Abstract
Hodgkin's lymphoma (HL) is characterized by the presence of malignant so-called Hodgkin's/Reed-Sternberg (HRS) cells, which display resistance to certain apoptotic stimuli, including a lack of sensitivity to Fas-mediated cell death. However, the mechanisms responsible for their resistance to apoptosis inducers have not been elucidated. Here we confirm that both HL-derived cell lines and the HRS cells of primary HL tissues express Fas ligand (FasL) along with the inhibitory c-FLIP protein. Down-regulation of cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein (c-FLIP) through the use of specific small inhibitory RNAs (siRNAs) leads to reduced viability of the L428 and L591 HL-derived cell lines. To determine whether endogenous FasL was responsible for the reduction in cell viability observed after down-regulation of c-FLIP, L428 and L591 cells were treated with c-FLIP-specific siRNAs with and without siRNAs directed to FasL. Treatment of these cells with both c-FLIP- and FasL-specific siRNAs in combination restored cell viability to near control levels. Our results provide a mechanism whereby HRS cells are protected from autonomous FasL-mediated cell death while preserving their ability to evade immunosurveillance. Targeting c-FLIP could provide a novel approach to the treatment of HL.
Keywords: Fas ligand, apoptosis, small interfering RNA
The malignant Hodgkin's/Reed-Sternberg (HRS) cells of Hodgkin's lymphoma (HL) frequently harbor a high load of somatic mutations in the variable region of the immunoglobulin (Ig) genes, suggesting their germinal center (GC) or post-GC B cell origin (1, 2). During the GC reaction, B cells with self-reactive or low-affinity antibody die by Fas (CD95/APO-1)-mediated apoptosis, whereas cells that express Ig with increased affinity for the corresponding antigen are stimulated to proliferate and exit the GC as memory B cells or plasma cells (3-5). In contrast, although HRS cells have rearranged Ig genes, they do not express a functional B cell receptor (BCR), either because of crippling mutations in their somatically mutated Ig genes (1, 2) or because of the loss of transcription factors important for Ig transcription, namely Oct2 and Bob.1 (6-8). Thus, a fundamental pathogenetic event in the development of HL is the ability of HRS cells to escape the Fas-mediated apoptosis that would be the normal fate of GC B cells lacking functional BCR expression.
Fas-induced death involves clustering of Fas at the cell membrane and recruitment of the Fas-associated death domain (DD)-containing protein (FADD) to the trimerized intracellular DD of the receptor. FADD in turn recruits the proenzymatic form of caspase-8/FADD-like IL-1β-converting enzyme (FLICE), leading to the formation of a multimolecular signaling platform known as the death-inducing signaling complex (DISC). After the formation of the DISC, caspase-8 is activated by autoproteolytic cleavage and initiates the apoptotic cascade. Early studies demonstrated that cultured HRS cells were generally resistant to Fas-mediated apoptosis (9) despite high levels of CD95/Fas expression (9-11). There have also been a number of reports of the expression of Fas ligand (FasL) by HRS cells (11-13); however, there has been some concern over the specificity of antibodies used to detect FasL in general. Expression of FasL by HRS cells would suggest that these cells not only have to contend with the incoming FasL expressed on immune effector cells but also require an internal protective mechanism to prevent their self-destruction through engagement of their own Fas receptor with endogenous FasL.
The rare occurrence of mutations in the Fas gene in HL tumors (14, 15) suggests that the general resistance of HRS cells to Fas-induced death is due to defects further downstream in the Fas pathway. One of the most proximal regulators of the CD95-induced death program is the FLICE-inhibitory protein (FLIP). FLIP was originally identified as a virus-encoded apoptosis-inhibitory protein, but its cellular homologue (c-FLIP) also has the capacity to interfere with formation of the DISC (16) and has a key role in the regulation of GC B cell apoptosis. Alternative splicing generates two isoforms of c-FLIP: a long form (c-FLIPL), which contains a caspase-like domain but is devoid of caspase catalytic activity, and a short form (c-FLIPS) lacking the caspase-like domain.
Although c-FLIP expression has been previously demonstrated in HRS cells (15, 17), its contribution to protection from Fas-mediated apoptosis in these cells has not been studied. Here we show that down-regulation of c-FLIP in HL cells leads to dramatically reduced cell viability, which is not diminished further by the addition of an exogenously supplied Fas stimulus. Furthermore, we confirm that HRS cells express FasL, and concomitant down-regulation of FasL and c-FLIP restores the viability of L428 and L591 cells to control levels. The down-regulation of c-FLIP in HRS cells appears to unmask a latent mechanism that allows self-inflicted Fas-mediated cell death. Targeting c-FLIP could therefore provide a novel approach to the treatment of HL.
Materials and Methods
Cell Lines and Tissue Samples. HL-derived cell lines, KM-H2, L428, and L591 and the T cell leukemia cell line Jurkat were maintained in RPMI medium 1640 supplemented with 10% FCS, 2 mM l-glutamine, and 1% penicillin/streptomycin solution. KM-H2 was established from the pleural effusion of a patient with mixed cellularity HL (18). L428 was derived from a pleural effusion that was histologically confirmed as HL (19). L591 is a cell line established from pleural effusion of a female patient with histologically confirmed nodular sclerosis HL (20).
Snap-frozen and paraffin-embedded primary tumor tissues of HL patients were obtained from Birmingham Heartland's Hospital, U.K., and from Birmingham Children's Hospital, U.K. The Epstein-Barr virus status of all tumors was determined by immunohistochemistry for LMP1 as previously described (21).
Induction of Apoptosis. Cells (5 × 105 cells per ml) were cultured for 48 h in the presence of 1 μg/ml mouse anti-human Fas antibody, CH11 (Coulter-Immunotech), with and without 200 ng/ml cycloheximide (CHX), a protein synthesis inhibitor. Cells were harvested, resuspended in 0.4 ml saline (prewarmed to 37°C), and incubated in 250 nM SYTO 16 (Molecular Probes) for 1 h at room temperature, after which 5 μg/ml propidium iodide was added.
Cell viability was assessed on a Coulter EPICS XL flow cytometer. A two-dimensional dot plot was generated of SYTO 16 fluorescence versus propidium iodide fluorescence.
Only viable cells take up SYTO 16, and propidium iodide stains only permeabilized cells, therefore live, apoptotic, and necrotic cells can be differentiated.
RT-PCR and Southern Blotting. Total RNA was extracted from cell lines by using the NucleoSpin RNA II kit (Macherey & Nagel), according to the protocol of the supplier. Three micrograms of RNA was used in a cDNA synthesis reaction with the reverse gene-specific primer (see below) using avian myeloblastosis virus reverse transcriptase.
c-FLIPL transcripts were amplified by using the forward and reverse primers 5′-CATACTGAGATGCAAGAATT-3′ and 5′-GCTGAAGTCATCCATGAGGT-3′, respectively. PCR was performed by using “hot start” with Red Hot Taq DNA Polymerase (Abgene, Epsom, U.K.), and involved an initial 2-min denaturation at 94°C, followed by 30 cycles, consisting of a denaturing step for 30 s at 94°C, an annealing step for 1 min at 45°C, and an extension for 1 min at 72°C. PCR products (542 bp) were visualized on 2% agarose gels and confirmed by Southern blotting using the c-FLIP-specific probe, 5′-GGAGCAGGGACAAGTTACAG-3′. Negative (no RNA, no DNA) and positive (Jurkat cells) controls were performed in parallel.
FasL transcripts were amplified by using the forward and reverse primers 5′-GGTCCATGCCTCTGGAATGG-3′ and 5′-CACATCTGCCCAGTAGTGCA-3′, respectively.
PCR products (249 bp) were confirmed by Southern blotting, using the FasL-specific probe 5′-ATGAGGAACTCTAAGTATCC-3′.
Western Blot Analysis. HL-derived cell lines were washed in cold PBS and lysed in 100 μl of lysis buffer (20 mM Tris·HCl,pH7.4/150 mM NaCl/1 mM EDTA/1 mM EGTA/1% Triton X-100).
Protein concentration was determined by using the DC protein assay (Bio-Rad), and 30 μg of lysate was separated by SDS/10% PAGE. To detect c-FLIP, proteins were transferred to poly(vinylidene difluoride) membranes (PVDF). After a 1-h incubation in blocking solution (5% dry milk in PBS/0.01% Tween 20), membranes were incubated for 1 h in secondary antibody (1:5000 dilution, goat anti-rabbit IgG) and normal goat serum (0.2%) in PBS/0.01% Tween 20/5% dry milk. Horseradish peroxidase was deactivated by using chromogen and hydrogen peroxide (Vector substrate, Vector Laboratories). After washing in PBS/Tween 20, blots were probed overnight with rabbit anti-c-FLIP monoclonal antibody (diluted 1:100). After washing in PBS, the secondary peroxidase-labeled antibody was added at a 1:2000 dilution. c-FLIP protein was visualized with Supersignal chemiluminescence technique.
For the detection of FasL, proteins were transferred to nitrocellulose membranes. After a 1-h incubation in blocking solution (5% dry milk in PBS/0.01% Tween 20), blots were probed overnight with polyclonal rabbit IgG anti-FasL antibody (N-20, Santa Cruz Biotechnology; diluted 1:200). After washing in PBS, the secondary peroxidase-labeled antibody was added at 1:2000. FasL was visualized with the enhanced chemiluminescence (ECL) technique (Amersham Pharmacia).
Lysates of Jurkat cells were used as a positive control for the detection of both FasL and c-FLIP proteins.
Laser-Capture Microdissection and Single-Cell RT-PCR. Frozen sections of biopsies from seven HL patients were prepared at 7 μmon uncoated RNase-free glass slides and were stained with hematoxylin under strict RNase-free conditions. HRS cells identified by their characteristic morphology were microdissected by using the PixCell II LCM System (Arcturus, Mountain View, CA). Several samples were rich in HRS cells, including that from patient 1, in which clusters of tumor cells free of any infiltrating nontumor cells were present; HRS cells were collected from these clusters. The absence of contaminating T cells from these clusters was confirmed by the staining of adjacent sections for the T cell marker CD3. A laser beam diameter of 7.5 μm was used in all investigations and microdissection was performed within the confines of the cell to ensure only the collection of tissue from HRS cells. Laser power typically ranged between 30 and 100 mW and laser pulse duration was set at 50 ms. The Arcturus High Sensitivity System that avoids contact between the transfer film and the tissue section during microdissection was used throughout to reduce the risk of contamination by nonneoplastic cells. We also examined both caps and sections after capture to ensure that material from other cells had not been collected. RNA was extracted from microdissected cells by using the Absolutely RNA Microprep Kit (Stratagene).
RT-PCR was performed according to the method described above, with the exception that maximum volumes of RNA/cDNA were added to reactions at the expense of water, and 40 cycles of amplification were used.
Immunohistochemistry. Paraffin-embedded tissues were cut at 5 μm on to adhesive-coated slides. Cytospin preparations of cell lines were fixed for 20 min in 10% formal saline solution. After dewaxing of paraffin sections, all slides were incubated for 10 min in 3% hydrogen peroxide in methanol to block endogenous peroxidase activity. Antigens were retrieved by incubation overnight in EDTA (1 mM, pH 8.0)/Tween 20 (0.1%) buffer on a hot-plate stirrer at 65°C.
The expression of c-FLIP was determined in HL tissues and cell lines by using a c-FLIP monoclonal antibody at a dilution of 1:80. Bound primary antibody was detected by using the peroxidase-based Chemicon IHC Select kit (Chemicon International).
Small Interfering RNAs (siRNAs) Directed to c-FLIP and FasL. The siRNA sequences directed to c-FLIP (forward 5′-CUGCUCUACAGAGUGAGGC-3′ and reverse 3′GACGAGAUGUCUCACUCCG-5′), FasL (forward 5′-CUCAAGGUCCAUGCCUCUG-3′ and reverse 3′-GAGUUCCAGGUACGGAGAC-5′) and an irrelevant/control siRNA (forward 5′-UUUGCACGGACAGGCAUUG-3′ and reverse 3′-AAACGUGCCUGUCCGUAAC-5′) were designed and manufactured by Eurogentec (Brussels). To assess transfection efficiency, c-FLIP and FasL sequences were labeled with the fluorescent dyes 5′-fluorescein (6-FAM) and 5′-TAMRA (rhodamine), respectively. Before transfection, 30 μl of each of the RNA oligonucleotide solutions (50 μM) was combined with 15 μl of annealing buffer (final buffer concentration 100 mM potassium acetate/30 mM Hepes-KOH, pH 7.4/2 mM magnesium acetate). These were then incubated for 1 min at 90°C, briefly centrifuged, and then incubated for a further 1 h at 37°C. The siRNAs (20 μM) prepared in this way were stored at -20°C until transfection.
L428 and L591 cells were diluted with medium to a density of 2.5 × 105 cells per 250 μl in 24-well plates. Three microliters of the transfection reagent RiboJuice (Novagen) was added to 47 μl of serum-free medium for each sample; after gentle Vortex mixing these samples were incubated for 5 min at room temperature. siRNAs were then added to this solution (final concentration 2 μM) and, after a further 15-min incubation, the mixture was added dropwise to the cell suspension. Plates were left overnight at 37°C, under a 5% CO2/95% air atmosphere, and samples were then centrifuged and resuspended in 1 ml of culture medium containing CH11 (1 μg/ml) where appropriate.
Semiquantitative RT-PCR. After transfection with siRNA, cells were harvested for RNA extraction. RNA was quantified from each cell sample and equivalent concentrations were added to cDNA reactions before semiquantitative RT-PCR. RT-PCR for c-FLIP and FasL mRNA detection was performed as described above but for only 10, 15, 20, and 25 cycles.
Trypan Blue Cell Viability Assay. Cell viability after siRNA transfection was assessed in triplicate by using the trypan blue reagent. One hundred microliters of cell suspension was removed from plates and mixed with 100 μl of trypan blue reagent for 2 min. Cell viability was determined by direct counting of unstained cells in a hemocytometer.
Results
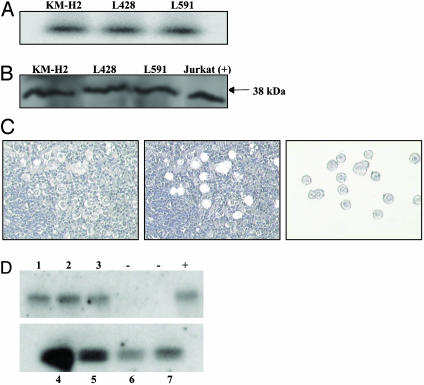
FasL Is Expressed by HL-Derived Cell Lines and by HRS Cells of Primary HL Biopsies. Previous studies have demonstrated that Fas is expressed on HRS cells and we confirmed this in the HL-derived cell lines by immunohistochemistry using well-established monoclonal antibodies (data not shown). We confirmed that HRS cells also express FasL, initially by using RT-PCR analysis of HL-derived cell lines, KM-H2, L428, and L591 (Fig. 1A), and expression was confirmed by the detection of the membrane-bound form of FasL protein in all three lines by immunoblotting (Fig. 1B). To verify that tumor cells in primary tissue samples expressed FasL, HRS cells were microdissected from tissue sections of a total of seven HL patients, (including three adults and four children), four of these samples were positive for Epstein-Barr virus. In one set of experiments HRS cells were collected from patients and pooled (up to 20 cells per patient; Fig. 1C); FasL transcripts were detected in all seven patients (data not shown). In a separate series of experiments single HRS cells from each patient were analyzed. Fig. 1D shows the detection of FasL transcription in a single HRS cell microdissected from each of these seven patients. Samples from several of these patients, including no. 1, were rich in HRS cells; in these cases microdissection was restricted to clusters of HRS cells that were free of any infiltrating nontumor cells.
Fig. 1.
FasL is expressed by HRS cells. (A and B) RT-PCR (A) and Western blot (B) demonstrate FasL expression in the HL-derived cell lines KM-H2, L428, and L591. (C) Microdissection of HRS cells from HL tumors before (Left) and after (Center) capture. Right shows procured cells on the cap ready for analysis. (D) RT-PCR analysis of HRS cells microdissected from the HL biopsies of seven patients, 1-7. There is evidence of FasL transcription in all cases. Positive control (+) for this experiment was microdissected T lymphocytes. Negative controls were water-only PCR controls.
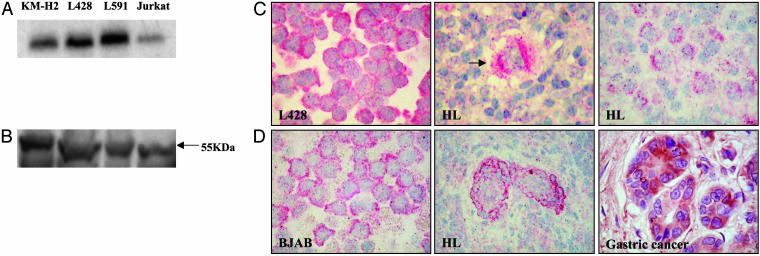
c-FLIP Is Expressed by HL Cell Lines and by HRS Cells of Primary HL Tissues. The ability of both HRS cells and HL-derived cell lines to survive and proliferate in the presence of endogenous FasL implies that these cells are inherently protected from Fas-mediated cell death, and this implication led us to explore the possibility that c-FLIP, a protein that interferes with the formation of DISC, is responsible. Analysis of HL-derived cell lines by RT-PCR identified c-FLIP transcripts in KM-H2, L428, and L591 cell lines (Fig. 2A). Furthermore, the long form of c-FLIP protein (c-FLIPL) was also detectable in all of these lines by immunoblotting and immunohistochemistry (Fig. 2 B and C).
Fig. 2.
c-FLIP expression by HL-derived cell lines and HRS cells of primary HL tissues. (A and B) RT-PCR (A) and Western blot (B) demonstrate c-FLIPL expression in HL-derived cell lines KM-H2, L428, and L591. Jurkat cells were used as a positive control. (C) Expression of c-FLIP protein in HL cells as shown by immunohistochemistry. Left shows staining of L428 cells for c-FLIP, Center and Right show a single HRS cell (arrowed) and a cluster of HRS cells, respectively, from two separate HL patients. (D) Controls for c-FLIP immunostaining. Left shows BJAB cells, Center shows endothelial cells from an HL patient, and Right shows gastric cancer cells.
Ten paraffin-embedded and 11 snap-frozen primary HL tumor samples were investigated for expression of c-FLIP protein by immunohistochemistry. Three of 10 paraffin-embedded tumors and 9 of 11 frozen tumors showed strong cytoplasmic c-FLIP protein expression within malignant HRS cells (Fig. 2C). Positivity was unrelated to the Epstein-Barr virus status of the tumors. We used various positive controls for the c-FLIP staining, including BJAB cells which have previously been shown to express c-FLIP (17), tonsil, where we demonstrated strong staining in the GC B cells, as has been previously described (17), and cells from several patients with gastric cancer, which are often c-FLIP-positive (22). Furthermore, we were also able to demonstrate c-FLIP expression in endothelial cells of HL tissues; endothelial cells are consistently positive for c-FLIP (23). Examples of immunostaining in control cells and tissues are shown in Fig. 2D.
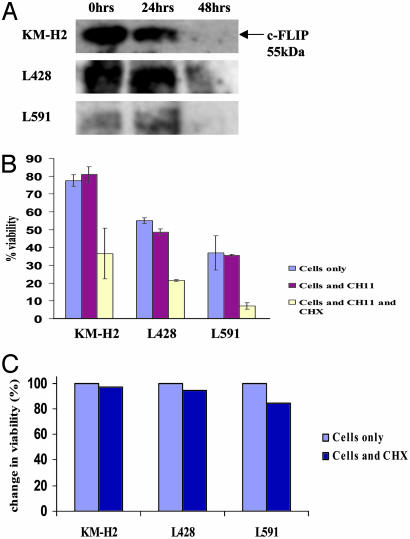
Down-Regulation of c-FLIP in the Presence of CHX Coincides with Increased Susceptibility to Fas-Mediated Apoptosis. Treatment of each of the HL-derived cell lines in the presence of the protein synthesis inhibitor CHX resulted in a reduction in c-FLIP protein expression, which was evident after 48 h (Fig. 3A). The down-regulation of c-FLIP protein coincided with the increased susceptibility of these cells to Fas-mediated apoptosis, particularly in the presence of the Fas-agonistic monoclonal antibody CH11. Thus, HL-derived cell lines were incubated in the presence of CH11 with and without addition of CHX. Fig. 3B shows the mean change in cell viability across three such experiments. Cells treated with CH11 alone showed no significant reduction in viability, with the exception of L428 cells, which showed a small fall in viability. However, treatment of the HL-derived cells, KM-H2, L591, and L428, with CH11 in the presence of CHX resulted in dramatic falls in cell viability in all three lines. This reduction in cell viability was not due to the toxic effects of CHX because incubation of cells resulted in only a small fall in viability (Fig. 3C) compared with cells treated with a combination of CH11 and CHX. The inability of CHX treatment alone to unmask the apoptotic effects of endogenous FasL is probably due to the effect of CHX treatment on FasL expression, and it highlighted the need for more specific approaches to down-regulate c-FLIP expression.
Fig. 3.
c-FLIP down-regulation in HRS cells after treatment with CHX is associated with reduced viability. (A) Western blot analysis of c-FLIPL protein in HL-derived cell lines after treatment with CHX. Down-regulation of c-FLIP protein is evident after 48-h treatment with CHX. (B) Cells treated with CH11 alone showed no significant (error bars show standard errors calculated from three replicates) reduction in viability, with the exception of L428 cells, which showed a small fall in viability. However, treatment of HL cells with CH11 in the presence of CHX resulted in dramatic falls in cell viability in all three cell lines. (C) Change in viability of HL cells after treatment with CHX. Treatment of cells with CHX resulted in only a small fall in viability compared with that seen when cells were incubated with CHX and CH11 (B).
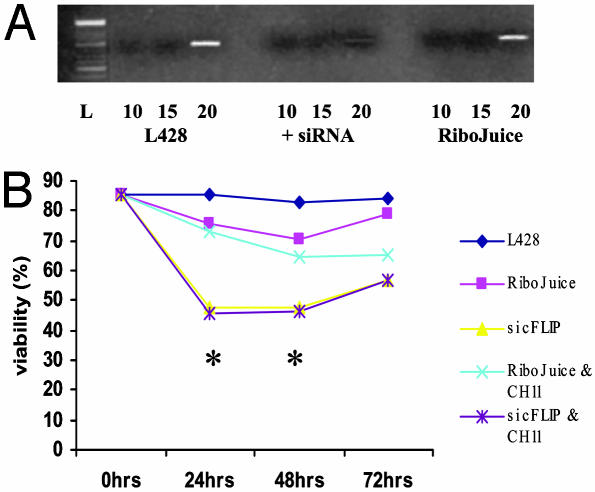
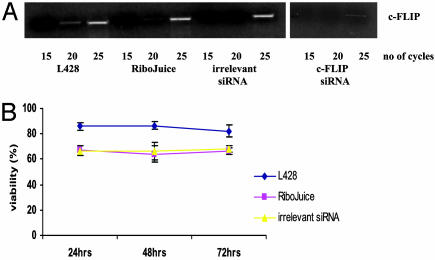
Down-Regulation of c-FLIP Expression Results in Reduced Viability of L428 Cells. Treatment of L428 cells with c-FLIP-specific siRNAs resulted in the down-regulation of c-FLIP transcription, which reached a maximum around 24 h after transfection (Fig. 4A). Approximately 85% of L428 cells were successfully transfected as assessed by immunofluorescence microscopy (data not shown). c-FLIP transcription was unaffected when cells were treated with the RiboJuice transfection reagent alone (Fig. 4A). Treatment with c-FLIP-specific siRNAs did not result in a general down-regulation of mRNA, because GAPDH transcription was unaffected by the siRNA treatment (data not shown). Furthermore, cells treated with c-FLIP-specific siRNAs showed significantly reduced viability compared with control cells at all time points (Fig. 4B), although cell viability began to rise at around 48 h, consistent with the transient nature of the siRNA knock-down. Surprisingly, when the siRNA-treated cells were challenged with CH11 antibody no further cell death was induced. Transfection of L428 cells with irrelevant siRNAs had no effect on c-FLIP transcription or cell viability (Fig. 5).
Fig. 4.
Down-regulation of c-FLIP by specific siRNAs results in a fall in viability of L428 cells. (A) Semiquantitative RT-PCR (over 10, 15, and 20 cycles) demonstrates down-regulation of c-FLIP mRNA in L428 cells after 24-h treatment with c-FLIP-specific siRNAs, but not in L428 cells treated with the RiboJuice transfection reagent alone. (B) Inhibition of c-FLIP transcription resulted in reduced viability of L428 cells compared with controls. No further reduction in cell viability was seen when transfected cells were challenged with the Fas agonist antibody, CH11, suggesting that endogenous FasL was sufficient to induce maximum cell death. *, Significant differences in mean viability between cells treated with c-FLIP-specific siRNAs and controls (RiboJuice only).
Fig. 5.
Treatment of L428 cells with irrelevant siRNAs has no effect on c-FLIP transcription or viability. (A) (Left) Semiquantitative RT-PCR for c-FLIP mRNA in L428 cells after transfection with irrelevant siRNAs. No difference in c-FLIP transcription was detected between L428 cells treated with the irrelevant siRNAs and control cells treated with RiboJuice transfection reagent alone. Also shown is the down-regulation of c-FLIP mRNA after treatment with c-FLIP-specific siRNAs (Right). (B) Viability of cells treated with irrelevant siRNAs remained unchanged compared with controls.
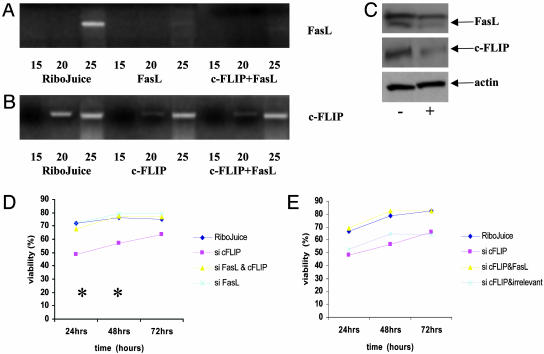
c-FLIP Protects L428 Cells from FasL-Mediated Apoptosis. To confirm that FasL was responsible for the cell death that resulted from down-regulation of c-FLIP, L428 cells were coincubated with c-FLIP- and FasL-specific siRNAs. Transfection efficiency with the FasL-specific siRNAs was comparable to that observed for c-FLIP siRNAs (data not shown). L428 cells transfected with FasL siRNAs either alone or together with c-FLIP siRNAs showed down-regulation of FasL mRNA that was maximal around 24 h after transfection (Fig. 6A). Fig. 6B shows that down-regulation of c-FLIP mRNA was successful in dually transfected cells. Furthermore, we were able to demonstrate down-regulation of c-FLIP and FasL protein in cells transfected with both siRNAs (Fig. 6C).
Fig. 6.
Down-regulation of FasL and c-FLIP restores viability in L428 cells. (A and B) Semiquantitative RT-PCR for FasL mRNA (A) and c-FLIP mRNA (B) in L428 cells 24 h after transfection with FasL- and c-FLIP-specific siRNAs, either alone or in combination. Down-regulation of FasL and c-FLIP mRNA was observed after either single or cotransfection with siRNAs. (C) Down-regulation of both FasL and c-FLIP protein was also observed in dually transfected cells (+) compared with control cells (-) treated with RiboJuice reagent only. (D) Transfection with c-FLIP siRNAs resulted in significant cell death as previously shown. However, cotransfection with FasL- and c-FLIP-specific siRNAs reversed this fall in cell viability. *, Significant differences in mean viability between cells treated with c-FLIP-specific siRNAs and cells treated with both siRNAs in combination. (E) Transfection of irrelevant siRNAs together with c-FLIP-specific siRNAs was not able to reverse the decrease in viability.
As shown previously, when c-FLIP-specific siRNAs were introduced alone this resulted in significant cell death. However, this effect was neutralized when cells treated with c-FLIP siRNAs were cotransfected with FasL-specific siRNAs (Fig. 6D). Cotransfection of c-FLIP siRNAs with irrelevant siRNAs was not able to reverse the fall in viability (Fig. 6E).
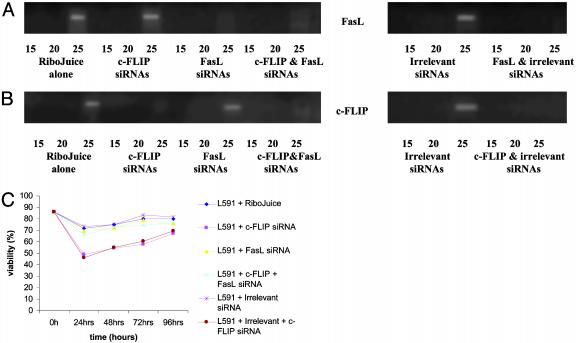
The c-FLIP down-regulation and the co-down-regulation of c-FLIP and FasL was also studied in the L591 HL cell line (Fig. 7). Down-regulation of c-FLIP by siRNA treatment led to similarly reduced viability of L591 cells. Transfection of L591 cells with both c-FLIP- and FasL-specific siRNAs in combination also restored cell viability to control levels. Furthermore, viability of L591 cells dually transfected with c-FLIP-specific and FasL-specific siRNAs was reduced from a mean of 73% (cells treated with siRNAs to c-FLIP and FasL) to 57.8% (cells treated with siRNAs to c-FLIP and FasL plus CH11), confirming that the Fas pathway was intact in these cells (data not shown).
Fig. 7.
Down-regulation of c-FLIP in L591 cells results in a fall in viability, which is restored after down-regulation of FasL. (A and B) Semiquantitative RT-PCR for FasL mRNA (A) and c-FLIP mRNA (B) in L591 cells 24 h after transfection with FasL- and c-FLIP-specific siRNAs, either alone or in combination. Down-regulation of FasL and c-FLIP mRNA was observed after either single or cotransfection with siRNAs. No difference in c-FLIP or FasL transcription was detected in L591 cells treated with the irrelevant siRNAs. Transfection of irrelevant siRNAs together with c-FLIP- or FasL-specific siRNAs did not interfere with the down-regulation of either c-FLIP or FasL. (C) Transfection with c-FLIP siRNAs resulted in significant cell death as previously shown in L428 cells. Cotransfection with FasL- and c-FLIP-specific siRNAs reversed this fall in cell viability.
Discussion
In this study we confirm, through the use of expression analysis of cell lines and microdissected tumor cells obtained from primary tissues, that HRS cells express both Fas and FasL, suggesting the existence of a pathway in which HRS cells are primed to undergo Fas-mediated death. Furthermore, we have verified that the anti-apoptotic factor c-FLIP is also expressed by HL-derived cell lines and by HRS cells of the majority of patients with HL. The failure to detect c-FLIP in some of the paraffin-embedded tissues was most likely due to a failure of the paraffin processing to preserve the c-FLIP epitope, because analysis of frozen sections in almost all cases demonstrated c-FLIP expression in HRS cells. Importantly, we have shown that the specific down-regulation of c-FLIP in the HL-derived cell lines, L428 and L591, results in increased cell death and that concomitant down-regulation of c-FLIP and FasL in both of these cell lines prevented the cell death that occurred when c-FLIP alone was down-regulated. Interestingly, the viability of HL cells after c-FLIP down-regulation was not further reduced by treatment of these cells with the Fas-agonistic antibody CH11, suggesting that the endogenous FasL-Fas pathway is sufficient to induce maximal cell death in the absence of c-FLIP.
c-FLIPL is a critical regulator of GC B-cell apoptosis. In vitro experiments conducted on isolated human GC B cells have shown that they have a preformed inactive DISC containing Fas-associated death domain-containing protein (FADD), procaspase-8, and c-FLIPL. However, c-FLIPL disappears rapidly from the DISC when GC B cells are removed from their microenvironment. The stimuli responsible for the sustained expression of cFLIPL and protection from apoptosis in GC B cells include the ligation of the BCR or ligation of CD40 (24, 25). Thus, the maintenance of GC B cell survival seems to correlate with the potential to prevent the disappearance of cFLIPL. c-FLIPL expression in the GC B cells appears to play the role of a molecular switch turning the preformed CD95 signaling complex on or off.
In the majority of cases HRS cells are believed to derive from GC B cells that have lost the capacity to express functional BCR (1, 2, 6-8). Ordinarily these cells would die by apoptosis, but they are presumably rescued by a transforming event that enables them to evade cell death in the absence of the normal physiological survival signals. Our data point to the aberrant expression of c-FLIP as a key event that enables HRS cell survival in the absence of BCR expression and suggests that, like normal GC B cells, HRS cells are also primed to undergo Fas-mediated apoptosis. An alternative signal to the BCR could be provided by CD40 ligation. HRS cells are known to express high levels of CD40 and are frequently surrounded by CD40-ligand-expressing T cells (26, 27). However, c-FLIP up-regulation after CD40 stimulation of normal B cells is only transient, and prolonged CD40 stimulation results in only low or undetectable levels of c-FLIP (28, 29). A much more plausible explanation for the continued expression of c-FLIP in HRS cells and their associated protection from Fas-mediated death is the constitutive activation of the NF-κB pathway, which has already been shown to be a major factor in HRS cell survival and is responsible for regulating c-FLIP expression (30-32). We were able to confirm the dependence of c-FLIP expression on NF-κB activity in L428 HL cells as well as the ability of NF-κB inhibition to promote apoptosis in these cells (data not shown).
Our studies have revealed that, like GC B cells, HRS cells are primed to undergo Fas-mediated apoptosis. However, unlike GC B cells, they express c-FLIP in the absence of normal physiological BCR signals. This aberrant c-FLIP expression is most probably the result of their constitutively high NF-κB activity. Furthermore, the source of the stimulus for Fas-induced death after removal of c-FLIP may be different between HRS cells and normal GC B cells. Here we have demonstrated expression of FasL not only by HL-derived cell lines but also by the HRS cells of primary tumor samples. Furthermore, in HRS cells the Fas-induced death that results after c-FLIP down-regulation appears dependent on this endogenous FasL. In contrast, it has been shown that GC B cells express neither the transcript nor the protein for FasL and that in these cells ligand-independent oligomerization of CD95 could be responsible for the activation of the Fas pathway (24). FasL transcription is controlled by a number of transcriptional regulators, including NF-κB (33). The expression of FasL by HRS cells, therefore, might be a consequence of constitutive NF-κB activity in these cells. Tumor cells have been shown to acquire the expression of FasL and utilize it to deliver death signals to activated Fas-positive T cells (34). Thus, the expression of FasL by HRS cells might contribute to tumor cell survival by the elimination of activated Fas-expressing cytotoxic T lymphocytes from the tumor microenvironment of HL. Such a mechanism could be particularly relevant to the pathogenesis of those HL cases where the Epstein-Barr virus genome is carried by HRS cells.
In summary, our results demonstrate that HRS cells are similar to GC B cells in being primed to undergo Fas-induced cell death. However, the transformed HRS cells differ from GC B cells in two important respects. First, HRS cells constitutively express c-FLIP, which is responsible for preventing their Fas-induced apoptosis, and second they express FasL, which in the absence of exogenously supplied FasL is sufficient to induce cell death when c-FLIP is down-regulated. Therefore, c-FLIP down-regulation in HRS cells can unmask a preexisting mechanism that allows for the autonomous death of HRS cells through Fas-mediated induced apoptosis. Targeting c-FLIP could provide a novel approach to the treatment of HL.
Acknowledgments
We are grateful to the Leukemia Research Fund and the Medical Research Council U.K. for financial support.
Abbreviations: HRS cells, Hodgkin's/Reed-Sternberg cells; HL, Hodgkin's lymphoma; GC, germinal center; BCR, B cell receptor; DISC, death-inducing signaling complex; FasL, Fas ligand; c-FLIP, cellular homologue of FLICE-inhibitory protein; CHX, cycloheximide; siRNA, small interfering RNA.
References
- 1.Kanzler, H., Küppers, R., Hansmann, M. L. & Rajewsky, K. (1996) J. Exp. Med. 184, 1495-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Küppers, R. & Rajewsky, K. (1998) Annu. Rev. Immunol. 16, 471-493. [DOI] [PubMed] [Google Scholar]
- 3.Rajewsky, K. (1996) Nature. 381, 751-758. [DOI] [PubMed] [Google Scholar]
- 4.Rothstein, T. L., Wang, J. K, Panka, D. J., Foote, L. C., Wang, Z., Stanger, B., Cui, H. & Marshak-Rothstein, A. (1995) Nature 374, 163-165. [DOI] [PubMed] [Google Scholar]
- 5.Schattner, E. & Friedman, S. M. (1996) Immunol. Res. 15, 246-257. [DOI] [PubMed] [Google Scholar]
- 6.Marafioti, T., Hummel, M., Foss, H. D., Laumen, H., Korbjuhn, P., Anagnostopoulos, I., Lammert, H., Demel, G., Theil, J., Wirth, T. & Stein, H. (2000) Blood 95, 1443-1450. [PubMed] [Google Scholar]
- 7.Re, D., Müschen, M., Ahmadi, T., Wickenhauser, C., Staratschek-Jox, A., Holtick, U., Diehl, V. & Wolf, J. (2001) Cancer Res. 61, 2080-2084. [PubMed] [Google Scholar]
- 8.Stein, H., Marafioti, T., Foss, H. D., Laumen, H., Hummel, M., Anagnostopoulos, I., Wirth, T., Demel, G. & Falini, B. (2001) Blood 97, 496-501. [DOI] [PubMed] [Google Scholar]
- 9.Re, D., Hofmann, A., Wolf, J., Diehl, V. & Staratschek-Jox, A. (2000) Exp. Hematol. 28, 31-35. [DOI] [PubMed] [Google Scholar]
- 10.Xerri, L., Carbuccia, N., Parc, P., Hassoun, J. & Birg, F. (1995) Histopathology 27, 235-241. [DOI] [PubMed] [Google Scholar]
- 11.Metkar, S. S., Naresh, K. N., Redkar, A. A., Soman, C. S., Advani, S. H. & Nadkarni, J. J. (1999) Leuk. Lymphoma 33, 521-530. [DOI] [PubMed] [Google Scholar]
- 12.Verbeke, C. S., Wenthe, U., Grobholz, R. & Zentgraf, H. (2001) Am. J. Surg. Pathol. 25, 388-394. [DOI] [PubMed] [Google Scholar]
- 13.Sakuma, I., Yoshino, T., Omonishi, K., Nishiuchi, R., Teramoto, N., Yanai, H., Kawahara, K., Kubonishi, I., Matsuo, Y. & Tadaatsu, A. (1999) Pathol. Int. 49, 103-109. [DOI] [PubMed] [Google Scholar]
- 14.Muschen, M., Re, D., Brauninger, A., Wolf, J., Hansmann, M. L., Diehl, V., Küppers, R. & Rajewsky, K. (2000) Cancer Res. 60, 5640-5643. [PubMed] [Google Scholar]
- 15.Maggio, E. M., Van Den Berg, A., de Jong, D., Diepstra, A. & Poppema, S. (2003) Am. J. Pathol. 162, 29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tschopp, J., Irmler, M. & Thome, M. (1998) Curr. Opin. Immunol. 10, 552-558. [DOI] [PubMed] [Google Scholar]
- 17.Thomas, R. K., Kallenborn, A., Wickenhauser, C., Lugwig Schutz, J., Draube, A., Vockerodt, M., Re, D., Diehl, V. & Wolf, J. (2002) Am. J. Pathol. 160, 1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamesaki, H., Fukuhara, S., Tatsumi, E., Uchino, H., Yamabe, H., Miwa, H., Shirakawa, S., Hatanaka, M. & Honjo, T. (1986) Blood 68, 285-292. [PubMed] [Google Scholar]
- 19.Schaadt, M., Diehl, V., Stein, H., Fonatsch, C. & Kirchner, H. H. (1980) Int. J. Cancer 15, 723-731. [DOI] [PubMed] [Google Scholar]
- 20.Diehl, V., Kirchner, H. H, Burrichter, H., Stein, H., Fonatsch, C., Gerdes, J., Schaadt, M., Heit, W., Uchanska-Ziegler, B., Ziegler, A., et al. (1982) Cancer Treat. Rep. 66, 615-632. [PubMed] [Google Scholar]
- 21.Murray, P. G., Swinnen, L. J., Constandinou, C. M., Pyle, J. M., Carr, T. J., Hardwick, J. M. & Ambinder, R. F. (1996) Blood 87, 706-711. [PubMed] [Google Scholar]
- 22.Lee, S. H., Kim, H. S., Kim, S. Y., Lee, Y. S., Park, W. S., Kim, S. H., Lee, J. Y. & Yoo, N. J. (2003) APMIS 111, 309-314. [DOI] [PubMed] [Google Scholar]
- 23.Imanishi, T., McBride, J., Ho, Q., O'Brien, K. D., Schwartz, S. M. & Han, D. K. (2000) Am. J. Pathol. 156, 125-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hennino, A., Berard, M., Krammer, P. H. & Defrance, T. (2001) J. Exp. Med. 193, 447-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Eijk, M., Medema, J. P. & de Groot, C. M. (2001) J. Immunol. 166, 6473-6476. [DOI] [PubMed] [Google Scholar]
- 26.Gruss, H. J., Hirschstein, D., Wright, B., Ulrich, D., Caligiuri, M. A., Barcos, M., Strockbine, L., Armitage, R. J. & Dower, S. K. (1994) Blood 84, 2305-2314. [PubMed] [Google Scholar]
- 27.Carbone, A., Gloghini, A., Gattei, V., Aldinucci, D., Degan, M., De Paoli, P., Zagonel, V. & Pinto, A. (1995) Blood 85, 780-789. [PubMed] [Google Scholar]
- 28.Van Parijs, L., Refaeli, Y., Abbas, A. K. & Baltimore, D. (1999) Immunity 11, 763-770. [DOI] [PubMed] [Google Scholar]
- 29.Hennino, A., Berard, M., Casamayor-Palleja, M., Krammer, P. H. & Defrance, T. (2000) J. Immunol. 165, 3023-3030. [DOI] [PubMed] [Google Scholar]
- 30.Bargou, R. C., Leng, C., Krappmann, D., Emmerich, F., Mapara, M. Y., Bommert, K., Royer, H. D., Scheidereit, C. & Dorken, B. (1996) Blood 87, 4340-4347. [PubMed] [Google Scholar]
- 31.Bargou, R. C., Emmerich, F., Krappmann, D., Bommert, K., Mapara, M. Y., Arnold, W., Royer, H. D., Grinstein, E., Greiner, A., Scheidereit, C. & Dorken, B. (1997) J. Clin. Invest. 100, 2961-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Micheau, O., Lens, S., Gaide, O., Alevizopoulos, K. & Tschopp, J. (2001) Mol. Cell. Biol. 21, 5299-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavurma, M. M. & Khachigian, L. M. (2003) Cell Death Differ. 10, 36-44. [DOI] [PubMed] [Google Scholar]
- 34.O'Connell, J., Bennett, M. W., O'Sullivan, G. C., Collins, J. K. & Shanahan, F. (1999) Immunol. Today 20, 46-52. [DOI] [PubMed] [Google Scholar]