Abstract
Gut mucosal enterochromaffin (EC) cells are regarded as key regulators of intestinal motility and fluid secretion via secretion of serotonin (5HT), are increased in numbers in mucosal inflammation and located in close proximity to immune cells. We examined whether interleukin (IL)1β and Escherichia coli lipopolysaccharide (LPS) induced EC cell 5HT release through Toll-like/IL-1 (TIL) receptor activation, nuclear factor kappa B (NFκB) and mitogen-activated protein kinase (MAPK) phosphorylation and evaluated whether somatostatin could inhibit this phenomenon. Pure (>98%) human intestinal EC cells were isolated by fluorescent activated cell sorting from preparations of normal (n = 5) and Crohn's colitis (n = 6) mucosa. 5HT release was measured (ELISA), and NFκB and ERK phosphorylation quantitated (ELISA) in response to IL1β and LPS. 5HT secretion was increased by both E. coli LPS (EC50 = 5 ng mL−1) and IL1β (EC50 = 0.05 pmol L−1) >2-fold (P < 0.05) in Crohn's EC cells compared with normal EC cells. Secretion was reversible by the TLR4 antagonist, E. coli K12 LPS (IC50 = 12 ng mL−1) and the IL1β receptor antagonist (ILRA; IC50 = 3.4 ng mL−1). IL1β caused significant (P < 0.05) NFκB and MAPK phosphorylation (40–55%). The somatostatin analogue, lanreotide inhibited IL1b-stimulated secretion in Crohn's (IC50 = 0.61 nmol L−1) and normal EC cells (IC50 = 1.8 nmol L−1). Inter-leukins (IL1β) and bacterial products (E. coli LPS) stimulated 5HT secretion from Crohn's EC cells via TIL receptor activation (TLR4 and IL1β). Immune-mediated alterations in EC cell secretion of 5HT may represent a component of the pathogenesis of abnormal bowel function in Crohn's disease. Inhibition of EC cell-mediated 5HT secretion may be an alternative therapeutic strategy in the amelioration of inflammatory bowel disease symptomatology.
Keywords: Crohn's, enterochromaffin cell, IL1β, lipopolysaccharide, serotonin.
The enterochromaffin (EC) cell is the principal sensor and effector cell of the gut diffuse neuroendocrine system which is responsible for the regulation of gut secretion, absorption and motility. Enterochromaffin cells sense the gut luminal milieu through microvilli and upon activation, participate in the regulation of intestinal motility and fluid secretion via release of serotonin (5HT) to adjacent mucosa cells and nerve endings in the submucosa.1,2 Enterochromaffin cells also interact with contiguous immune cells in the gut mucosa3 and secretory products from CD4+ T cells enhance EC cell production of 5HT in enteric infection.4 Furthermore, mature lymphocytes express 5HT receptors 5HT1A and 5-HT2A, and 5HT induces activation and proliferation of naïve lymphocytes through the 5-HT7 receptor.5
Crohn's disease is a complex disease which exhibits abnormal immune mechanisms and inflammation-mediated mucosal damage,6 while its clinical manifestations include substantial secretory and motility abnormalities as well as visceral pain.6,7 Increases in colonic EC cells have been observed in models of postinfectious diarrhoea, in 2,4,6-trinitrobenzene sulfonic acid-mediated colitis,8,9 and in inflammatory bowel disease (IBD).10,11 Furthermore, increased 5HT immunoreactivity occurs in the ileal enteric nervous system of patients with Crohn's disease.12 It thus seems as if conditions associated with gut mucosal inflammation are accompanied by an altered (usually increased) EC cell number and hypersecretion of 5HT. The close proximity between EC cells and immune cells in the mucosa, and the recent knowledge showing that secretory products from immune cells can activate EC cell secretion,4 indicate that an activation of the gut neuroendocrine cell system may be responsible for aspects of Crohn's disease pathophysiology. An explanation for the altered gut motility and secretion might thus reflect activation of EC cell secretion. Specifically, we hypothesized that increased 5HT synthesis and secretion occurs either as a response to cytokines, e.g. IL1β13–16 and bacterial lipopolysaccharides (LPS)17 which are known to be increased in Crohn's mucosa, or EC cells exhibit a decreased inhibitory response to somatostatin, levels of which can be reduced in Crohn's mucosa.18,19 These hypotheses could not previously be addressed in detail as there existed no methodology to study isolated human EC cell function. We have developed a technique to isolate and maintain EC cells from the human bowel in short-term culture.20,21 These cells are morphologically intact, fully functional in that they exhibit both excitatory and inhibitory receptor activity, have normal expression of tryptophan hydroxylase-1 (Tph-1) and secretion of 5HT.20,21
In the current study, EC cells from surgically resected Crohn's mucosa were studied. We hypothesized that Crohn's EC cells would exhibit increased 5HT secretion and be more sensitive than normal EC cells to the cytokine IL1β and Escherichia coli LPS.14,22–33 To confirm that these responses were evident in the EC cell, we identified and quantified Toll-like/IL-1 (TIL) receptor activation (TLR4 and IL1β) and signalling through nuclear factor kappa B (NFκB) nuclear targeting34,35 and mitogen-activated protein kinase (MAPK) phosphorylation which both transduce LPS/cytokine-mediated cellular events. To evaluate whether the somatostatin inhibitory response was intact in EC cells, we determined whether a somatostatin analogue, lanreotide, could inhibit IL1β-mediated 5HT release.
MATERIALS AND METHODS
Tissue specimens
Tissue was collected from 17 patients [M : F = 9 : 8; median age (range) = 49 years (22–63)]. Crohn's tissue (n = 9) was obtained from patients who had undergone hemi- or colectomies for Crohn's ileitis (n = 3) or colitis (n = 6). Only grossly affected tissue was studied. Macroscopically ‘normal’ tissue was obtained from patients undergoing surgery for diverticulitis (n = 4) or hemicolectomies for colon cancer (small intestine: n = 3; colon: n = 1). All tissue was collected between 2006 and 2007 at the Yale University Department of Surgery.
5HT and IL1β ELISAs
Serotonin and IL1b levels (active form) were measured in normal mucosa (ileum: n = 3; colon: n = 3) and in macroscopically affected mucosa from patients with Crohn's ileitis (n = 3) and Crohn's colitis (n = 6) using commercially available ELISA assays (BA 10-0900 Rocky Mountain Diagnostics, Colorado Springs, CO, USA and DLB50; R&D Systems, Minneapolis, MN, USA respectively) as described.21
Enterochromaffin cell isolation
Crohn's colitis (n = 6) and normal colon (n = 5) mucosa were used for the isolation and study of short-term cultured EC cells. EC cells were isolated from colon following mucosal stripping and enzymatic digestion using a combination of Nycodenz gradient fractionation and acridine orange uptake, and cell fluorescent activated cell sorting (FACS) sorted as described.21 In previous studies we obtained preparations of >98% pure EC cells.21 Approximately 1 × 106 cells were obtained per mucosal sample, a quantity sufficient for short-term culture, secretion and signalling studies.21
Measurement of Tph-1, SERT and TIL/somatostatin receptors transcripts in isolated normal and Crohn's mucosal EC cells
Total RNA was extracted from 5 × 105 isolated EC cells using TRIZOL® (Invitrogen, Carlsbad, CA, USA) extraction and cleaned up with the Qiagen RNeasy kit in conjunction with the DNeasy Tissue kit (Qiagen Inc., Germantown, MD, USA) to ensure no contaminating genomic DNA.21 RNA was converted to cDNA (High Capacity cDNA Archive Kit; Applied Biosystems, Foster City, CA, USA). Real-time PCR was performed (ABI 7900 Sequence Detection System) in triplicate using Assays-on-Demand primers as described.21 Amplification of Tph-1, SERT, TLR4 and IL1βR was compared with three reference genes (ALG9, TFCP2 and ZNF410) using a geNorm protocol as previously described.36
Enterochromaffin cell culture
FACS-sorted cells were divided into aliquots of ~5 × 104 cells 100 μL−1 culture media (Ham's F12 medium; Invitrogen) supplemented with foetal calf serum (10%) and antibiotics (100 U penicillin mL−1 + 100 μg streptomycin mL−1; Sigma-Aldrich, St Louis, MO, USA), seeded into 96-well collagen I-coated plates (Becton Dickinson, Franklin Lakes, NJ, USA), and maintained in a humidified atmosphere.21
Secretion experiments
Two hours after FACS sorting and short-term culture, normal or Crohn's EC cells were stimulated with pathophysiological concentrations of toxigenic E. coli LPS (0.01–100 ng mL−1, serotype 0127:B8, Group 2 serotype; Sigma-Aldrich)37 or human recombinant IL1β (0.6 × 10−15–0.6 × 10−12 mol L−1 equivalent to 0.01–10 pg mL−1; 201-LB-005; R&D Systems)38 for 60 min as described.20,21 These experiments were repeated in triplicate for each concentration. To confirm specificity of the EC cell secretory response to LPS and IL1β, we examined the effects of preincubating cells with either the TLR4 antagonist E. coli K12 LPS (0.5–500 ng mL−1; InvivoGen, San Diego, CA, USA) or the human recombinant IL1 receptor antagonist, IL1RA (0.5–500 ng mL−1; 280-RA; R&D Systems) prior to agonist (EC50) stimulation. The effects of signalling inhibitors on IL1β-mediated normal and Crohn's EC cell 5HT release were evaluated by preincubating cells for 20 min with IL1RA (3–25 ng mL−1), SN50 (Calbiochem, San Diego, CA, USA) (10 μmol L−1) or PD98059 (Sigma-Aldrich) (1 μmol L−1) prior to IL1β (EC50 = 0.05 pmol L−1 or 0.8 pg mL−1) challenge. The inhibitory effects of somatostatin analogue (lanreotide) on IL1β-mediated normal and Crohn's EC cell 5HT release were evaluated by preincubating cells for 20 min prior to IL1β (EC50 = 0.05 pmol L−1 or 0.8 pg mL−1) challenge. Media was collected and frozen at −80 ° C until ELISA analysis using a commercially available ELISA (BA 10-0900; Rocky Mountain Diagnostics).20,21
Signalling studies
After 2 h of culture, Crohn's or normal EC cells were stimulated with EC50 values of IL1β for 60 min and NFκB/MAPK (ERK) signal activity determined using SuperArray CASE™ ELISA kits (NFκB – FE-005; ERK – FE-002). The NFκB kit measures phosphorylation of the p65/RelA protein at s536 while the ERK kit measures phosphorylation at T202/Y204. We have previously used this approach to measure signal activation in normal and neoplastic small intestinal EC cells.39 To confirm specificity, cells were preincubated with IL1RA (3–25 ng mL−1), SN50 (10 μmol L−1) or PD98059 (1 μmol L−1) prior to IL1β (EC50: 0.03– 0.05 pmol L−1) stimulation. Stimulated cells were fixed (4% formaldehyde), and stained with either phosphorylated or nonphosphorylated antibodies (60 min, RT). After washing, and secondary antibody application (60 min, RT), cells were incubated with colour developer (10 min, RT) and plates read at 450 nm. Protein was then assayed in each well (reading at 595 nm). Results were calculated as antibody (450 nm)/protein concentration (595 nm) and normalized to unstimulated cells. Phosphorylated signal was compared with the total signal for the pathway for IL1β in normal and Crohn's EC cells respectively.
Data analysis
Results are expressed as mean ± standard error (SEM). All statistical analyses were performed using Prism 4 (GraphPad Software, San Diego, CA, USA). Dose–response curves were calculated for LPS or IL1β and each of the inhibitors and the EC50/ECmax or IC50/ICmax. Results were compared between normal and Crohn's EC cells using the Mann–Whitney test. P < 0.05 was considered significant.
RESULTS
Identification of 5-HT and the active form of IL1β in normal and Crohn's mucosa
Serotonin levels were significantly ( ~7-fold, P = 0.026) increased in Crohn's mucosa compared with normal mucosa (Fig. 1A) while IL1β levels were also elevated (threefold, P = 0.05) (Fig. 1B). No significant differences were noted between small intestine and colon levels for either 5HT or IL1β (P > 0.75, Mann–Whitney test).
Figure 1.
Serotonin (5HT) and active form IL1β mucosal levels in normal colon mucosa and in Crohn's tissue. Levels of 5HT were ~7-fold higher level in grossly affected Crohn's (IBD mucosa) tissue compared with normal mucosa (A) while levels of the activated cytokine were significantly increased ~3-fold in Crohn's tissue compared with normal mucosa. Mean ± SEM, n = 6 (normal mucosa), n = 9 (Crohn's tissue). *P < 0.05.
Isolation of a pure preparation of Crohn's EC cells
Enterochromaffin cells were isolated to >98% purity from both normal and inflamed colonic mucosa (Fig. 2A). Microscopy of FACS-sorted Crohn's EC cells (dual-stained with acridine orange and anti-Tph-1) demonstrated co-localization of Tph-1 and acridine orange within the cytoplasm (Fig. 2B). 99.6 ± 1.7% cells were Chromogranin A-positive and 98 ± 1.4% Tph-1 positive, indicating that the methodology produced cells of neuroendocrine origin (CgA positive) and an EC cell phenotype (Tph-1 positive). Using PCR analysis, we confirmed Tph-1 transcription and demonstrated that the EC cell-enriched preparations were also positive for the 5HT transporter SERT, substance P, guanylin and neurotensin but negative for somatostatin, cholecystokinin and enteroglucagon. Expression of Tph-1 (Fig. 2C) but not SERT (Fig. 2D) was significantly higher ( ~2-fold, P = 0.034) in Crohn's EC cells. These results confirm the EC cell origin of the preparation and indicate that the 1% contamination is due to neurotensin-producing endocrine cells. The absence of dual neurotensin/5HT double-positive cells in the human intestine makes it likely that this is a separate cell population.40
Figure 2.
Isolation methodology for enterochromaffin (EC) cells from normal and Crohn's mucosa. (A) Histogram showing the shift to the right (P4) in FITC-labelled EC cells after staining of live preparations with acridine orange (0.02 μmol L−1). Marker P4 was set to collect positive cells. P5 reflects poorly-positive cells. Doublet and debris discrimination were performed prior to sorting using standard procedures (data not shown). (B) Dual immunostaining of colonic EC cells (stained with Cy5 anti-Tph-1 – red) and acridine orange (green). Dual staining (vesicles – yellow) signifies overlapping Tph-1 and acidic vesicle localization. 98 ± 1.4% of FACS-sorted cells were Tph-1 positive. These EC-enriched cell preparations were PCR positive for Tph-1, the serotonin transporter SERT, substance P and guanylin further confirming their EC cell origin.72 The 1% contamination is due to neurotensin-producing endocrine cells. (C) Expression of Tph-1 transcripts (normalized using geNorm and three house-keeping genes: ALG9, TFCP2 and ZNF410 – GeNormATZ−36 was significantly increased ~2-fold in Crohn's-derived EC cells compared with normal EC cells. (D) SERT transcripts were not significantly altered. Mean ± SEM, n = 4 (normal EC cells), n = 5 (Crohn's EC cells). *P < 0.03.
Demonstration and confirmation of TOLL, interleukin and somatostatin receptor mRNA in EC cells
Real-time PCR of normal human colonic EC cells (n = 4) confirmed the presence of both TLR4 and IL1β type I receptor mRNA. Both TLR4 (fivefold, P = 0.03) (Fig. 3A) and the IL1β receptor (>10-fold, P = 0.006) (Fig. 3B) were over-expressed in Crohn's EC cells (n = 5) compared with normal EC cells. Somatostatin receptors are present on many neuroendocrine cells41 but their profile on inflamed human EC cells has not been defined. In this study, we confirm that normal EC cells expressed somatostatin subtypes 2 and 521 whereas Crohn's-derived EC cells expressed subtypes 1, 2, 3 and 5 (Fig. 3C). Subtype 4 was not expressed in either cell type.
Figure 3.
TLR4, IL1β and somatostatin receptor expression in normal and Crohn's-derived enterochromaffin (EC) cells by real-time PCR (normalized as described in Fig. 2).36 (A) Expression of TLR4 was significantly increased ( ~5-fold) in Crohn's-derived EC cells (IBD; n = 5) compared with normal EC cells (N; n = 4). (B) IL1R was significantly increased ( ~10-fold) in Crohn's-derived EC cells. (C) Expression of somatostatin subtypes 2 (SSTR2) and 5 (SSTR5) were identified in normal EC cells. Crohn's-derived EC cells, in addition, expressed transcript for types 1 and 3. Type 4 was not evident in either EC cell phenotype. Significantly elevated transcripts were noted for SSTR1, SSTR3 and SSTR5 in Crohn's EC cells. Mean ± SEM, n = 4 (normal EC cells), n = 5 (Crohn's EC cells). *P < 0.03.
Basal 5HT secretion in normal and Crohn's-derived EC cells
In order to evaluate whether there were any alterations in Crohn's EC cell secretion, basal 5HT secretion in Crohn's EC cells was compared with 5HT secretion from normal and neoplastic EC cells. Basal 5HT secretion from normal human EC cells was ~5 × 10−8 mol L−1 h−1,21 levels adequate for effective in vivo activation of downstream secretory and neural pathways.42–48 In cultured Crohn's EC cells, basal 5HT release was <7 × 10−8 mol L−1 h−1, similar to normal EC cells. In contrast, in cultured neoplastic EC cells (KRJ-I cell line which constitutively secretes 5HT),21,49 basal 5HT release ranged between 3 × 10−7 and 7 × 10−7 mol L−1. This is 6–13 times more 5HT release than normal EC cells per equivalent cell concentration (50 000 cells). We interpreted these data as indicating that Crohn's EC cells are similar to normal EC cells in that they do not exhibit constitutively activated 5HT release. The demonstration of similar SERT transcripts in normal and Crohn's EC cells (Fig. 2D) suggests that 5HT reuptake may be similar under normal and inflamed conditions.
Stimulation of 5HT secretion in normal and Crohn's-derived EC cells by E. coli LPS and IL1β
We evaluated 5HT secretory responses to toxigenic E. coli LPS and IL1β respectively. In normal EC cells, a small, insignificant increase in 5HT release (20% effective secretion at 10 ng mL−1: Fig. 4A) was evident in response to LPS. In contrast, in Crohn's EC cells, a significant dose-dependent increase in 5HT release over 60 min (EC50 = 4.5 ng mL−1) and a maximal effect at 10 ng mL−1 of 2.04 ± 0.13 fold (P < 0.01 vs control, unstimulated cells, paired t-test) was noted (Fig. 4A). In normal EC cells, IL1β stimulated 5HT release dose-dependently with an ~EC50 = 0.03 pmol L−1 (0.56 pg mL−1) and maximal effect at 0.06 pmol L−1 (1 pg mL−1) of 1.38 ± 0.03 fold (P = 0.05 vs control, unstimulated cells, paired t-test) (Fig. 4B). The effect of IL1b on Crohn's-derived EC cells demonstrated a dose-dependent increase in 5HT release over 60 min with an EC50 = 0.05 pmol L−1 (0.83 pg mL−1) and maximal effect at 0.6 pmol L−1 (10 pg mL−1) of 2.2 ± 0.06 fold (P < 0.05 compared to stimulated normal EC cells, Mann–Whitney test) (Fig. 4B). These results demonstrate that normal EC cells possess an activatable cytokine-mediated 5HT secretory system and the response to inflammatory mediators is accentuated in Crohn's-derived EC cells.
Figure 4.
Serotonin (5HT) secretion from isolated normal enterochromaffin (EC) cells and Crohn's EC cells stimulated with lipopolysaccharide (LPS) or IL1β. (A) LPS had no significant effect on normal EC cells (O) but increased 5HT secretion >2-fold in Crohn's-derived EC cells (•), half-maximal effect was seen at 4.5 ng mL−1. (B) The half-maximal effect for IL1β on normal EC cells was 0.03 pmol L−1 (0.56 pg mL−1) and 0.05 pmol L−1 (0.83 pg mL−1) for Crohn's-derived EC cells. (C) The TLR4 antagonist, Escherichia coli K12 LPS, inhibited LPS-mediated secretion with a half-maximal effect of 12 ng mL−1. (D) The half-maximal inhibitory effect of IL1RA was 3.4 ng mL−1 on IL1β (0.05 pmol L−1)-stimulated 5HT secretion. Mean ± SEM, n = 3. *P < 0.05 vs normal EC cells.
To exclude any cytotoxic effect, EC cell viability was assessed by Trypan blue exclusion, lactate dehydrogenase release and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide uptake. No significant alteration in any parameter in normal or Crohn's EC cells was evident during the 60 min time course in response to either maximal concentrations of LPS (10 ng mL−1) or IL1β (6 pmol L−1 or 100 pg mL−1) (data not shown).
Confirmation of specificity of LPSβ-mediated 5HT secretory responses in normal and Crohn's-derived EC cells
To confirm the specificity of the EC cell secretory response to LPS and IL1β, we examined the effects of preincubating cells with either E. coli K12 LPS or human recombinant IL1 receptor antagonist prior to agonist stimulation. Preincubation with K12 LPS inhibited LPS-induced secretion in Crohn's-derived EC cells (IC50 = 12 ng mL−1) after 20 min (Fig. 4C). Similarly, preincubation with IL1RA inhibited IL1β-stimulated secretion in Crohn's EC cells with IC50 = 3 ng mL−1 after 20 min (Fig. 4D).
Somatostatin analogue inhibits IL1β-stimulated 5HT secretion in normal and Crohn's-derived EC cells
To examine whether IL1β-mediated EC cell 5HT release could be inhibited through activation of somatostatin receptors, normal and Crohn's EC cells were preincubated with a somatostatin analogue, lanreotide. Lanreotide inhibited secretion with an IC = 1.8 × 10−9 mol L−1 (Fig. 5) in normal cells and 6.1 × 10−10 mol L−1 in Crohn's-derived EC cells. These results demonstrate that an intact somatostatin-mediated inhibitory system exists in Crohn's-derived EC cells and that these cells are twice as sensitive as normal EC cells to somatostatin receptor activation.
Figure 5.
Effect of the somatostatin receptor analogue, lanreotide, on IL1β-stimulated serotonin (5HT) release in normal enterochromaffin (EC) cells (•) and Crohn's-derived EC cells (□). The half-maximal inhibitory effect on normal cells was 1.8 × 10−9 and 6 × 10−10 mol L−1 for Crohn's-derived EC cells. Mean ± SEM, n = 3.
Demonstration of IL1β-mediated TIL signalling
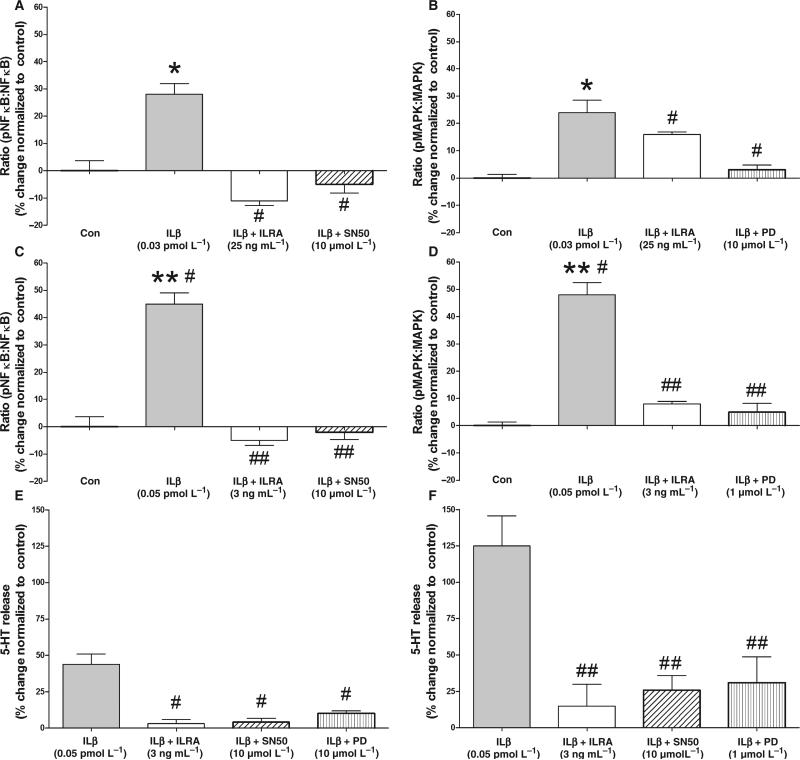
Interleukin-1β signalling is associated with phosphor-ylation and translocation of NFκB p6550 which can be identified using an ELISA approach. Nuclear factor kappa B phosphorylation in response to IL1β (0.03 pmol L−1 or 0.5 pg mL−1 – normal EC cells and 0.05 pmol L−1 or 0.8 pg mL−1 – Crohn's EC cells), demonstrated a significant 30% increase (P < 0.05 vs unstimulated normal cells) in phosphorylated NFκB in normal cells and a 45% increase (P < 0.01 vs unstimulated Crohn's EC cells) in Crohn's EC cells. These results demonstrate that IL1b activated TIL signalling through this transcription factor in both normal and Crohn's-derived EC cells (Fig. 6A,C). A comparison of NFκB phosphorylation in normal and Crohn's EC cells demonstrated that this effect was more pronounced (P < 0.05) in the latter cell type. Preincubation with IL1RA (20 min – 25 ng mL−1 – normal cells; or 3 ng mL−1 – Crohn's EC cells) completely inhibited NFκB phosphorylation and 5-HT release (Fig. 6E, F) as did SN50 (10 μmol L−1). This demonstrates that the effects of IL1β are mediated at least via this pathway in these cells and that inhibiting NFκB attenuates normal and Crohn's EC cell 5-HT release.
Figure 6.
Effect of IL1β on NFκB (A) and MAPK (ERK) (B) phosphorylation in isolated normal enterochromaffin (EC) cells (A/B) and Crohn's-derived EC cells (C/D) and effect of inhibiting these signalling pathways on serotonin (5-HT) release (normal EC cells: E; Crohn's derived EC cells: F). (A) IL1β (0.03 pmol L−1/0.5 pg mL−1) significantly (P < 0.05) stimulated NFκB phosphorylation by 30% in normal EC cells. This was completely reversed by ILRA (25 ng mL−1) and the specific NFκB inhibitor SN50 (10 μmol L−1). (B) IL1β (0.03 pmol L−1/0.5 pg mL−1) significantly (P < 0.05) stimulated MAPK phosphorylation by 25% which was inhibited by ILRA (25 ng mL−1) and the MEK inhibitor, PD98059 (1 μmol L−1). (C) IL1β (0.05 pmol L−1/0.8 pg mL−1) significantly (P < 0.01) stimulated NFκB phosphorylation by 45% in Crohn's-derived EC cells. This was completely reversed by ILRA (3 ng mL−1) and SN50 (10 μmol L−1). (D) IL1β (0.05 pmol L−1/0.8 pg mL−1) significantly (P < 0.01) stimulated MAPK phosphorylation by 55% which was inhibited by ILRA (3 ng mL−1) and PD98059 (1 μmol L−1). (E) IL1β (0.03 pmol L−1/0.5 pg mL−1)-stimulated 5-HT release was significantly inhibited (P < 0.05) by ILRA (25 ng mL−1), SN50 (10 μmol L−1) and PD98059 (1 μmol L−1). (F) IL1β (0.05 pmol L−1/0.8 pg mL−1)-stimulated 5-HT release was significantly inhibited (P < 0.05) by ILRA (3 ng mL−1), SN50 (10 μmol L−1) and PD98059 (1 μmol L−1). NFκB and MAPK phosphorylation in response to IL1β stimulation were both significantly (P < 0.05) elevated in Crohn's EC cells compared with normal EC cells. Mean ± SEM, n = 4. *P < 0.05 vs unstimulated cells, **P < 0.01 vs unstimulated cells, P < 0.05 vs IL1β-stimulated normal EC cells, ##P < 0.05 vs IL1β-stimulated Crohn's EC cells.
Increased MAPK phosphorylation (25–55%, P < 0.05–0.01 vs unstimulated cells) was noted in response to IL1β (Fig. 6B,D), effects which could be reversed (~50–90%) by ILRA or PD98059 (1 μmol L−1) preincubation. The effects of IL1β on MAPK phosphor-ylation were significantly (P < 0.01) increased in Crohn's EC cells, similar to that noted for NFκB phosphorylation. Inhibiting the IL1β-mediated pathway with PD98059 also inhibited 5-HT release (Fig. 6-E,F), confirming that MAPK phosphorylation is associated with EC cell secretion.
DISCUSSION
The role of the neuroendocrine system in IBD has not been investigated in a functional or mechanistic fashion despite the fact that EC cells regulate many of the events that are part of the pathogenesis of this disease.1,51 We have proposed that the mediators of inflammation – a key component of Crohn's disease – may be implicated in the genesis of symptomatology by activation of bowel neuroendocrine EC cell secretion. The investigation of inflammatory cytokines and bacterial products that induce inflammation, e.g. LPS, has yielded mechanistic information regarding the pathobiology of Crohn's and provided some insight into abnormal immune mechanisms and inflammation-mediated mucosal damage that are associated with secretory, motility abnormalities and pain.6,7
In the current study, we derived pure preparations of normal and Crohn's disease EC cells and examined the effects of E. coli LPS and interleukins (IL1β) on these EC cells. We demonstrated that: (i) 5HT and the active form of IL1β were increased in grossly affected Crohn's mucosa; (ii) EC Crohn's cells exhibited significantly elevated transcripts for Tph-1, IL1Rβ and TLR4, and somatostatin subtypes 1, 3 and 5; (iii) both E. coli LPS-and IL1β stimulated significantly more 5HT release from Crohn's EC cells than normal cells; (iv) this secretory response was not due to cell damage; (v) this was specific and reversible by TIL receptor antagonists ILRA and E. coli K12 LPS; (vi) this occurred through NFκB phosphorylation and MAPK activation; and (vii) this could be inhibited by the somatostatin analogue, lanreotide.
In human studies, Crohn's disease is characterized by an exaggerated immune response at the gut lymphoreticular tissue level while bacterial LPS has been detected in the plasma of Crohn's patients, and an abnormal microflora and/or an increased intestinal permeability have been invoked as cofactors responsible for endotoxemia.52 Crohn's patients have increased serum levels of LPS and its receptor, soluble CD14.53 Lipopolysaccharide stimulates inflammatory cytokine production in colonic explants.54 These studies are supported by the observation that TLR4 expression, while weakly expressed in normal gastrointestinal mucosa, is substantially increased in Crohn's mucosa55 and that an association of TLR4 polymorphisms with Crohn's disease has been noted in selective populations.56–58 The current study demonstrates that neuroendocrine EC cells in Crohn's colitis tissue have increased TLR4 expression. The presence of this receptor suggests a mechanism by which bacterial products like LPS may affect EC cell function.
While E. coli LPS had no effect on normal EC cell 5HT secretion, it caused a twofold increase in 5HT release from Crohn's EC cells. This effect was not due to cell damage and mimics the effect that LPS has on gastric EC-like cells37 which release histamine in response to both E. coli and Helicobacter pylori LPS. Direct stimulation by bacterial LPS of the luminal-sensing EC cell is therefore a mechanism by which 5HT release can be modified in inflamed Crohn's mucosa.
Activation of lymphocytes, monocytes, macrophages, enterocytes and endothelial cells occurs as a consequence of inflammation,22,59 and proinflammatory cytokines such as IL1β are increased in mucosal tissue of Crohn's patients.16 Although the expression levels of IL1RA that blocks IL1β is also elevated in Crohn's tissue,16 the IL1β:IL1RA ratio is markedly increased in Crohn's mucosa and is closely correlated with disease activity,13,14,29,30 consistent with the proposal that alterations in IL1β play a role in the pathophysiology of Crohn's disease. In vitro studies confirm that IL1β is increased in the intestinal supernatants of tissue extracted from Crohn's patients,22,60 indicating that this cytokine is actively secreted and can stimulate mucosal cells.
In the current study, the active form of IL1β was increased ~3-fold in grossly affected tissue compared to normal mucosa, confirming earlier results and suggesting this cytokine may regulate EC cell secretion. When studied in vitro, IL1β dose-dependently stimulated 5HT release with an ~EC50 = 0.03 pmol L−1 (0.56 pg mL−1) and maximal effect of 1.4-fold in EC cells from normal tissue. The effect of IL1β on Crohn's-derived EC cells was >2.2-fold greater with a similar potency (EC50 = 0.05 pmol L−1). IL1β has also been shown to play a role in modulating gastric neuroendocrine EC-like cell function whereby IL1β receptor activation results in increased histamine secretion and subsequent parietal cell activation.61 The current study suggests that an interaction of inflammatory mediators and the EC cell system may occur in Crohn's disease and is capable of producing a biological mediator (5HT) that can generate much of the pathophysiological symptoms (secretory/motility disturbances) associated with this type of IBD.
We have demonstrated that components of the TIL signal transduction pathway – IL-1 receptor–associated kinase and NFκB – are present in the normal human EC cell transcriptome.21 Both LPS and IL1β activate the common TIL (TLR4/IL1β) signalling pathway: nuclear targeting of NFκB with transcriptional activation.34,35 In addition, TIL also signals through MAPK34,62 a regulator of neuroendocrine cell secretion.39,63 It seems plausible therefore that LPS and cytokines in the Crohn's mucosal microenvironment might activate 5HT secretion through the TIL/MAPK pathway. To investigate whether TIL signalling occurred in EC cells, we studied the effects of IL1β on NFκB activity and MAPK phosphorylation and demonstrated a functionally intact TIL pathway with higher activity in Crohn's EC cells compared with normal EC cells. This observation provides evidence that there is a functional mechanism by which cytokines can regulate EC cell 5HT release (through NFκB/MAPK activation).
In Crohn's mucosa, somatostatin levels are decreased,64,65 and in an experimental colitis model, the somatostatin subtype 2 receptor analogue, octreo-tide, attenuated colonic mucosal damage.66 This suggests that somatostatin may have a protective role in Crohn's inflammation and the observation was interpreted as providing evidence of a regulatory target for cells expressing these receptors. We have demonstrated that normal intestinal EC cells express somatostatin subtypes 2 and 5,21 and in the current studies we confirmed that colonic EC cells have the same profile and demonstrate that Crohn's-derived EC cells express subtypes 1, 2, 3 and 5. The reasons for the altered somatostatin receptor profile remain to be explained but the promoter regions of both sst1/sst3 are known to include consensus motifs for NFκB and cAMP response elements.67,68 The elevated expression of pNFκB and pMAPK (activated by increases in intracellular cAMP) in response to IL1β identified in Crohn's EC cells (Fig. 6C,D) suggests one potential mechanism for increased sst1/3 expression.
As one possible cause of increased EC cell secretion was loss of inhibitory regulation, the demonstration of somatostatin receptors on Crohn's-derived EC cells as well as their functional ability renders this unlikely. In fact, given the known inhibitory effect of somatostatin on neuroendocrine cell secretion,41,69 our results provide evidence that EC cell secretion in Crohn's disease can be inhibited, particularly by somatostatin analogues that bind multiple receptor subtypes. In the current study, lanreotide (sst2,3,5 target) was two times more effective in the inhibition of 5HT secretion in Crohn's-derived EC cells than in normal cells. In a clinical study, decreased bowel action frequency was noted in IBD patients treated with octreotide + 5-ASA compared to single therapy with 5-ASA70 The expression of sst1,3 in Crohn's EC cells suggests that a pan-receptor analogue,71 e.g. SOM-230, may be a more effective inhibitor of Crohn's EC cell hypersecretion.
In summary, we demonstrate that interleukins (IL1β) and bacterial products (E. coli LPS) are more potent stimulators of 5HT release in Crohn's EC cells compared to normal EC cells. The mechanism of this secretion is via NFκB and MAPK phosphorylation and can be inhibited by an IL1β receptor antagonist and a TLR4 antagonist as well as a somatostatin analogue. Thus, inflammatory mucosa-derived EC cells are more sensitive to cytokine-mediated activation and possess intact somatostatin receptor inhibitory mechanisms.
We propose that the identification of abnormal EC cell function may be of relevance in the elucidation of the pathogenesis of the hypersecretion and dysmotility features of Crohn's disease, and may also facilitate the definition of future potential targets for therapeutic intervention.
ACKNOWLEDGMENTS
This work was supported by the Bruggeman Medical Foundation, the Norwegian Research Council and the Oddrun Mjåland Foundation.
Footnotes
COMPETING INTERESTS
The authors have no competing interests.
REFERENCES
- 1.Crowell MD, Shetzline MA, Moses PL, Mawe GM, Talley NJ. Enterochromaffin cells and 5-HT signaling in the pathophysiology of disorders of gastrointestinal function. Curr Opin Investig Drugs. 2004;5:55–60. [PubMed] [Google Scholar]
- 2.Thompson A, Keelman M, Thiesen A, Clandinin M, Ropeleski M, Wild G. Small bowel review: normal physiology part 2. Dig Dis Sci. 2001;46:2588–607. doi: 10.1023/a:1012746622735. [DOI] [PubMed] [Google Scholar]
- 3.Yang GB, Lackner AA. Proximity between 5-HT secreting enteroendocrine cells and lymphocytes in the gut mucosa of rhesus macaques (Macaca mulatta) is suggestive of a role for enterochromaffin cell 5-HT in mucosal immunity. J Neuroimmunol. 2004;146:46–9. doi: 10.1016/j.jneuroim.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Steeds J, Motomura Y, et al. CD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut. 2007;56:949–57. doi: 10.1136/gut.2006.103226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leon-Ponte M, Ahern GP, O'Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007;109:3139–46. doi: 10.1182/blood-2006-10-052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 7.Gershon M. Review article: Serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20(Suppl. 7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 8.Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863–70. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 9.Linden D, Foley K, McQuoid C, Simpson J, Sharkey K, Mawe G. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil. 2005;17:565–74. doi: 10.1111/j.1365-2982.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- 10.Kyosola K, Penttila O, Salaspuro M. Rectal mucosal adrenergic innervation and enterochromaffin cells in ulcerative colitis and irritable colon. Scand J Gastroenterol. 1977;12:363–7. doi: 10.3109/00365527709180942. [DOI] [PubMed] [Google Scholar]
- 11.El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–9. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- 12.Belai A, Boulos PB, Robson T, Burnstock G. Neurochemical coding in the small intestine of patients with Crohn's disease. Gut. 1997;40:767–74. doi: 10.1136/gut.40.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22:382–9. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- 14.Casini-Raggi V, Kam L, Chong YJ, Fiocchi C, Pizarro TT, Cominelli F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol. 1995;154:2434–40. [PubMed] [Google Scholar]
- 15.Minderhoud IM, Oldenburg B, Schipper ME, Ter Linde JJ, Samsom M. Serotonin synthesis and uptake in symptomatic patients with Crohn's disease in remission. Clin Gastroenterol Hepatol. 2007;5:714–20. doi: 10.1016/j.cgh.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto T, Nakahigashi M, Umegae S, Kitagawa T, Matsumoto K. Impact of elemental diet on mucosal inflammation in patients with active Crohn's disease: cytokine production and endoscopic and histological findings. Inflamm Bowel Dis. 2005;11:580–8. doi: 10.1097/01.mib.0000161307.58327.96. [DOI] [PubMed] [Google Scholar]
- 17.Fort MM, Mozaffarian A, Stover AG, et al. A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J Immunol. 2005;174:6416–23. doi: 10.4049/jimmunol.174.10.6416. [DOI] [PubMed] [Google Scholar]
- 18.Reubi JC, Mazzucchelli L, Laissue JA. Intestinal vessels express a high density of somatostatin receptors in human inflammatory bowel disease. Gastroenterology. 1994;106:951–9. doi: 10.1016/0016-5085(94)90754-4. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe T, Kubota Y, Sawada T, Muto T. Distribution and quantification of somatostatin in inflammatory disease. Dis Colon Rectum. 1992;35:488–94. doi: 10.1007/BF02049408. [DOI] [PubMed] [Google Scholar]
- 20.Kidd M, Modlin I, Eick G, Champaneria M. Isolation, purification and functional characterization of the Mastomys EC cell. Am J Physiol. 2006;291:G778–91. doi: 10.1152/ajpgi.00552.2005. [DOI] [PubMed] [Google Scholar]
- 21.Modlin IM, Kidd M, Eick G, Champaneria M. The functional characterization of normal and neoplastic EC cells. J Clin Endocrinol Metab. 2006;91:2340–8. doi: 10.1210/jc.2006-0110. [DOI] [PubMed] [Google Scholar]
- 22.Stevens C, Walz G, Singaram C, et al. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci. 1992;37:818–26. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- 23.Gross V, Andus T, Leser HG, Roth M, Scholmerich J. Inflammatory mediators in chronic inflammatory bowel diseases. Klin Wochenschr. 1991;69:981–7. doi: 10.1007/BF01645143. [DOI] [PubMed] [Google Scholar]
- 24.Fiocchi C, Hilfiker ML, Youngman KR, Doerder NC, Finke JH. Interleukin 2 activity of human intestinal mucosa mononuclear cells. Decreased levels in inflammatory bowel disease. Gastroenterology. 1984;86:734–42. [PubMed] [Google Scholar]
- 25.Reinecker HC, Steffen M, Witthoeft T, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–81. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breese EJ, Michie CA, Nicholls SW, et al. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106:1455–66. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 27.Reimund JM, Wittersheim C, Dumont S, et al. Increased production of tumour necrosis factor-alpha interleukin-1 beta, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn's disease. Gut. 1996;39:684–9. doi: 10.1136/gut.39.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raab Y, Gerdin B, Ahlstedt S, Hallgren R. Neutrophil mucosal involvement is accompanied by enhanced local production of interleukin-8 in ulcerative colitis. Gut. 1993;34:1203–6. doi: 10.1136/gut.34.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cominelli F, Pizarro TT. Interleukin-1 and interleukin-1 receptor antagonist in inflammatory bowel disease. Aliment Pharmacol Ther. 1996;10(Suppl. 2):49–53. doi: 10.1046/j.1365-2036.1996.22164020.x. discussion 54. [DOI] [PubMed] [Google Scholar]
- 30.Dionne S, D'Agata ID, Hiscott J, Vanounou T, Seidman EG. Colonic explant production of IL-1 and its receptor antagonist is imbalanced in inflammatory bowel disease (IBD). Clin Exp Immunol. 1998;112:435–42. doi: 10.1046/j.1365-2249.1998.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitsuyama K, Sasaki E, Toyonaga A, et al. Colonic mucosal interleukin-6 in inflammatory bowel disease. Digestion. 1991;50:104–11. doi: 10.1159/000200747. [DOI] [PubMed] [Google Scholar]
- 32.Waidmann M, Bechtold O, Frick JS, et al. Bacteroides vulgatus protects against Escherichia coli-induced colitis in gnotobiotic interleukin-2-deficient mice. Gastroenterology. 2003;125:162–77. doi: 10.1016/s0016-5085(03)00672-3. [DOI] [PubMed] [Google Scholar]
- 33.Kim SC, Tonkonogy SL, Albright CA, et al. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005;128:891–906. doi: 10.1053/j.gastro.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Dunne A, O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2003;2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- 35.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–90. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 36.Kidd M, Nadler B, Mane SM, et al. GeneChip, geNorm and Gastrointestinal tumors: novel reference genes for real-time PCR. Physiol Genomics. 2007;30:363–70. doi: 10.1152/physiolgenomics.00251.2006. [DOI] [PubMed] [Google Scholar]
- 37.Kidd M, Miu K, Tang LH, et al. Helicobacter pylori lipopolysaccharide stimulates histamine release and DNA synthesis in rat enterochromaffin-like cells. Gastroenterology. 1997;113:1110–7. doi: 10.1053/gast.1997.v113.pm9322505. [DOI] [PubMed] [Google Scholar]
- 38.Grider JR. Interleukin-1 beta selectively increases substance P release and augments the ascending phase of the peristaltic reflex. Neurogastroenterol Motil. 2003;15:607–15. doi: 10.1046/j.1350-1925.2003.00445.x. [DOI] [PubMed] [Google Scholar]
- 39.Kidd M, Modlin I, Gustafsson B, Drozdov I, Hauso O, Pfragner R. The luminal regulation of normal and neo-plastic EC cell serotonin release is mediated by bile salts, amines, tastants and olfactants. Am J Physiol. 2008;295:G260–72. doi: 10.1152/ajpgi.00056.2008. [DOI] [PubMed] [Google Scholar]
- 40.Sjolund K, Sanden G, Hakanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–30. [PubMed] [Google Scholar]
- 41.Barnett P. Somatostatin and somatostatin receptor physiology. Endocrine. 2003;20:255–64. doi: 10.1385/ENDO:20:3:255. [DOI] [PubMed] [Google Scholar]
- 42.Coughlin SR, Moskowitz MA, Antoniades HN, Levine L. Serotonin receptor-mediated stimulation of bovine smooth muscle cell prostacyclin synthesis and its modulation by platelet-derived growth factor. Proc Natl Acad Sci USA. 1981;78:7134–8. doi: 10.1073/pnas.78.11.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchheit KH, Engel G, Mutschler E, Richardson B. Study of the contractile effect of 5-hydroxytryptamine (5-HT) in the isolated longitudinal muscle strip from guinea-pig ileum. Evidence for two distinct release mechanisms. Naunyn Schmiedebergs Arch Pharmacol. 1985;329:36–41. doi: 10.1007/BF00695189. [DOI] [PubMed] [Google Scholar]
- 44.Siriwardena A, Kellum JM., Jr A 5-HT2 receptor mediates serotonin-induced electrolyte transport in rat left colon. J Surg Res. 1993;55:323–9. doi: 10.1006/jsre.1993.1149. [DOI] [PubMed] [Google Scholar]
- 45.Bobker DH, Williams JT. Serotonin-mediated inhibitory postsynaptic potential in guinea-pig prepositus hypoglossi and feedback inhibition by serotonin. J Physiol. 1990;422:447–62. doi: 10.1113/jphysiol.1990.sp017994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarado-Alvarez R, Arechiga H, Garcia U. Serotonin activates a Ca(2+)-dependent K(+) current in identified peptidergic neurons from the crayfish. J Exp Biol. 2000;203:715–23. doi: 10.1242/jeb.203.4.715. [DOI] [PubMed] [Google Scholar]
- 47.Sidhu M, Cooke HJ. Role for 5-HT and ACh in submucosal reflexes mediating colonic secretion. Am J Physiol. 1995;269:G346–51. doi: 10.1152/ajpgi.1995.269.3.G346. [DOI] [PubMed] [Google Scholar]
- 48.Cooke HJ, Wang YZ, Frieling T, Wood JD. Neural 5-hydroxytryptamine receptors regulate chloride secretion in guinea pig distal colon. Am J Physiol. 1991;261:G833–40. doi: 10.1152/ajpgi.1991.261.5.G833. [DOI] [PubMed] [Google Scholar]
- 49.Kidd M, Eick GN, Modlin IM, Pfragner R, Champaneria MC, Murren J. Further delineation of the continuous human neoplastic enterochromaffin cell line, KRJ-I, and the inhibitory effects of lanreotide and rapamycin. J Mol Endocrinol. 2007;38:181–92. doi: 10.1677/jme.1.02037. [DOI] [PubMed] [Google Scholar]
- 50.Kitaoka Y, Munemasa Y, Nakazawa T, Ueno S. NMDA-induced interleukin-1beta expression is mediated by nuclear factor-kappa B p65 in the retina. Brain Res. 2007;1142:247–55. doi: 10.1016/j.brainres.2007.01.097. [DOI] [PubMed] [Google Scholar]
- 51.Lomax AE, Linden DR, Mawe GM, Sharkey KA. Effects of gastrointestinal inflammation on enteroendocrine cells and enteric neural reflex circuits. Auton Neurosci. 2006;127:250–7. doi: 10.1016/j.autneu.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Caradonna L, Amati L, Magrone T, Pellegrino NM, Jirillo E, Caccavo D. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res. 2000;6:205–14. [PubMed] [Google Scholar]
- 53.Pastor Rojo O, Lopez San Roman A, Albeniz Arbizu E, de la Hera Martinez A, Ripoll Sevillano E, Albillos Martinez A. Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:269–77. doi: 10.1002/ibd.20019. [DOI] [PubMed] [Google Scholar]
- 54.Dionne S, Laberge S, Deslandres C, Seidman EG. Modulation of cytokine release from colonic explants by bacterial antigens in inflammatory bowel disease. Clin Exp Immunol. 2003;133:108–14. doi: 10.1046/j.1365-2249.2003.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franchimont D, Vermeire S, El Housni H, et al. Deficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299gly polymorphism is associated with Crohn's disease and ulcerative colitis. Gut. 2004;53:987–92. doi: 10.1136/gut.2003.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oostenbrug LE, Drenth JP, de Jong DJ, et al. Association between Toll-like receptor 4 and inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:567–75. doi: 10.1097/01.mib.0000161305.81198.0f. [DOI] [PubMed] [Google Scholar]
- 58.Gazouli M, Mantzaris G, Kotsinas A, et al. Association between polymorphisms in the Toll-like receptor 4, CD14, and CARD15/NOD2 and inflammatory bowel disease in the Greek population. World J Gastroenterol. 2005;11:681–5. doi: 10.3748/wjg.v11.i5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sandborn W, Targan S. Biologic therapy of inflammatory bowel disease. Gastroenterology. 2002;122:1592–608. doi: 10.1053/gast.2002.33426. [DOI] [PubMed] [Google Scholar]
- 60.Ligumsky M, Simon PL, Karmeli F, Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease – enhanced production during active disease. Gut. 1990;31:686–9. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prinz C, Neumayer N, Mahr S, Classen M, Schepp W. Functional impairment of rat enterochromaffin-like cells by interleukin 1 beta. Gastroenterology. 1997;112:364–75. doi: 10.1053/gast.1997.v112.pm9024290. [DOI] [PubMed] [Google Scholar]
- 62.Doyle SL, O'Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006;72:1102–13. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 63.Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA. 2007;104:15069–74. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koch TR, Carney JA, Morris VA, Go VL. Somatostatin in the idiopathic inflammatory bowel diseases. Dis Colon Rectum. 1988;31:198–203. doi: 10.1007/BF02552546. [DOI] [PubMed] [Google Scholar]
- 65.Eliakim R, Karmeli F, Rachmilewitz D. Decreased somatostatin (SS) generation by colonic mucosa in active ulcerative colitis. Gastroenterology. 1991;100:A578. [Google Scholar]
- 66.Eliakim R, Karmeli F, Okon E, Rachmilewitz D. Octreo-tide effectively decreases mucosal damage in experimental colitis. Gut. 1993;34:264–9. doi: 10.1136/gut.34.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Redmann A, Rasch A, Tourne H, Mann K, Petersenn S. Characterization and transcriptional regulation of the human somatostatin receptor subtype 1 gene. Horm Metab Res. 2007;39:359–65. doi: 10.1055/s-2007-976540. [DOI] [PubMed] [Google Scholar]
- 68.Baumeister H, Meyerhof W. Gene regulation of somatostatin receptors in rats. J Physiol Paris. 2000;94:167–77. doi: 10.1016/s0928-4257(00)00201-1. [DOI] [PubMed] [Google Scholar]
- 69.Hofland LJ, van Hagen PM, Lamberts SW. Functional role of somatostatin receptors in neuroendocrine and immune cells. Ann Med. 1999;31(Suppl. 2):23–7. [PubMed] [Google Scholar]
- 70.van Bergeijk JD, Wilson JH, Nielsen OH, et al. Octreotide in patients with active ulcerative colitis treated with high dose corticosteroids (OPUS 1). Eur J Gastroenterol Hepatol. 2002;14:243–8. doi: 10.1097/00042737-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 71.van der Hoek J, van der Lelij AJ, Feelders RA, et al. The somatostatin analogue SOM230, compared with octreo-tide, induces differential effects in several metabolic pathways in acromegalic patients. Clin Endocrinol (Oxf) 2005;63:176–84. doi: 10.1111/j.1365-2265.2005.02322.x. [DOI] [PubMed] [Google Scholar]
- 72.Nilsson AH. The gut as the largest endocrine organ in the body. Ann Oncol. 2001;12(Suppl. 2):S63–8. doi: 10.1093/annonc/12.suppl_2.s63. [DOI] [PubMed] [Google Scholar]