of PEDF (Protein Data Bank ID code 1IMV) and ACT (Protein Data Bank ID code 1QMN) are shown (b). PEDFpep and ACTpep are highlighted as light blue ribbons in the corresponding regions of PEDF and ACT, respectively. The four amino acid substitutions between PEDFpep and CHIMERApep are highlighted in dark blue and labeled in red. The numbering for both proteins begins at the secretory signal peptide. VEGF was injected in one eye, and VEGF plus CHIMERApep was injected in the contralateral eye. CHIMERApep (equimolar to PEDFpep in Fig. 3a) had no discernible effect on VEGF-induced retinal vascular permeability by fluorescein angiogram (c) or by Evans blue assay (n = 27) (d).
Intravitreal Injection to Assess Bioactivity on Vascular Permeability. C57BL/6J mice (National Cancer Institute, National Institutes of Health), 6-8 weeks of age, were cared for in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and protocols were approved by the Johns Hopkins University Animal Care and Use Committee. For anesthesia, the mice each received an intramuscular injection of ketamine (20 mg/kg; Phoenix Pharmaceuticals, St. Joseph, MO), xylazine (20 mg/kg, Phoenix Pharmaceuticals), and urethane (800 mg/kg, Sigma) in 0.3-0.4 ml of PBS (1.06 mM KH2PO4/0.15 M NaCl/3.00 mM Na2HPO4, pH 7.4). As supplemental topical anesthesia, each eye received one drop of 0.5% tetracaine (Wilson Ophthalmics, Mustang, OK). Under ×10 magnification, 1 μl of recombinant murine VEGF164 solution (12.6 ng/μl in PBS; R & D Systems) was delivered through a 20° beveled glass pipette, with a tip diameter of 13-20 μm. The contralateral eye received an equal volume of PBS alone or PBS containing 12.6 ng of VEGF164 and a 20-fold molar excess of PEDF (232 ng), ACT (278 ng), HSP47 (278 ng), PEDFpep (28.1 ng), ACTpep (29.7 ng), or CHIMERApep (28.2 ng). Routinely, the right eye received VEGF164 plus the putative modulatory agent; the left eye received VEGF164.
Fluorescein Angiography. Twenty hours after intravitreal injections, each pupil was dilated with one drop of 1% atropine sulfate. Ten minutes after the instillation, 0.1 ml of 25% fluorescein (AK-FLUOR 25% AMP, Akorn, Decatur, IL) was injected i.p. Successive photographs of the right and left retinas were taken with a Kowa Genesis small animal fluorescein angiography fundus camera (Tokyo) and Kodak Elite Chrome 400 film. The first photograph was taken when the fluorescence was evident in the eye, typically within 20 s of the i.p. fluorescein injection. Time elapsed between the alternating right and left eye retinal photographs averaged 10 s. Fluorescein leakage manifests as indistinct vascular borders progressing to diffusely hazy fluorescence.
Evans Blue Assay. We used a modification of the method described by Qaum et al. (29) Briefly, each mouse received intravitreal injections of proteins or peptides, as described above. After 24 h, the mouse was anesthetized again in the same manner, and Evans blue (30 mg/ml in PBS, 45 mg/kg mouse weight; Sigma) was injected through the jugular vein. After 2 h, 200 μl of blood was taken and assayed for Evans blue. Each mouse was perfused with 1% paraformaldehyde in 50 mM sodium citrate, pH 3.5, at 37°C to clear the vasculature of Evans blue. The anterior chamber was incised longitudinally. A Crile forceps was used to exert gentle pressure behind the globe and extrude the retina. The retinas were dissected free from the lens, vitreous, and any adherent retinal pigment epithelium. Each retina was dried in a pre-weighed vessel, and dry weight was calculated. The Evans blue dye was extracted with 100 μl of formamide, followed by ultracentrifugation.
To assess the Evans blue-albumin concentration, the absorbance of the retinal extract and plasma samples was measured at 620 nm and 740 nm. The retinal vasopermeability was calculated as the quantity of retinal Evans blue normalized to retinal dry weight, plasma Evans blue concentration, and circulation time by using the formula previously described (29, 30). As all animals in this report had one eye injected with VEGF alone, the retinal permeability in the VEGF-injected eyes was normalized to the VEGF-injected eye in the set of animals where one eye received PBS. The VEGF-induced increase in permeability was taken to be 100%.
Statistical Analysis. All results are expressed as mean ± SE. The paired Student t test was used for comparison of eyes from the same animal. Groups were analyzed for differences by one-way ANOVA. Differences were considered statistically significant when P < 0.05.
Results
Because VEGF promotes angiogenesis and vascular leakage (31) and PEDF is an antagonist to VEGF's angiogenic activity, we asked whether PEDF could also counteract VEGF's vascular permeability activity. To answer the question, we injected VEGF164 (the murine ortholog of human VEGF165) into the left eye and VEGF164 in combination with PEDF into the right eye. If PEDF were a VEGF antagonist, one would expect a decrease in vascular permeability of the right eye compared with the left eye.
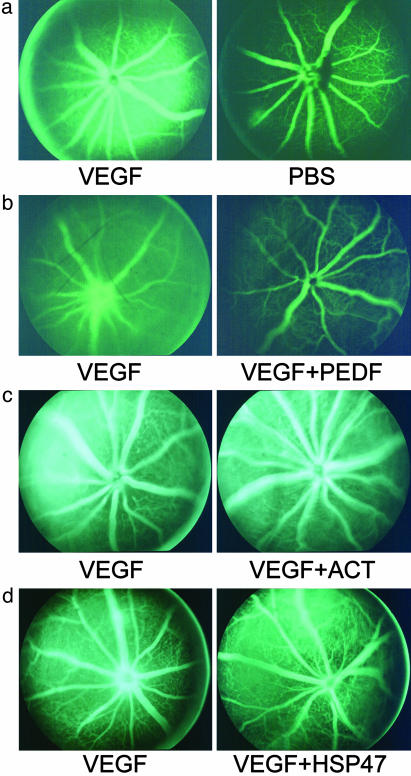
PEDF Inhibits VEGF-Induced Retinal Vascular Permeability Qualitatively. Fluorescein angiography, a clinical diagnostic technique, allows us to see photographically the effect of factors that modulate VEGF-induced permeability. Decreased fluorescence of one eye relative to the contralateral eye can be attributed to different agents injected into the two eyes. Because VEGF promotes vascular permeability, there was, as expected, increased fluorescein leakage in the eye receiving VEGF164 compared with the saline-injected contralateral eye (Fig. 1a). The VEGF-induced vascular permeability was not observed when PEDF was coinjected with VEGF164 (Fig. 1b).
Fig. 1.
PEDF qualitatively inhibits VEGF-induced retinal vascular permeability. Recombinant mouse VEGF164 (VEGF) was injected into one eye and the test reagents coinjected into the contralateral eye. Fluorescein angiography revealed the degree of leakage into the retina and vitreous. VEGF induced vascular leakage to a much higher degree than that observed with PBS (a). Other reagents were coinjected with VEGF: recombinant human PEDF (PEDF) (b), ACT (c), and HSP47 (d). All photographs are characteristic of the results of four or more mice.
To show that the antivasopermeability activity was specific to PEDF, we tested the effect of ACT and HSP47 in the same assay. ACT and HSP47 are from two subfamilies of the serpin super-family (32), distinct from the subfamily to which PEDF belongs. Despite the high level of structural conservation among serpins (33, 34), ACT and HSP47 had no effect on VEGF-induced fluorescein leakage in mouse retina (Fig. 1 c and d). Thus, the inhibitory effect of PEDF on VEGF-induced vascular permeability is specific to PEDF.
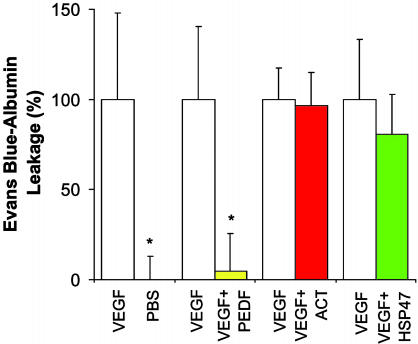
PEDF Inhibits VEGF-Induced Retinal Vascular Permeability Quantitatively. To quantify and confirm PEDF's ability to inhibit VEGF-induced vascular permeability, we used a modified Evans blue assay (30). Mice, injected intravitreally as in the fluorescein angiography experiments, received intravascular Evans blue 24 h later. PEDF nearly abolished (95.6 ± 21.2%) the VEGF-induced permeability, whereas ACT and HSP47 had no discernible effect (inhibition of 3.4 ± 18.2% and 19.4 ± 22.3%, respectively) (Fig. 2). These data corroborate quantitatively what we observed qualitatively by fluorescein angiography: PEDF inhibits VEGF-induced retinal vascular permeability.
Fig. 2.
PEDF quantitatively inhibits VEGF-induced retinal vascular permeability. Twenty-four hours after intravitreous injection of recombinant mouse VEGF164 (VEGF) into one eye and test reagents into the contralateral eye, the amount of retinal Evans blue characterizes vascular leakage. The amount of VEGF-induced vascular leakage above control (PBS) was set to 100%. Vascular leakage with PBS injection was set to 0% (n = 29). A second reagent was coinjected with VEGF to test its effect on vascular permeability; human PEDF (n = 26), but not ACT (n = 27) or HSP47 (n = 28), obliterated the VEGF-induced vascular permeability. Data are means ± SE, with n representing the number of mice in each group. *, P < 0.05 compared with vascular permeability induced by VEGF.
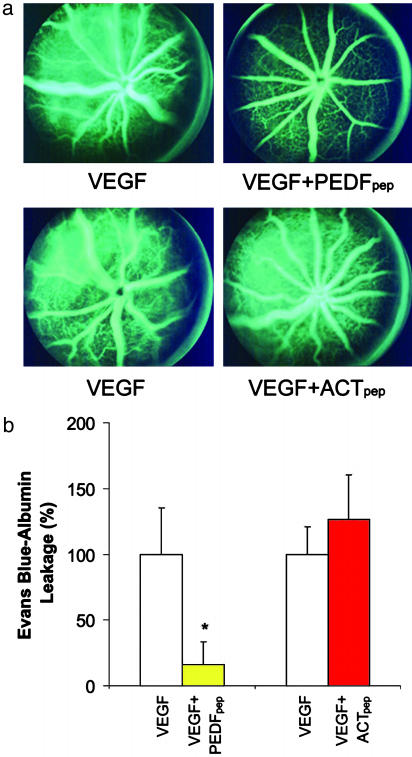
PEDFpep Inhibits VEGF-Induced Vascular Permeability. Because PEDF's neurotrophic/neuroprotective activity has been attributed to a 44-aa region (24, 25), we asked whether this region also possesses the permeability modulating activity. PEDFpep, which consists of amino acid residues 78-121 of human PEDF, was injected intravitreally in place of, and in equimolar amounts as, full-length PEDF. The peptide effectively inhibited VEGF-induced vascular permeability in the fluorescein angiographic assay (Fig. 3a). A 46-aa peptide from the corresponding region of ACT (positions 73-118, designated ACTpep) had no effect on VEGF-induced vascular permeability.
Fig. 3.
A 44-aa peptide from human PEDF, PEDFpep, effectively inhibits VEGF-induced retinal vascular permeability. (a) PEDFpep coinjection effectively inhibits VEGF-induced fluorescein leakage from the retinal vasculature (Upper). Mouse eye injected with both VEGF and ACTpep, a peptide from ACT in the corresponding region of PEDFpep, showed no discernible difference from the eye injected with VEGF alone (Lower). (b) PEDFpep coinjection effectively inhibits VEGF-induced vascular permeability quantitatively by Evans blue assay. The VEGF-induced increase in Evans blue was effectively inhibited with coinjection of PEDFpep (n = 26) with VEGF. No inhibition of VEGF-induced vascular permeability was observed with ACTpep coinjection (n = 28), in equimolar amounts as PEDFpep. Data are means ± SE, with n representing the number of mice in each group. *, P < 0.05 compared with vascular permeability induced by VEGF.
The Evans blue assay corroborated the fluorescein angiographic findings (Fig. 3b). PEDFpep blocked 83.7 ± 17.1% of VEGF-induced retinal vascular permeability to Evans blue-albumin. Similar to full-length ACT, ACTpep did not inhibit VEGF-induced vascular permeability (-26.4 ± 34.3%). Full-length PEDF and PEDFpep at equimolar concentrations were similarly potent. Analysis by one-way ANOVA showed no significant difference between their efficacies. The 44-aa region near the N terminus of PEDF confers the inhibitory activity of PEDF on VEGF-induced vascular permeability.
Four Amino Acid Residues Within PEDFpep Are Necessary for Inhibiting VEGF-Induced Vascular Permeability Activity. To identify the amino acid residues essential for the bioactivity, we compared the sequences and crystallographic structures of ACT, HSP47, and PEDF (Fig. 4 a and b) and selected four candidate amino acid residues within PEDF for evaluation as the key moieties. Previous work (24, 25) and our preliminary studies pointed to residues 78-121 of PEDF as the active site. From the crystal structure, the 44-aa region includes the complete secondary structural elements s6B, hB, and hC, one turn of hD, and the connecting loops (34). Both s6B and hB are buried in the interior of PEDF. The elements hC, hD, and the loop connecting them are largely exposed, forming an accessible surface. For this reason, we focused on residues 99-121, which contain hC, the connecting loop, and one turn of hD.
We reasoned that the key amino acids should be residues divergent between PEDF and the two serpins devoid of vascular permeability modulating activity (ACT and HSP47) (Fig. 4a). On this basis, six amino acids were identified. Two of these six amino acid residues were excluded. Arginine-99 was excluded because its change to alanine did not modify PEDF bioactivity (unpublished results). Proline-116 was excluded because of proline's role in maintaining the structure of the peptide backbone. The other four residues in PEDFpep (glutamate-101, isoleucine-103, leucine-112, and serine-115) were modified to create CHIMERApep. Analogously to alanine scanning, glutamate-101 was replaced with alanine, the corresponding residue in HSP47. Isoleucine-103, leucine-112, and serine-115 were replaced with glutamate, the corresponding residues in ACT. At these three residues, ACT and HSP47 share similar electron-rich side groups (glutamine or aspartate in HSP47).
In both the fluorescein angiographic assay and the Evans blue assay, CHIMERApep failed to inhibit VEGF-induced vascular permeability (Fig. 4 c and d). CHIMERApep had no significant effect on VEGF-induced Evans blue-albumin leakage (16.0 ± 27.8%). In a one-way ANOVA test, CHIMERApep was significantly less effective than PEDF in the inhibition of VEGF-induced vascular permeability.
Discussion
We report a previously undocumented activity of PEDF: its ability to counteract VEGF-induced vascular permeability. We found that a 44-aa fragment of PEDF (PEDFpep) was sufficient to render the activity. We identified four amino acid residues within PEDFpep that are important for this activity.
The relationships between the various activities of PEDF and VEGF are not entirely clear. Initial studies showed that PEDF induced neurite outgrowth (13), and VEGF promoted angiogenesis and vascular permeability (1-3, 35-37). The report of PEDF's antiangiogenic activity revealed an antagonistic relationship between PEDF and VEGF. In various types of neuronal cells, PEDF and VEGF share similar activities: both are neurotrophic and neuroprotective (5, 25, 38). Thus, VEGF has a triad of activities: (i) promoting angiogenesis, (ii) promoting neuronal survival and growth, and (iii) promoting vascular permeability. Because PEDF has effects on the first two of the three activities, we asked whether PEDF modulates vascular permeability. Preliminary data suggested that PEDF indeed possesses antivasopermeability activity (39).
Vascular permeability plays a key pathophysiologic role not only in nonproliferative diabetic retinopathy but also in many other disease states. We chose the retinal vasculature as a model system to study PEDF's potential effect on vascular permeability because the retinal vessels are easily observed through the clear optical system of the eye. In nonproliferative diabetic retinopathy, one of the most common causes of vision loss, increased vascular permeability is the sine qua non of diabetic retinal edema. The gold standard diagnostic test for diabetic retinal edema is fluorescein angiography, a test used to demonstrate VEGF's central role in the pathophysiology of diabetic retinopathy (40). The mouse eye injected with VEGF has increased vascular permeability resulting in increased fluorescein leakage. In our study, this increase was counteracted when PEDF was coinjected, a finding confirmed with the quantitative Evans blue assay. Thus, PEDF, like VEGF, also possesses a triad of activities. PEDF not only functions as an antiangiogenic and neurotrophic/neuroprotective agent, it also inhibits pathologically increased vascular permeability. Furthermore, PEDF is naturally present in the eye in significant quantities, and thus these activities may help maintain the normally tight blood-retina barrier of the eye.
The amounts of VEGF and PEDF used in this study were above physiological concentrations. We used a quantity of VEGF that was similar to the amount previously reported to stimulate retinal vascular leakage (31). If the injected VEGF and PEDF were evenly distributed throughout the vitreous cavity, the intravitreal concentrations would be increased by 0.55 μg/ml and 13 μg/ml, respectively. In comparison with intravitreal levels in patients with proliferative diabetic retinopathy, the injected amounts of VEGF (41) and PEDF (42, 43) would result in concentrations that are, respectively, 2 and 1 orders of magnitude higher. Estimation of the level of VEGF and PEDF at or near the vasculature and comparison of the VEGF level in our studies to those from clinical specimens are complicated. The temporal and spatial distributions of VEGF and PEDF are not quantitatively known, and their levels from preproliferative diabetic macular edema, the best suited clinical correlate, are not available. To simulate pathologic vascular leakage, a greater amount of VEGF may be required when the agent is given in a single bolus rather than the steady-state elevation expected in a chronic disease. Given that the relative balance of provasopermeability and antivasopermeability agents may be more important than their absolute quantities (44), a higher PEDF level would be required to thwart the action of the greater amount of injected VEGF.
Because the neurotrophic/neuroprotective function of the triad of PEDF activities is likely to be receptor-mediated (24, 25), is the antivasopermeability effect also receptor mediated? The localization of the active site of both the motoneuron survival and the antivasopermeability activities to the same 44-aa region suggests that the activities are mediated by the same or similar receptors.
To further refine the localization of the active site within PEDFpep, we prepared CHIMERApep with the hypothesis that the bioactivity would be abolished if the key amino acid residues in PEDFpep were replaced by corresponding residues from ACT or HSP47 (highlighted in yellow in Fig. 4a). When the four candidate amino acid residues were mutated in PEDFpep, bioactivity was lost. CHIMERApep, identical to PEDFpep, with the exception of these four amino acid residues, is inactive in antagonizing VEGF-induced vascular permeability by fluorescein angiography or by Evans blue assay. Analysis of full-length PEDF with mutations of subsets of the four amino acids will allow us to assess the relative contribution of each residue to antivasopermeability activity.
In addition to the identification of the neurotrophic/neuroprotective region of PEDF within amino acid residues 78-121, a number of other binding sites on PEDF have been mapped: the acidic heparin binding domain, the collagen binding domain within β-sheet A strands 2 and 3 and helix F, and the serpin-exposed loop at residues 367-387 (34, 45). We analyzed the binding site responsible for antivasopermeability activity and showed that amino acid residues glutamine-101, isoleucine-103, leucine-112, and serine-115 include the necessary components for the activity. Whether the active sites for the three activities are identical or extremely similar remains to be deciphered. Identity would suggest that either a single receptor or multiple receptors with the same binding specificities serve the three activities. An example of multiple receptors having distinct functions, but with extremely similar binding specificities, is the two mannose-6-phosphate receptors (46-48).
Our study has limitations. Because the important amino acid residues were identified in a peptide, we do not know whether the mutated full-length PEDF protein would yield the same functional data as the peptide. Because we mutated four of the residues with exposed amino acid side chains, the possibility that only a subset of the four residues, rather than all four, is necessary for activity cannot be ruled out. Although deemed unlikely, we do not have evidence supporting or refuting a direct interaction between PEDF and VEGF as the mechanism of PEDF inhibition of VEGF-induced activities. Despite these limitations, our findings and further studies of PEDF promise to lead to therapies to abate diabetic macular edema. PEDF or an agent mimicking the PEDF active site may have other potential therapeutic effects relating to vasopermeability, angiogenesis, or motoneuron survival.
We report a previously undocumented bioactivity of PEDF, the inhibition of VEGF-induced vascular permeability. Vascular permeability is an important component in a number of diseases. Furthermore, we identified four amino acid residues important to PEDF function as an initial step in understanding how PEDF exerts its effects. Our findings suggest that PEDF may be an important therapeutic adjunct in the treatment of life-threatening and sight-threatening diseases where increased vascular permeability is a pathophysiologic mechanism. The refinement of the active site for PEDF allows for development of pharmacologic agents mimicking the active site of PEDF.
Acknowledgments
We thank Marta Marsh for assistance with the statistical analysis, Amir Rattner and Hui Sun for helpful discussions, and Jennifer Macke for editorial assistance. The work was supported by the Michael and Mary Kathryn Panitch Fund, and through the generosity of Rick and Sandy Forsythe and Jack and Viki Thompson.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PEDF, pigment epithelium-derived factor; VEGF, vascular endothelial growth factor; ACT, α1-antichymotrypsin; HSP47, heat shock protein 47.
References
- 1.Senger, D. R., Galli, S. J., Dvorak, A. M., Perruzzi, C. A., Harvey, V. S. & Dvorak, H. F. (1983) Science 219, 983-985. [DOI] [PubMed] [Google Scholar]
- 2.Connolly, D. T., Heuvelman, D. M., Nelson, R., Olander, J. V., Eppley, B. L., Delfino, J. J., Siegel, N. R., Leimgruber, R. M. & Feder, J. (1989) J. Clin. Invest. 84, 1470-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keck, P. J., Hauser, S. D., Krivi, G., Sanzo, K., Warren, T., Feder, J. & Connolly, D. T. (1989) Science 246, 1309-1312. [DOI] [PubMed] [Google Scholar]
- 4.Oosthuyse, B., Moons, L., Storkebaum, E., Beck, H., Nuyens, D., Brusselmans, K., Van Dorpe, J., Hellings, P., Gorselink, M., Heymans, S., et al. (2001) Nat. Genet. 28, 131-138. [DOI] [PubMed] [Google Scholar]
- 5.Sondell, M., Lundborg, G. & Kanje, M. (1999) J. Neurosci. 19, 5731-5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wick, A., Wick, W., Waltenberger, J., Weller, M., Dichgans, J. & Schulz, J. B. (2002) J. Neurosci. 22, 6401-6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waltenberger, J., Claesson-Welsh, L., Siegbahn, A., Shibuya, M. & Heldin, C. H. (1994) J. Biol. Chem. 269, 26988-26995. [PubMed] [Google Scholar]
- 8.Gille, H., Kowalski, J., Li, B., LeCouter, J., Moffat, B., Zioncheck, T. F., Pelletier, N. & Ferrara, N. (2001) J. Biol. Chem. 276, 3222-3230. [DOI] [PubMed] [Google Scholar]
- 9.Bernatchez, P. N., Soker, S. & Sirois, M. G. (1999) J. Biol. Chem. 274, 31047-31054. [DOI] [PubMed] [Google Scholar]
- 10.Steele, F. R., Chader, G. J., Johnson, L. V. & Tombran-Tink, J. (1993) Proc. Natl. Acad. Sci. USA 90, 1526-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becerra, S. P., Sagasti, A., Spinella, P. & Notario, V. (1995) J. Biol. Chem. 270, 25992-25999. [DOI] [PubMed] [Google Scholar]
- 12.Dafforn, T. R., Della, M. & Miller, A. D. (2001) J. Biol. Chem. 276, 49310-49319. [DOI] [PubMed] [Google Scholar]
- 13.Tombran-Tink, J. & Johnson, L. V. (1989) Invest. Ophthalmol. Visual Sci. 30, 1700-1707. [PubMed] [Google Scholar]
- 14.Tombran-Tink, J., Chader, G. G. & Johnson, L. V. (1991) Exp. Eye Res. 53, 411-414. [DOI] [PubMed] [Google Scholar]
- 15.Becerra, S. P., Palmer, I., Kumar, A., Steele, F., Shiloach, J., Notario, V. & Chader, G. J. (1993) J. Biol. Chem. 268, 23148-23156. [PubMed] [Google Scholar]
- 16.Seigel, G. M., Tombran-Tink, J., Becerra, S. P., Chader, G. J., Diloreto, D. A., Jr., del Cerro, C., Lazar, E. S. & del Cerro, M. (1994) Growth Factors 10, 289-297. [DOI] [PubMed] [Google Scholar]
- 17.Dawson, D. W., Volpert, O. V., Gillis, P., Crawford, S. E., Xu, H., Benedict, W. & Bouck, N. P. (1999) Science 285, 245-248. [DOI] [PubMed] [Google Scholar]
- 18.Stellmach, V., Crawford, S. E., Zhou, W. & Bouck, N. (2001) Proc. Natl. Acad. Sci. USA 98, 2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duh, E. J., Yang, H. S., Suzuma, I., Miyagi, M., Youngman, E., Mori, K., Katai, M., Yan, L., Suzuma, K., West, K., et al. (2002) Invest. Ophthalmol. Visual Sci. 43, 821-829. [PubMed] [Google Scholar]
- 20.Gao, G., Li, Y., Fant, J., Crosson, C. E., Becerra, S. P. & Ma, J. X. (2002) Diabetes 51, 1218-1225. [DOI] [PubMed] [Google Scholar]
- 21.Gao, G., Li, Y., Gee, S., Dudley, A., Fant, J., Crosson, C. & Ma, J. X. (2002) J. Biol. Chem. 277, 9492-9497. [DOI] [PubMed] [Google Scholar]
- 22.Gao, G., Li, Y., Zhang, D., Gee, S., Crosson, C. & Ma, J. (2001) FEBS Lett. 489, 270-276. [DOI] [PubMed] [Google Scholar]
- 23.Ohno-Matsui, K., Morita, I., Tombran-Tink, J., Mrazek, D., Onodera, M., Uetama, T., Hayano, M., Murota, S. I. & Mochizuki, M. (2001) J. Cell. Physiol. 189, 323-333. [DOI] [PubMed] [Google Scholar]
- 24.Alberdi, E., Aymerich, M. S. & Becerra, S. P. (1999) J. Biol. Chem. 274, 31605-31612. [DOI] [PubMed] [Google Scholar]
- 25.Bilak, M. M., Becerra, S. P., Vincent, A. M., Moss, B. H., Aymerich, M. S. & Kuncl, R. W. (2002) J. Neurosci. 22, 9378-9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao, W., Tombran-Tink, J., Chen, W., Mrazek, D., Elias, R. & McGinnis, J. F. (1999) J. Neurosci. Res. 57, 789-800. [PubMed] [Google Scholar]
- 27.Taniwaki, T., Becerra, S. P., Chader, G. J. & Schwartz, J. P. (1995) J. Neurochem. 64, 2509-2517. [DOI] [PubMed] [Google Scholar]
- 28.Stratikos, E., Alberdi, E., Gettins, P. G. & Becerra, S. P. (1996) Protein Sci. 5, 2575-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qaum, T., Xu, Q., Joussen, A. M., Clemens, M. W., Qin, W., Miyamoto, K., Hassessian, H., Wiegand, S. J., Rudge, J., Yancopoulos, G. D., et al. (2001) Invest. Ophthalmol. Visual Sci. 42, 2408-2413. [PubMed] [Google Scholar]
- 30.Xu, Q., Qaum, T. & Adamis, A. P. (2001) Invest. Ophthalmol. Visual Sci. 42, 789-794. [PubMed] [Google Scholar]
- 31.Derevjanik, N. L., Vinores, S. A., Xiao, W. H., Mori, K., Turon, T., Hudish, T., Dong, S. & Campochiaro, P. A. (2002) Invest. Ophthalmol. Visual Sci. 43, 2462-2467. [PubMed] [Google Scholar]
- 32.Gettins, P. G. (2002) Chem. Rev. 102, 4751-4804. [DOI] [PubMed] [Google Scholar]
- 33.Becerra, S. P. (1997) Adv. Exp. Med. Biol. 425, 223-237. [PubMed] [Google Scholar]
- 34.Simonovic, M., Gettins, P. G. & Volz, K. (2001) Proc. Natl. Acad. Sci. USA 98, 11131-11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conn, G., Soderman, D. D., Schaeffer, M. T., Wile, M., Hatcher, V. B. & Thomas, K. A. (1990) Proc. Natl. Acad. Sci. USA 87, 1323-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gospodarowicz, D., Abraham, J. A. & Schilling, J. (1989) Proc. Natl. Acad. Sci. USA 86, 7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung, D. W., Cachianes, G., Kuang, W. J., Goeddel, D. V. & Ferrara, N. (1989) Science 246, 1306-1309. [DOI] [PubMed] [Google Scholar]
- 38.Gettins, P. G., Simonovic, M. & Volz, K. (2002) Biol. Chem. 383, 1677-1682. [DOI] [PubMed] [Google Scholar]
- 39.Clermont, A. C., Cahill, M. T., Bursell, S. E., Bouck, N. & Aiello, L. P. (2001) Invest. Ophthalmol. Visual Sci. 42, S92. [Google Scholar]
- 40.Aiello, L. P. & Wong, J. S. (2000) Kidney Int. Suppl. 77, S113-S119. [DOI] [PubMed] [Google Scholar]
- 41.Aiello, L. P., Avery, R. L., Arrigg, P. G., Keyt, B. A., Jampel, H. D., Shah, S. T., Pasquale, L. R., Thieme, H., Iwamoto, M. A., Park, J. E., et al. (1994) N. Engl. J. Med. 331, 1480-1487. [DOI] [PubMed] [Google Scholar]
- 42.Ogata, N., Tombran-Tink, J., Nishikawa, M., Nishimura, T., Mitsuma, Y., Sakamoto, T. & Matsumura, M. (2001) Am. J. Ophthalmol. 132, 378-382. [DOI] [PubMed] [Google Scholar]
- 43.Spranger, J., Osterhoff, M., Reimann, M., Mohlig, M., Ristow, M., Francis, M. K., Cristofalo, V., Hammes, H. P., Smith, G., Boulton, M., et al. (2001) Diabetes 50, 2641-2645. [DOI] [PubMed] [Google Scholar]
- 44.Hanahan, D. & Folkman, J. (1996) Cell 86, 353-364. [DOI] [PubMed] [Google Scholar]
- 45.Meyer, C., Notari, L. & Becerra, S. P. (2002) J. Biol. Chem. 277, 45400-45407. [DOI] [PubMed] [Google Scholar]
- 46.Roberts, D. L., Weix, D. J., Dahms, N. M. & Kim, J. J. (1998) Cell 93, 639-648. [DOI] [PubMed] [Google Scholar]
- 47.Tong, P. Y., Gregory, W. & Kornfeld, S. (1989) J. Biol. Chem. 264, 7962-7969. [PubMed] [Google Scholar]
- 48.Tong, P. Y. & Kornfeld, S. (1989) J. Biol. Chem. 264, 7970-7975. [PubMed] [Google Scholar]