Abstract
Osteoporosis is characterized by a low bone-mineral density associated with skeletal fractures. The decrease in bone-mineral density is the consequence of an unbalanced bone-remodeling process, with higher bone resorption than bone formation. The orchestration of the bone-remodeling process is under the control of the most abundant cell in bone, the osteocyte. Functioning as an endocrine cell, osteocytes are also a source of soluble factors that not only target cells on the bone surface, but also target distant organs. Therefore, any drugs targeting the osteocyte functions and signaling pathways will have a major impact on the bone-remodeling process. This review discusses potential advances in drug therapy for osteoporosis, including novel osteocyte-related antiresorptive and anabolic agents that may become available in the coming years.
Keywords: drug therapy, osteocyte, osteoporosis
Introduction
The mammalian skeleton is a greatly active tissue that undergoes continuous remodeling throughout childhood and adult life. Bone remodeling is needed for microfracture consolidation, and skeleton adaptation to mechanical use, and also for calcium homeostasis [Dallas et al. 2013]. This bone remodeling implicates the coupling of osteoclastic bone resorption and osteoblastic bone formation. Common diseases, such as osteoporosis, multiple myeloma, Paget’s disease, and other bone-metastasized cancers, are characterized by imbalances between the formation and resorption processes [Devogelaer, 2000; Daci et al. 2002; Shankar et al. 2013]. As these pathologies contribute to increase morbidity and mortality worldwide, there is great concern towards improving our understanding of the processes that regulate bone remodeling [Shoback, 2007]. Osteoporosis, which occurs mainly in postmenopausal women, is characterized by excessive bone resorption compared with the formation of new bone. Osteoporosis is thus characterized by a loss of bone strength, a decrease in bone mass, and a worsening in bone quality, leading to an increased risk of fracture [Mosley, 2000].
In addition to the well-known behavior of mature osteoblasts and osteoclasts, and their respective precursor cells, on the bone-remodeling process, there is increasing evidence that osteocytes play important roles in detecting imperfections or microfractures and initiating a targeted bone remodeling [Verborgt et al. 2000; Kogianni and Noble, 2007; Heino et al. 2009]. Osteocytes originate from mesenchymal stem cells through osteoblast lineage differentiation, with only 10–20% of osteoblasts differentiating into osteocytes [Aubin and Turksen, 1996]. During this differentiation, osteocytes become embedded in the bone matrix during the modeling and/or remodeling processes where the bone matrix is synthesized [Rochefort et al. 2010]. Osteocytes remain active in the bone-remodeling process by maintaining connections to the bone surface, to osteoblasts and osteoclasts, and to other osteocytes through an extensive canalicular network. Osteocytes are able to release nitric oxide, prostaglandin E2, and adenosine-triphosphate that activate bone formation, and sclerostin, Dickkopf-related protein 1 (DKK1), and frizzled-related protein 1 that inhibit bone formation. They are also able to release the receptor activator of the nuclear factor kappa-B ligand (RANKL) to support osteoclastogenesis, but also to secrete the bone-formation inhibitor sclerostin [Winkler et al. 2003; Van Bezooijen et al. 2004; Mulcahy et al. 2011; Nakashima et al. 2011; Moustafa et al. 2012]. Therefore, any drugs that target the remodeling cycle by affecting osteoblasts, osteoclasts, and osteocytes, and/or molecules that control the signaling pathways, will have a major impact on the targeted bone remodeling [Killock, 2011; Moriishi et al. 2012].
The cellular and molecular basis of osteoporosis
Osteoporosis is a systemic skeletal disorder characterized by low bone mass and micro-architectural deterioration of bone tissue with a consequent compromised bone strength and increased susceptibility to fracture. According to the clinical definition of osteoporosis proposed by the World Health Organization, a patient is osteoporotic when the dual-energy X-ray absorptiometry measurement of bone-mineral density is 2.5 standard deviations below the typical peak bone mass of young healthy women [Blake and Fogelman, 2007]. Osteoporosis occurs in both genders, at all ages, and can be separated into three types: (a) primary osteoporosis in which no underlying cause can be clearly identified, but often follows menopause in women and occurs later in life in men; (b) secondary osteoporosis in which the underlying cause is known (e.g. hyperparathyroidism, hypophosphatasia, diabetes types 1 and 2, alcoholism, glucocorticosteroid use, etc.); (c) more rare forms of the disease, such as juvenile, pregnancy-related, and postpartum osteoporosis [Taxel and Kenny, 2000; Schnatz et al. 2010; Cook et al. 2013].
Osteoporosis is associated with typical fractures (e.g. lumbar spine, femoral neck or distal radius, vertebral fractures, and any fracture resulting from a low trauma in the elderly) that are associated with an increase in morbidity and mortality [Lindsay, 1996; Garnero, 2008; Hopkins et al. 2013]. Therefore, the goal of osteoporosis therapies is to prevent these fractures by inhibiting bone resorption and/or by stimulating bone formation [Sun et al. 2013]. While antiresorptive drugs lower bone turnover [Lewiecki, 2013], anabolic therapies increase bone modeling and/or remodeling osteoblastic activity [Khan and Khan, 2006].
Osteoporosis treatments
The most widely prescribed and first-line drugs for bone diseases are the bisphosphonates, such as alendronate, risedronate, ibandronate, or zoledronic acid [Fleisch, 2002]. These molecules are generally considered to be safe drugs, with the clinical benefits surpassing the risks associated with treatment. Due to their widespread usage in many patients suffering from different diseases, several adverse effects have been reported, including nausea, abdominal pain, ocular inflammation, difficulty in swallowing, and the risk of an inflamed esophagus or esophageal ulcers. However, the relationship between the drug and adverse events is often difficult to establish because clear correlations are often missing due to comedication or comorbidities. One of the most severe adverse events of bisphosphonate treatment is jawbone osteonecrosis, defined as an exposed area of bone in the maxillofacial region after a tooth extraction in which a section of jawbone persists for at least 8 weeks [Khosla et al. 2007; Rizzoli et al. 2008], dies, and deteriorates [Aspenberg, 2006]. Incidence of this rare side effect ranges from 1 per 10,000–110,000 patient years to approximately 10% in patients with myeloma [Khosla et al. 2007; Rizzoli et al. 2008]. The occurrence of jawbone osteonecrosis is often connected to underlying dental problems. In addition, bisphosphonate treatment has been linked to stress and atypical fractures of the femoral shaft, but data are not always significant [Schilcher et al. 2011]. These stress fractures seem to occur through inhibition of bone remodeling. When a patient is undergoing long-term bisphosphonate treatment, bone microdamages are not always repaired, and thus accumulate, and eventually lead to fractures occurring on compact bones at sites of high tensional stress.
Hormone-replacement therapies are also an established approach to the treatment and prevention of osteoporosis, with significant improvement in bone-mineral density, and reduction in hip and vertebral fracture. Estrogen started soon after menopause helps to maintain bone density. However, estrogen therapy in women may increase blood clots, and risk of endometrial cancer, breast cancer, and possibly heart disease [Maclean et al. 2008]. Treatment mimicking estrogen, such as raloxifene, has significant beneficial effects on bone density in postmenopausal women, without some of the side effects associated with estrogen, such as breast cancer. Osteoporosis in men may be related to a gradual age-related decrease in testosterone levels that may be treated by testosterone-replacement therapy, which has a lower impact on bone density than direct osteoporosis medications [Maclean et al. 2008].
In addition to these treatments, other bone anabolic pathways can be targeted, such as the powerful parathyroid hormone (PTH) analogous teriparatide that stimulates new bone growth and is more dependent on increasing the activation frequency, or the monoclonal antibody denosumab that binds to RANKL, a protein involved in the formation, function, and survival of bone-resorption osteoclasts. New treatments under development are aimed at targeting sclerostin and the canonical wingless-int (Wnt) signaling, which is more dependent on increasing bone modeling [Bringhurst, 2002; Deal, 2009; Lim and Clarke, 2012].
The osteocyte within bone tissue
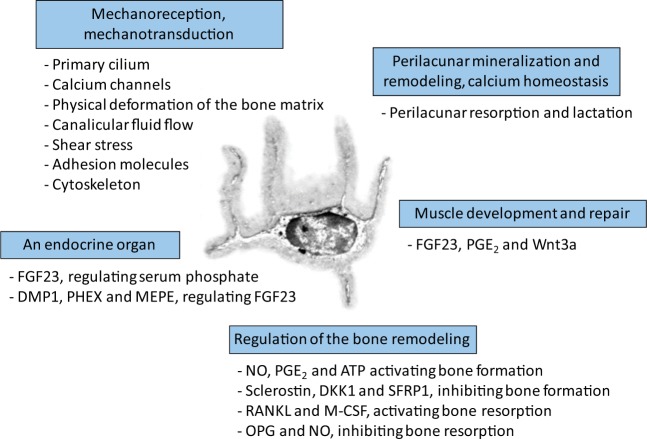
Osteocytes, the most abundant cells in bone, represent 90–95% of all cells in the adult skeleton [Rochefort et al. 2010]. They are able to live for several decades within the bone matrix, whereas osteoblasts and osteoclasts have a lifespan of only a few days or weeks [Bonewald, 2011]. Osteocytes represent the terminal differentiation of osteoblasts [Klein-Nulend et al. 2003]. These cells send out long dendritic processes (the dendrites) through fine channels within the bone matrix (the canaliculi), thus forming a large dendritic network connecting these cells with each other and with osteoblasts, lining cells, and osteoclasts [Rosser and Bonewald, 2012]. Osteocytes and their dendritic processes are bathed in an interstitial fluid (the bone fluid flow) [Bivi et al. 2012]. The osteocytic dendrites are particularly important in the mechanical sensitivity of these cells [Burra et al. 2010], as well as the mechanical signals recorded by the cilia [Hoey et al. 2011; Uzbekov et al. 2012]. More sensitive than osteoblasts, osteocytes are able to respond to mechanical stimulation, particularly shear stress forces [Klein-Nulend et al. 2002], by secreting several molecules, including insulin-like growth factors, osteocalcin, sclerostin, c-fos, prostanoids, and nitric oxide [Uzbekov et al. 2012; Dallas et al. 2013]. Among all the functions assigned to this cell (see an overview in Figure 1) [Dallas et al. 2013]), osteocyte mechanoreception may stimulate the Wnt-signaling pathway as a negative regulator of sclerostin secretion, itself acting as a negative regulator of bone formation [Ozcivici et al. 2010; Post et al. 2013].
Figure 1.
Functions assigned to osteocytes.
The osteocyte has several functions: (a) it senses and integrates mechanical signals (mechanoreception), and converts them into a biological message (mechanotransduction); (b) it directs the differentiation and activity of osteoblasts through the release of NO, PGE2, and ATP that activates bone formation, and sclerostin, DKK1, and SFRP1 that inhibits bone formation; (c) it directs the differentiation and activity of osteoclasts through the secretion of RANKL and M-CSF that activates bone resorption, and OPG and NO that inhibits bone resorption; (d) it controls the local mineralization of the surrounding bone matrix and calcium homeostasis that it can lyse locally to release calcium into the systemic bloodstream during periods of high demand (e.g. lactation); (e) it has an endocrine function by releasing into the bloodstream a specific endocrine factor, FGF23 and its related regulating factors DMP1, PHEX, and MEPE, to modulate phosphate homeostasis; (f) it may modulate the proliferation and tone of skeletal striated muscle cells through the expression of FGF23, PGE2,and Wnt3a. (Adapted with the publisher’s permission from Dallas et al. [2013] and Rochefort and Benhamou [2013].) ATP, adenosine-triphosphate; DKK1, Dickkopf-related protein 1; DMP1, dentin matrix protein 1; FGF23, fibroblast growth factor 23; M-CSF, macrophage-colony stimulating factor; MEPE, matrix extracellular phosphoglycoprotein; NO, nitric oxide; OPG, osteoprotegerin; PGE2, prostaglandin E2; PHEX, phosphate-regulating gene with homologies to endopeptidases on the X chromosome; RANKL, receptor activator of nuclear factor kappa-B ligand; SFRP1, frizzled-related protein 1.
PTH-related therapies
The mechanism of action of the recombinant human PTH drug is still under investigation, but it probably affects multiple signaling pathways and alters the biological activity of several bone cells, including osteoblasts, lining cells, osteoclasts, and osteocytes [O’Brien et al. 2008: Bellido et al. 2013]. The PTH stimulates bone formation by increasing the number of osteoblasts [Wang et al. 2007]. The PTH effects are mediated by a G-protein-coupled receptor, the PTH receptor 1 [Maeda et al. 2013; Van Der Lee et al. 2013]. Different recombinant peptides, mimicking this PTH receptor 1, have different anabolic effects. Thus, the cyclic amino-terminal fragment may have a more anabolic profile than the PTH1–34 or PTH1–84 fragments [Fraher et al. 1999; Whitfield, 2006; Henriksen et al. 2013]. Selected amino-acid substitutions at various positions in the PTH1–28 fragment have also revealed an increased activity of this recombinant hormone [Yang et al. 2007].
The release regulation of PTH, and the related regulation of calcium homeostasis, is under the influence of the calcium-sensing receptor, a G-protein-coupled, seven-pass transmembrane molecule present in the parathyroid gland, the kidney, and in osteoblasts and osteocytes [Brown, 2007; Fromigue et al. 2009; Xue et al. 2012]. Allosteric modulators of this calcium-sensing receptor can affect the secretion of PTH [Trivedi et al. 2008; Riccardi, 2012]. Positive calcium-sensing receptor agonists, called calcimimetics, such as cinacalcet, can reduce PTH secretion in patients with hyperparathyroidism and renal disease [Li et al. 2013; Tsuruta et al. 2013], whereas negative antagonists of this receptor, called calcilytics, can inhibit the receptor function thus inducing the release of a PTH pulse [Cabal et al. 2013]. Therefore, these molecules may represent new targets in the treatment of osteoporosis [Fraser et al. 2004; Nemeth, 2004; John et al. 2011]. However, to be useful as anabolic agents, calcilytic agents must induce the release of sufficient PTH to be anabolic, they must have a short half-life since sustained activation would result in prolonged PTH secretion and a catabolic state (hyperparathyroidism), and they should not deplete the parathyroid gland, and not result in hyperplasia [Avlani et al. 2013]. Recently, a calcilytic agent, called ronacaleret, has shown a strong PTH response, with a short half-life, and an increase in both cortical and trabecular bone formation in rodents [Balan et al. 2009; Atchison et al. 2011]. However, a recent clinical trial involving ronacaleret in humans showed a small, nondose-dependent increase in bone-mineral density in the lumbar spine at 6 months [Fitzpatrick et al. 2012].
Recent studies demonstrated that some actions of PTH on the skeleton are mediated by direct effects on osteocytes [Bellido et al. 2013]. PTH thus down-regulates the expression of the SOST gene, encoding the potent inhibitor of bone formation sclerostin expressed in osteocytes (see below) [Bellido et al. 2005; Keller and Kneissel, 2005]. Furthermore, PTH increases the expression of fibroblast growth factor 23, a hormone expressed in osteocytes (and osteoblasts) that regulates phosphate reabsorption in kidney and contributes to mineral homeostasis [Lavi-Moshayoff et al. 2010; Bellido et al. 2013].
Wnt signaling, sclerostin, and DKK1
Wnt proteins are a large family of extracellular (secreted) cysteine-rich glycoproteins that regulate bone remodeling, but are also involved in several other physiopathological situations, including prostate adenocarcinoma [Yu et al. 2011], renal cancer [Banumathy and Cairns, 2010], most sporadic colorectal cancers [Scholer-Dahirel et al. 2011], melanoma [Lucero et al. 2010], breast cancer [Bu et al. 2008], as well as parathyroid carcinoma [Svedlund et al. 2010], and glioma [Liu et al. 2011].
The biological action of Wnt passes through canonical and noncanonical pathways. Canonical Wnt signaling employs extracellular Wnt ligands that bind frizzled and lipoprotein receptor-related protein (LRP) 5/6 coreceptors at the cell surface to transduce a signal that results in the intracellular activation of β-catenin (Figure 2). This canonical Wnt pathway regulates production of the β-catenin transcription factor by inhibiting its phosphorylation, ubiquitination, and degradation. Noncanonical Wnt signaling is defined as Wnt-initiated or frizzled-initiated signaling that is independent of β-catenin transcriptional function [Lee et al. 2010; Baron and Kneissel, 2013]. Noncanonical Wnt pathways are diverse and include Wnt-cGMP/Ca2+ signaling, Wnt-ROR2 signaling, Wnt-RYK signaling, Wnt-mTOR signaling, etc. [Lee et al. 2010; Sassi et al. 2013].
Figure 2.
Wnt pathway, sclerostin, and DKK1; effects of monoclonal antibodies on bone.
(a) Scl and DKK1 bind Wnt coreceptors LRP5/6 to inhibit Wnt binding and signaling to decrease bone formation. Scl and DKK1 bind the first β-sheet of LRP5 and LRP6 to inhibit Wnt-1 signaling. DKK1 also binds the third β-sheet to inhibit Wnt-3a signaling. DKK1 and Scl can also utilize coreceptors, such as Kremen-1 or -2 receptors, to augment Wnt inhibitory activity resulting in the internalization of the complex. (b) Scl-Ab and DKK1-Ab prevent the interaction of these molecules with LRP5 and LRP6, thereby allowing Wnt-1 and Wnt-3a to bind the first and third β-sheet of LRP5/6, respectively. Actions of these antibodies on bone are summaries. Wnt proteins (either Wnt-1 or Wnt-3a) form a complex with FRZ receptors and LRP5/6 to transduce an intracellular signal leading to increased bone formation. (For an in-depth review see Ke et al. [2012]). DKK1, Dickkopf-related protein 1; DKK1-Ab, Dickkopf-1 antibody; FRZ, frizzled; LRP, lipoprotein receptor-related protein; Scl, sclerostin; Scl-Ab, sclerostin antibody.
The Wnt protein also allows the activation of a protein complex consisting of axin, adenomatous polyposis coli, and glycogen synthase kinase 3 activating an intracellular signal. When the Wnt protein is absent, the membrane receptors frizzled and LRP5/6 are dissociated and glycogen synthase kinase 3 phosphorylates β-catenin, which is then degraded through the ubiquitin/proteasome pathway [Boudin et al. 2013]. When the Wnt protein is present, the frizzled membrane receptors are associated with LRP5/6, the protein complex is disrupted, and the phosphorylation of β-catenin does not occur. Therefore β-catenin accumulates, and is then translocated to the cell nucleus, and binds to transcription factors that can affect the transcription of genes related to bone formation [Lee et al. 2010; Baron and Kneissel, 2013; Boudin et al. 2013].
Elements of the Wnt-signaling pathway are well conserved in evolution and are found in primitive metazoans, such as cnidarians [Lengfeld et al. 2009]. The Wnt antagonist DKK1 is expressed by early invertebrates, such as the Hydra [Guder et al. 2006], whereas the expression of sclerostin is not found until the emergence of bony vertebrates, indicating a more specific role for sclerostin in the development and maintenance of the skeleton and a broader role for DKK1.
In addition to binding LRP5/6, both sclerostin and DKK1 can bind other transmembrane molecules, such as LRP4, also known as multiple epidermal growth factor-like domains 7 (Megf7) to increase their inhibitory activity on the Wnt-signaling pathway (Figure 2) [Choi et al. 2009]. Indeed, Megf4 KO mice exhibit limb abnormalities with polysyndactyly [Simon-Chazottes et al. 2006], whereas loss-of-function mutations of this factor in humans, also known as Cenani–Lenz syndrome, cause syndactyly, kidney malformations, and bone overgrowth [Li et al. 2010b]. This indicates that LRP4 acts as a negative regulator of LRP5/6 signaling by increasing the inhibitory activity of sclerostin on the Wnt pathway, without affecting the activity of DKK1 [Leupin et al. 2011].
DKK1 may also bind to a two-member family of proteins referred to as Kremen-1 and Kremen-2, leading to the removal of LRP5/6 from the cell surface (Figure 2) [Mao et al. 2002]. Invalidation of both Kremen-1 and -2 in mice lead to subtle patterning defects in the forelimb with increased bone formation that were additionally enhanced by the deletion of a single DKK1 allele, indicating the importance of these factors in the regulation of bone mass through modulation of LRP5/6 [Ellwanger et al. 2008; Schulze et al. 2010]. However, the homozygous deletion of either Kremen-1 or -2 lead to normal bone formation and bone mass, suggesting a functional redundancy of Kremen-1 and -2 [Schulze et al. 2010].
Inhibitors of the Wnt pathway can target either frizzled (serum frizzled-related proteins), Wnt (Wnt inhibitory factors), or LRP5/6 (DKK1 and the osteocyte-released sclerostin) (Figure 2) [Rybchyn et al. 2011]. These agonist molecules can prevent Wnt from activating the frizzled LRP5/6 signaling pathway, inducing a decrease in Wnt signaling, and thus a significant bone decrease in bone formation [Glantschnig et al. 2011]. On the other hand, deficiencies in these inhibitors or antibodies targeting these inhibitors induce an increase of Wnt signaling and, therefore, induce an increase in bone formation [Glantschnig et al. 2010].
Complexity of Wnt signaling has increased since the recent description of distinct ligand-binding domains on LRP5/6 receptors that recognize different classes of Wnt proteins and inhibitors [Bourhis et al. 2010; Ettenberg et al. 2010; Gong et al. 2010]. The Wnt1 class, comprising Wnt 1, 2, 6, 7a, 7b, 9a, 9b, and 10b, was reported to bind the first β-sheet of LRP5/6, whereas the Wnt3 class, including Wnt3 and 3a, was shown to bind the third β-sheet of LRP5/6 (Figure 2). DKK1 was revealed to bind indifferently the first and third β-sheets of LRP5/6 [Bourhis et al. 2010], whereas sclerostin bound only the first β-sheet of LRP5/6 (Figure 2) [Ettenberg et al. 2010]. Therefore, DKK1 inhibited both the Wnt1 and Wnt3 classes, and sclerostin inhibited the Wnt1 class and enhanced the Wnt3 class [Ettenberg et al. 2010].
The sclerostin protein is encoded by the human SOST gene [Winkler et al. 2003; Robling et al. 2008; Cohen-Kfir et al. 2011]. Homozygous mutation of loss of expression of the SOST gene is responsible for two rare genetic disorders, sclerosteosis and van Buchem disease, which are associated with general progressive skeletal overgrowth and sclerosis of the axial and appendicular skeleton [Brunkow et al. 2001; Bhadada et al. 2013]. In sclerosteosis, bone formation is stimulated by the absence or decreased synthesis of sclerostin in humans, whereas bone resorption is not (or only mildly) affected [Van Lierop et al. 2011]. Van Buchem disease is a disorder resembling sclerosteosis but distinguished by its less severe phenotype and absence of hand malformations, such as syndactyly [Staehling-Hampton et al. 2002]. The difference between these two disorders might be explained by the fact that the deleted genomic region in van Buchem disease includes no regulatory elements required for sclerostin expression during the embryologic steps of digit formation [Brunkow et al. 2001; Uitterlinden et al. 2004]. Finally, heterozygous mutations in the SOST gene cause a mild increase in bone mass and fewer skeletal complications [Van Lierop et al. 2011].
SOST mRNA is expressed in many tissues during embryogenesis. However, the sclerostin protein is found only postnatally in terminally differentiated cells embedded within a mineralized matrix, including osteocytes, mineralized hypertrophic chondrocytes, and cementocytes [Ohyama et al. 2004]. In humans, SOST mRNA is detectable in tissues such as heart, aorta, liver, odontoblasts, and kidney, whereas sclerostin protein has never been detected in any organs other than bone [Balemans et al. 2001; Moester et al. 2010]. Since the sclerostin protein is almost exclusively produced by osteocytes in adult murine and human bone, antibodies targeting this offer a way to target specifically bone formation [Winkler et al. 2003; Van Bezooijen et al. 2005; Robling et al. 2008]. Sclerostin antibodies thus increase bone formation in osteopenic estrogen-deficient rats [Keller and Kneissel, 2005; Li et al. 2009]. A single subcutaneous dose of a sclerostin antibody in postmenopausal women resulted in an increase in bone density and bone formation markers, without any modification of bone-resorption markers [Papapoulos, 2011; Lewiecki, 2013].
Next to sclerostin, mutations in the gene encoding LRP5/6 inducing a gain of function cause an increase in bone mass [Kim et al. 2007; Hoeppner et al. 2009]. These mutations induce impairment of the binding of DKK1 to frizzled LRP5/6, thus allowing an increase in the Wnt-signaling pathway, and an increase in bone formation [Choi et al. 2009]. Antibodies targeting DKK1 and/or LRP5/6 induce the increase in bone mass, volume, and formation in rodents [Van Dinther et al. 2013]. Therefore, antibodies targeting DKK1 could also be used as anabolic or antiresorptive agents for the treatment of patients with low bone mass [Ahn et al. 2011].
Effects of monoclonal antibodies on bone
The pharmacologic inhibition of the sclerostin protein, using monoclonal antibody, has confirmed efficacy in animal models of bone diseases, including estrogen deficiency-induced bone loss [Li et al. 2009], age-related, or androgen deficiency-induced bone loss [Li et al. 2010a], disuse/immobilization-induced bone loss [Tian et al. 2011], glucocorticoid-induced bone loss [Marenzana et al. 2011], chronic inflammation-induced bone loss [Eddleston et al. 2009], bone loss associated with type 2 diabetes mellitus [Gaudio et al. 2012], as well as in a rodent model of osteogenesis imperfecta [Sinder et al. 2013], and fracture healing [Gamie et al. 2012; Cui et al. 2013]. Effects of sclerostin monoclonal antibody have also been reported in human preclinical models of bone loss, including osteogenesis imperfecta, fracture healing, implant diseases. and other bone disorders (Figure 2) [Eddleston et al. 2009; Li et al. 2009, 2010a]. The use of sclerostin antibody in these conditions has demonstrated a consistent ability to increase bone formation, bone mass, and bone strength [Ke et al. 2012].
A human antisclerostin antibody, known as AMG 785 or romosozumab, is being developed by Amgen (Thousand Oaks, CA, USA) and UCB Inc. (Smyrna, GA, USA), and a phase l randomized, double-blinded, placebo-controlled study using this antibody has been conducted on men and postmenopausal women [Padhi et al. 2011]. In this trial, antibody injection was associated with substantial increases in bone-formation markers and reductions in bone-resorption markers, as well as a dose-dependent increase in bone-mineral density at the lumbar spine and total hip after 3 months. Results from the phase ll trial on postmenopausal osteoporosis have not been published at this time, but a recent press release by Amgen and UCB Inc. reported positive results with the cohort using the monoclonal antisclerostin antibody, including a significant increase in bone-mineral density of the lumbar spine at 12 months compared with placebo. Furthermore, this antisclerostin antibody was positively compared with teriparatide and alendronate. Positive phase ll results of this antibody in patients with postmenopausal osteoporosis have been announced by Amgen and UCB Inc. (NCT00896532, May 2009), whereas phase lll programs on fracture healing, and in patients with postmenopausal osteoporosis are currently ongoing (NCT01631214, May 2012; NCT01796301, February 2013).
In the same manner, the pharmacologic inhibition of DKK1, using monoclonal antibody, has also demonstrated efficacy in animal models of bone diseases, including gonad-intact rodents [Li et al. 2006], rodent models of fracture healing [Li et al. 2011], and implant fixation [Agholme et al. 2011]. However, unlike Scl-Ab, DKK1 demonstrated no efficacy in adult ovariectomized rats [Li et al. 2006], and a modest improvement in ovariectomized rhesus monkeys [Glantschnig et al. 2011; Li et al. 2011]. DKK1 antibody demonstrated efficacy in rodent models of rheumatoid arthritis [Diarra et al. 2007], ankylosing spondylitis [Uderhardt et al. 2010], and multiple myeloma [Fulciniti et al. 2009].
Conclusion
Treatments of low bone mass and osteoporosis have advanced significantly beyond hormone therapy administered at menopause. These advances are mainly the result of increased understanding of the mechanisms underlying osteoblast, osteoclast, and osteocyte biology. Novel anabolic and antiresorptive agents, affecting osteocyte-associated sclerostin, the calcium-sensing receptor, or Wnt signaling, offer promise for the treatment of bone disorders. Additional therapies treating patients with established fractures are needed next to reduce the burden of this other disease.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflict of interest in preparing this article.
References
- Agholme F., Isaksson H., Kuhstoss S., Aspenberg P. (2011) The effects of Dickkopf-1 antibody on metaphyseal bone and implant fixation under different loading conditions. Bone 48: 988–996 [DOI] [PubMed] [Google Scholar]
- Ahn V., Chu M., Choi H., Tran D., Abo A., Weis W. (2011) Structural basis of Wnt signaling inhibition by Dickkopf binding to LRP5/6. Dev Cell 21: 862–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspenberg P. (2006) Osteonecrosis of the jaw: what do bisphosphonates do? Expert Opin Drug Saf 5: 743–745 [DOI] [PubMed] [Google Scholar]
- Atchison D., Harding P., Beierwaltes W. (2011) Hypercalcemia reduces plasma renin via parathyroid hormone, renal interstitial calcium, and the calcium-sensing receptor. Hypertension 58: 604–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin J., Turksen K. (1996) Monoclonal antibodies as tools for studying the osteoblast lineage. Microsc Res Tech 33: 128–140 [DOI] [PubMed] [Google Scholar]
- Avlani V., Ma W., Mun H., Leach K., Delbridge L., Christopoulos A., et al. (2013) Calcium-sensing receptor-dependent activation of CREB phosphorylation in HEK293 cells and human parathyroid cells. Am J Physiol Endocrinol Metab 304: E1097–E1104 [DOI] [PubMed] [Google Scholar]
- Balan G., Bauman J., Bhattacharya S., Castrodad M., Healy D., Herr M., et al. (2009) The discovery of novel calcium sensing receptor negative allosteric modulators. Bioorg Med Chem Lett 19: 3328–3332 [DOI] [PubMed] [Google Scholar]
- Balemans W., Ebeling M., Patel N., Van Hul E., Olson P., Dioszegi M., et al. (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10: 537–543 [DOI] [PubMed] [Google Scholar]
- Banumathy G., Cairns P. (2010) Signaling pathways in renal cell carcinoma. Cancer Biol Ther 10: 658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R., Kneissel M. (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19: 179–192 [DOI] [PubMed] [Google Scholar]
- Bellido T., Ali A., Gubrij I., Plotkin L., Fu Q., O’Brien C., et al. (2005) Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146: 4577–4583 [DOI] [PubMed] [Google Scholar]
- Bellido T., Saini V., Pajevic P. (2013) Effects of PTH on osteocyte function. Bone 54: 250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadada S., Rastogi A., Steenackers E., Boudin E., Arya A., Dhiman V., et al. (2013) Novel SOST gene mutation in a sclerosteosis patient and her parents. Bone 52: 707–710 [DOI] [PubMed] [Google Scholar]
- Bivi N., Nelson M., Faillace M., Li J., Miller L., Plotkin L. (2012) Deletion of Cx43 from osteocytes results in defective bone material properties but does not decrease extrinsic strength in cortical bone. Calcif Tissue Int 91: 215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake G., Fogelman I. (2007) Role of dual-energy X-ray absorptiometry in the diagnosis and treatment of osteoporosis. J Clin Densitom 10: 102–110 [DOI] [PubMed] [Google Scholar]
- Bonewald L. (2011) The amazing osteocyte. J Bone Miner Res 26: 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudin E., Fijalkowski I., Piters E., Van Hul W. (2013) The role of extracellular modulators of canonical Wnt signaling in bone metabolism and diseases. Semin Arthritis Rheum 43: 220–240 [DOI] [PubMed] [Google Scholar]
- Bourhis E., Tam C., Franke Y., Bazan J., Ernst J., Hwang J., et al. (2010) Reconstitution of a frizzled8.Wnt3a.LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6. J Biol Chem 285: 9172–9179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringhurst F. (2002) PTH receptors and apoptosis in osteocytes. J Musculoskelet Neuronal Interact 2: 245–251 [PubMed] [Google Scholar]
- Brown E. (2007) The calcium-sensing receptor: physiology, pathophysiology and CaR-based therapeutics. Subcell Biochem 45: 139–167 [DOI] [PubMed] [Google Scholar]
- Brunkow M., Gardner J., Van Ness J., Paeper B., Kovacevich B., Proll S., et al. (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68: 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G., Lu W., Liu C., Selander K., Yoneda T., Hall C., et al. (2008) Breast cancer-derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: implication for breast cancer osteolytic bone metastases. Int J Cancer 123: 1034–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burra S., Nicolella D., Francis W., Freitas C., Mueschke N., Poole K., et al. (2010) Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci U S A 107: 13648–13653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabal A., Mehta K., Ross D., Shrestha R., Comisar W., Denker A., et al. (2013) A semimechanistic model of the time-course of release of PTH into plasma following administration of the calcilytic JTT-305/MK-5442 in humans. J Bone Miner Res 28: 1830–1836 [DOI] [PubMed] [Google Scholar]
- Choi H., Dieckmann M., Herz J., Niemeier A. (2009) Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS One 4: e7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kfir E., Artsi H., Levin A., Abramowitz E., Bajayo A., Gurt I., et al. (2011) Sirt1 is a regulator of bone mass and a repressor of SOST encoding for sclerostin, a bone formation inhibitor. Endocrinology 152: 4514–4524 [DOI] [PubMed] [Google Scholar]
- Cook F., Mumm S., Whyte M., Wenkert D. (2013) Pregnancy-associated osteoporosis with a heterozygous deactivating LDL receptor-related protein 5 (LRP5) mutation and a homozygous methylenetetrahydrofolate reductase (MTHFR) polymorphism. J Bone Miner Res. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Cui L., Cheng H., Song C., Li C., Simonet W., Ke H., et al. (2013) Time-dependent effects of sclerostin antibody on a mouse fracture healing model. J Musculoskelet Neuronal Interact 13: 178–184 [PubMed] [Google Scholar]
- Daci E., Van Cromphaut S., Bouillon R. (2002) Mechanisms influencing bone metabolism in chronic illness. Horm Res 58(Suppl. 1): 44–51 [DOI] [PubMed] [Google Scholar]
- Dallas S., Prideaux M., Bonewald L. (2013) The osteocyte: an endocrine cell … and more. Endocr Rev 34: 658–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal C. (2009) Potential new drug targets for osteoporosis. Nat Clin Pract Rheumatol 5: 20–27 [DOI] [PubMed] [Google Scholar]
- Devogelaer J. (2000) Treatment of bone diseases with bisphosphonates, excluding osteoporosis. Curr Opin Rheumatol 12: 331–335 [DOI] [PubMed] [Google Scholar]
- Diarra D., Stolina M., Polzer K., Zwerina J., Ominsky M., Dwyer D., et al. (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13: 156–163 [DOI] [PubMed] [Google Scholar]
- Eddleston A., Marenzana M., Moore A., Stephens P., Muzylak M., Marshall D., et al. (2009) A short treatment with an antibody to sclerostin can inhibit bone loss in an ongoing model of colitis. J Bone Miner Res 24: 1662–1671 [DOI] [PubMed] [Google Scholar]
- Ellwanger K., Saito H., Clement-Lacroix P., Maltry N., Niedermeyer J., Lee W., et al. (2008) Targeted disruption of the Wnt regulator Kremen induces limb defects and high bone density. Mol Cell Biol 28: 4875–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg S., Charlat O., Daley M., Liu S., Vincent K., Stuart D., et al. (2010) Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc Natl Acad Sci U S A 107: 15473–15478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick L., Dabrowski C., Cicconetti G., Gordon D., Fuerst T., Engelke K., et al. (2012) Ronacaleret, a calcium-sensing receptor antagonist, increases trabecular but not cortical bone in postmenopausal women. J Bone Miner Res 27: 255–262 [DOI] [PubMed] [Google Scholar]
- Fleisch H. (2002) Development of bisphosphonates. Breast Cancer Res 4: 30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraher L., Avram R., Watson P., Hendy G., Henderson J., Chong K., et al. (1999) Comparison of the biochemical responses to human parathyroid hormone-(1–31)NH2 and hPTH-(1–34) in healthy humans. J Clin Endocrinol Metab 84: 2739–2743 [DOI] [PubMed] [Google Scholar]
- Fraser W., Ahmad A., Vora J. (2004) The physiology of the circadian rhythm of parathyroid hormone and its potential as a treatment for osteoporosis. Curr Opin Nephrol Hypertens 13: 437–444 [DOI] [PubMed] [Google Scholar]
- Fromigue O., Hay E., Barbara A., Petrel C., Traiffort E., Ruat M., et al. (2009) Calcium sensing receptor-dependent and receptor-independent activation of osteoblast replication and survival by strontium ranelate. J Cell Mol Med 13: 2189–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulciniti M., Tassone P., Hideshima T., Vallet S., Nanjappa P., Ettenberg S., et al. (2009) Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 114: 371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamie Z., Korres N., Leonidou A., Gray A., Tsiridis E. (2012) Sclerostin monoclonal antibodies on bone metabolism and fracture healing. Expert Opin Investig Drugs 21: 1523–1534 [DOI] [PubMed] [Google Scholar]
- Garnero P. (2008) Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol Diagn Ther 12: 157–170 [DOI] [PubMed] [Google Scholar]
- Gaudio A., Privitera F., Battaglia K., Torrisi V., Sidoti M., Pulvirenti I., et al. (2012) Sclerostin levels associated with inhibition of the Wnt/beta-catenin signaling and reduced bone turnover in type 2 diabetes mellitus. J Clin Endocrinol Metab 97: 3744–3750 [DOI] [PubMed] [Google Scholar]
- Glantschnig H., Hampton R., Lu P., Zhao J., Vitelli S., Huang L., et al. (2010) Generation and selection of novel fully human monoclonal antibodies that neutralize Dickkopf-1 (DKK1) inhibitory function in vitro and increase bone mass in vivo. J Biol Chem 285: 40135–40147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantschnig H., Scott K., Hampton R., Wei N., Mccracken P., Nantermet P., et al. (2011) A rate-limiting role for Dickkopf-1 in bone formation and the remediation of bone loss in mouse and primate models of postmenopausal osteoporosis by an experimental therapeutic antibody. J Pharmacol Exp Ther 338: 568–578 [DOI] [PubMed] [Google Scholar]
- Gong Y., Bourhis E., Chiu C., Stawicki S., Dealmeida V., Liu B., et al. (2010) Wnt isoform-specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLoS One 5: e12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guder C., Pinho S., Nacak T., Schmidt H., Hobmayer B., Niehrs C., et al. (2006) An ancient Wnt-Dickkopf antagonism in Hydra. Development 133: 901–911 [DOI] [PubMed] [Google Scholar]
- Heino T., Kurata K., Higaki H., Vaananen H. (2009) Evidence for the role of osteocytes in the initiation of targeted remodeling. Technol Health Care 17: 49–56 [DOI] [PubMed] [Google Scholar]
- Henriksen K., Andersen J., Riis B., Mehta N., Tavakkol R., Alexandersen P., et al. (2013) Evaluation of the efficacy, safety and pharmacokinetic profile of oral recombinant human parathyroid hormone [rhPTH(1–31)NH(2)] in postmenopausal women with osteoporosis. Bone 53: 160–166 [DOI] [PubMed] [Google Scholar]
- Hoeppner L., Secreto F., Westendorf J. (2009) Wnt signaling as a therapeutic target for bone diseases. Expert Opin Ther Targets 13: 485–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey D., Kelly D., Jacobs C. (2011) A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem Biophys Res Commun 412: 182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R., Tarride J., Leslie W., Metge C., Lix L., Morin S., et al. (2013) Estimating the excess costs for patients with incident fractures, prevalent fractures, and nonfracture osteoporosis. Osteoporos Int 24: 581–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John M., Widler L., Gamse R., Buhl T., Seuwen K., Breitenstein W., et al. (2011) ATF936, a novel oral calcilytic, increases bone mineral density in rats and transiently releases parathyroid hormone in humans. Bone 49: 233–241 [DOI] [PubMed] [Google Scholar]
- Ke H., Richards W., Li X., Ominsky M. (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33: 747–783 [DOI] [PubMed] [Google Scholar]
- Keller H., Kneissel M. (2005) SOST is a target gene for PTH in bone. Bone 37: 148–158 [DOI] [PubMed] [Google Scholar]
- Khan A., Khan A. (2006) Anabolic agents: a new chapter in the management of osteoporosis. J Obstet Gynaecol Can 28: 136–141 [DOI] [PubMed] [Google Scholar]
- Khosla S., Burr D., Cauley J., Dempster D., Ebeling P., Felsenberg D., et al. (2007) Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 22: 1479–1491 [DOI] [PubMed] [Google Scholar]
- Killock D. (2011) Bone: osteocyte RANKL in bone homeostasis: a paradigm shift? Nat Rev Rheumatol 7: 619. [DOI] [PubMed] [Google Scholar]
- Kim J., Leucht P., Lam K., Luppen C., Ten Berge D., Nusse R., et al. (2007) Bone regeneration is regulated by wnt signaling. J Bone Miner Res 22: 1913–1923 [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J., Nijweide P., Burger E. (2003) Osteocyte and bone structure. Curr Osteoporos Rep 1: 5–10 [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J., Sterck J., Semeins C., Lips P., Joldersma M., Baart J., et al. (2002) Donor age and mechanosensitivity of human bone cells. Osteoporos Int 13: 137–146 [DOI] [PubMed] [Google Scholar]
- Kogianni G., Noble B. (2007) The biology of osteocytes. Curr Osteoporos Rep 5: 81–86 [DOI] [PubMed] [Google Scholar]
- Lavi-Moshayoff V., Wasserman G., Meir T., Silver J., Naveh-Many T. (2010) PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol 299: F882–889 [DOI] [PubMed] [Google Scholar]
- Lee D., Kim H., Ku S., Kim S., Choi Y., Kim J. (2010) Association between polymorphisms in Wnt signaling pathway genes and bone mineral density in postmenopausal Korean women. Menopause 17: 1064–1070 [DOI] [PubMed] [Google Scholar]
- Lengfeld T., Watanabe H., Simakov O., Lindgens D., Gee L., Law L., et al. (2009) Multiple Wnts are involved in Hydra organizer formation and regeneration. Dev Biol 330: 186–199 [DOI] [PubMed] [Google Scholar]
- Leupin O., Piters E., Halleux C., Hu S., Kramer I., Morvan F., et al. (2011) Bone overgrowth-associated mutations in the LRP4 gene impair sclerostin facilitator function. J Biol Chem 286: 19489–19500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewiecki E. (2013) Monoclonal antibodies for the treatment of osteoporosis. Expert Opin Biol Ther 13: 183–196 [DOI] [PubMed] [Google Scholar]
- Li D., Shao L., Zhou H., Jiang W., Zhang W., Xu Y. (2013) The efficacy of cinacalcet combined with conventional therapy on bone and mineral metabolism in dialysis patients with secondary hyperparathyroidism: a meta-analysis. Endocrine 43: 68–77 [DOI] [PubMed] [Google Scholar]
- Li J., Sarosi I., Cattley R., Pretorius J., Asuncion F., Grisanti M., et al. (2006) Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39: 754–766 [DOI] [PubMed] [Google Scholar]
- Li X., Grisanti M., Fan W., Asuncion F., Tan H., Dwyer D., et al. (2011) Dickkopf-1 regulates bone formation in young growing rodents and upon traumatic injury. J Bone Miner Res 26: 2610–2621 [DOI] [PubMed] [Google Scholar]
- Li X., Ominsky M., Warmington K., Morony S., Gong J., Cao J., et al. (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24: 578–588 [DOI] [PubMed] [Google Scholar]
- Li X., Warmington K., Niu Q., Asuncion F., Barrero M., Grisanti M., et al. (2010a) Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res 25: 2647–2656 [DOI] [PubMed] [Google Scholar]
- Li Y., Pawlik B., Elcioglu N., Aglan M., Kayserili H., Yigit G., et al. (2010b) LRP4 mutations alter Wnt/beta-catenin signaling and cause limb and kidney malformations in Cenani–Lenz syndrome. Am J Hum Genet 86: 696–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim V., Clarke B. (2012) New therapeutic targets for osteoporosis: beyond denosumab. Maturitas 73: 269–272 [DOI] [PubMed] [Google Scholar]
- Lindsay R. (1996) The menopause and osteoporosis. Obstet Gynecol 87: 16S-19S [DOI] [PubMed] [Google Scholar]
- Liu C., Tu Y., Sun X., Jiang J., Jin X., Bo X., et al. (2011) Wnt/beta-catenin pathway in human glioma: expression pattern and clinical/prognostic correlations. Clin Exp Med 11: 105–112 [DOI] [PubMed] [Google Scholar]
- Lucero O., Dawson D., Moon R., Chien A. (2010) A re-evaluation of the “oncogenic” nature of Wnt/beta-catenin signaling in melanoma and other cancers. Curr Oncol Rep 12: 314–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean C., Newberry S., Maglione M., Mcmahon M., Ranganath V., Suttorp M., et al. (2008) Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 148: 197–213 [DOI] [PubMed] [Google Scholar]
- Maeda A., Okazaki M., Baron D., Dean T., Khatri A., Mahon M., et al. (2013) Critical role of parathyroid hormone (PTH) receptor-1 phosphorylation in regulating acute responses to PTH. Proc Natl Acad Sci U S A 110: 5864–5869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B., Wu W., Davidson G., Marhold J., Li M., Mechler B., et al. (2002) Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417: 664–667 [DOI] [PubMed] [Google Scholar]
- Marenzana M., Greenslade K., Eddleston A., Okoye R., Marshall D., Moore A., et al. (2011) Sclerostin antibody treatment enhances bone strength but does not prevent growth retardation in young mice treated with dexamethasone. Arthritis Rheum 63: 2385–2395 [DOI] [PubMed] [Google Scholar]
- Moester M., Papapoulos S., Lowik C., Van Bezooijen R. (2010) Sclerostin: current knowledge and future perspectives. Calcif Tissue Int 87: 99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriishi T., Fukuyama R., Ito M., Miyazaki T., Maeno T., Kawai Y., et al. (2012) Osteocyte network; a negative regulatory system for bone mass augmented by the induction of Rankl in osteoblasts and SOST in osteocytes at unloading. PLoS One 7: e40143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley J. (2000) Osteoporosis and bone functional adaptation: mechanobiological regulation of bone architecture in growing and adult bone, a review. J Rehabil Res Dev 37: 189–199 [PubMed] [Google Scholar]
- Moustafa A., Sugiyama T., Prasad J., Zaman G., Gross T., Lanyon L., et al. (2012) Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int 23: 1225–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy L., Taylor D., Lee T., Duffy G. (2011) RANKL and OPG activity is regulated by injury size in networks of osteocyte-like cells. Bone 48: 182–188 [DOI] [PubMed] [Google Scholar]
- Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J., et al. (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17: 1231–1234 [DOI] [PubMed] [Google Scholar]
- Nemeth E. (2004) Calcimimetic and calcilytic drugs: just for parathyroid cells? Cell Calcium 35: 283–289 [DOI] [PubMed] [Google Scholar]
- O’Brien C., Plotkin L., Galli C., Goellner J., Gortazar A., Allen M., et al. (2008) Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One 3: e2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama Y., Nifuji A., Maeda Y., Amagasa T., Noda M. (2004) Spaciotemporal association and bone morphogenetic protein regulation of sclerostin and osterix expression during embryonic osteogenesis. Endocrinology 145: 4685–4692 [DOI] [PubMed] [Google Scholar]
- Ozcivici E., Luu Y., Adler B., Qin Y., Rubin J., Judex S., et al. (2010) Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol 6: 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhi D., Jang G., Stouch B., Fang L., Posvar E. (2011) Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26: 19–26 [DOI] [PubMed] [Google Scholar]
- Papapoulos S. (2011) Targeting sclerostin as potential treatment of osteoporosis. Ann Rheum Dis 70(Suppl. 1): i119–i122 [DOI] [PubMed] [Google Scholar]
- Post T., Schmidt S., Peletier L., De Greef R., Kerbusch T., Danhof M. (2013) Application of a mechanism-based disease systems model for osteoporosis to clinical data. J Pharmacokinet Pharmacodyn 40: 143–156 [DOI] [PubMed] [Google Scholar]
- Riccardi D. (2012) Antagonizing the calcium-sensing receptor: towards new bone anabolics? Curr Mol Pharmacol 5: 182–188 [DOI] [PubMed] [Google Scholar]
- Rizzoli R., Burlet N., Cahall D., Delmas P., Eriksen E., Felsenberg D., et al. (2008) Osteonecrosis of the jaw and bisphosphonate treatment for osteoporosis. Bone 42: 841–847 [DOI] [PubMed] [Google Scholar]
- Robling A., Niziolek P., Baldridge L., Condon K., Allen M., Alam I., et al. (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283: 5866–5875 [DOI] [PubMed] [Google Scholar]
- Rochefort G., Benhamou C. (2013) Osteocytes are not only mechanoreceptive cells. Int j numer method biomed eng 29: 1082–1088 [DOI] [PubMed] [Google Scholar]
- Rochefort G., Pallu S., Benhamou C. (2010) Osteocyte: the unrecognized side of bone tissue. Osteoporos Int 21: 1457–1469 [DOI] [PubMed] [Google Scholar]
- Rosser J., Bonewald L. (2012) Studying osteocyte function using the cell lines MLO-Y4 and MLO-A5. Methods Mol Biol 816: 67–81 [DOI] [PubMed] [Google Scholar]
- Rybchyn M., Slater M., Conigrave A., Mason R. (2011) An Akt-dependent increase in canonical Wnt signaling and a decrease in sclerostin protein levels are involved in strontium ranelate-induced osteogenic effects in human osteoblasts. J Biol Chem 286: 23771–23779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi N., Laadhar L., Allouche M., Zandieh-Doulabi B., Hamdoun M., Klein-Nulend J., et al. (2013) The roles of canonical and non-canonical Wnt signaling in human de-differentiated articular chondrocytes. Biotech Histochem 13: 384–392 [DOI] [PubMed] [Google Scholar]
- Schilcher J., Michaelsson K., Aspenberg P. (2011) Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 364: 1728–1737 [DOI] [PubMed] [Google Scholar]
- Schnatz P., Marakovits K., O’Sullivan D. (2010) Assessment of postmenopausal women and significant risk factors for osteoporosis. Obstet Gynecol Surv 65: 591–596 [DOI] [PubMed] [Google Scholar]
- Scholer-Dahirel A., Schlabach M., Loo A., Bagdasarian L., Meyer R., Guo R., et al. (2011) Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A 108: 17135–17140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze J., Seitz S., Saito H., Schneebauer M., Marshall R., Baranowsky A., et al. (2010) Negative regulation of bone formation by the transmembrane Wnt antagonist Kremen-2. PLoS One 5: e10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar Y., Misra S., Vineet D., Baskaran P. (2013) Paget disease of bone: a classic case report. Contemp Clin Dent 4: 227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoback D. (2007) Update in osteoporosis and metabolic bone disorders. J Clin Endocrinol Metab 92: 747–753 [DOI] [PubMed] [Google Scholar]
- Simon-Chazottes D., Tutois S., Kuehn M., Evans M., Bourgade F., Cook S., et al. (2006) Mutations in the gene encoding the low-density lipoprotein receptor LRP4 cause abnormal limb development in the mouse. Genomics 87: 673–677 [DOI] [PubMed] [Google Scholar]
- Sinder B., Eddy M., Ominsky M., Caird M., Marini J., Kozloff K. (2013) Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J Bone Miner Res 28: 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehling-Hampton K., Proll S., Paeper B., Zhao L., Charmley P., Brown A., et al. (2002) A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet 110: 144–152 [DOI] [PubMed] [Google Scholar]
- Sun G., Guo T., Chen Y., Xu B., Guo J.H., Zhao J. (2013) Significant pathways detection in osteoporosis based on the bibliometric network. Eur Rev Med Pharmacol Sci 17: 1–7 [PubMed] [Google Scholar]
- Svedlund J., Auren M., Sundstrom M., Dralle H., Akerstrom G., Bjorklund P., et al. (2010) Aberrant WNT/beta-catenin signaling in parathyroid carcinoma. Mol Cancer 9: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxel P., Kenny A. (2000) Differential diagnosis and secondary causes of osteoporosis. Clin Cornerstone 2: 11–21 [DOI] [PubMed] [Google Scholar]
- Tian X., Jee W., Li X., Paszty C., Ke H. (2011) Sclerostin antibody increases bone mass by stimulating bone formation and inhibiting bone resorption in a hindlimb-immobilization rat model. Bone 48: 197–201 [DOI] [PubMed] [Google Scholar]
- Trivedi R., Mithal A., Chattopadhyay N. (2008) Recent updates on the calcium-sensing receptor as a drug target. Curr Med Chem 15: 178–186 [DOI] [PubMed] [Google Scholar]
- Tsuruta Y., Okano K., Kikuchi K., Akiba T., Nitta K. (2013) Effects of cinacalcet on bone mineral density and bone markers in hemodialysis patients with secondary hyperparathyroidism. Clin Exp Nephrol 17: 120–126 [DOI] [PubMed] [Google Scholar]
- Uderhardt S., Diarra D., Katzenbeisser J., David J., Zwerina J., Richards W., et al. (2010) Blockade of Dickkopf (DKK)-1 induces fusion of sacroiliac joints. Ann Rheum Dis 69: 592–597 [DOI] [PubMed] [Google Scholar]
- Uitterlinden A., Arp P., Paeper B., Charmley P., Proll S., Rivadeneira F., et al. (2004) Polymorphisms in the sclerosteosis/van Buchem disease gene (SOST) region are associated with bone-mineral density in elderly whites. Am J Hum Genet 75: 1032–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzbekov R., Maurel D., Aveline P., Pallu S., Benhamou C., Rochefort G. (2012) Centrosome fine ultrastructure of the osteocyte mechanosensitive primary cilium. Microsc Microanal 18: 1430–1441 [DOI] [PubMed] [Google Scholar]
- Van Bezooijen R., Roelen B., Visser A., Van Der Wee-Pals L., De Wilt E., Karperien M., et al. (2004) Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199: 805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bezooijen R., Ten Dijke P., Papapoulos S., Lowik C. (2005) SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev 16: 319–327 [DOI] [PubMed] [Google Scholar]
- Van Der Lee M., Verkaar F., Wat J., Van Offenbeek J., Timmerman M., Voorneveld L., et al. (2013) Beta-arrestin-biased signaling of PTH analogs of the type 1 parathyroid hormone receptor. Cell Signal 25: 527–538 [DOI] [PubMed] [Google Scholar]
- Van Dinther M., Zhang J., Weidauer S., Boschert V., Muth E., Knappik A., et al. (2013) Anti-sclerostin antibody inhibits internalization of sclerostin and sclerostin-mediated antagonism of Wnt/LRP6 signaling. PLoS One 8: e62295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lierop A., Hamdy N., Hamersma H., Van Bezooijen R., Power J., Loveridge N., et al. (2011) Patients with sclerosteosis and disease carriers: human models of the effect of sclerostin on bone turnover. J Bone Miner Res 26: 2804–2811 [DOI] [PubMed] [Google Scholar]
- Verborgt O., Gibson G., Schaffler M. (2000) Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res 15: 60–67 [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu Y., Rowe D. (2007) Effects of transient PTH on early proliferation, apoptosis, and subsequent differentiation of osteoblast in primary osteoblast cultures. Am J Physiol Endocrinol Metab 292: E594–E603 [DOI] [PubMed] [Google Scholar]
- Whitfield J. (2006) Osteoporosis-treating parathyroid hormone peptides: What are they? What do they do? How might they do it? Curr Opin Investig Drugs 7: 349–359 [PubMed] [Google Scholar]
- Winkler D., Sutherland M., Geoghegan J., Yu C., Hayes T., Skonier J., et al. (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22: 6267–6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., Xiao Y., Liu J., Karaplis A., Pollak M., Brown E., et al. (2012) The calcium-sensing receptor complements parathyroid hormone-induced bone turnover in discrete skeletal compartments in mice. Am J Physiol Endocrinol Metab 302: E841–E851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Singh R., Divieti P., Guo J., Bouxsein M., Bringhurst F. (2007) Contributions of parathyroid hormone (PTH)/PTH-related peptide receptor signaling pathways to the anabolic effect of PTH on bone. Bone 40: 1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Wang Y., Degraff D., Wills M., Matusik R. (2011) Wnt/beta-catenin activation promotes prostate tumor progression in a mouse model. Oncogene 30: 1868–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]