Abstract
The nucleus accumbens, a site within the ventral striatum, plays a prominent role in mediating the reinforcing effects of drugs of abuse, food, sex, and other addictions. Indeed, it is generally believed that this structure mandates motivated behaviors such as eating, drinking, and sexual activity, which are elicited by natural rewards and other strong incentive stimuli. This article focuses on sex addiction, but we hypothesize that there is a common underlying mechanism of action for the powerful effects that all addictions have on human motivation. That is, biological drives may have common molecular genetic antecedents, which if impaired, lead to aberrant behaviors. Based on abundant scientific support, we further hypothesize that dopaminergic genes, and possibly other candidate neurotransmitter-related gene polymorphisms, affect both hedonic and anhedonic behavioral outcomes. Genotyping studies already have linked gene polymorphic associations with alcohol and drug addictions and obesity, and we anticipate that future genotyping studies of sex addicts will provide evidence for polymorphic associations with specific clustering of sexual typologies based on clinical instrument assessments. We recommend that scientists and clinicians embark on research coupling the use of neuroimaging tools with dopaminergic agonistic agents to target specific gene polymorphisms systematically for normalizing hyper- or hypo-sexual behaviors.
Keywords: dopamine, mesolimbic systems, neurogenetics, reward deficiency syndrome (RDS), sexual addiction
The role of dopaminergic pathways in the reward circuitry of the brain is well established. Most drugs of abuse induce a preferential release of neuronal dopamine (DA) in the nucleus accumbens (NAc). Recent research suggests that many compulsive nondrug-related behaviors such as hypersexuality, pathological gambling, Internet gaming, overeating, and listening to music also induce NAc DA release (Blum et al. 2009; Cocores & Gold 2009; Comings et al. 1996). In this review we analyze current neurochemical and genetic research on the notion that sex, drugs, and rock ‘n’ roll have related underlying and biological influences and commonly acting mesolimbic dopaminergic loci. We further propose that increasing DA D2 receptors through direct or indirect activation seems prudent as one important therapeutic approach to attenuating sexual abuse and addiction.
Throughout, the common thread is the dopaminergic mesolimbic system of the brain. Its activation is accomplished by a variety of substances and activities, among which are drugs of abuse, sex, food (Cocores & Gold 2009), and music (Blum et al. 2010a). All of these cause the release of DA and fall under the rubric we term reward deficiency syndrome (RDS; Blum et al. 1996b). This article focuses mainly on sexual addiction. However, most addictions likely have a number of common features. Food addiction, for example, especially with salted or highly sweetened foods, acts similarly to drug and sex addictions and may involve addictive substances that stimulates opiate and DA receptors in brain reward and pleasure centers (Cocores & Gold 2009). They act in the brain like an opiate agonist, producing hedonic reward and withdrawal symptoms (i.e., strong preferences, urges, cravings, or hungers) similar to those in opiate abstinence. Moreover, the concept that food, drugs, and sex are common addictions, while receiving recent note, has been the subject of earlier investigation (Joranby, Frost-Pineda & Gold 2005). Of interest is the fact that individuals caught up in their specific addiction tend to interact with others having similar addictions including comorbidities of drug and food addictions. In the world of counseling, the well-known adage “birds of a feather flock together,” is not far from the truth, even genetically. Blum and colleagues (2009) provided genetic evidence that individuals presenting high RDS risk behaviors marry other individuals having these behaviors, and most importantly also carrying dopaminergic risk alleles. In two independent RDS families followed for five generations, 100% of individuals in one family married a partner carrying the DRD2 A1 allele. This and other studies support the concept of commonality in addictions.
SEXUAL ADDICTION
Sexual addiction has been defined by Patrick Carnes (IITAP 2011) as “any sexually-related, compulsive behavior which interferes with normal living and causes severe stress on family, friends, loved ones, and one's work environment.”
Sexual addiction has been called sexual dependency and sexual compulsivity. By any name, it is a compulsive behavior that completely dominates the addict's life. Sexual addicts make sex a priority more important than family, friends, and work. Sex becomes the organizing principle of addict's lives. They are willing to sacrifice what they cherish most in order to preserve and continue their unhealthy behavior.
No single behavior pattern defines sexual addiction. These behaviors, when they have taken control of addicts’ lives and become unmanageable, include: compulsive masturbation, compulsive heterosexual and homosexual relationships, pornography, prostitution, exhibitionism, voyeurism, indecent phone calls, child molesting, incest, rape, and violence. Even the healthiest forms of human sexual expression can turn into self-defeating behaviors.
In contrast, Dr. Carnes (IITAP 2011) defines sexual anorexia (a repetitive behavior) as “an obsessive state in which the physical, mental, and emotional task of avoiding sex dominates one's life.” Like self-starvation with food or compulsive dieting or hoarding of money, deprivation of sex can make one feel powerful and defended against all hurts.
As with any other altered states of consciousness such as those brought on by chemical use, compulsive gambling, eating, or any other addiction process, the preoccupation with the avoidance of sex can seem to obliterate one's life problems. The obsession can then become a way to cope with all stress and all life difficulties. Yet, as with other addictions and compulsions, the costs are great. In this case, sex becomes a furtive enemy to be continually kept at bay, even at the price of annihilating a part of oneself.
IS SEX LIKE A PSYCHOACTIVE DRUG?
Regarding our hypothesis of a common underlying mechanism for addictions, the question we raise relates to the simple notion that sex in its purist form is like any psychoactive drug having reward properties. The main premise is that dopaminergic gene polymorphisms, among many other neurotransmitter genes involved in the “brain reward cascade” (Blum et al. 1996a, b; Blum & Kozlowski 1990; Blum & Briggs 1988), and their interactions with environmental elements influence mesolimbic reward activation of sexual stimulation response. This notion is predicated on the well-known effect (DiChiara & Imperato 1988) of addictive drugs preferentially releasing DA at the NAc. Evidence for the role of DA and related mechanisms continues to support the association of dopaminergic activity and sexual drive (Krüger, Hartmann & Schedlowski 2005). For example, an increase in DA turnover was noted in the external layer of the median eminence up to 30 minutes post-coitus (Bensch et al. 1975).
It is noteworthy that DA not only is involved in sex drive, it has been shown in rodents to influence continued copulation, where social odor sensing influences female reproductive status thereby affecting neuroendocrine cascades. The odor of male mouse urine can induce ovulation or block pregnancy in mice within three days post coitus. Females avoid the action of such olfactory stimuli after embryonic implantation. The mechanisms underlying these changes are unknown. Serguera and colleagues (2008) reported that shortly after mating, a surge in DA in the mouse main olfactory bulb impaired the perception of social odors contained in male urine. Treatment of females at 6.5 days post coitus with a DA D2 receptor antagonist restored social odor sensing and favored disruption of pregnancy by inhibition of prolactin release, when administered in the presence of alien male urine odors. These results show that an active sensory barrier blocks social olfactory cues detrimental to pregnancy, consistent with the main olfactory bulb being a major relay through which social odor modulates reproductive status. This report further supports the role of DA in sexual activity and provides clues to sexual dysfunction in both genders.
In terms of molecular studies involving catecholamines and gene expression during coitus, a number of findings have generated interest. Studies have shown that coitus in female rabbits induced a gonadotropin-releasing hormone surge that was preceded by an increase in hypothalamic norepinephrine release (Yang et al. 1996). Additional findings of an enhanced tyrosine hydroxylase mRNA expression in the female brainstem after coitus, together with the appropriate topographic distribution of tyrosine hydroxylase and dopamine-beta-hydroxylase, led scientists to hypothesize that coital signals are relayed to neurons that secrete hypothalamic gonadotropin-releasing hormone via brainstem norepinephrine-containing perikarya. Additionally, coitally activated areas in the brainstem induced an increase in tyrosine hydroxylase mRNA expression, which remained for 60 minutes postcoitus, further supporting a role of catecholamines in sexual activity (Caba et al. 2000).
MULTIPLE ADDICTIONS: A COMMON MESOLIMBIC INTERACTION
Individuals caught up with addictive behaviors tend to move from one addiction to another over their lifetime (see Rastegar & Fingerhood 2005). Many children of alcoholics become obsessed with food and ultimately become obese. There are a number of studies showing the role of food as a stimulus of DA in the central nervous system. While compulsive overeating may not have mechanisms in common with substance-seeking behavior, binge eating disorder is a phenotype particularly well suited to such a conceptualization, and sound clinical and scientific evidence supports this viewpoint (Davis & Carter 2009). Related neurochemical changes commonly observed with drugs of abuse, including changes in DA and acetylcholine release in the NAc, can also be found with bingeing on sugar (Rada, Avena & Hoebel 2009). Specifically, sucrose during the first hour of daily access observed over 21 days increased extracellular DA to 130% of baseline as measured in the NAc shell by microdialysis (Avena, Rada & Hoebel 2009; Rada, Avena & Hoebel 2005).
Polydrug abuse is a worldwide problem and the combination of morphine with methamphetamine is common among addicts. The commonality of these two drugs involves the preferential release of DA in the ventral tegmental area (VTA)-NAc (Lan et al. 2009). This is equally true for alcohol, cocaine, marijuana, and nicotine (DiChiara & Imperato 1988). Numerous studies have provided evidence for common association of drug use and sexual activity. Results of these studies revealed that proximal drug use was associated with sexual aggression severity. Moreover, increased drug use predicted increased severity of sexual aggression across time (Swartout & White 2010). In Australia, it was determined that in street-based female sex workers, cocaine dependence was associated with engaging in sex and injecting risk behaviors (Roxburgh et al. 2008). It has been conjectured that drugs that stimulate the activation of hypothalamic DA or that blunt endocannabinoid or serotonin release and/or postsynaptic binding may be effective in stimulating sexual desire in humans and nonhuman animals alike.
There is a relationship between increase in sexual activity, risk behaviors, and music, sexual lyrics, and sounds fostered by hip-hop clubs (Muñoz-Laboy et al. 2008). Artistic creativity forms the basis of music culture and the music industry. Composing, improvising, and arranging music are complex creative functions of the human brain, with still unknown biological value. Ukkola and colleagues (2009) hypothesized that practicing music is social communication that needs musical aptitude and creativity. In order to understand the neurobiological basis of music in human evolution and communication, they analyzed polymorphisms of the arginine vasopressin receptor 1A (AVPR1A), serotonin transporter (SLC6A4), catecol-O-methyltranferase (COMT), DA receptor D2 (DRD2) and tyrosine hydroxylase 1 (TPH1) in 19 Finnish families with professional musicians and/or active amateurs (n = 343 members). All of these genes are associated with social bonding and cognitive functions. They showed for the first time that creative functions in music had a strong genetic component (h(2) = .84; composing h(2) = .40; arranging h(2) = .46; improvising h(2) = .62) in Finnish multigenerational families.
Most importantly, using functional magnetic resonance imaging (fMRI), Menon and Levitin (2005) reported that listening to music strongly modulates activity in a network of mesolimbic structures involved in reward processing. These areas include the NAc and the VTA, as well as the hypothalamus and insula, which are thought to be involved in regulating autonomic and physiological responses to rewarding and emotional stimuli. Importantly, responses in the NAc and VTA were strongly correlated, pointing to an association between DA release and NAc response to music. Listening to pleasant music induced a strong response and significant activation of the VTA-mediated interaction of the NAc with the hypothalamus, insula, and orbitofrontal cortex (Blum et al. 2009).
The commonality of the mesolimbic interaction with sex, drugs, and rock ‘n’ roll provides the basis for therapeutic targets. Malfunctioning of the brain's reward center is increasingly understood to underlie all addictive behaviors. Composed of mesolimbic incentive salience circuitry, the reward center governs all behaviors in which motivation has a central role, including acquiring food, nurturing young, and having sex. To the detriment of normal functioning, basic survival activities can pale in importance when challenged by the allure of addictive substances or behaviors. DA is the neurotransmitter driving both normal and addictive behavior. Other neurotransmitters modulate the amount of DA released in response to a stimulus, with the salience determined by the intensity of the DA pulse. Opiates (either endogenous or exogenous) exemplify such modulators.
It is very interesting that in terms of common therapeutic approaches, the narcotic antagonist naltrexone has been used to block opiate receptors thereby reducing DA release. This compound has been successful to some degree in the treatment of alcohol and opiate abuse. Supporting the commonality hypothesis as proposed herein, it is of interest that because of the mechanism of action of naltrexone in the reward center, this substance has been shown to suppress a euphorically compulsive and interpersonally devastating addiction to Internet pornography (Bostwick & Bucci 2008). Interestingly there is a relationship between narcotic antagonism, the DRD2 gene, and Internet addiction. The DRD2A1 allele has been associated with Internet addiction in Korean adolescents (Kim et al. 2006), and naltrexone was found to have less binding affinity to opiate receptors in the brain in carriers of the DRD2 A1 allele compared to carriers of the A2 allele (Ritchie & Noble 1996).
Most recently, using the neurochemical specificity of [(11)C]raclopride positron emission tomography scanning combined with psychophysiological measures of autonomic nervous system activity, Salimpoor and colleagues (2011) found endogenous DA release in the striatum at peak emotional arousal during music listening. To examine the time course of DA release, they used fMRI with the same stimuli and listeners, and found a functional dissociation: the caudate was more involved during the anticipation and the NAc was more involved during the experience of peak emotional responses to music. These results indicated that intense pleasure in response to music could lead to DA release in the striatal system. Notably, the anticipation of an abstract reward can result in DA release in an anatomical pathway distinct from that associated with the peak pleasure itself. This result helps to explain why music is of such high value across all human societies, and is associated with sexual activity.
NEUROCHEMISTRY OF LOVE: WHY “I GET A KICK OUT OF YOU”
When the flush of love works its magic, a cocaine-like reaction occurs in the brain, which really is the chemistry of romance (Bartels 2004; Bartels & Zeki 2000). It may take the fun out of it but that is a fact. Theories about love's purpose range from the biologically practical to the biologically complex. Anthropologists proclaim that love has sustained our species through 150,000 years as seen in the tracing of reproduction of our genes (Olson 2002). However, attachment therapists suggest that it is a byproduct of our relations with our childhood caregivers. The role of attachment style, self-esteem, and relationship attributions as possible mediators between abusive childhood experiences and difficulties in establishing supportive love relationships in adulthood were investigated in a sample of women known to be at risk of experiencing relationship problems. Participants who had experienced child abuse were found to be six times more likely to be experiencing difficulties in the domain of adult love relationships than those who had not. Self-esteem and relationship attributions were found not to be related to child abuse (McCarthy & Taylor 1999).
Now neuroscientists are exploring through the use of imaging tools what happens in the brain as a romantic relationship progresses. Mammals and birds regularly express mate preferences and make mate choices. Data on mate choice among mammals suggest that this behavioral attraction system is associated with dopaminergic reward pathways in the brain. It has been proposed that intense romantic love, a human cross-cultural universal, is a developed form of this attraction system. To examine neural mechanisms associated with romantic attraction in humans, Fisher and colleagues (2006) used fMRI to study 17 people who were intensely in love. Activation specific to the beloved occurred in the brainstem right VTA and right posterodorsal body of the caudate nucleus. These and other results suggest that dopaminergic reward and motivation pathways contribute to aspects of romantic love. Other studies by Aron and colleagues (2005) have suggested that dopaminergic reward pathways contribute to the general arousal component of romantic love. Romantic love is distinct from the sex drive, and it shares biobehavioral similarities with mammalian attraction. Early-stage romantic love can induce euphoria, is a cross-cultural phenomenon, and is possibly a developed form of a mammalian drive to pursue preferred mates. It has an important influence on social behaviors that have reproductive and genetic consequences. In studies from Aron's laboratory, brain scans using fMRI on people newly in love indicated that after a first date activation in the left VTA was correlated with facial attractiveness scores. Activation in the right anteromedial caudate was correlated with questionnaire scores that quantified intensity of romantic passion. In the left insula-putamen-globus pallidus, activation correlated with trait affect intensity. The results suggest that romantic love uses subcortical reward and motivation systems to focus on a specific individual, that limbic cortical regions process individual emotion factors, and that there is localization heterogeneity for reward functions in the human brain.
Simply, DA is released in the mesolimbic portion of the brain while one is engaged in highly pleasurable activities like having sex, doing cocaine, or eating chocolate (which contains a tetrahydroisoquinoline, which acts at opiate receptors; Blum 1988; Blum et al. 1978). Moreover, it is well known that after the DA surge, both oxytocin and vasopressin are also released (Young 2009). Oxytocin is released in humans during intimate moments of prolonged eye contact, hugging, and sex, as well as having a role in maternal bonding (Lee et al. 2009). Succu and colleagues (2007) found that stimulation of DA receptors (mainly of the D2 to D4 subtype) in the paraventricular nucleus induces the release of oxytocin in brain areas that influence the activity of mesolimbic dopaminergic neurons mediating the appetitive and reinforcing effects of sexual activity. This provides evidence for a role of oxytocin in neural circuits that integrate the activity of neural pathways controlling the consummatory aspects of sexual behavior (e.g., penile erection) with those controlling sexual motivation and sexual arousal.
Vasopressin, a peptide related to oxytocin, is linked to bonding in prairie voles, which may have similarity to bonding in men (Wang & Aragona 2004). Responding to this clue, others have attempted to investigate the role of the vasopressin receptor gene (AVPR1A) and infidelity (Cherkas et al. 2004). While the investigators were unsuccessful in associating AVPRIA and infidelity and multiple partners, they did demonstrate that infidelity and number of sexual partners were both under moderate genetic influence (41% and 38% heritable, respectively) and the genetic correlation between these two traits was strong (47%). Conversely, attitudes towards infidelity were driven by shared and unique environmental, but not genetic, influences. A pharmacological “cure” for Casanova type behavior has not yet appeared (Kirn 2004).
The consensus of the literature supports the notion that all chemicals released in the brain in new love ensure that we as Homo sapiens mate and stay together long enough to reproduce or form partnerships for the long term. What happens when these love inducing messengers subside over time? It has been assumed that most couples eventually settle down and are happy in a more intimate and compassionate kind of love with greater commitment but fewer thrills. Unpublished fMRI work by Blanco Aceveo at the University of California at Santa Barbara found that brain scans of couples even after 20 years of marriage, who claimed to be intensely in love with a strong bond, showed the same dopaminergic activation as they would if they had just fallen in love.
Finally, it has been hypothesized that cerebral neuro-transmitters such as DA and serotonin could play a role in human romantic bonding. Emanuele and colleagues (2007) looked for associations between markers in neuro-transmitter genes (the serotonin transporter gene, 5-HTT; the serotonin receptor 2A, 5HT2A; the DA D2 receptor gene, DRD2; and the DA D4 receptor gene, DRD4) and the six styles of love (eros, ludus, storge, pragma, mania, and agape). There was a significant association between the DRD2 TaqI A genotypes and eros (a loving style characterized by a tendency to develop intense emotional experiences based on the physical attraction to the partner), as well as between the C516T 5HT2A polymorphism and mania (a possessive and dependent romantic attachment, characterized by self-defeating emotions). These associations were present in both sexes and remained significant even after adjustment for potential confounders. These data provide strong evidence of a possible genetic loading on human loving styles.
PATHWAYS OF SEXUAL DESIRE
It is theorized that the NAc will produce great pleasure when stimulated electrically, and it has been called the brain's pleasure center. Some references state that the septum pellucidium is generally considered to be a pleasure center (Walsh 1991), while others mention the hypothalamus when referring to the pleasure center for intracranial stimulation (Giuliano & Allard 2001; Auriacombe et al. 1990). Certain chemicals are known to stimulate pleasure centers of the brain. These include DA and various endorphins. Physical exertion can release opioid peptides in what is called the “runner's high,” and equally, it has been found that chocolate and certain spices, such as those from the chili family, can cause the release of psychoactive chemicals similar to those released during sexual acts. Interestingly, the vanilloid receptor (VR1) is a nonselective cation channel that is most abundant in peripheral sensory fibers but also is found in several brain nuclei. VR1 is gated by protons, heat, and the pungent ingredient of hot chili peppers, capsaicin. A search for an endogenous compound with potency at this receptor comparable to that of capsaicin has led Huang and colleagues (2002) to investigate N-arachidonoyl-dopamine as an endogenous capsaicin-like substance in mammalian nervous tissues. They found the highest concentrations of N-arachidonoyl-dopamine in the striatum, hippocampus, and cerebellum, and the lowest concentrations in the dorsal root ganglion. N-arachidonoyldopamine activates both human and rat VR1 over expressed in human embryonic kidney (HEK)293 cells, with potency (EC(50) approximately 50 nM) and efficacy similar to those of capsaicin. This finding may have value in the future as a potential target to anhedonia or loss of sexual desire.
In order to understand the pathways of sexual desire, Pfaus (2009) provided insight by defining the pathways involved in hyposexual desire. Sexual desire is controlled by brain systems involved in sexual excitation and inhibition. Hyposexual desire may result from hypofunctional excitation, hyperfunctional inhibition, or some mix of the two. Pfaus pointed out that brain DA systems (incertohypothalamic and mesolimbic), which link the hypothalamus and limbic system, appear to form the core of the excitatory system. This system also includes melanocortins, oxytocin, and norepinephrine. Brain opioid, endocannabinoid, and serotonin systems are activated during periods of sexual inhibition, and blunt the ability of excitatory systems to be activated (Pfaus 2009). Pfaus supported our proposed hypothesis: drugs that stimulate the activation of hypothalamic DA or that blunt endocannabinoid or serotonin release and/or postsynaptic binding may be effective in stimulating sexual desire in animals and humans.
Sexually arousing visual stimuli activate the human reward system and trigger sexual behavior. While the assumption that men respond more than women to visual sexual stimuli is generally empirically supported, previous reports of gender differences are confounded by the variable content of the stimuli presented and measurement techniques. Rupp and Wallen (2008) concluded, based on the literature, that content characteristics might differentially produce higher levels of sexual arousal in men and women. Specifically, men appear more influenced by the sex of the actors depicted in the stimuli while women's response may differ with the context presented.
To dissociate gender of the stimulus from sexual preference, others studied male and female heterosexual and homosexual volunteers while they viewed sexual and non-sexual control stimuli. The ventral striatum and the centromedian thalamus showed a stronger neuronal response to preferred relative to non-preferred stimuli. Likewise, the ventral premotor cortex, which is a key structure for imitative (mirror neurons also in music activation) and tool-related actions (canonical neurons), showed a bilateral sexual preference-specific activation, suggesting that viewing sexually aroused genitals of the preferred sex triggers action representations of sexual behavior (Ponseti et al. 2006). This finding has been further documented in a number of related studies showing that men exhibited much higher levels of genital and subjective arousal to sexual stimuli containing their preferred sex than they do to stimuli containing only the non-preferred sex (Safron et al. 2007). Both homosexual and heterosexual men exhibited category-specific arousal in brain activity. Within the amygdala, greater preference-related activity was observed in homosexual men, but it is unclear whether this is a cause or a consequence of their sexuality.
Scientists have pondered the potential differences between men and women's responses to audio-visual sexual stimuli and what brain regions are responsible for these differences. In one fMRI experiment from Wallen's group (Hamann et al. 2004), it was found that men and women showed similar activation patterns across multiple brain regions, including ventral striatal regions involved in reward. Their findings indicate that the amygdala mediates sex differences in responsiveness to appetitive and biologically salient stimuli. The human amygdala may also mediate the reportedly greater role of visual stimuli in male sexual behavior, paralleling prior animal findings (Hamann et al. 2004). Specifically, in rodents, reproductively relevant pheromonal cues are detected by receptors in the vomeronasal organ, which in turn transmit this information centrally via the accessory olfactory bulb, the medial nucleus of the amygdala, the posterior medial bed nucleus of the stria terminalis and the medial preoptic area. In the rat, more neurons are present in males than in females at virtually every relay in this vomeronasal projection circuit. Using Fos immunoreactivity as a marker of neuronal activation, chemosensory stimulation augmented Fos immunoreactivity in the NAc shell and core, two regions receiving dopaminergic afferents, which further supports our hypothesis implicating DA in sexual reward (Bressler & Baum 1996). It should be pointed out that NAc physiology is very complex and it is not prudent simply to target DA without considering other linked receptors such as the N-methyl-D-aspartate receptor (Kelley, Smith-Roe & Holahan 1997).
Moreover, there is a plethora of evidence for the role of activation of the VTA in association with sexual stimuli. However, neurobiological mechanisms of deviant sexual preferences such as pedophilia are largely unknown. While this review does not focus specifically on deviant sexual activity like pedophilia, we believe that neurophysiologic information on this topic may shed light on normal sexual function and potential therapeutic targets.
According to the Diagnostic and Statistical Manual of Mental Disorders-IV (APA 1994), pedophilia is a form of paraphilia in which a person has at least six months of recurrent, sexually arousing fantasies and urges or behaviors involving prepubescent children (Geoghegan 2009; Laws & O'Donohue 2008; Emmers-Sommer et al. 2004; Fagan et al. 2002). Researchers studying brain structure and function detected several differences in structural magnetic resonance imaging (MRI) of the brains of pedophilic (and hebephilic) men, including a lower volume of white matter than a control group. Additionally, they were found to have a greater probability of having suffered childhood head injuries resulting in unconsciousness, lower IQs, poorer memory tests scores, greater rates of left-handedness, school grade failure, and less physical height (Cantor et al. 2008; Schiltz et al. 2007). They also report that one or more neurological characteristics present at birth increased the likelihood of being pedophilic. That, together with evidence of familial transmission signifies that genetic factors might be important in the development of pedophilia (Gaffney, Lurie & Berlin 1984).
Research using fMRI has shown that compared with nonpedophilic controls, pedophiles viewing sexually arousing pictures of adults demonstrated reduced activation of the hypothalamus (Walter et al. 2007). Cognitive dysfunction in sexual arousal processing, possibly associated with stimulus controlled sexual compulsive behaviors, may result from a disturbance noted (using fMRI) in the prefrontal networks of heterosexual pedophile inpatients (Schiffer et al. 2008). Homosexual pedophiles on the other hand, showed similar central processing of visual sexual stimuli compared to that in nonpedophile control subjects. However, when compared with homosexual control subjects, pedophile activation patterns referred more strongly to the subcortical regions involved in processing reward signals, the brain region that plays an important role in addictive and stimulus-controlled behaviors (Blanchard, Cantor & Robichaud 2006; Blanchard et al. 2002).
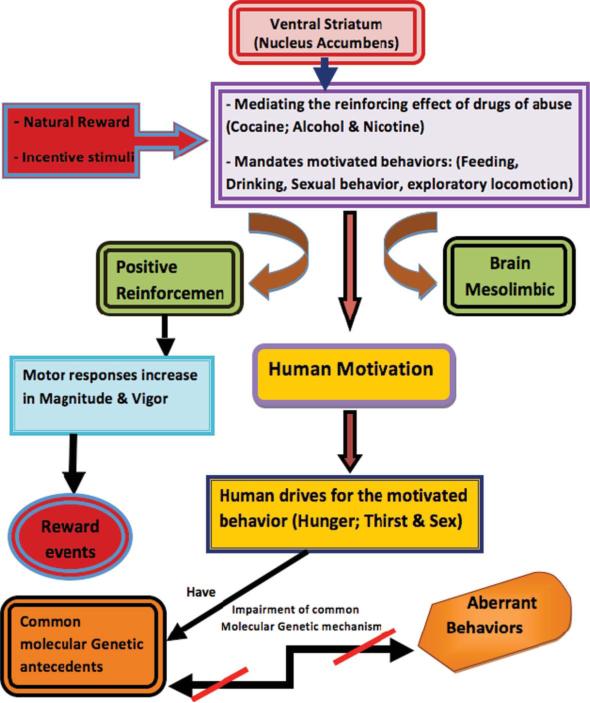
There are numerous neuroimaging studies involving sexual learning behavior, cerebral blood flow during sexual stimuli, compulsive sexual activity and clitoral stimulation providing additional evidence for central process and reward activation (Michels et al. 2010; Zhu et al. 2010; Georgiadis et al. 2009; Klucken et al. 2009). Figure 1 provides a schematic summarization of the various interactions of the pathways of sexual desire.
FIGURE 1. Summary of a Model of Common Mesolimbic Activation as a Function of Reward Gene Polymorphisms (color figure available online).
The nucleus accumbens, a site within the ventral striatum, mediates motivated behaviors such as feeding, drinking, and sexual behavior, which are elicited by natural rewards or incentive stimuli. A basic rule of positive reinforcement is that motor responses will increase in magnitude and vigor if followed by a rewarding event. Here we hypothesize a common mechanism of action that all addictions have on human motivation, with common molecular genetic antecedents, which if impaired, lead to aberrant behaviors.
Comorbid psychiatric illnesses like substance use disorder and personality disorders are risk factors for acting on pedophilic urges (Blanchard, Cantor & Robichaud 2006). Additionally Blanchard and colleagues (2002) found that the mothers of pedophiles are more likely to have undergone psychiatric treatment. This suggests that their off-spring are therefore likely to carry a genetic risk for psychiatric illnesses. It is quite possible that dopaminergic activation over a long period of time may have important treatment relevance and positive outcome value.
With this background, we present a proposed gene map specific for sexual problems, showing the basic relationship of gene polymorphisms as risk alleles for both hedonia (hyper-sexuality) and anhedonia (hypo-sexuality). (See Table 1.)
TABLE 1 Proposed Gene Map for Hyper-Sexual and Hypo-Sexual Function
| Gene Name | Hypersexual | Hyposexual | Gender | Polymorphism |
|---|---|---|---|---|
| Dopamine D2 Receptor | Applicable | N/A | M/F | Taq A1 allele |
| Vasopressin Receptor | Applicable | N/A | M/F | AVPR1A repeat polymorphisms (RS3) |
| 5hydroxytrypta-mine Receptor 2A (HTR2A) | Applicable | N/A | M/F | C516T |
| Oxytocin Receptor (OXTR) | Applicable | N/A | F | rs1042778 |
| Estrogen Receptor (ER) | N/A | Applicable | F | XbaI and PvuII |
| MTHFR | Applicable | N/A | F | 1298C allele or 677TT |
| Dopamine D4 Receptor | Applicable | N/A | M/F | Any 3 R Not 4 R |
| 5hydroxytrypta-mine Receptor 2A (HTR2A) | N/A | Applicable | M/F | T102C |
| Nitric Oxide Synthase (eNOS) | N/A | Applicable | M | G894T |
| GRIK4 (Glutamate Receptor) | N/A | Applicable | M/F | rs1954787 |
| Serotonin Transporter Gene | N/A | Applicable | M | LL variant |
THE NEUROGENTICS OF SEXUAL PROCLIVITY: SEXY GENES
It is our hypothesis that both hedonic and anhedonic behaviors are outcomes in-part of an individual's risk alleles for these behaviors and that treatment resides in appropriately targeting these identified polymorphisms. Moreover, treatment response also depends on these risk alleles and provides important rationale for pharmacogenetic testing and pharmacogenetic/nutrigenomic solutions.
Following the controversial initial finding by our group (Blum et al. 1990), whereby we provided the first evidence for an association of the DRD2 A1 allele and severe alcoholism, there have been 3,101 articles in PUBMED (7/23/11) on this most widely studied psychiatric gene polymorphism and associated behaviors and physiology. However, there is a paucity of data linking sexual activity (Miner et al. 2009) to this and other related genes in spite of the overwhelming evidence of mesolimbic activation, especially in dopaminergic pathways and neuronal loci related to sexual stimuli and activity. It is noteworthy that Blum and Noble (1994) correctly classified the DRD2 gene as a generalized reward gene independent of any particular drug of abuse or reward deficiency syndrome (RDS) behavior. In fact, Bayesian theorem analysis indicates that carriers of the Taq A1 allele will have a 74% chance that over their lifetime they will have a rendezvous with one or more RDS behaviors including sexual addiction (Blum et al. 1996a, b).
MEN ARE FROM MARS AND WOMEN ARE FROM VENUS
The first association of any gene polymorphism and sexual activity did not occur until Miller and colleagues (1999) evaluated a number of dopaminergic genes. The basic finding is that the dopaminergic system in the brain seems to play an important role in the regulation of sexual behavior. The relationship between genes for the D1, D2, and D4 DA receptors and age at first sexual intercourse was examined in a sample consisting of 414 men and women (non-Hispanic, European-American). A significant association was observed between a DRD2 allele and age at first sexual intercourse, and an even stronger association was found when both the DRD1 and DRD2 alleles were incorporated into the statistical model. A constrained regression model was constructed predicting age at first sexual intercourse, using sex and a group of nine psychosocial variables as predictors. Adding the DRD2 and the DRD2-by-DRD1 predictors to this model increased the explained variance by 23% and 55%, respectively. The fact that these findings suggest a stronger association among males than among females is in agreement with the recent work of others showing higher sexual stimuli response in males than in females (Hamann et al. 2004). So maybe “men are from Mars and women are from Venus,” and this even may be true for cocaine abuse (Quiñones-Jenab 2006). Specifically, both preclinical and clinical studies have shown sexually dimorphic patterns in behavioral responses to cocaine in all phases of the cocaine addiction process (induction, maintenance, and relapse). Thus, a clear picture is emerging that suggests that the biological basis of sex-specific differences in cocaine addiction resides in the disparate regulation of the central nervous system by male and female gonadal hormones, and may be predictive on the basis of DRD2 gene polymorphisms (Noble et al. 1993). Moreover, it is known that genetic associations between COMT and various psychiatric phenotypes frequently show differences between men and women. The functional Val(158)Met polymorphism in COMT is associated with obsessive-compulsive disorder in men, with anxiety phenotypes in women, and has a greater impact on cognitive function in boys than girls (Harrison & Tunbridge 2008; Delorme et al. 2009).
While Miller and colleagues (1999) did not find an association of the polymorphisms and the DRDD4 gene and age at first sexual intercourse, others found signifi-cant associations in certain ethnic groups. Specifically, their analysis of the polymorphisms in DRD4 indicated that those with any-3R genotype experienced a significantly higher risk of early onset of first sexual intercourse than those with other (or any-4R) genotype in a large sample consisting of Asian, White, and Hispanic ethnicities. Interestingly, the risk of first sex did not differ between the two genotypes in the African-American sample, raising the question of cultural upbringing (Guo & Tong 2006).
It is noteworthy that sexual experience, like repeated drug use, produces long-term changes including sensitization in the NAc and dorsal striatum. Bradley and colleagues (2005) while studying hamsters using microarray analysis found that the sexual experience in either male or female animals differentially up- or down-regulated mRNA expression of a series of genes in the NAc. They found that in comparison with sexually naive animals, sexually experienced hamsters receiving a stimulus male on week seven exhibited an increase in a large number of genes. Conversely, sexually experienced female hamsters not receiving a stimulus male on week seven exhibited a reduction in the expression of many genes. According to the authors, this first ever gene profiling in female hamsters may provide insight into the mechanisms by which both motivated behaviors (sex) and drugs of abuse induce long-term changes in the mesolimbic and nigrostriatal DA pathways.
It is of further interest that chronic self-stimulating reward experiences in self-stimulation-experienced rats (measured through bipolar electrodes, stereotaxically implanted bilaterally in the lateral hypothalamus and substantia nigra-VTA), have been found to induce a significant increase in the number of synapses in the CA3 region of hippocampus and the molecular layer of the motor cortex. In essence, chronic brain stimulation induced long-term potentiation, which is known to increase new synaptic connections (Rao, Raju & Meti 1999). Similarly, a single exposure to cocaine in naive animals was sufficient to trigger sustained changes on VTA glutamatergic synapses that resemble activity-dependent long-term potentiation in other brain regions. This cocaine-induced long-term potentiation appears to be mediated via DA D(5) receptor activation of N-methyl-D-aspartate receptors and requires protein synthesis (Heshmati 2009). This again supports the premise proposed herein that drugs and sex might have common neurochemical substrates.
Empirical research has revealed a positive relationship between the number of sex partners and involvement in antisocial behaviors. Most attempts to explain this association have taken an evolutionary perspective and argued that the same traits (e.g., impulsiveness, shortsightedness, and aggressiveness) that are related to a large number of sex partners are also related to criminal involvement. However, there is also reason to believe that the covariation between sex partners and crime behaviors can be partially explained by a common genetic pathway, where genes that are related to sex partners are also related to antisocial conduct. Using the above described rationale, Beaver and colleagues (2008) found that a strong positive association between numbers of sex partners and antisocial behavior and polymorphisms of the DA transporter gene (DAT1) explained variation in both number of sexual partners and in criminal conduct for males. The polymorphic effect of the DAT1 gene and the number of sexual partners may be due to an association found between certain polymorphisms and male premature penile ejaculation. Carriers of the 10R10R genotype had scores indicating a lower threshold to ejaculate on each of the indicators compared to the combined 9R9R/9R10R carrier group (Santtila et al. 2010). Polymorphisms of the DAT1 gene, specifically the 10R10R genotype, have been found in juvenile delinquents attending a school for pathological aggressive behaviors including antisocial behavior (Chen et al. 2007, 2005). A positive correlation of the DRD2 and of the DAT1 polymorphisms was observed with pathological violence in adolescents in a blinded clinical trial. Moreover, though initially conceptualized as resulting from peer imitation of child-onset or life-course-persistent youth, there is mounting evidence from twin studies that adolescent-onset or adolescent-limited antisocial behavior may also be genetically influenced. Burt and Mikolajewski (2008) not only confirmed these findings with the DAT1 gene but extended the findings to include the His452Tyr variant of the gene encoding the 5-HT2A receptor as well.
GENETIC AND MEME EVOLUTION: HUMAN PROCREATION-GENE BOMB CONCEPT
In his writings about sexuality and personality, Eysenck (2006, 1997) proposed a positive correlation between extraversion and intensified sexual behavior and between neuroticism and problems in sexual behavior (antisocial behavior). An earlier study with married persons did not show any of these correlations. It was hypothesized that this connection exists only for unmarried persons who are not engaged in long-lasting relationships, because in the latter the quality of the relationship determines the sexual interaction. Within a sample of young unmarried men there was a positive correlation between extraversion and items in which the person described earlier sexual activity with more persons and in higher frequency. No correlation was found with neuroticism. There were also slight correlations with other personality and social attitude scales. Because of the correlation with an acting out personality scale, the findings were interpreted from a social-psychological perspective. In our culture, young men are expected to take the initiative in sexual interactions, and extraverted young men can accomplish this better than introverted men (Addad & Lesiau 1989). This is in direct agreement with Richard Brodie's (1996) idea on selfish genes of the mind. When considering DNA, anthropologists would agree that humans must “go forth and multiply.” However, biological evolutionary advances are slow compared with meme evolution in which an idea can mutate in the time it takes to read a sentence. If there are genes that give people the tendency to take on memes that limit their number of off-spring, they will die out in a few generations in favor of genes that give people a tendency to acquire children. Unfortunately a number of studies suggest that Homo sapiens having lower IQs have been increasing in the twentieth century. The rate of decline is at least 0.8 IQ points per generation (Herrnstein & Murray 1994).
Extraordinarily, it turns out that since extraversion is linked to increased sexual activity, especially in males, and quantitative geneticists estimate the heritability of extraverted personality to be around 40% to 60%, it was intuitive that Smillie and associates (2010) studied and found that one copy of the DRD2 gene A1 allele was associated with significantly higher extraversion. This raises an interesting question in terms of human procreation first suggested by Comings (1996). Because of their marked effect on reproductive behavior, the learning disorders as well as impulsive, compulsive, aggressive, and addictive disorders (DRD2 A1 associated) have the ability to result in progressive and permanent changes in the frequency of the associated genes; a potential consequence could be a genetic meltdown of the species.
In his book, The Gene Bomb, Comings (1996) provided evidence that people with addictive-disruptive behaviors have children early in their lives, which can impact the selection of addiction genes like the DRD2 A1 allele. For example, if individuals carrying this disruptive risk allele have children at 20 years of age, and individuals without this allele have children at age 25, the mutant gene will reproduce faster, namely every 20 years, while the normal form of the gene will reproduce every 25 years. The ratio of 25/20 is 1.25; thus, the rate at which a gene that has a 1.25-fold selective advantage will increase in frequency from generation to generation. A difference of five years in the age of mothers or fathers when they have their first children is sufficient to result in a significant and relatively rapid selection for genes carried by group initiating childbearing at an earlier age. Increases in a number of RDS behaviors have been documented from 1955 to the present. These increases include adolescent behavior syndrome (drugs, sex, teen pregnancies and delinquent behaviors, smoking), conduct disorder, crime, drug abuse, alcoholism, unprotected sexual behavior, school drop outs and expulsions, as well as concomitant decreases in IQ. These results are based on the Berkeley study utilizing longitudinal data from the Child Health and Developmental studies and the National Longitudinal Surveys of Youth. Utilizing this information, Comings suggested that from 1955 to 2015, there will be a doubling of the frequency of, for example, the DRD2 A1 allele, therefore increasing the prevalence of RDS behaviors including precocious sexual intercourse (Guo & Tong 2006; Miller et al. 1999).
PRACTICE MAKES PERFECT
Precocious sexual intercourse often leads to multiple partners and increased frequency of sexual activity. The old adage “practice make perfect” makes sense when it comes to the sexual experience of females. In what we consider to be a nonhuman animal model of RDS, Kohlert and Meisel (1999) examined the effects of prior sexual experience on extracellular concentrations of DA in the NAc of female Syrian hamsters, known to drink abnormal amounts of alcohol when given a choice between alcohol and water solutions. The NAc DA was measured by in vivo microdialysis during mating in female hamsters that had previously been given either three or six prior sexual encounters with a male, or were sexually naive. They found females that received six prior sexual encounters had significantly elevated and prolonged increases in dialysate DA compared with those of the sexually naive females or females with only three prior sexual encounters with a male. The data indicated that the mesolimbic system could be sensitized by repeated experiences associated with a motivated behavior. This study seems to support the well documented notion held by sexologists that there is a powerful rise of NAc DA in males following sexual intercourse coupled with a rapid fall below baseline, whereas DA in the NAc in females increases more slowly but can be prolonged during increased sexual arousal leading to the potential of female multiple organisms (Chia & Chia 1991; Dunn & Trost 1989).
The importance of DA release in the NAc in both males and females in terms of sexual behavior has been further characterized by mouse studies initiated by Szczypka and colleagues (1998). In their studies, mice unable to synthesize DA in dopaminergic neurons were generated by gene targeting. These dopamine-deficient mice required daily administration of 3,4-dihydroxyphenylalanine (L-dopa) for survival beyond two to three weeks of age. This treatment stimulated mounting and aggressive behavior of adult dopamine-deficient males toward both male and female mice. They also found a number of interesting outcomes:
Non-specific DA agonist (apomorphine) stimulated aggression and mounting behavior.
Specific D1 agonist (SKF 81297) stimulated aggression and mounting behavior.
Specific D2 agonist stimulated aggression and mounting behavior.
DA-stimulated sexual behavior was testosterone dependent in mice.
DA is implicated in penile erection in rats through activation of D2-like receptors. However, the exact role of each subtype (D2, D3, and D4) of this receptor family in penile erection still is not clearly elucidated. There have been a few reports indicating that D4 agonists induce penile erection through a central mechanism, and polymorphisms of this specific gene are involved in human sexuality, desire, and arousal (Ben-Zion et al. 2006). Most recently Depoortère and colleagues (2009) reported data that did not support a major implication of either DA D3 or D4 receptors in the control of penile erection in rats, but indicated a preponderant role of DA D2 receptors.
Along these lines, specific dopaminergic genes seem to play a role in human sexual desire, arousal, and sexual function, and a number of genes have been associated with male and/or female sexual dysfunction. Complete sequencing of the human genome has made possible a new age of molecular medicine. The utilization of sophisticated genomic technologies has important implications for the understanding, diagnosis, and treatment of erectile dysfunction.
Briefly, preclinical evidence emerging from several laboratories indicating that gene therapy for erectile dysfunction may well provide the first safe and effective application of gene therapy to the treatment of human smooth muscle disease (Melman & Davies 2010). The molecular targets explored thus far have concentrated largely on manipulating various aspects of the nitric oxide/guanylate cyclase/cGMP system, although genetic modulation of growth factors, calcium sensitization mechanisms, and potassium channel expression also have been explored.
Additionally, cell-based gene therapy techniques are being explored. The apparent preclinical success of virtually all of these gene-based strategies reflects the multi-factorial nature of erectile disease, as well as the numerous regulatory mechanisms available for restoring erectile capacity. While technical hurdles remain with respect to the choice of delivery vectors, molecular target validation, and duration of efficacy, proof-of-concept has clearly been documented. An ultimate goal of gene therapy is to provide a safe, effective, and specific means for altering intracavernous pressure on demand, while simultaneously eliminating the necessity for other forms of therapy, and moreover, without altering resting penile function, or the physiology of other organ systems.
GENETICS OF SEXUAL DYSFUNCTION: BIRDS OF A FEATHER FLOCK TOGETHER
In the 1980s, Cocores and colleagues (1988) studied the relationship between sexual dysfunction and drugs of abuse. Because alcohol abuse and cocaine dependence often occurred together, and sexual dysfunction was not uncommon in either condition, they examined the sexual history of this dually addicted population. Sexual dysfunction was found in 62% of male cocaine and alcohol abusers consecutively admitted to a substance disorder treatment unit. Interestingly, the sexual dysfunction observed in cocaine and alcohol abusers was overcome by a specific D2 receptor DA agonist. Certainly, anhedonia and loss of interest in sex is common in end stage addictions (Gold 1988; Cocores, Dackis & Gold 1986). These studies confirmed the earlier work of Vijayasenan (1981), who found that 71% of alcoholic patients suffered from sexual dysfunction for a period more than 12 months prior to admission to a hospital. The disturbances noted were diminished sexual desire, impotence, premature ejaculation, and ejaculatory incompetence. A high proportion of the alcoholics showed signs of sexual deviations, such as performance of sexual crimes or having repeated thoughts of sexual crimes. It is important to acknowledge that sexual assaults are proven to be motivated by hostility, power, and control. They are not motivated by sexual desire. Alcohol does not cause sexual deviation. It is demonstrated to decrease inhibitions, thereby allowing people to act on prior thoughts, including sexual crimes or deviations.
Interestingly, Delmonico and Carnes (1999) reported on an overlap of cybersex and sexual addiction. This is not surprising when we consider the known relationship between the DA D2 A1 allele and hypersexuality (Miller et al. 1999) and addiction to Internet gaming, with special emphasis on mesolimbic DA release (Koepp et al. 1998). Specifically, excessive Internet video game play has emerged as a leading cause of behavioral and developmental problems in adolescents. Recent research has implicated the role of the striatal dopaminergic system in the behavioral maladaptations associated with such excessive Internet video game play. In a study by Han and colleagues (2007), 79 male excessive Internet video game playing (EIGP) adolescents and 75 age- and gender-matched comparison adolescents were recruited. Associations were tested with respect to the Reward Dependence scale in Cloninger's Temperament and Character Inventory and the frequencies of two DA polymorphisms: Taq1A1 allele of the DA D2 receptor (DRD2 Taq1A1) and Val158Met in the Catecholamine-O-Methyltransferase (COMT) genes. The Taq1A1 and low activity (COMTL) alleles were significantly more prevalent in the excessive gaming group relative to the comparison group. The present excessive gaming group had significantly higher Reward Dependence scores than controls. Within the EIGP group, the presence of the Taq1A1 allele correlated with higher RD scores. These findings suggested that excessive gaming subjects have higher reward dependency and an increased prevalence of the DRD2 Taq1A1 and COMTL alleles. In particular, the DRD2 Taq1A1 allele seems to be associated with reward dependence in adolescents who excessively play video games on the Internet. Since cybersex seems to constitute a distinct typological category in terms of sexual addiction, we wonder if individuals having this behavior may not have a conscious choice, and may be biologically tied to cyberspace and Internet addiction as a function of specific dopaminergic gene polymorphisms.
Exploring multiple genotypes and polymorphisms of neurotransmitter pathways, Gelernter's group at Yale University (Yang et al. 2008) found that of all genes involved in substance use disorders, the most robust association occurred with the DRD2/ANKKI gene loci. Specifically, the association with substance use disorder was between 10−7 to 10.−9 They concluded that variants in the chromosomal q11-23 cluster including TTC12 exon 3, NCAM1 exon 12, and the two 3’-ends of ANKK1 and DRD2 co-regulate risk for alcohol and drug dependence. Based on this and other work, Chen and colleagues (2011) proposed a candidate-gene panel map to follow drug addiction reward genes (see Table 2).
TABLE 2 Proposed Drug Addiction Reward Gene Panel
| Gene | Significant | Comment |
|---|---|---|
| ALDH2** | P = 5 × 10–37 | With alcoholism and alcohol-induced medical diseases. |
| ADH1B** | P = 2 × 10–21 | With alcoholism and alcohol-induced medical diseases. |
| ADH1C** | P = 4 × 10–33 | With alcoholism and alcohol-induce medical diseases. |
| DRD2* | P = 1 × 10–8 | With alcohol and drug abuse. |
| DRD4* | P = 1 × 10–2 | With alcohol and drug abuse. |
| SLC6A4** | P = 2 × 10–3 | With alcohol, heroin, cocaine, methamphetamine dependence |
| HTRIB* | P = 5 × 10–1 | With alcohol and drug abuse. |
| HTRI2A* | P = 5 × 10–1 | With alcohol and drug abuse. |
| TPH* | P = 2 × 10–3 | With alcohol and drug abuse. |
| MAOA* | P = 9 × 10–5 | With alcohol and drug abuse. |
| OPRD1** | P = 5 × 10–1 | With alcohol and drug abuse. |
| GABRG2** | P = 5 × 10–4 | With alcohol and drug abuse. |
| GABRA2* | P = 7 × 10–4 | With alcohol and drug abuse. |
| GABRA6** | P = 6 × 10–4 | With alcohol and drug abuse. |
| COMT* | P = 5 × 10–1 | With alcohol and drug abuse in Asians. |
| DAT1* | P = 5 × 10–1 | With alcohol and drug abuse in Asians. |
| CNR1* | P = 5 × 10–1 | With alcohol and drug abuse. |
| CYP2E1** | P = 7 × 10–2 | With alcohol liver disease. |
| ANKKI** | P = 5 × 10–6 | With alcohol and drug abuse. |
US and European patents issued
US & PTC pending
Reproduced from Chen et al. 2011. Independent analysis by Li, Zhao, and Galanter presented at XVII World Congress of Psychiatric Genetics Abstract (#ECI16) Nov 4–8, San Diego, CA 2009.
It has been found that deltaFosB gene in the NAc is critical for reinforcing effects of sexual reward. Pitchers and colleagues (2010) reported that sexual experience was shown to cause DeltaFosB accumulation in several limbic brain regions including the NAc, medial pre-frontal cortex, VTA, caudate, and putamen, but not the medial preoptic nucleus. Next, the induction of c-Fos, a downstream (repressed) target of DeltaFosB, was measured in sexually experienced and naive animals. The number of mating-induced c-Fos-IR cells was significantly decreased in sexually experienced animals compared to sexually naive controls. Finally, DeltaFosB levels and its activity in the NAc were manipulated using viral-mediated gene transfer to study its potential role in mediating sexual experience and experience-induced facilitation of sexual performance. Animals with DeltaFosB overexpression displayed enhanced facilitation of sexual performance with sexual experience relative to controls. In contrast, the expression of DeltaJunD, a dominant-negative binding partner of DeltaFosB, attenuated sexual experience-induced facilitation of sexual performance, and stunted long-term maintenance of facilitation compared to DeltaFosB overexpressing group. Together, these findings support a critical role for DeltaFosB expression in the NAc in the reinforcing effects of sexual behavior and sexual experience-induced facilitation of sexual performance.
Furthermore, both drug addiction and sexual addiction represent pathological forms of neuroplasticity along with the emergence of aberrant behaviors involving a cascade of neurochemical changes mainly in the brain's rewarding circuitry. The aberrant behavioral phenotypes can be assessed by an animal model of drug-induced behavioral sensitization, which is characterized by an initiation stage formed in the VTA, and a behavioral expression stage determined mainly in the NAc. Numerous studies during past decades have demonstrated that the mesocorticolimbic DA pathway plays an essential role in the development of behavioral sensitization. Moreover, a series of cellular signaling pathways and gene expression determine the severity of addictive behaviors. In addition to the well-characterized DA D1 receptor-mediated cAMP/protein kinase A up-regulation in the NAc, recent reports indicated the cellular mediator DA- and cAMP-regulated phospho-protein of 32 kDa (DARPP-32) and transcription regulator DeltaFosB are associated with the accumbal protein kinase A pathway to modulate the development of behavioral sensitization. The finding of cAMP-independent and DA D2 receptor-mediated Akt/GSK3 in activation in the NAc of behaviorally sensitized animals implies that a signal cascade down-stream of both DA D1 and D2 receptors comprises the mainstay of the addiction network. This review outlines the cellular pathways that have been demonstrated to participate in psychostimulant addiction, focused particularly in the NAc (Chen, Chen & Chiang 2009).
CASE REPORTS AND COMMENTARY
Individuals often develop sexual disorders or deviations based on their life experiences, which may influence specific gene expression(s). Utilizing case studies, we demonstrate some of the ramifications of individuals’ life experiences on the development of these deviations.
Roger
Roger is the owner of a restaurant/strip club. Roger initially contacted a mental health treatment center for treatment of his cocaine and alcohol abuse and depression. During group discussions of family relationships, he discussed how he started drinking in his early teens to deal with the stress of his mother's many abusive relationships with men. His mother had a string of live-in boyfriends, and many were physically violent toward her and Roger. Roger remembers many incidences where he would attempt to intervene during the abuse of his mother. This intervention often involved physical altercations, one of which sent Roger to an emergency ward for a broken arm. After this occurred, child protective services became involved. Faced with the possibility of losing custody of Roger, his mother broke up with that boyfriend and promised to find a father/husband who would treat them well. Eventually his mother met a man she married who, while not abusive, was extremely controlling of every aspect of their family life.
As an adult, Roger continued to drink and started using cocaine to help with alertness at work. He has never been married but has had a series of relationships with strippers who work in his club. As each of these relationships developed he would want them to stop stripping and move in with him, where he provided for all of their financial needs. Once a woman moved in, his interest would diminish, and he would begin flirtations with strippers currently working in the club. Eventually his frequent indiscretions would be discovered by his current girlfriend, causing arguments and culminating in a breakup.
Roger felt that he had “something missing.” He was always hopeful at the beginning of a relationship, and his drinking and substance abuse would briefly subside. During the cycle of rapid diminishment in his sexual interest, he would feel guilty and ashamed of his lack of ability to connect. He would start cheating and escalating his drinking and substance abuse. In this case, we asked the question as to parental rearing which can be related to genetic hard wiring. In a study by Keltikangas-Järvinen and others (2009), the DRD2 gene polymorphism was not directly associated with novelty seeking, but there was a statistically significant interaction between DRD2 and strict maternal disciplinary style in predicting novelty-seeking. The interaction showed that when the child-rearing environment was punitive, participants carrying any A1 allele of the DRD2 gene had higher scores on novelty seeking than carriers of the A2/A2 genotype. The genotype had no effect on novelty seeking when the childhood environment was more favorable. The findings suggest that the DRD2 may have an environmentally moderated impact on novelty-seeking and that the origins of such an association may result from abuse in childhood.
Amy
Amy was court ordered for inpatient treatment of her alcohol abuse after her second DUI offense. After Amy was in the hospital for a couple of days, her roommate went to one of the counselors and expressed concern because Amy was vomiting after every meal. The counselor encouraged the roommate to bring up her concerns in the next group session. Later that day in the group, Amy admitted that she was worried about how much more she was eating since being in treatment and she was trying to control her weight by vomiting. This was something she had done in the past whenever she felt that her weight was out of control.
The treatment team decided to expand Amy's individual therapy sessions to further explore the possibility that she may have an eating disorder. Several days later, while in a therapy session, Amy demanded to be discharged because she had been the victim of sexual misconduct by one of the male staff members. The facility medical director began interviewing Amy and all of the staff to investigate Amy's claim.
During her interview Amy was tearful and expressed a great deal of hostility towards the staff member who she claimed made sexual advances towards her. She said that he had made several sexually suggestive comments and tried to come into her room when he knew she was disrobed. Amy expressed her feelings that this was just another event in a long chain of victimization by men in her life including extreme verbal abuse by her father and date rape while in college. When the medical director tried to elicit specific details of what occurred with the staff member, Amy became completely overwrought and refused to answer any further questions.
During his interview, the staff member had a very different account of events. He claimed that Amy began flirting with him from first contact. She would make a point to touch his arm or shoulder whenever she was speaking with him and also made several personal remarks about his appearance including how fit he was. The staff member said that he ignored these incidents, hoping Amy would discontinue her actions.
On the day of the incident, the staff member said that he went into Amy's room to get her up for her therapy session, because her roommate said that she refused to get out of bed. When the staff member went into her room to try to wake her, Amy tried to kiss him. He immediately pushed her away and left the room. The staff member informed his supervisor what had occurred during the time that Amy was in her therapy session.
Subsequently, after an extended interview, Amy admitted that the staff member had not approached her and that she approached him. She said that she felt embarrassed and hurt by his rejection and she blamed him in order to retaliate.
Later during group sessions when the incident was discussed, Amy admitted that she did not know how to relate to men other than sexually and that she felt that there was something missing in her if she was not in a sexual relationship. She worried that she is incapable of ever being truly satisfied, since even when she had a long-term boyfriend she was always flirting and occasionally cheating to feel attractive and desirable.
This may be a case where “no satisfaction” could be lack of sufficient DA D2 receptor density. Interestingly it has been addressed by Vaske and colleagues (2010), using data from the National Longitudinal Study of Adolescent Health (also called Add Health) to investigate whether variants of a polymorphism in the DA D2 receptor gene (DRD2) distinguish between offenders who are violently victimized and offenders who are not violently victimized. The results showed that offenders who are violently victimized are more likely to carry the DRD2 (A1) risk allele than offenders who have not been violently victimized. This may have relevance to Amy's case and may be related potentially to her being a rape victim.
It is imperative for clinicians to understand that individuals with sexual disorders (a subclassification of RDS) often have other presenting complaints on their initial presentation for treatment. It is not uncommon for these individuals to present for treatment of mood disorders or substance abuse. An intake assessment necessitates inclusion of a thorough psychosocial history, including direct inquiries with regards to the patient's sexual history and behaviors. Even with this type of direct questioning, many patients will not fully disclose their activities and concerns during the first few appointments. Therefore, it remains important for clinicians to re-address the psychosexual history multiple times throughout treatment. Disclosure is the first step to being able to integrate past history and interpersonal interactions with the development of sexual disorder symptoms. Being able to integrate the two and helping the patient to identify the underlying connections is one of the first and most important tasks of treatment.
NEW ROMANTIC LOVE
Under normal conditions, it is not surprising that sexual activity is physiologically regulated by the reward circuitry of the brain, specifically by dopaminergic pathways (see Figure 1). Moreover, the early stages of a new, romantic relationship can be a powerful and absorbing experience. Individuals in new romantic relationships report feeling euphoric and energetic. They also become emotionally dependent on, desire closeness with, and have highly focused attention on their partner (Reynaud et al. 2010; Young 2009). Human neuroimaging studies have shown that feelings experienced during the early stages of a romantic relationship are associated with neural activations in several reward-system and affect-processing regions of the brain (Young 2009; Aron et al. 2005; Bartels & Zeki 2000; Mashek, Aron & Fisher 2000). Those studies displayed pictures of participants’ own romantic partners to evoke acute positive affects and self-reported feelings of love.
In one such fMRI study, Aron and colleagues (2005) instructed participants in new romantic relationships to view pictures of their partner and pictures of a familiar acquaintance who were the same age and sex as the participant's partner. Neural activations specific to viewing pictures of the romantic partner were observed in several reward-processing regions, such as the bilateral caudate nucleus and the right VTA. An earlier fMRI study using a similar protocol reported neural activations specific to the romantic partner pictures in reward regions such as the bilateral caudate nucleus and bilateral hippocampus (Bartels & Zeki 2000).
The activation of reward structures caused by viewing pictures of a romantic partner has also been confirmed in a Chinese sample, suggesting the phenomenon may be culturally universal (Xu et al. 2011). Collectively, these neuroimaging studies demonstrate that reward-system activation is a central component of self-reported feelings of love in new romantic relationships. While this may be true for normal romantic kind of love relationships, as detailed in this review, impairments of the mesolimbic system promote unhealthy sexual activity and potentially sexual abuse.
CONCLUSIONS
Drug addiction as well as sexual addiction are pathological forms of neuroplasticity that along with the emergence of aberrant behaviors involve a cascade of neurochemical changes mainly in the brain's reward circuitry. Aberrant behavioral phenotypes can be assessed by nonhuman animal models of drug-induced behavioral sensitization, which is characterized by an initiation stage formed in the VTA, and a behavioral expression stage determined mainly in the NAc. Numerous studies during past decades have demonstrated that the mesocorticolimbic DA pathway plays an essential role in the development of behavioral sensitization.
Being cognizant of this well-established mechanism, we believe that future research will continue to use research techniques such as neuroimaging and genotyping to unravel the biogenetic basis of human sexuality. We propose herein, that a challenge for sex therapists is to promote healing in those individuals caught up in sexual abuse, as either the victim or the victimizer, by finding therapeutic modalities that will enhance dopaminergic activity, overcoming hypodopaminergic function due in part to polymorphisms of known genes involved in the regulation of reward mechanisms and resultant dopaminergic function (Blum et al. 2010a, b).
Acknowledgments
The authors are indebted to the superb editorial work by Margaret Madigan and Paula J. Edge. The writing of this paper was supported in part by funds from the US Department of Health Services, NIAAA (R01-AA07112 and K05-AA00219) and the Medical Research Service of the US Department of Veterans Affairs (Marlene Oscar-Berman).
Footnotes
Conflict of interest: Kenneth Blum, PhD owns stock in LifeGen, Inc. San Diego, CA, a neurogentics company involved in developing natural DA D2 agonists and genetic testing.
REFERENCES
- Addad M, Lesiau A. Extraversion, neuroticism, immoral judgment and criminal behaviour. Medicine and Law. 1989;8(6):611–22. [PubMed] [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders-IV. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;94(1):327–37. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Auriacombe M, Tignol J, Le Moal M, Stinus L. Transcutaneous electrical stimulation with Limoge current potentiates morphine analgesia and attenuates opiate abstinence syndrome. Biological Psychiatry. 1990;28(8):650–56. doi: 10.1016/0006-3223(90)90451-7. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. Journal of Nutrition. 2009;139:623–28. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A. The neural correlates of maternal and romantic love. Neuroimage. 2004;21(3):1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829–34. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Beaver KM, Wright JP, Walsh A. A gene-based evolutionary explanation for the association between criminal involvement and number of sex partners. Biodemography and Social Biology. 2008;54(1):47–55. doi: 10.1080/19485565.2008.9989131. [DOI] [PubMed] [Google Scholar]
- Bensch C, Lescure H, Robert J, Faure JM. Catecholamine histofluorescence in the median eminence of female rabbits activated by mating. Journal of Neural Transmission. 1975;36(1):1–18. doi: 10.1007/BF01243433. [DOI] [PubMed] [Google Scholar]
- Ben-Zion IZ, Tessler R, Cohen L, Lerer E, Raz Y, Bachner-Melman R, Gritsenko I, Nemanov L, Zohar AH, Belmaker RH, Benjamin J, Ebstein RP. Polymorphisms in the dopamine D4 receptor gene (DRD4) contribute to individual differences in human sexual behavior: Desire, arousal and sexual function. Molecular Psychiatry. 2006;11(8):782–86. doi: 10.1038/sj.mp.4001832. [DOI] [PubMed] [Google Scholar]
- Blanchard R, Cantor JM, Robichaud LK. Biological factors in the development of sexual deviance and aggression in males. In: Barbaree HE, Marshall WL, editors. The Juvenile Sex Offender. Second Ed Guilford Press; New York: 2006. [Google Scholar]
- Blanchard R, Christensen BK, Strong SM, Cantor JM, Kuban ME, Klassen P, Dickey R, Blak T. Retrospective self-reports of childhood accidents causing unconsciousness in phallometrically diagnosed pedophiles. Archives of Sexual Behavior. 2002;31:511–26. doi: 10.1023/a:1020659331965. [DOI] [PubMed] [Google Scholar]
- Blum K. Narcotic antagonism of seizures induced by a dopamine-derived tetrahydroisoquinoline alkaloid. Experientia. 1988;44(9):751–53. doi: 10.1007/BF01959150. [DOI] [PubMed] [Google Scholar]
- Blum K, Noble EP. The sobering D2 story. Science. 1994;265(5177):1346–47. doi: 10.1126/science.7802784. [DOI] [PubMed] [Google Scholar]
- Blum K, Kozlowski GP. Ethanol and neuromodulator interactions: A cascade model of reward. In: Ollat H, Parves S;, Parvez H, editors. Progress in Alcohol Research, Vol. 2: Alcohol and Behavior. VSP; Utrecht, The Netherlands: 1990. [Google Scholar]
- Blum K, Briggs AH. Opioid peptides and genotype responses to ethanol. Biogenic Amines. 1988;5:527. [Google Scholar]
- Blum K, Chen TJ, Chen AL, Madigan M, Downs BW, Waite RL, Braverman ER, Kerner M, Bowirrat A, Giordano J, Henshaw H, Gold MS. Do dopaminergic gene polymorphisms affect mesolimbic reward activation of music listening response? Therapeutic impact on Reward Deficiency Syndrome (RDS). Medical Hypotheses. 2010a;74(3):513–20. doi: 10.1016/j.mehy.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Blum K, Chen TJ, Morse S, Giordano J, Chen AL, Thompson J, Allen C, Smolen A, Lubar L, Stice E, Downs W, Waite RL, Madigan MA, Kerner M, Fornari F, Braverman ER. Overcoming qEEG abnormalities and reward gene deficits during protracted abstinence in male psychostimulant and polydrug abusers utilizing putative dopamine D2 agonist therapy: Part 2. Postgrad Medicine. 2010b;122(6):214–26. doi: 10.3810/pgm.2010.11.2237. [DOI] [PubMed] [Google Scholar]
- Blum K, Chen ALC, Chen TJH, Bowirrat A, Downs BW, Waite RL, Reinking J, Kerner M, Braverman D, DiNubile N, Rhoads P, Braverman ER, Savarimuthu SM, Blum SH, Oscar-Berman M, Palomo T, Stice E, Gold M, Comings DE. Genes and happiness. Gene Therapy and Molecular Biology. 2009;13:92–129. [Google Scholar]
- Blum K, Cull JG, Braverman ER, Chen TJH, Comings DE. Reward Deficiency Syndrome: Neurobiological and genetic aspects. In: Blum K, Noble EP, editors. Handbook of Psychiatric Genetics. CRC Press; Boca Raton: 1996a. [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. Journal of the Royal Society of Medicine. 1996b;89(7):396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, Jagadeeswaran P, Nogami H, Briggs AH, Cohn JB. Allelic association of human dopamine D2 receptor gene in alcoholism. Journal of the American Medical Association. 1990;263(15):2055–60. [PubMed] [Google Scholar]
- Blum K, Briggs AH, Elston SF, DeLallo L, Sheridan PJ, Sar M. Reduced leucine-enkephalin-like immunoreactive substance in hamster basal ganglia after long-term ethanol exposure. Science. 1982;216(4553):1425–27. doi: 10.1126/science.7089531. [DOI] [PubMed] [Google Scholar]
- Blum K, Hamilton MG, Hirst M, Wallace JE. Putative role of isoquinoline alkaloids in alcoholism: A link to opiates. Alcoholism: Clinical and Experimental Research. 1978;2(2):113–20. doi: 10.1111/j.1530-0277.1978.tb04710.x. [DOI] [PubMed] [Google Scholar]
- Bostwick JM, Bucci JA. Internet sex addiction treated with naltrexone. Mayo Clinic Proceedings. 2008;83(2):226–30. doi: 10.4065/83.2.226. [DOI] [PubMed] [Google Scholar]
- Bradley KC, Boulware MB, Jiang H, Doerge RW, Meisel RL, Mermelstein PG. Changes in gene expression within the nucleus accumbens and striatum following sexual experience. Genes, Brain and Behavior. 2005;4(1):31–44. doi: 10.1111/j.1601-183X.2004.00093.x. [DOI] [PubMed] [Google Scholar]
- Bressler SC, Baum MJ. Sex comparison of neuronal Fos immunoreactivity in the rat vomeronasal projection circuit after chemosensory stimulation. Neuroscience. 1996;71(4):1063–72. doi: 10.1016/0306-4522(95)00493-9. [DOI] [PubMed] [Google Scholar]
- Brodie R. Virus of the Mind. Hay House, Inc.; New York: 1996. [Google Scholar]
- Burt SA, Mikolajewski AJ. Preliminary evidence that specific candidate genes are associated with adolescent-onset antisocial behavior. Aggressive Behavior. 2008;34(4):437–45. doi: 10.1002/ab.20251. [DOI] [PubMed] [Google Scholar]
- Caba M, Bao J, Pau KY, Spies HG. Molecular activation of noradrenergic neurons in the rabbit brainstem after coitus. Brain Research. Molecular Brain Research. 2000;77(2):222–231. doi: 10.1016/s0169-328x(00)00055-3. [DOI] [PubMed] [Google Scholar]
- Cantor JM, Kabani N, Christensen BK, Zipursky RB, Barbaree HE, Dickey R, Klassen PE, Mikulis DJ, Kuban ME, Blak T, Richards BA, Hanratty MK, Blanchard R. Cerebral white matter deficiencies in pedophilic men. Journal of Psychiatric Research. 2008;42:167–183. doi: 10.1016/j.jpsychires.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Chen JC, Chen PC, Chiang YC. Molecular mechanisms of psychostimulant addiction. Chang Gung Medical Journal. 2009;32(2):148–54. [PubMed] [Google Scholar]
- Chen TJ, Blum K, Chen AL, Bowirrat A, Downs WB, Madigan MA, et al. Neurogenetic and clinical evidence for the putative activation of the brain reward circuitry by a neuroadaptagen: Proposing an addiction candidate gene panel map. Journal of Psychoactive Drugs. 2011;43(2):108–27. doi: 10.1080/02791072.2011.587393. [DOI] [PubMed] [Google Scholar]
- Chen TJ, Blum K, Mathews D, Fisher L, Schnautz N, Braverman ER, et al. Preliminary association of both the dopamine D2 receptor (DRD2) [Taq1 A1 allele] and the dopamine transporter (DAT1) [480 bp allele] genes with pathological-aggressive behavior, a clinical subtype of Reward Deficiency Syndrome (RDS) in adolescents. Gene Therapy & Molecular Biology. 2007;1:93–112. [Google Scholar]
- Chen TJ, Blum K, Mathews D, Fisher L, Schnautz N, Braverman ER, Schoolfield J, Downs BW, Comings DE. Are dopaminergic genes involved in a predisposition to pathological aggression? Hypothesizing the importance of “super normal controls” in psychiatric genetic research of complex behavioral disorders. Medical Hypotheses. 2005;65(4):703–07. doi: 10.1016/j.mehy.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Cherkas LF, Oelsner EC, Mak YT, Valdes A, Spector TD. Genetic influences on female infidelity and number of sexual partners in humans: A linkage and association study of the role of the vasopressin receptor gene (AVPR1A). Twin Research. 2004;7:649–58. doi: 10.1375/1369052042663922. [DOI] [PubMed] [Google Scholar]
- Chia M, Chia M. Healing Love through the Tao: Cultivating Female Sexual Energy. Healing Tao Books; Huntington, NY: 1991. [Google Scholar]
- Cocores JA, Gold MS. The Salted Food Addiction Hypothesis may explain overeating and the obesity epidemic. Medical Hypotheses. 2009;73(6):892–99. doi: 10.1016/j.mehy.2009.06.049. [DOI] [PubMed] [Google Scholar]
- Cocores JA, Miller NS, Pottash AC, Gold MS. Sexual dysfunction in abusers of cocaine and alcohol. American Journal of Drug and Alcohol Abuse. 1988;14(2):169–73. doi: 10.3109/00952999809001544. [DOI] [PubMed] [Google Scholar]
- Cocores JA, Dackis CA, Gold MS. Sexual dysfunction secondary to cocaine abuse in two patients. Journal of Clinical Psychiatry. 1986;47(7):384–85. [PubMed] [Google Scholar]
- Comings DE. The Gene Bomb. Hope Press; Duarte, CA: 1996. [Google Scholar]
- Comings DE, Rosenthal RJ, Lesieur HR, Rugle LJ, Muhleman D, Chiu C, Dietz G, Gade R. A study of the dopamine D2 receptor gene in pathological gambling. Pharmacogenetics. 1996;6(3):223–34. doi: 10.1097/00008571-199606000-00004. [DOI] [PubMed] [Google Scholar]
- Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. 2009;53(1):1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Delmonico DL, Carnes PJ. Virtual sex addiction: When cybersex becomes the drug of choice. CyberPsychology & Behavior. 1999;2(5):457–63. doi: 10.1089/cpb.1999.2.457. [DOI] [PubMed] [Google Scholar]
- Delorme R, Betancur C, Chaste P, Kernéis S, Stopin A, Mouren MC, Collet C;, Bourgeron T;, Leboyer M, Launay J-M. Reduced 3-O-methyl-dopa levels in OCD patients and their unaffected parents is associated with the low activity M158 COMT allele. American Journal of Medical Genetics B. Neuropsychiatric Genetics. 2009;153B(2):542–48. doi: 10.1002/ajmg.b.31016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoortère R, Bardin L, Rodrigues M, Abrial E, Aliaga M, Newman-Tancredi A. Penile erection and yawning induced by dopamine D2-like receptor agonists in rats: Influence of strain and contribution of dopamine D2, but not D3 and D4 receptors. Behavioral Pharmacology. 2009;20(4):303–11. doi: 10.1097/FBP.0b013e32832ec5aa. [DOI] [PubMed] [Google Scholar]
- DiChiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences. 1988;85(14):5274–78. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn M, Trost J. Male multiple orgasms: A descriptive study. Archives of Sexual Behavior. 1989;18(50):385. doi: 10.1007/BF01541970. [DOI] [PubMed] [Google Scholar]
- Emanuele E, Brondino N, Pesenti S, Re S, Geroldi D. Genetic loading on human loving styles. Neuro Endocrinology Letters. 2007;28:815–21. [PubMed] [Google Scholar]
- Emmers-Sommer TM, Allen M, Bourhis J, Sahlstein E, Laskowski K, Falato WL, Ackerman J, Erian M, Barringer D, Weiner J, Corey J, Krieger J, Moramba G, Cashman L. A meta-analysis of the relationship between social skills and sexual offenders. Communication Reports. 2004;17:1–10. [Google Scholar]
- Eysenck HJ. Biological Basis of Personality. Transaction Publishers; Piscataway, NJ: 2006. [Google Scholar]
- Eysenck HJ. Dimensions of Personality. Transaction Publishers; Piscataway, NJ: 1997. [Google Scholar]
- Fagan PJ, Wise TN, Schmidt CW, Berlin FS. Pedophilia. Journal of the American Medical Association. 2002;288(19):2458–65. doi: 10.1001/jama.288.19.2458. [DOI] [PubMed] [Google Scholar]
- Fisher HE, Aron A, Brown LL. Romantic love: A mammalian brain system for mate choice. Philosophical Transactions of the Royal Society of London. B. Biological Science. 2006;361(1476):2173–86. doi: 10.1098/rstb.2006.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney GR, Lurie SF, Berlin FS. Is there familial transmission of pedophilia? Journal of Nervous and Mental Disease. 1984;172(9):546–48. doi: 10.1097/00005053-198409000-00006. [DOI] [PubMed] [Google Scholar]
- Geoghegan T. Are there women paedophiles? BBC NEWS Magazine; Apr 29, 2009. Available at: http://news.bbc.co.uk/2/hi/us_news/magazine/8022861.stm. [Google Scholar]
- Georgiadis JR, Farrell MJ, Boessen R, Denton DA, Gavrilescu M, Kortekaas R, Renken RJ, Hoogduin JM, Egan GF. Dynamic subcortical blood flow during male sexual activity with ecological validity: A perfusion fMRI study. Neuroimage. 2009;50(1):208–16. doi: 10.1016/j.neuroimage.2009.12.034. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Allard J. Dopamine and male sexual function. European Urology. 2001;40:601–08. doi: 10.1159/000049844. [DOI] [PubMed] [Google Scholar]
- Gold MS. Alcohol, drugs, and sexual dysfunction. Alcoholism & Addiction. 1988;9(2):13. [Google Scholar]
- Guo G, Tong Y. Age at first sexual intercourse, genes, and social context: Evidence from twins and the dopamine D4 receptor gene. Demography. 2006;43(4):747–69. doi: 10.1353/dem.2006.0029. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nature Neuroscience. 2004;7(4):411–16. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Han DH, Lee YS, Yang KC, Kim EY, Lyoo IK, Renshaw PF. Dopamine genes and reward dependence in adolescents with excessive Internet video game play. Journal of Addiction Medicine. 2007;1(3):133–38. doi: 10.1097/ADM.0b013e31811f465f. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): A gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2008;33(13):3037–45. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- Herrnstein RJ, Murray C. The Bell Curve. The Free Press; New York: 1994. [Google Scholar]
- Heshmati M. Cocaine-induced LTP in the ventral tegmental area: New insights into mechanism and time course illuminate the cellular substrates of addiction. Journal of Neurophysiology. 2009;101(6):2735–37. doi: 10.1152/jn.00127.2009. [DOI] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proceedings of the National Academy of Sciences. 2002;99(12):8400–05. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Institute for Trauma and Addiction Professionals (IITAP) FAQs About Sexual Addiction. 2011 Available at http://www.sexhelp.com/sex-education/what-is-sex-addiction-faqs.
- Jorandby L, Frost-Pineda K, Gold MS. Addiction to food and brain reward systems. Sexual Addiction and Compulsivity. 2005;12(2–3):201–17. [Google Scholar]
- Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-D-aspartate receptor activation in the nucleus accumbens core. Proceedings of the National Academy of Sciences. 1997;94(22):12174–79. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltikangas-Järvinen L, Pulkki-Råback L, Elovainio M, Raitakari OT, Viikari J, Lehtimäki T. DRD2 C32806T modifies the effect of child-rearing environment on adulthood novelty seeking. American Journal of Medical Genetics B. Neuropsychiatric Genetics. 2009;150B(3):389–94. doi: 10.1002/ajmg.b.30830. [DOI] [PubMed] [Google Scholar]
- Kim EY, Lee YS, Han DH, Suh DS, Kee BS. Temperament and genetic polymorphism in Korean male adolescents with Internet addiction tendency. Journal of the Korean Neuropsychiatric Association. 2006;45(5):468–75. [Google Scholar]
- Kirn W. Curing Casanova: A pharmacological infidelity inhibitor just may be on its way. Should we welcome it? New York Times Magazine. 2004;18(13):14. [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Merz CJ, Tabbert K, Walter B, Kagerer S, Vaitl D, Stark R. Neural activations of the acquisition of conditioned sexual arousal: Effects of contingency awareness and sex. Journal of Sex Medicine. 2009;6(11):3071–85. doi: 10.1111/j.1743-6109.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Brooks DJ, Bench CJ, Grasby PM. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–68. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Kohlert JG, Meisel RL. Sexual experience sensitizes mating-related nucleus accumbens dopamine responses of female Syrian hamsters. Behavioural Brain Research. 1999;99(1):45–52. doi: 10.1016/s0166-4328(98)00068-0. [DOI] [PubMed] [Google Scholar]
- Krüger TH, Hartmann U, Schedlowski M. Prolactinergic and dopaminergic mechanisms underlying sexual arousal and orgasm in humans. World Journal of Urology. 2005;23(2):130–38. doi: 10.1007/s00345-004-0496-7. [DOI] [PubMed] [Google Scholar]
- Lan KC, Chang AC, Liu SH, Ho IK, Lin-Shiau SY. Enhancing effects of morphine on methamphetamine-induced reinforcing behavior and its association with dopamine release and metabolism in mice. Journal of Neurochemistry. 2009;109(2):382–92. doi: 10.1111/j.1471-4159.2009.05998.x. [DOI] [PubMed] [Google Scholar]
- Laws DR, O'Donohue WT. Sexual Deviance: Theory, Assessment, and Treatment. Guilford Press; New York: 2008. [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd. Oxytocin: The great facilitator of life. Progress in Neurobiology. 2009;88:127–51. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashek D, Aron A, Fisher H. Identifying, evoking, and measuring intense feelings of romantic love. Representative Research in Social Psychology. 2000;24:48–55. [Google Scholar]
- McCarthy G, Taylor A. Avoidant/ambivalent attachment style as a mediator between abusive childhood experiences and adult relationship difficulties. Journal of Child Psychology and Psychiatry. 1999;40(3):465–77. [PubMed] [Google Scholar]
- Melman A, Davies K. Gene therapy for erectile dysfunction: What is the future? Current Urology Reports. 2010;11(6):421–26. doi: 10.1007/s11934-010-0145-1. [DOI] [PubMed] [Google Scholar]
- Menon V, Levitin DJ. The rewards of music listening: Response and physiological connectivity of the mesolimbic system. Neuroimage. 2005;28(1):175–84. doi: 10.1016/j.neuroimage.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Michels L, Mehnert U, Boy S, Schurch B, Kollias S. The somatosensory representation of the human clitoris: An fMRI study. Neuroimage. 2010;49(1):177–84. doi: 10.1016/j.neuroimage.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Miller WB, Pasta DJ, MacMurray J, Chiu C, Wu H, Comings DE. Dopamine receptor genes are associated with age at first sexual intercourse. Journal of Biosocial Science. 1999;31(1):43–54. doi: 10.1017/s0021932099000437. [DOI] [PubMed] [Google Scholar]
- Miner MH, Raymond N, Mueller BA, Lloyd M, Lim KO. Preliminary investigation of the impulsive and neuroanatomical characteristics of compulsive sexual behavior. Psychiatry Research. 2009;174(2):146–51. doi: 10.1016/j.pscychresns.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Laboy MA, Castellanos DH, Haliburton CS, del Aguila EV, Weinstein HJ, Parker RG. Condom use and hip hop culture: The case of urban young men in New York City. American Journal of Public Health. 2008;98(6):1081–85. doi: 10.2105/AJPH.2007.119198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EP, Blum K, Khalsa ME, Ritchie T, Montgomery A, Wood RC, Fitch RJ, Ozkaragoz T, Sheridan PJ, Anglin MD, Paredes A, Treiman LJ, Sparkes RS. Allelic association of the D2 dopamine receptor gene with cocaine dependence. Drug and Alcohol Dependence. 1993;33(3):271–85. doi: 10.1016/0376-8716(93)90113-5. [DOI] [PubMed] [Google Scholar]
- Olson S. Mapping Human History. Mariner Books; Boston: 2002. [Google Scholar]
- Parker RG. Bodies and pleasures: On the construction of erotic meanings in contemporary Brazil. Anthropology & Humanism Quarterly. 1989;14(2):58–64. [Google Scholar]
- Pfaus JG. Pathways of sexual desire. Journal of Sex Medicine. 2009;6(6):1506–33. doi: 10.1111/j.1743-6109.2009.01309.x. [DOI] [PubMed] [Google Scholar]
- Pitchers KK, Frohmader KS, Vialou V, Mouzon E, Nestler EJ, Lehman MN, Coolen LM. DFosB in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes Brain and Behavior. 2010;9(7):831–40. doi: 10.1111/j.1601-183X.2010.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponseti J, Bosinski HA, Wolff S, Peller M, Jansen O, Mehdorn HM, Büchel C, Siebner HR. A functional endophenotype for sexual orientation in humans. Neuroimage. 2006;33(3):825–833. doi: 10.1016/j.neuroimage.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Quiñones-Jenab V. Why are women from Venus and men from Mars when they abuse cocaine? Brain Research. 2006;1126(1):200–03. doi: 10.1016/j.brainres.2006.08.109. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134(3):737–44. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Rao BS, Raju TR, Meti BL. Increased numerical density of synapses in CA3 region of hippocampus and molecular layer of motor cortex after self-stimulation rewarding experience. Neuroscience. 1999;91(3):799–803. doi: 10.1016/s0306-4522(99)00083-4. [DOI] [PubMed] [Google Scholar]
- Rastegar DA, Fingerhood MI. Addiction Medicine: An Evidence-Based Handbook. Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- Reynaud M, Karila L, Blecha L, Benyamina A. Is love passion an addictive disorder? American Journal of Drug and Alcohol Abuse. 2010;36(5):261–67. doi: 10.3109/00952990.2010.495183. [DOI] [PubMed] [Google Scholar]
- Ritchie T, Noble EP. [3H]naloxone binding in the human brain: Alcoholism and the TaqI A D2 dopamine receptor polymorphism. Brain Research. 1996;718(1–2):193–97. doi: 10.1016/0006-8993(96)00068-6. [DOI] [PubMed] [Google Scholar]
- Roxburgh A, Degenhardt L, Copeland J, Larance B. Drug dependence and associated risks among female street-based sex workers in the greater Sydney area, Australia. Substance Use and Misuse. 2008;43(8–9):1202–17. doi: 10.1080/10826080801914410. [DOI] [PubMed] [Google Scholar]
- Rupp HA, Wallen K. Sex differences in response to visual sexual stimuli: A review. Archives of Sexual Behavior. 2008;37(2):206–18. doi: 10.1007/s10508-007-9217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safron A, Barch B, Bailey JM, Gitelman DR, Parrish TB, Reber PJ. Neural correlates of sexual arousal in homosexual and heterosexual men. Behavioral Neuroscience. 2007;121(2):237–48. doi: 10.1037/0735-7044.121.2.237. [DOI] [PubMed] [Google Scholar]
- Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nature Neuroscience. 2011;14:257–62. doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- Santtila P, Jern P, Westberg L, Walum H, Pedersen CT, Eriksson E, Sandnabba NK. The dopamine transporter gene (DAT1) polymorphism is associated with premature ejaculation. Journal of Sex Medicine. 2010;7(4 Pt 1):1538–46. doi: 10.1111/j.1743-6109.2009.01696.x. [DOI] [PubMed] [Google Scholar]
- Schiffer B, Paul T, Gizewski E, Forsting M, Leygraf N, Schedlowski M, Krüger THG. Functional brain correlates of heterosexual paedophilia. Neuroimage. 2008;41(1):80–91. doi: 10.1016/j.neuroimage.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Schiltz K, Witzel J, Northoff G, Zierhut K, Gubka U, Fellman H, Kaufmann J, Tempelmann C, Wiebking C, Bogerts B. Brain pathology in pedophilic offenders: Evidence of volume reduction in the right amygdala and related diencephalic structures. Archives of General Psychiatry. 2007;64:737–46. doi: 10.1001/archpsyc.64.6.737. [DOI] [PubMed] [Google Scholar]
- Serguera C, Triaca V, Kelly-Barrett J, Banchaabouchi MA, Minichiello L. Increased dopamine after mating impairs olfaction and prevents odor interference with pregnancy. Nature Neuroscience. 2008;11(8):949–56. doi: 10.1038/nn.2154. [DOI] [PubMed] [Google Scholar]
- Smillie LD, Cooper AJ, Proitsi P, Powell JF, Pickering AD. Variation in DRD2 dopamine gene predicts extraverted personality. Neuroscience Letters. 2010;468(3):234–37. doi: 10.1016/j.neulet.2009.10.095. [DOI] [PubMed] [Google Scholar]
- Succu S, Sanna F, Melis T, Boi A, Argiolas A, Melis MR. Stimulation of dopamine receptors in the paraventricular nucleus of the hypothalamus of male rats induces penile erection and increases extra-cellular dopamine in the nucleus accumbens: Involvement of central oxytocin. Neuropharmacology. 2007;52:1034–43. doi: 10.1016/j.neuropharm.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Swartout KM, White JW. The relationship between drug use and sexual aggression in men across time. Journal of Interpersonal Violence. 2010;25(9):1716–35. doi: 10.1177/0886260509354586. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Zhou QY, Palmiter RD. Dopamine-stimulated sexual behavior is testosterone dependent in mice. Behavioral Neuroscience. 1998;112(5):1229–35. doi: 10.1037//0735-7044.112.5.1229. [DOI] [PubMed] [Google Scholar]
- Ukkola LT, Onkamo P, Raijas P, Karma K, Järvelä I. Musical aptitude is associated with AVPR1A-haplotypes. PLoS One. 2009;4(5):e5534. doi: 10.1371/journal.pone.0005534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaske J, Wright JP, Beaver KM. A dopamine gene (DRD2) distinguishes between offenders who have and have not been violently victimized. International Journal of Offender Therapy and Comparative Criminology. 2010;55(2):251–67. doi: 10.1177/0306624X10361583. [DOI] [PubMed] [Google Scholar]
- Vijayasenan ME. Alcohol and sex. New Zealand Medical Journal. 1981;93(675):18–20. [PubMed] [Google Scholar]
- Walsh A. The Science of Love—Understanding Love and its Effects on Mind and Body. Prometheus Books; Amherst: 1991. [Google Scholar]
- Walter M, Witzel J, Wiebking C, Gubka U, Rotte M, Schiltz K, Bermpohl C, Tempelmann C, Bogerts B, Heinze HJ, Northoff G. Pedophilia is linked to reduced activation in hypothalamus and lateral prefrontal cortex during visual erotic stimulation. Biological Psychiatry. 2007;62(6):698–701. doi: 10.1016/j.biopsych.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Wang Z, Aragona BJ. Neurochemical regulation of pair bonding in male prairie voles. Physiology & Behavior. 2004;83:319–28. doi: 10.1016/j.physbeh.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Xu X, Aron A, Brown L, Cao G, Feng T, Weng X. Reward and motivation systems: A brain mapping study of early-stage intense romantic love in Chinese participants. Human Brain Mapping. 2011;32(2):249–57. doi: 10.1002/hbm.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BZ, Kranzler HR, Zhao H, Gruen JR, Luo X, Gelernter J. Haplotypic variants in DRD2, ANKK1, TTC12, and NCAM1 are associated with comorbid alcohol and drug dependence. Alcoholism: Clinical and Experimental Research. 2008;32(12):2117–27. doi: 10.1111/j.1530-0277.2008.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Pau F, Hess SL, Spies HG. Sexual dimorphism in secretion of hypothalamic gonadotropin-releasing hormone and norepinephrine after coitus in rabbits. Endocrinology. 1996;137(7):2683–93. doi: 10.1210/endo.137.7.8770887. [DOI] [PubMed] [Google Scholar]
- Young LJ. Being human: love: Neuroscience reveals all. Nature. 2009;457(7226):148. doi: 10.1038/457148a. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang X, Parkinson C, Cai C, Gao S, Hu P. Brain activation evoked by erotic films varies with different menstrual phases: An fMRI study. Behavioral Brain Research. 2010;206(2):279–85. doi: 10.1016/j.bbr.2009.09.027. [DOI] [PubMed] [Google Scholar]