Abstract
Tissue and nerve damage can result in chronic pain. Yet, chronic pain after cesarean delivery is remarkably rare in women and hypersensitivity from peripheral nerve injury in rats resolves rapidly if the injury occurs in the puerperium. Little is known regarding the mechanisms of this protection except for a reliance on central nervous system oxytocin signaling. Here we show that density of inhibitory noradrenergic fibers in the spinal cord is greater when nerve injury is performed in rats during the puerperium, whereas expression of the excitatory regulators dynorphin A and neuregulin-1 in the spinal cord is reduced. The puerperium did not alter spinal cord microgial and astrocyte activation. Astrocyte activation, as measured by GFAP expression, was not evident in female rats with injury, regardless of delivery status suggesting sex differences in spinal astrocyte activation after injury. These results suggest a change in the descending inhibitory/facilitating balance on spinal nociception neurotransmission during the puerperium, as mechanisms for its protective effect against injury-induced hypersensitivity.
Keywords: Nerve injury, puerperium, spinal cord plasticity
Introduction
There is a strikingly low incidence of chronic pain from childbirth even though it can be accompanied by considerable tissue and potentially nerve damage (Eisenach et al., 2012). This low incidence is unexpected, given the enhanced excitability, enhanced TRPV1 expression, and sprouting of sensory afferents innervating the lower uterine segment and cervix at the end of pregnancy (Tong et al., 2006). In addition, labor is associated with an inflammation-like response (Tornblom et al., 2005) in these tissues, which results in their increased compliance to allow passage of the fetus. This inflammation-like response also sensitizes peripheral afferents. Despite these changes in afferent sensitization peripheral nerve injury-induced hypersensitivity, as a model of neuropathic pain, resolves rapidly in rats when surgery is performed surrounding the time of delivery (Gutierrez et al., 2012). This protection appears to be an effect of the post-delivery period rather than pregnancy per se and is not dependable on circulating progesterone and estrogen which decline rapidly following delivery and involves oxytocin signaling in the central nervous system. The primary goal of the current study is to better understand the mechanisms by which the puerperium hastens resolution of hypersensitivity after peripheral nerve injury.
Peripheral nerve injury alters both primary afferent and second order spinal cord neurons, resulting in sensitization and abnormal responses to peripheral stimuli. Glia in the spinal cord also reacts to peripheral nerve injury, with an initial wave of activation of microglia, followed by a more sustained activation of astrocytes resulting in release of pronociceptive cytokines and neuronal sensitization (Gao et al., 2009; Zhang and De Koninck, 2006). The increased immunoreactivity to the ionized calcium binding adaptor molecule (IBA1) and the glial fibrillary acidic proteins (GFAP) are good indicators of microglia and astrocyte activation, respectively and were used in the current study to determine the effect of the puerperium on spinal cord glial activation following peripheral nerve injury.
Spinal cord neurons are also modulated by descending pathways from the pons and the medulla, as proposed in the original description of the gate control theory of pain (Melzack and Wall, 1965). The balance between descending inhibition and facilitation has been proposed to be disrupted after peripheral nerve injury, leading to spinal sensitization, increased ascending nociceptive signaling, and consequently pain. A key inhibitory pathway, noradrenergic fibers descending from the pons, sprout after peripheral nerve injury in mice and rats (Hayashida et al., 2008; Ma and Eisenach, 2003) and presence of this system may play an inhibitory role in glial activation in the spinal cord following peripheral nerve injury (Hayashida et al., 2012). On the other hand, a key facilitatory pathway from the nucleus raphe magnus releases serotonin into the spinal cord, resulting in increased synthesis of dynorphin (Hentall et al., 2006; Kondo et al., 1993). This endogenous opioid peptide paradoxically drives injury-induced hypersensitivity (Gardell et al., 2002; Wang et al., 2001) by actions on n-methyl-d-aspartate receptors (Laughlin et al., 1997). Another aim of the current study was to determine the effect of the puerperium on noradrenergic fiber density, using immunostaining for dopamine β-hydroxylase (DβH) and dynorphin content in the spinal cord after nerve injury.
Finally, most previous work on mechanisms of neuropathic pain or hypersensitivity has been performed in male animals, despite a predominance of many common chronic pain conditions in women. We therefore asked whether the glial and neuronal plasticity after nerve injury, classically defined in male rats, applied to female rats. As part of this effort, we focused on the role of the puerperium on neuregulin-1 (NRG1), which has shown to be important in maintaining persistent pain among female rats (Lacroix-Fralish et al., 2008).
Experimental procedures
Animals
Sprague-Dawley rats (250–350 g) from Harlan Industries (Indianapolis, IN, USA), housed under a 12-h light-dark cycle with food and water ad libitum, were used. All experiments were approved by Animal Care and Use Committee at Wake Forest University, School of Medicine (Winston Salem, NC, USA
Tissues from a total of 108 (98 virgin females and 10 males) age matched Sprague-Dawley rats (age=16–17 weeks, weigh=250–350 g) were used in this study. The behavioral data from 28/108 animals were reported previously (Gutierrez et al., 2012) and the tissue collected from those animals was used in the immunocytochemistry analysis (n=7 in each group). Withdrawal thresholds were not determined in the remaining 80 animals (10 males (non-breeders) and 70 females). The tissue collected from these 80 animals was used in Western blots (n=7 in each group of females and n=5 in each group of males) and enzyme immune-assay (n=7 in each group of postpartum and n=4 in each group of virgin females).
Surgical procedures
Within 24 hours after the delivery of the last pup and when virgin females and males rats were 16–17 weeks old (to match the age of postpartum rats at the time of delivery), spinal nerve ligation (SNL) surgery was performed as previously described (Hayashida et al., 2012). Animals were anesthetized with 2% isoflurane in oxygen and SNL or sham surgery was performed. For SNL, the right L6 transverse process was removed and the right L5 and L6 spinal nerves were tightly ligated using 5.0 silk sutures. Sham surgery consisted of exposure of the L5 and L6 spinal nerves, but no ligation. After surgery, animals were housed with their pups (postpartum) or pair housed (males and virgin females) in plastic cages in a climate-controlled room under a 12 h–12 h light-dark cycle, with free access to food and water.
Tissue preparation for Immunocytochemistry
Seventeen days following SNL or sham surgery, rats were anesthetized with sodium pentobarbital (i.p; 100 mg/kg), the thorax was opened, and fixative (4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4) was perfused through the left ventricle with a peristaltic pump (20 ml/min) for 15 minutes. The spinal cord L4–L6 segments were then identified based on anatomy (lumbar enlargement and identification of the L4–L6 spinal nerves), removed and immersed in fixative (postfixation) for 3 hours at 4°C. After post-fixation a needle was inserted in the ventral dorsal horn, ipsilateral to SNL surgery and the spinal cord immersed in 30% sucrose at 4°C for cryoprotection until sectioned. Transverse sections (40 μm) were cut on a cryostat. At least three experiments were performed per marker (IBA1, GFAP and DβH). Sections from 2–3 animals per group from the L5–L6 segments were processed simultaneously in each experiment. Antibodies for IBA-1, GFAP and DβH were used to examine glia, astrocytes and noradrenergic fibers (Hayashida et al., 2008; Zhang and De Koninck, 2006). To analyze these aspects, the sections were incubated over night at 4°C with the primary antibody rabbit anti-IBA1 (1:1000, #019-19741, Wako Chemicals, Richmond, VA, USA); rabbit anti-GFAP (1:5000, #RZ0334, Dako, Carpinteria, CA, USA) or mouse monoclonal anti-dopamine β hydroxylase (DβH, 1:500, #MAB308, Chemicon International Inc., Temecula, CA, USA) followed by the corresponding biotinylated donkey anti-rabbit or anti-mouse IgG (1:500, Vector Laboratories, Burlingame, CA, USA) in 1.5 % normal donkey serum (NDS, Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA), 0.1% Triton X-100 in 0.01M phosphate buffer saline (PBS). Then the sections were reacted using Elite Vectastain ABC kit (Vector Laboratories, AC, USA). The Elite Vectastain ABC kit was used to link the antigen antibody complex to horseradish peroxidase (HRP; ABC Elite, Vector), which was then visualized with 3, 3-diaminobenzidinetetrahydrochloride (DAB) histochemistry. Finally, the sections were washed thoroughly in PBS, mounted on plus-slides, air-dried, dehydrated in ethanol, cleared in xylene, and cover slipped with DPX.
Image analysis
Coded numbered slides with the sections were examined with brightfield illumination on a Nikon E600 epifluorescence microscope and images were captured with a CCD digital camera attached to the microscope using a 10X objective. Images of ipsilateral L5/6 dorsal horn were captured, corresponding to projection sites of denervated regions of the spinal cord. Quantification for each image was performed calculating the average from three to five randomly selected spinal cord sections per rat. The images were quantified using Image J (U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2011). In order to conduct the regional analysis of the spinal cord, a macro was defined using a digital template to contain laminae I to IV of the dorsal horn. The number of pixels occupied by immunoreactive processes with intensity above a fixed threshold (used for the analysis of all sections) and within the defined area was counted automatically. The average was then calculated per animal and per group. The experimenter performing image analysis was blinded to group.
Tissue preparation for enzyme immune-assay (EIA) and Western Blots
Animals were euthanized 17 days after SNL or sham surgery by deep halothane anesthesia followed by decapitation. The spinal cord was removed, the segment containing in the lumbar enlargement dissected, cut (coronal plane) and ipsi and contralateral dorsal quarters were rapidly dissected on ice, then frozen in dry ice-cooled 2-methylbutane and stored at −80° C.
Preparation of spinal cord extracts for dynorphin A EIA
Dynorphin A content of spinal cord was determined as previously described with minor modifications (Parra et al., 2002). The dissected segments of the spinal cord were sonicated on ice in 1N acetic acid and a protease inhibitor cocktail (1:1000, Sigma, St Louis, MO, USA), centrifuged and the supernatants were collected. The samples were further processed using the C18 Sep-Column Extraction Method following the manufacturer’s instructions in the EIA kit (EIA kit, S-1203, Peninsula Laboratories, CA, USA). The samples were applied to equilibrated Sep-Pak C18 3cc cartridges (#20805, Millipore, Mass, USA), washed and the peptides eluted. The eluant was then concentrated using a speed vac concentrator and the residue dissolved in assay buffer (EIA buffer). Dynorphin A was measured using the EIA kit. Briefly, after preparing duplicates of dynorphin A standards and samples, the antiserum and biotinylated tracer were added to the wells following the manufacturer’s instructions. The antiserum and tracer were added to the samples and standards, mixed and incubated at room temperature for two hours. Following washing, sample solutions were incubated with the streptavidin-HRP at room temperature for one hour, washed and reacted with the substrate for 30 minutes. The reaction was then terminated by adding 2N HCl and the absorbance was read at 450 nm within ten minutes with a spectrophotometer (Epoch microplate spectrophotometer, BioTek Instruments, Inc, Vermont, USA). The standard curve was generated using Microplate Data Collection & Analysis Software Gen 5 (BioTek Instruments, Inc, Vermont, US 2006–2009), then the dynorphin A concentration calculated. The limit of sensitivity of the assay was 0.02ng/ml and the coefficient of variation at 0.08 ng/ml protein was 10.9%.
The final values per surgery group (postpartum and virgin) were then normalized to the sham groups, respectively.
Preparation of spinal cord extracts for Western Blots (NRG1 and GFAP)
The dissected segments of the spinal cord were sonicated on ice in homogenization buffer (mammalian cell lysis kit, Sigma MCL-1) and a protease inhibitor cocktail (1:1000, Sigma, St Louis, MO), centrifuged and the supernatants collected and used for the immunoblotting assays. Protein concentration was determined by Bradford protein assay (Bio-Rad laboratories, CA, USA, (Bradford, 1976)). The samples (25 μg total protein for GFAP and 15 μg total protein NRG1) were combined with gel loading buffer, heated to 95°C for 5 min and separated on 10% Tris-HCl gels (#345-0010, Bio-Rad laboratories, CA, USA). The proteins were transferred to polyvinylidene membranes (PVDF membrane, Bio-Rad laboratories, CA, USA), blocked in 5% dry milk in PBS and incubated overnight at 4°C with rabbit anti-GFAP(1:40000) or rabbit anti-NRG1(1:100, #sc-28916, Santa Cruz, CA, USA) and rabbit monoclonal anti-GAPDH (1:1000, # 21118, Cell Signaling technology, MA, USA). After washing, the membranes were incubated with horseradish peroxidase-conjugated donkey anti-rabbit (1:40000 or 1:30000, respectively, Jackson ImmunoResearch Laboratories, PA, US). The GFAP or NRG1 signal was visualized using SuperSignal (West Pico Chemiluminiscence Substrate (#34080, Thermo Scientific, IL, USA) and quantified using Image J software and normalized to GAPDH (Wang et al., 2011). The ratio of NRG1 or GFAP to GAPDH was calculated for each lane and values of these ratios were normalized to the sham group.
Statistical analysis
Immunocytochemical data are expressed as number of pixels occupied by immunoreactive cell bodies and processes with intensity above the threshold. Western blot data is expressed as the GFAP/GAPDH ratio, normalized to the median value for the Sham male group. EIA data are expressed as pg of dynorphin A per ml. Statistical analysis was conducted using SigmaStat software (Version 3.1).
Data that were (or were not) normally distributed are presented as mean (or median) ± standard deviation (SD or 25th and 75th percentiles).
Immunocytochemical and biochemical data were normally distributed (IBA1, DβH, NRG-1 and dynorphin A) and were analyzed using two way analysis of variance followed by all pairwise multiple comparison procedures (Bonferroni’s test).
Immunocytochemical data and biochemical data that were not normally distributed (GFAP immunocytochemistry and GFAP western blot) were analyzed using Mann Whitney Rank Sum test and Kruskal-Wallis One Way Analysis of Variance on Ranks, respectively. The criterion for statistical significance was P< 0.05.
Results
All animals recovered from surgery without evidence of infection, and all deliveries occurred spontaneously with an average litter size of 11.
Glial activation
Compared to sham surgery, SNL resulted in a significant ipsilateral increase in IBA1 immunoreactivity in the spinal dorsal horn of postpartum rats and control virgin females, (Fig 1, p<0.05). This 2–3 fold increase is similar to that previously observed by us and others in males (Hayashida et al., 2012; Vega-Avelaira et al., 2007), so male rat tissue was not examined for this marker. IBA1 immunoreactivity did not differ between postpartum and virgin female rats in either Sham or SNL conditions (Fig 1).
Figure 1.
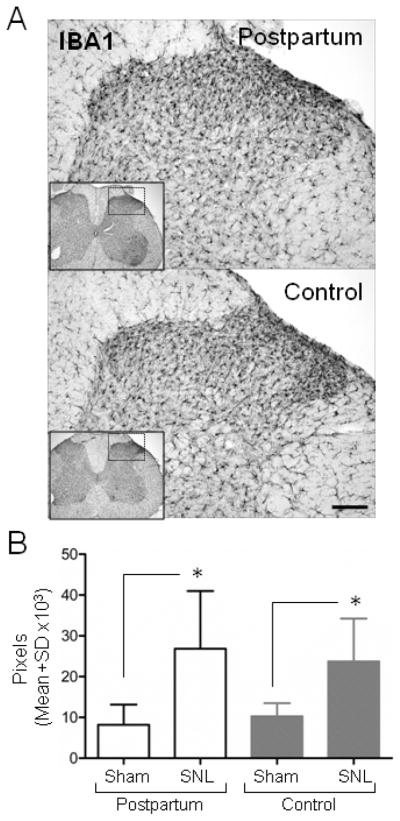
Spinal nerve ligation (SNL) activates microglia in the ipsilateral spinal dorsal horn 17 days after surgery (A) Representative IBA1 immunoreactive sections (L5) from postpartum (PP) and control rats (virgin females) (top and bottom panel, respectively) visualized by avidin-biotin complex method (B) SNL but not Sham surgery increases IBA1 immunoreactivity in the ipsilateral spinal dorsal horn of postpartum and control rats (virgin females). Scale bar=100 μm. Data are presented as mean ± SD, n=7 in each group, Groups were analysis by Two way ANOVA, p (Surgery)<0.001, p (Group)<0.887, p (Surgery x Group)=0.466, Bonferroni’s Multiple Comparison Test, *p <0.05 compared to PP Sham or Control.
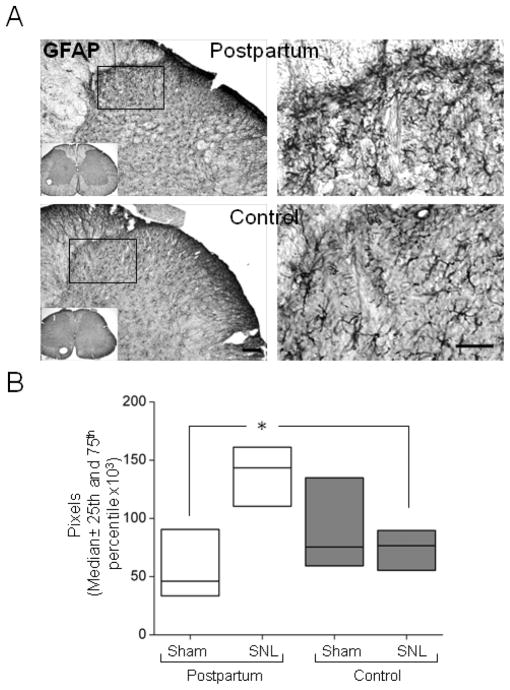
Astrocyte activation, as measured by GFAP immunoreactivity was similar after sham surgery between postpartum and virgin rats, but was greater after SNL surgery in postpartum rats (Fig 2, p < 0.05).
Figure 2.
Lack of astrocyte activation in the ipsilateral spinal dorsal horn of female control virgin rats, 17 days after SNL surgery. (A) Representative GFAP immunoreactive sections from postpartum and control (virgin females) rats (top and bottom panel, respectively) and detail of the superficial laminas of the spinal cord visualized by avidin-biotin complex method. (B) GFAP immunoreactivity increases in the ipsilateral spinal dorsal horn of postpartum (PP) rats but not control (virgin females) rats after SNL surgery. Scale bar=100μm (left panels) and 50 μm (right panels). Data are presented as median (horizontal line) ± 25th and 75th percentiles, n=7 in each group, Rank sum Test, *p <0.05 compared to PP Sham and to control SNL.
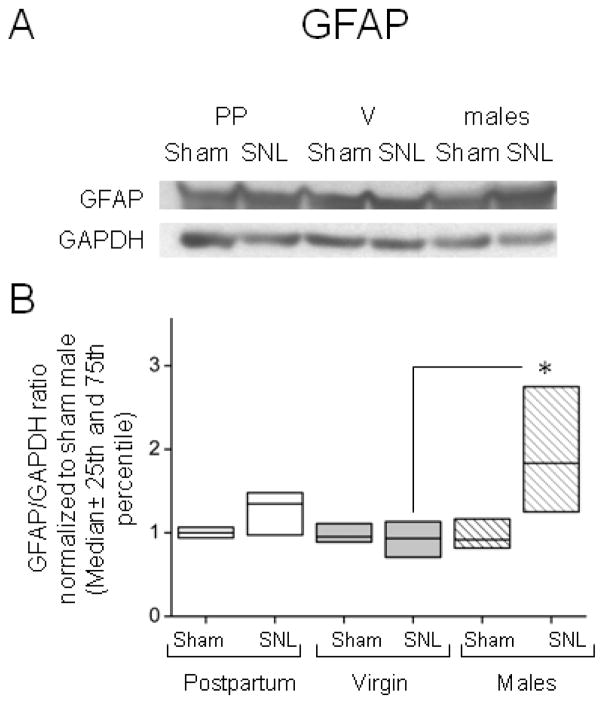
Quantitative Western analysis for GFAP content in the spinal dorsal horn showed no increase in GFAP protein after SNL surgery in either virgin (p=0.511), postpartum female (p=0.165) or male rats (p=0.056) when compared to sham surgery, although the expression of this protein was greater after SNL surgery in males compared to virgin females (Fig 3, p<0.01).
Figure 3.
Sexual dimorphic differences in the GFAP expression after SNL surgery determined by western blot analysis. (A) Representative immunoblot for GFAP and GAPDH from ipsilateral dorsal lumbar spinal cord homogenizates. (B) The expression of GFAP increases after SNL surgery in male rats (stripes) when compared to virgin females rats (V (light grey)). Data represent the GFAP/GAPDH ratio, normalized to the median value for the Sham male group. Data are presented as median (horizontal line) ± 25th and 75th percentiles; n=7 in each group of females and n=5 in each group of males, Kruskal-Wallis one way analysis of Variance on Ranks (p=0.029), using Pairwise multiple comparison procedures (Dunn’s method), *p <0.05 compared to VSNL.
Noradrenergic fiber density
Noradrenergic fibers were present throughout the spinal cord dorsal horn of female rats Fig 4A), consistent with previous reports in males (Hayashida et al., 2008; Ma and Eisenach, 2003). Although postpartum and virgin females did not differ significantly after sham surgery in density of DβH immunoreactive fibers, this fiber density was increased significantly following SNL surgery only in postpartum animals (Fig 4B, p<0.05).
Figure 4.
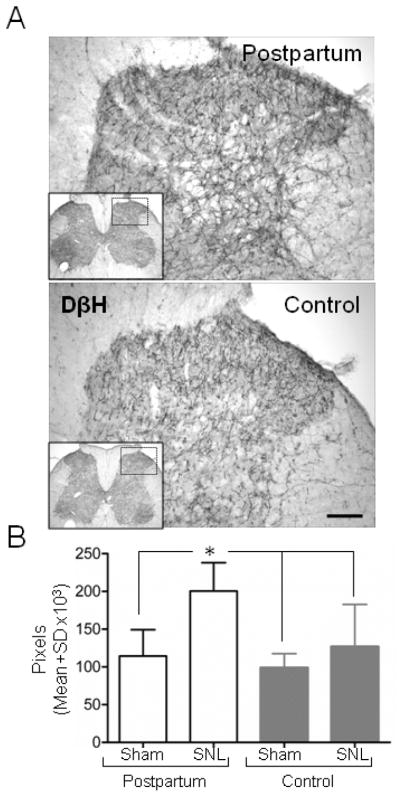
Increased SNL-induced noradrenergic sprouting in the ipsilateral spinal dorsal horn during the postpartum period. (A) DβH immunoreactive axons are observed in postpartum and virgin females (top and bottom panel, respectively) visualized by the use of avidin-biotin complex method. (B) DβH immunoreactivity increases in the ipsilateral spinal dorsal horn postpartum (PP) after SNL but not after Sham surgery. Scale bar=100 μm. Data are presented as mean ± SD, n=7 in each group, Groups were analysis by Two way ANOVA, p (Surgery)<0.0001, p (Group)=0.006 p (Surgery x Group)=0.061, Bonferroni’s Multiple Comparison Test, *p <0.05 comparing PP after Sham and SNL surgery, and to control after Sham and SNL surgery.
Neuregulin-1
SNL surgery induced an increase in the spinal cord dorsal quadrant protein expression of neuregulin-1 in virgin rats (Fig 5 p<0.05). In contrast, the expression of this protein was reduced in postpartum rats after SNL surgery.
Figure 5.
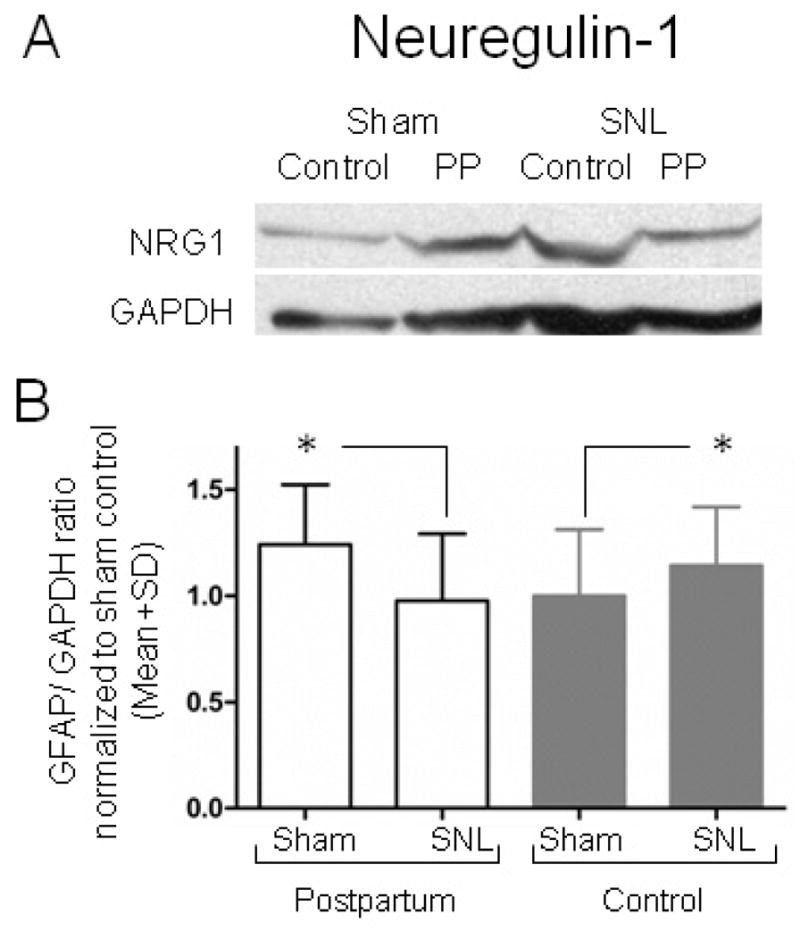
The expression of Neuregulin-1 (NRG1) decreases after SNL surgery in the ipsilateral dorsal lumbar spinal cord homogenizates of postpartum rats using western blot analysis. (A) Representative immunoblots for NRG1 and GAPDH from spinal cord homogenizates. (B) The expression NRG1 decreases in postpartum (PP) and increases in controls (virgin females) rats after SNL surgery when compared to Sham controls. Data represent the GFAP/GAPDH ratio, normalized to the mean value for the Sham control group. Mean ± SD; n=7 in each group. Groups were analysis by Two way ANOVA, p (Surgery)<0.001 p (Group)=0.887, p (GroupxSurgery)=0.466. Bonferroni’s Multiple Comparison Test, *p <0.05 compared to PP Sham, and to control after Sham surgery.
Dynorphin A
SNL surgery resulted in an increase in dynorphin -A protein concentration in dorsal horn quadrants ipsilateral to surgery compared to sham, but only in virgin controls (Fig 6, p < 0.05). Postpartum and virgin females did not differ in spinal cord dynorphin A protein concentration after sham surgery (Fig 6).
Figure 6.
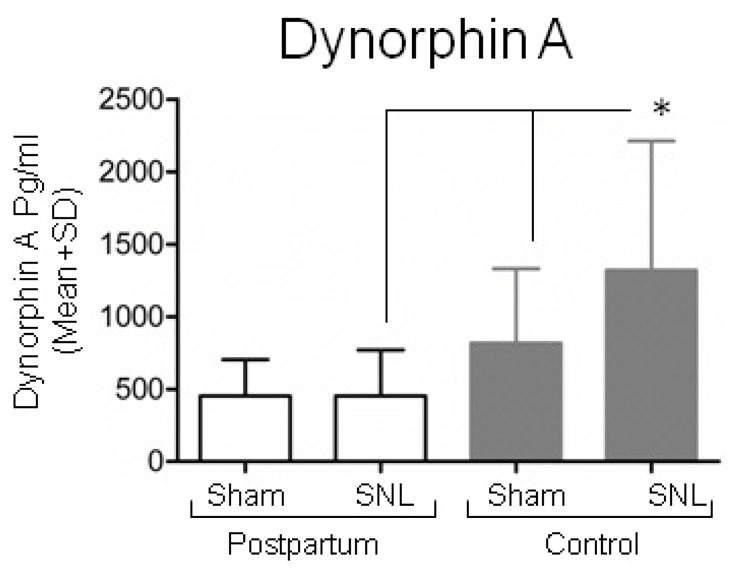
Dynorphin A content is similar after SNL or Sham surgery in the ipsilateral dorsal quadrant of the lumbar spinal cord of postpartum rats (PP) analyzed by enzyme immune-assay. Data are expressed as Mean ± SD; n=7 in each group of postpartum (PP) rats and n=14 in each group of control (virgin females) rats. Groups were analysis by Two way ANOVA, p (Group)=0.004, p (Surgery)=0.228, p (Group x Surgery)=0.224. Bonferroni’s Multiple Comparison Test, *p <0.05 compared to control SNL, #p <0.05 compared to control Sham.
Discussion
One approach to better understand chronic pain after injury is to examine natural conditions where injury fails to result in as high a prevalence of chronic pain as expected. Childbirth appears to be one of those conditions, both from epidemiologic studies in humans (Eisenach et al., 2012) and from surgically induced nerve injury studies in rodents (Gutierrez et al., 2012). We previously showed that central nervous system signaling by oxytocin was partially responsible for this protective effect after delivery (Gutierrez et al., 2012), but the pleiotropic actions of oxytocin could affect many responses to injury. For that reason, we focused in the current study on factors which are thought to contribute to post-injury hypersensitivity, including bulbospinal descending pathways, spinal cord glial activation, and proteins which are upregulated in the spinal cord in females only (Lacroix-Fralish et al., 2008). We found three injury induced changes which were modified in the puerperium – increased sprouting of inhibitory descending noradrenergic fibers, decreased expression of dynorphin A, which is driven by descending facilitatory pathways, and decreased expression of NRG-1, a protein important to synaptic plasticity that plays an important role in maintaining persistent hypersensitivity in female rats with injury. In addition, we noted a much smaller or absent activation of spinal cord astrocytes, as measured by GFAP expression, in female rats with injury compared to males. These results suggest an altered balance after childbirth between descending inhibition and facilitation as well as a reduced responsivity of spinal cord glia which may underlie protection from injury induced chronic pain at this time in the life cycle.
Norepinephrine, released into the spinal cord from fibers descending from the pons, has long been recognized to inhibit transmission of pain, both by reducing release of excitatory neurotransmitters from nociceptor terminals (Kuraishi et al., 1985; Li and Eisenach, 2001) and by reducing the response of second order neurons in the spinal cord dorsal horn (North and Yoshimura, 1984; Sonohata et al., 2004). Peripheral nerve injury increases the density of DβH fiber staining in the dorsal horn, accompanied by an increase in norepinephrine content (Hayashida et al., 2008). Two consequences of this apparent sprouting of noradrenergic fibers after injury have been proposed, an increased capacity to inhibit neurotransmission (Nakajima et al., 2012) and an increase in tonic activity, which reduces the degree of hypersensitivity and activation of glia in the spinal cord that would otherwise occur (Hayashida et al., 2012). We did not test these mechanisms in the current study since we did not examine the action of analgesic drugs which activate or enhance descending noradrenergic inhibition (opioids, gabapentin, monoamine reuptake inhibitors). Nor did we test the mechanisms which induce noradrenergic sprouting, thought to be reliant on BDNF release from either astrocytes or primary afferents (Coull et al., 2005; Gao and Ji, 2010). However, the increased noradrenergic sprouting when injury occurs in the puerperium is consistent with increased tonic noradrenergic activity and hence hypersensitivity during this time.
The role of dynorphins in pain states is been considered paradoxical because the release of this endogenous opioid peptide results in hypersensitivity (Gardell et al., 2004; Wang et al., 2001). Dynorphin A is upregulated in the spinal cord dorsal horn following peripheral nerve injury- and chronic opioid-induced hypersensitivity, where it contributes to sensitization by actions on n-methyl-d-aspartate receptors (Gardell et al., 2004; Laughlin et al., 1997; Wang et al., 2001). This upregulation is due to activation of descending facilitatory pathways from the rostral ventromedial medulla, which are in turn activated by ascending input from second order spinal cord neurons receiving nociceptive input (Vera-Portocarrero et al., 2007). It is thus conceivable that the lack of dynorphin A increase in the spinal cord of rats injured during the puerperium could have reflected reduced stimulation of second order spinal cord neurons, reduced responsiveness of neurons in the rostral ventromedial medulla, or reduced responsiveness of dynorphin A expressing elements in the spinal cord.
Astrocyte activation in the spinal cord after peripheral nerve injury classically follows microglial activation, is much longer lasting than the latter, and is thought to play an important role in hypersensitivity (Gao and Ji, 2010; Gao et al., 2009). The similar degree of microglial activation from nerve injury in postpartum and control female rats in the current study suggests that this does not underlie the reduction in hypersensitivity after delivery.
We observed a very modest effect of nerve injury on immunostaining for GFAP in the spinal cord of female rats, but a large effect in males. Quantitative Western analysis, including tissue from male rats after SNL, also confirms these findings, with no significant different increase in expression of GFAP in virgin females or postpartum rats and the large, previously described increase in males. The reasons for this considerable microglial activation but lack of subsequent astrocyte activation among female rats are currently under investigation.
Regardless of the cause, these results, if confirmed by a more complete assessment of astrocyte activation state, would suggest an important sex difference in spinal cord response to peripheral nerve injury and potentially differences in targets for treatment of chronic pain after injury between men and women.
Finally, others have shown an increase in NRG1 mRNA in virgin female, but not male rats following SNL and reduction in hypersensitivity when NRG1 signaling is blocked in females after injury (Lacroix-Fralish et al., 2006, 2008). We observed a modest but significant increase in NRG1 protein only in virgin females after SNL surgery in contrast to postpartum rats, with decreased NRG1 expression after nerve injury. The cause of this difference is uncertain, but could reflect reductions in progesterone since it is important to the increased NRG1 expression in response to injury (Lacroix-Fralish et al., 2006).
In summary, we examined several aspects of spinal cord plasticity following nerve injury-induced hypersensitivity in order to determine mechanisms for the protective effect when injury occurs surrounding the time of delivery. Increased noradrenergic fiber density and decreased dynorphin A and neuregulin-1 expression are consistent with a shift in the descending inhibitory/facilitating balance on spinal nociception neurotransmission towards inhibition. Additionally, despite a vigorous activation of microglia, spinal astrocytes are minimally activated in female rats, including postpartum rats, after nerve injury. Taken together, these results are consistent with multiple sites of protection from chronic pain at the time of childbirth and point towards novel, sex-specific responses which drive central sensitization after injury.
Highlights.
There are sex differences in spinal astrocyte activation after nerve injury
Nerve injury during puerperium results in greater noradrenergic fiber density in the spinal cord
Pain facilitating molecules, dynorphin and neuregulin-1, are reduced in the cord in this setting
Acknowledgments
We wish to thank to Mario D Boada, PhD, Assistant Professor of Anesthesiology, WFSM, Winston Salem, NC, USA for his helpful comments on the manuscript and to Renee Parker, Technician III of Anesthesiology, WFSM, Winston-Salem, NC, USA for her excellent technical assistance. This work was supported from grant GM48085 from the National Institutes of Health.
Abbreviations
- IBA1
ionized calcium binding adaptor molecule
- GFAP
glial fibrillary acidic protein
- NRG1
neuregulin-1
- DβH
anti-dopamine β hydroxylase
- NDS
normal donkey serum
- PBS
phosphate buffer saline
- DAB
diaminobenzidinetetrahydrochloride
- EIA
enzyme immune-assay
- SNL
spinal nerve ligation
- PP
postpartum
- V
virgin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Eisenach JCPP, Smiley RM, Lavand’homme P, Landau R, Houle TT. Resolution of pain after childbirth. Anesthesiology. 2012 doi: 10.1097/ALN.0b013e318278ccfd. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010;7:482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, Burgess SE, Dogrul A, Ossipov MH, Malan TP, Lai J, Porreca F. Pronociceptive effects of spinal dynorphin promote cannabinoid-induced pain and antinociceptive tolerance. Pain. 2002;98:79–88. doi: 10.1016/s0304-3959(01)00475-4. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Ibrahim M, Wang R, Wang Z, Ossipov MH, Malan TP, Jr, Porreca F, Lai J. Mouse strains that lack spinal dynorphin upregulation after peripheral nerve injury do not develop neuropathic pain. Neuroscience. 2004;123:43–52. doi: 10.1016/j.neuroscience.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Gutierrez SLB, Hayashida K, Houle TT, Eisenach JC. Reversal of Peripheral Nerve Injury-induced Hypersensitivity in the Postpartum Period:Role of Spinal Oxytocin. Anesthesiology. 2012 doi: 10.1097/ALN.0b013e318278cd21. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Clayton BA, Johnson JE, Eisenach JC. Brain derived nerve growth factor induces spinal noradrenergic fiber sprouting and enhances clonidine analgesia following nerve injury in rats. Pain. 2008;136:348–355. doi: 10.1016/j.pain.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Peters CM, Gutierrez S, Eisenach JC. Depletion of endogenous noradrenaline does not prevent spinal cord plasticity following peripheral nerve injury. J PAIN. 2012;13:49–57. doi: 10.1016/j.jpain.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentall ID, Pinzon A, Noga BR. Spatial and temporal patterns of serotonin release in the rat’s lumbar spinal cord following electrical stimulation of the nucleus raphe magnus. Neuroscience. 2006;142:893–903. doi: 10.1016/j.neuroscience.2006.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Ogawa N, Asanuma M, Hirata H, Tanaka K, Kawada Y, Mori A. Regional changes in neuropeptide levels after 5,7-dihydroxytryptamine-induced serotonin depletion in the rat brain. J Neural Transm Gen Sect. 1993;92:151–157. doi: 10.1007/BF01244874. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Hirota N, Sato Y, Kaneko S, Satoh M, Takagi H. Noradrenergic inhibition of the release of substance P from the primary afferents in the rabbit spinal dorsal horn. Brain Res. 1985;359:177–182. doi: 10.1016/0006-8993(85)91426-x. [DOI] [PubMed] [Google Scholar]
- Lacroix-Fralish ML, Tawfik VL, Nutile-McMenemy N, DeLeo JA. Progesterone mediates gonadal hormone differences in tactile and thermal hypersensitivity following L5 nerve root ligation in female rats. Neuroscience. 2006;138:601–608. doi: 10.1016/j.neuroscience.2005.11.048. [DOI] [PubMed] [Google Scholar]
- Lacroix-Fralish ML, Tawfik VL, Nutile-McMenemy N, Deleo JA. Neuregulin 1 is a pronociceptive cytokine that is regulated by progesterone in the spinal cord: implications for sex specific pain modulation. Eur J Pain. 2008;12:94–103. doi: 10.1016/j.ejpain.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Laughlin TM, Vanderah TW, Lashbrook J, Nichols ML, Ossipov M, Porreca F, Wilcox GL. Spinally administered dynorphin A produces long-lasting allodynia: involvement of NMDA but not opioid receptors. Pain. 1997;72:253–260. doi: 10.1016/s0304-3959(97)00046-8. [DOI] [PubMed] [Google Scholar]
- Li X, Eisenach JC. alpha2A-adrenoceptor stimulation reduces capsaicin-induced glutamate release from spinal cord synaptosomes. J Pharmacol Exp Ther. 2001;299:939–944. [PubMed] [Google Scholar]
- Ma W, Eisenach JC. Chronic constriction injury of sciatic nerve induces the up-regulation of descending inhibitory noradrenergic innervation to the lumbar dorsal horn of mice. Brain Res. 2003;970:110–118. doi: 10.1016/s0006-8993(03)02293-5. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Obata H, Iriuchijima N, Saito S. An increase in spinal cord noradrenaline is a major contributor to the antihyperalgesic effect of antidepressants after peripheral nerve injury in the rat. Pain. 2012;153:990–997. doi: 10.1016/j.pain.2012.01.029. [DOI] [PubMed] [Google Scholar]
- North RA, Yoshimura M. The actions of noradrenaline on neurones of the rat substantia gelatinosa in vitro. J Physiol. 1984;349:43–55. doi: 10.1113/jphysiol.1984.sp015141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra MC, Nguyen TN, Hurley RW, Hammond DL. Persistent inflammatory nociception increases levels of dynorphin 1-17 in the spinal cord, but not in supraspinal nuclei involved in pain modulation. J PAIN. 2002;3:330–336. doi: 10.1054/jpai.2002.125185. [DOI] [PubMed] [Google Scholar]
- Sonohata M, Furue H, Katafuchi T, Yasaka T, Doi A, Kumamoto E, Yoshimura M. Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J Physiol. 2004;555:515–526. doi: 10.1113/jphysiol.2003.054932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C, Conklin D, Clyne BB, Stanislaus JD, Eisenach JC. Uterine cervical afferents in thoracolumbar dorsal root ganglia express transient receptor potential vanilloid type 1 channel and calcitonin gene-related peptide, but not P2X3 receptor and somatostatin. Anesthesiology. 2006;104:651–657. doi: 10.1097/00000542-200604000-00007. [DOI] [PubMed] [Google Scholar]
- Tornblom SA, Klimaviciute A, Bystrom B, Chromek M, Brauner A, Ekman-Ordeberg G. Non-infected preterm parturition is related to increased concentrations of IL-6, IL-8 and MCP-1 in human cervix. Reprod Biol Endocrinol. 2005;3:39. doi: 10.1186/1477-7827-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Avelaira D, Moss A, Fitzgerald M. Age-related changes in the spinal cord microglial and astrocytic response profile to nerve injury. Brain Behav Immun. 2007;21:617–623. doi: 10.1016/j.bbi.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Zhang ET, King T, Ossipov MH, Vanderah TW, Lai J, Porreca F. Spinal NK-1 receptor expressing neurons mediate opioid-induced hyperalgesia and antinociceptive tolerance via activation of descending pathways. Pain. 2007;129:35–45. doi: 10.1016/j.pain.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Mei XP, Wei YY, Zhang MM, Zhang T, Xu LX, Wu SX, Li YQ. Neuronal NR2B-containing NMDA receptor mediates spinal astrocytic c-Jun N-terminal kinase activation in a rat model of neuropathic pain. Brain Behav Immun. 2011;25:1355–1366. doi: 10.1016/j.bbi.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP, Jr, Lai J, Porreca F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci. 2001;21:1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]