Abstract
Alcoholism results from an interplay between genetic and environmental factors, and is linked to brain defects and associated cognitive, emotional, and behavioral impairments. A confluence of findings from neuroimaging, physiological, neuropathological, and neuropsychological studies of alcoholics indicate that the frontal lobes, limbic system, and cerebellum are particularly vulnerable to damage and dysfunction. An integrative approach employing a variety of neuroscientific technologies is essential for recognizing the interconnectivity of the different functional systems affected by alcoholism. In that way, relevant experimental techniques can be applied to assist in determining the degree to which abstinence and treatment contribute to the reversal of atrophy and dysfunction.
Keywords: Alcoholism, Frontal lobes, Limbic system, Cerebellum, Right hemisphere
Alcoholic beverages contain ethanol, a psychoactive drug with relaxant and euphoric effects, consumed by people throughout the world. In general, the effects of alcohol intoxication follow a biphasic time course as the initial feelings of relaxation and exuberance give way to hangover, exhaustion, and depression, or vomiting and loss of consciousness in cases of higher doses (Nagoshi and Wilson 1989). Criteria for classifying someone as an alcoholic vary (Abel et al. 1999; Eckardt et al. 1998), but it is thought that excessive alcohol use and alcoholism exist along a continuum of alcohol-disorders associated with increased frequency of a harmful drinking pattern (Helzer et al. 2006).
Risky drinking patterns for men are defined as consuming more than 14 drinks per week, or more than four drinks in a single day at least once a month; for women, the limits are more than seven drinks per week and three drinks per day (Dawson et al. 2005). Individuals who abuse alcohol or are alcohol-dependent are considered to have alcohol use disorder (Grant et al. 2004). Alcohol abuse, as described by the American Psychiatric Association (APA 1994), is a psychiatric condition whereby alcoholic beverages are consumed despite negative consequences for health, well being, and interpersonal relationships. Alcohol dependence has additional physiological consequences such as increased tolerance for alcohol consumed, and withdrawal symptoms upon cessation of drinking. Studies assessing alcoholism-related neurobehavioral decline commonly require their participants to have had a history of at least 5 years of drinking 21 or more drinks per week (Eckardt et al. 1998; Oscar-Berman et al. 2004). However, problem-based criteria, as well as several endophenotypes (e.g., metabolic factors, and neuronal or behavioral disinhibition), should be considered when identifying alcohol use disorders, not just quantity and frequency of consumption (Lancaster 1995; NIAAA 1997; Nolen-Hoeksema and Hilt 2006; Wuethrich 2001). Alcohol abuse and alcohol dependence are responsible for failure in everyday life roles and high costs to society for disability and health expenditures (APA 1994; NIAAA 1997).
Alcoholism has devastating consequences, but not all alcoholics are equally at risk for brain changes and neurobehavioral deficits. Nearly half of the estimated 18 million people in the USA who are problem drinkers (NIAAA 1997) appear to be free of cognitive, sensory, or motor impairments. By contrast, upwards of 2 million alcoholics develop permanent and debilitating conditions that require lifetime custodial care (Oscar-Berman and Evert 1997; Rourke and Løberg 1996). However, most problem drinkers have mild neuropsychological difficulties, which improve within a year of abstinence (Bartsch et al. 2007; Ende et al. 2005; Fein et al. 2006b).
Even though structural and functional brain damage is partially reversible after several weeks of abstinence (Crews et al. 2005; Nixon 2006; Rosenbloom et al. 2003), the underlying mechanisms are poorly understood. It is clear, however, that the locus and extent of brain damage, as well as the type and degree of impairment, differ across individuals. Such differences suggest that certain factors increase the likelihood of developing cognitive, sensory, or motor impairments with alcohol misuse. Among the important factors that must be considered are demographic variables (e.g., age, gender, socioeconomic background, and education), genetics and family history of alcoholism, alcohol use patterns (e.g., the age of onset of alcohol consumption, the type and amount of alcohol consumed, severity and duration of the dependency, duration of abstinence, nutritional status during periods of consumption), and the use or abuse of other psychoactive substances and nicotine (Gazdzinski et al. 2005; Oscar-Berman and Marinkovic 2003). Additionally, overall physical and mental health are important factors, because comorbid medical, neurological, and psychiatric conditions not only can interact to aggravate alcoholism’s effects on the brain and behavior, but they also can contribute to further drinking (Petrakis et al. 2002).
Factors that Contribute to Alcoholism and its Sequelae: Age, Gender, Health, and Family History
Alcoholism’s effects on the brain and behavior are diverse, and are moderated or mediated by many factors (Oscar-Berman and Bowirrat 2005; Parsons 1996). The most commonly studied variables are age, gender, health, and family history.
Age
Normal chronological aging is associated with a number of physiological changes suggesting increased sensitivity to alcohol. For example, with declining body water content, older people who drink alcohol tend to have increased blood alcohol concentration compared to younger people (Dufour and Fuller 1995), and aging interferes with the body’s ability to metabolize alcohol (Kalant et al. 1998). Neuroanatomical changes seen in aging are similar to those associated with chronic alcoholism (Courville 1966; Harper 1998; Pfefferbaum et al. 2005; Wilkinson and Carlen 1982). In both, cerebral atrophy is most prominent in the frontal lobes. Other effects include greater than normal ventricular enlargement and widening of the cerebral sulci of alcoholics in relation to increasing age (Pfefferbaum et al. 1997; Sullivan 2000). Given the observed morphological similarities in the brains of alcoholic and aging nonalcoholic individuals, researchers sought to characterize parallels in functional decline associated with alcoholism and aging (Gansler et al. 2000), and some investigators proposed that alcoholism is associated with premature aging.
The premature aging hypothesis
has been put forth in two versions (reviewed by Ellis and Oscar-Berman 1989; Oscar-Berman and Schendan 2000). It was initially formulated as the “accelerated aging” or “cumulative effects” model, purporting that alcoholism is accompanied by the precocious onset of neuroanatomical and behavioral changes typically associated with advancing age. According to this model, alcoholics at all ages are impaired compared to age-matched controls, becoming cognitively old before their time. The second version placed the timing of the changes somewhat differently. The “increased vulnerability” interpretation suggests that an aging brain is more vulnerable to the influences of toxic substances, including ethanol, than is the brain of a younger person. This version proposes that only older alcoholics (over age 50) are impaired compared with age-matched controls; younger alcoholics remain cognitively intact.
Taken together, most of the evidence from neuropathological and neuroimaging investigations supports the increased vulnerability model of premature aging (Oscar-Berman and Marinkovic 2003). That is, certain brain structures show greater reduction in size (or blood flow) in older alcoholics than in younger alcoholics: the cerebral cortex (Di Sclafani et al. 1995; Harris et al. 1999; Pfefferbaum et al. 1997), the corpus callosum (Pfefferbaum et al. 1996, 2006; Schulte et al. 2005), the hippocampus (Laakso et al. 2000; Sullivan et al. 1995), and the cerebellum (Harris et al. 1999; Sullivan et al. 2000).
At the microstructural level, diffusion tensor imaging (DTI) measures of neuronal fibers in the corpus callosum have provided supporting evidence for a detrimental interaction between recent alcoholism history and age. That is, Pfefferbaum et al. (2006) reported significant negative relationships with age, not only in the size of the genu and splenium of the corpus callosum of older alcoholics, but also in the microstructure of the callosal fibers (i.e., functional anisotropy, a measure of orientational coherence of neuronal fibers, and bulk mean diffusivity, an index of the amount of water motility). The neuroanatomical indices also correlated with neuropsychological and psychomotor deficits in older alcoholics: Modest abnormalities in the genu were associated with diminished working memory scores, and abnormalities of the splenium were associated with diminished visuospatial ability. Measures of gait and balance showed nonspecific relationships with global measures of callosal size and neuronal structure. Although the only region displaying age–alcohol interactions in functional anisotropy was the callosal body, bulk mean diffusivity was affected in more callosal regions by the interaction of age and alcoholism.
Results of neurobehavioral investigations tend to support the view that aging increases one’s vulnerability to alcoholism-related decline (Oscar-Berman and Marinkovic 2003). Significant correlations have been reported between age and regional MRI and DTI measures and performance on working memory, visuospatial ability, and gait and balance (Pfefferbaum et al. 2006), as well as in interhemispheric processing speed (Schulte et al. 2005). The latter findings provide evidence for the functional ramifications of an interaction of age and alcoholism in exerting compounded abnormalities in the functioning of the corpus callosum. Pfefferbaum and Sullivan (2005) suggested that decreased orientational coherence of brain white matter in alcoholism is attributable, at least in part, to the accumulation of intracellular and extracellular fluid in excess of that occurring in aging, and that the differential influence of these fluid compartments can vary across brain regions.
Gender
In the past decade, there has been an increasing interest in alcoholism-related gender differences with respect to possible changes in brain and behavior (Lancaster 1995; NIAAA 1997; Nolen-Hoeksema and Hilt 2006; Wuethrich 2001). However, the degree to which men and women differ with respect to these changes remains controversial. For example, in a recent cross-sectional, population-based study in which gender differences in cognitive performance were explored in relation to alcohol consumption (Yonker et al. 2005), drinking data were collected from men and women between 35 and 85 years of age, and the participants were classified into non, light, moderate, and heavy drinking subgroups. There were clear gender differences in episodic memory (favoring women) and visuospatial tasks (favoring men). When these gender differences were examined by drinking group, visuospatial performance favoring men disappeared for the moderate to heavy drinking groups, but higher performance by women on episodic memory tasks was consistent across all levels of alcohol consumption. The results suggested that moderate alcohol intake may be less detrimental to women than to men.
In other studies, Sullivan and her colleagues administered an extensive battery of neuropsychological tests to recently detoxified alcoholic men (Sullivan et al. 2000) and women (Sullivan et al. 2002) compared with nonalcoholic control men and women. In alcoholic men and women alike, there were deficits in visuospatial abilities and balance. The alcoholic men, but not the women, had deficits in executive functions and gait, and the alcoholic women, but not the men, had additional impairments in short-term memory and fluency. In both gender groups, there was relative preservation of declarative memory and upper limb mobility. Parsons (1994) reported that although alcoholic men and women showed impaired performance on neuropsychological tests relative to same-sex nonalcoholic control participants, only the alcoholic men differed from their controls on a measure of visually evoked event-related brain potentials. Other investigators found that alcoholic men and women displayed similar electrophysiological abnormalities (Hill and Steinhauer 1993).
Neuroimaging studies that measured gender differences in alcoholics’ brain functioning have yielded contradictory evidence, with some studies showing women to be more susceptible than men to brain impairments, and other studies showing no such distinction. Using functional magnetic resonance imaging (fMRI), Tapert et al. (2001) found decreased activity in parietal and frontal cortex, particularly in the right hemisphere, in alcohol-dependent women during performance of a spatial working memory task. Other studies, however, did not find functional differences based on gender (Wang et al. 1998), or even found that alcohol intoxication decreased brain metabolism in men more than in women as measured with positron emission tomography (PET; Wang et al. 2003). Using structural MRI, Kroft et al. (1991) found that the average ventricular volume in alcoholic women was within the typical range found in MRI studies of nonalcoholic women of similar ages. Another MRI study reported that although age and alcoholism interacted adversely in both sexes, alcoholic men, but not alcoholic women, had abnormal cortical white matter and sulcal volumes compared to same sex healthy comparison groups (Pfefferbaum et al. 2001b). In contrast, Hommer et al. (2001) reported clear gender differences in the brain structure of alcoholics. In that study, alcoholic men and women had smaller volumes of gray and white matter, as well as greater volumes of sulcal and ventricular CSF, than nonalcoholic men and women, but these differences were largest for the women. Comparisons between alcoholic men and women on the proportion of intracranial contents occupied by gray matter indicated smaller size in alcoholic women than in alcoholic men. Using computerized tomography (CT) scans to measure brain atrophy, another group found evidence of a similar degree of brain shrinkage in men and women, despite shorter drinking histories in the women (Mann et al. 1992). MRI-based volumetric measures of the corpus callosum (Hommer et al. 1996) indicated that alcoholic women had smaller callosal areas than alcoholic men and nonalcoholic controls; alcoholic men did not differ from nonalcoholic control men. Abnormalities in the structure of the corpus callosum can occur as a consequence of diffuse cortical damage and subsequent degeneration of cortical axons. Furthermore, the size of the corpus callosum is notably reduced with age in alcoholic men (Pfefferbaum et al. 1996).
Pfefferbaum et al. (2002) measured white-matter brain macrostructure in women alcoholics to determine whether observed abnormalities interact with age. Although the alcoholic women did not differ from controls in any brain measures, greater length of sobriety was associated with more cortical white matter. Based on the results of a similar study employing DTI (a technique highly sensitive to microstructure damage) on separate subject groups, Pfefferbaum and Sullivan (2002) suggested that alcohol use by women causes white matter microstructural disruption that is not detectable with gross measures of white matter mass, and may antedate its appearance.
Clearly, many questions remain concerning the nature and extent of gender differences in the effects of alcoholism on brain and behavior. These potential differences deserve close scrutiny in the context of other variables such as age, drinking history, perceived social sanctions for drinking, impulsivity, genetic risk factors, etc. (Nolen-Hoeksema and Hilt 2006).
Health
Medical conditions concomitant with alcoholism that are most likely to influence neurobiological and neurobehavioral functioning include liver disease, cardio-vascular disease, and malnutrition. Thus, poor liver function (Stranges et al. 2004a) and hypertension (Stranges et al. 2004b) have been associated with drinking outside of meals, and certain arrhythmias have been associated with binge drinking (Klatsky 2007). Thiamine (vitamin B1) deficiency, a consequence of poor diet, can contribute to Alcohol-Induced Persisting Amnestic Disorder (Korsakoff’s syndrome), a severe disorder characterized by permanent cognitive and emotional deficits (Oscar-Berman and Evert 1997; Oscar-Berman et al. 2004). Other common neurological conditions in alcoholics are head injury, encephalopathy, and fetal alcohol syndrome (or fetal alcohol effects), all of which can have an impact on neurobehavioral outcome.
Regarding the influence of mental health factors, a comprehensive 12-month epidemiological study (Kessler et al. 2005) showed a prevalence of 44% comorbidity of alcohol abuse and dependence with externalizing disorders (e.g., conduct disorder). Other significant correlations with alcoholism were phobias, generalized anxiety disorders, and posttraumatic stress disorder. Frequently occurring comorbid psychiatric conditions also include depression, schizophrenia, and the use of other drugs, including nicotine (Durazzo et al. 2006; Petrakis et al. 2002). Interestingly, a twin study examining risk factors for common substance abuse and associated psychiatric disorders suggested that the underlying structure of genetic risk factors is similar for men and women, and genetic loadings for alcohol dependence are disorder specific (Kendler et al. 2003). However, the presence of a lifetime psychiatric diagnosis in mood, anxiety, and externalizing disorder domains in abstinent long-term alcoholics does not militate against achieving sustained abstinence, and abstinence can be maintained in the presence of a current mood or anxiety disorder (Di Sclafani et al. 2007).
These various findings emphasize that consideration must be given to the potential influence of a host of psychiatric and medical conditions on neurocognitive functioning in studies of alcoholism as the primary condition. However, it is difficult to quantify the significance of comorbid conditions, whether they are secondary or tertiary illnesses, into a single, predictive variable that measures neurobehavioral or neurobiological outcomes in alcoholism. Instead, comorbid conditions deserve independent consideration, in addition to examining multivariate effects and interactions. Furthermore, as an adjunct to using symptomatology that meets criteria for a specific psychiatric diagnosis, the use of continuous measures of psychological abnormalities can yield a more accurate picture of psychiatric illness co-occurring with alcoholism (Fein et al. 2007).
Family history
Results of twin, family, and adoption studies have shown that hereditary factors influence vulnerability to alcoholism (Begleiter and Porjesz 1999; Dick and Foroud 2003; Schuckit et al. 2004; Whitfield et al. 2004). Additionally, the pharmacogenomics of alcohol response is well established, and genetic variants for the principal enzymes of alcohol metabolism are thought to influence drinking behavior and protect against alcoholism (Dickson et al. 2006; Enoch 2003). Convergent evidence supports the view that vulnerability to alcoholism is likely to be due to multiple interacting genetic loci of small to modest effects (Johnson et al. 2006).
In an attempt to clarify the nature of genetic factors in relation to alcoholism, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) sponsored a multi-institutional program: Collaborative Studies on Genetics of Alcoholism (COGA) in 1989. Since then, COGA investigators have successfully recruited thousands of individuals from hundreds of extended families densely affected by alcoholism (Begleiter and Porjesz 1999; Bierut et al. 2002). The investigators have collected detailed and extensive clinical, neuropsychological, electrophysiological, biochemical, and genetic data. Evidence from these studies has led to the identification of chromosomal regions containing genes that influence alcoholism risk and related phenotypes (Edenberg and Foroud 2006). Subsequently, single nucleotide polymorphisms (SNPs) have been geno-typed in positional candidate genes located within the linked chromosomal regions, and analyzed for association with these phenotypes. Using this sequential approach, COGA investigators have reliably detected and identified associations with specific genes contributing to the risk for alcoholism (Edenberg and Foroud 2006).
Other studies have revealed an association between certain dopaminergic gene polymorphisms and a number of reward dependent behaviors including addictive, compulsive, and impulsive tendencies (Blum et al. 2000). Alcohol and other drugs of abuse cause activation and neuronal release of brain dopamine, which can decrease negative feelings and satisfy abnormal cravings (Bowirrat and Oscar-Berman 2005). A deficiency or absence of the D2 receptors then predisposes individuals to a high risk for multiple maladaptive behaviors (Koob 2003). Other neurotransmitters, e.g. glutamate, gamma-aminobutyric acid (GABA; Dick et al. 2004), serotonin (Goldman et al. 1992), and enkephalins (Comings et al. 1999) also may be important in determining the rewarding and stimulating effects of ethanol, but dopamine may be critical for initiating drug use and for reinstating drug use during protracted abstinence (Bowirrat and Oscar-Berman 2005; Connor et al. 2002).
As noted by Dick and Foroud (2003), sequencing of the human genome has facilitated the development of a catalog of human genes. Based on the findings from this catalog, researchers can identify candidate genes to determine the degree to which they are associated with alcoholism. Once replicable associations are established, the next step will be to identify the causative genetic variants responsible for the role of those genes in alcohol dependence. It also will be important to understand how the relevant genes influence patterns of alcohol use and metabolism, as well as the manner in which the genes may contribute to comorbidity of alcoholism with other psychiatric disorders. Genotype and environmental risk factors act and interact through complex pathways to influence alcohol dependence. Findings from COGA studies have exemplified the significance of careful screening and selection of the research participants when considering the influence of factors such as family history, age, gender, and health variables.
Acute Effects of Ethanol Ingestion
Studies using acute alcohol challenges contribute toward understanding dose-and task-related parameters of ethanol’s effects on the brain. Furthermore, studies of acute alcohol challenge are valuable in disclosing the types of functions and the neural circuits that underlie impairments due to alcohol intoxication. In concert with studies on chronic alcoholics and populations at risk, studies using acute alcohol challenge are important since they may help to parse out the effects of alcohol neurotoxicity, genetic susceptibility, and environmental factors. The importance of such evidence derives from its direct applicability to driving situations, work-related hazards, and other societally-relevant concerns.
Alcohol’s effects on the brain and behavior depend upon an individual’s blood alcohol concentration (BAC). Low doses can have a stimulating effect, and higher levels can have depressant effects. In addition, effects can differ depending on the time lapsed since ingestion; the same BAC may result in different effects on the ascending versus descending limbs of the BAC curve (Pohorecky and Brick 1977). Individuals also differ in their tolerance to acute intoxication. Even when people are subjected to the same environmental conditions, their responses to a given dose of alcohol vary significantly on metabolic, physiological, subjective, cognitive, motor, and other measures (Reed 1985). The pharmacokinetics (time course of absorption, distribution, metabolism, and excretion of ethanol) varies significantly when alcohol is administered orally, but much less so when alcohol is given intravenously (Grant et al. 2000).
Impairments in mental functions such as attention and vigilance can be detected at BAC levels much lower than the legal intoxication levels, such as 0.02–0.03% (Koelega 1995). Alcohol intoxication disrupts neurophysiological indices of stimulus processing in attentional (Grillon et al. 1995; Jääskeläinen et al. 1999; Marinkovic et al. 2001; Porjesz and Begleiter 1985), semantic, (Marinkovic et al. 2001, 2004), and psychomotor domains (Ridderinkhof et al. 2002). Furthermore, consistent with the evidence obtained from chronic alcoholics, acute intoxication results in a disproportionate impairment of executive functions such as planning, working memory, or complex behavioral control (Peterson et al. 1990).
Intoxication and behavioral control
It is a common belief that alcohol ingestion leads to aggression and reduced impulse control, and there is high association of alcohol intoxication with violent crimes (Murdoch et al. 1990). Results of laboratory research have shown that alcohol intoxication increases the likelihood of aggressive behaviors (Bushman and Cooper 1990; Hoaken and Stewart 2003). However, there is a dearth of careful studies of the complex interactions between alcohol intoxication and the multifaceted construct of aggression. This is due, in part, to ambiguities in the terminology. For instance, a behavior labeled as “aggressive” could include combinations of impulsivity, disinhibition, social or sexual inappropriateness, impairments in decision-making and executive functions, or some other feature. Itself a complex construct, impulsivity has been commonly operationalized with tasks measuring inhibitory control of responses, i.e., go/no-go tasks, which require subjects to refrain from responding on some trials. Some evidence suggests that alcohol may have disinhibitory effects on behavior. Rather low alcohol doses (peak BAC of ~0.04%) decrease the latency of arousal to sexually explicit stimuli (Wilson and Niaura 1984). Alcohol-induced disinhibition is also reflected in premature motor preparation based on incomplete stimulus evaluation (Marinkovic et al. 2000). The disinhibitory effects could result from the psychomotor stimulant properties of alcohol (Wise 1988), or may reflect a disruption in the inhibitory control of behavior subserved by prefrontal regions (Peterson et al. 1990). Indeed, alcohol decreases inhibitory control under the conditions of stop-signal imperative stimuli (Mulvihill et al. 1997) and a demanding vigilance task (Dougherty et al. 1999), as demonstrated by moderately intoxicated subjects who are impaired in withholding responses to inappropriate stimuli.
In a series of studies using a cued go/no-go task, Fillmore and colleagues have found a dose-related increase in commission errors and slower response times to the no-go signals that were falsely preceded by a “go” cue (Fillmore and Weafer 2004; Marczinski et al. 2005; Marczinski and Fillmore 2003). Similarly, alcohol decreases inhibitory control on the stop-signal task (de Wit et al. 1990; Mulvihill et al. 1997) and on a continuous performance task (Dougherty et al. 2000). Alcohol-induced disinhibition is reflected in premature motor preparation based on incomplete stimulus evaluation as measured by event-related potentials (ERPs; Marinkovic et al. 2000). Furthermore, these disinhibitory effects of alcohol are correlated with personality traits related to impulsivity and hyperactivity (Dougherty et al. 2000; Marinkovic et al. 2000). A cluster of traits termed “antisocial personality disorder” inclusive of impulsivity, hyperactivity, and sensation/novelty seeking correlates with the early-onset alcoholism, increased drinking (Brown et al. 1996; Finn et al. 2000; Mazas et al. 2000), and chronic alcohol use and dependence (Hesselbrock et al. 1985; Regier et al. 1990) and may reflect a disruption in the inhibitory control of behavior subserved by prefrontal regions (Deckel et al. 1996; Peterson et al. 1990). Recent models of vulnerability to alcoholism emphasize the importance of executive functions in mediating, as well as moderating the effects of alcohol (Finn 2002; Giancola 2004).
According to the DSM-IV (APA 1994), dysregulation of impulse control is one of the diagnostic criteria for diverse psychiatric disorders inclusive of substance abuse and antisocial personality disorder. In a broader sense, the underlying symptom concerns an inability to resist engaging in activity that one declares to be unwanted or even harmful. Alcoholics also have decision-making deficits, including the likelihood of making poor decisions regarding their alcohol consumption (Fein et al. 2004; 2006a). The inability to maintain inhibitory control over drinking has been considered by some researchers to be fundamental to alcohol abuse (Fillmore and Weafer 2004; Finn et al. 2000; Jentsch and Taylor 1999; Lyvers 2000). Evidence suggests that the vulnerability to alcoholism may share a common genetic component with antisocial personality disorder which, as a premorbid trait, may predispose individuals to a spectrum of conduct disorders including alcohol dependence (Begleiter and Porjesz 1999; Bowirrat and Oscar-Berman 2005; Dick et al. 2004; Heinz et al. 2001; Pihl et al. 1993; Schuckit et al. 2004). Furthermore, alcohol intoxication affects cognitive evaluation of the situation and impairs finding the most suitable response strategies. It may result in disinhibited behaviors, poor-self control, and inability to desist drinking. Thus, excessive alcohol use impairs the executive and motivational functions that determine self-regulation and goal-directed behavior and can, in turn, result in a further increase in alcohol intake, tolerance, and dependence. Consequently, impulsivity seems to mediate alcohol abuse both as a dispositional risk factor and as a consequence of excessive drinking.
Improvement with abstinence
Neurobehavioral functioning may improve within 3 to 4 weeks of abstinence (Crews et al. 2005; Sullivan 2000), accompanied by at least partial reversal of brain shrinkage (O’Neill et al. 2001; Pfefferbaum et al. 1995; Shear et al. 1994) and some recovery of metabolic functions in the frontal lobes (Johnson-Greene et al. 1997) and cerebellum (Ende et al. 2005; Martin et al. 1995; Seitz et al. 1999). Frontal lobe blood flow continues to increase with abstinence, returning to approximately normal levels within 4 years (Gansler et al. 2000). Relapse to drinking leads to resumption of shrinkage (Pfefferbaum et al. 1995, 1998), continued declines in metabolism and cognitive function (Johnson-Greene et al. 1997), and evidence of neuronal cell damage (Martin et al. 1995). Abstinence up to 7 years resolves many neurocognitive deficits associated with alcoholism, except for the suggestion of lingering deficits in spatial processing (Fein et al. 2006b).
Neural Systems Affected and Concomitant Neurobehavioral Deficits
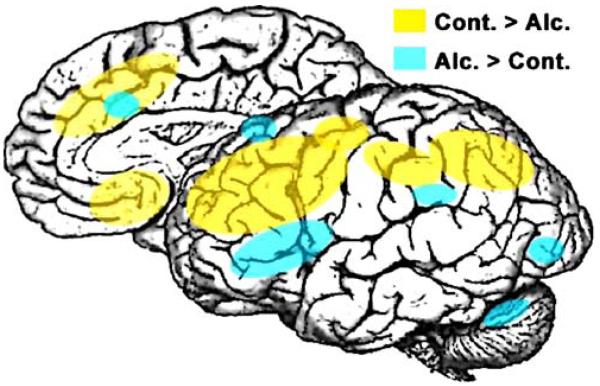
Results of research employing a variety of different techniques have determined that the brain structures most vulnerable to the effects of alcoholism are the neocortex (especially the frontal lobes), the limbic system, and the cerebellum (reviewed by Moselhy et al. 2001; Oscar-Berman and Hutner 1993; Oscar-Berman and Marinkovic 2003; Sullivan 2003). Each of these brain systems, and the functions affected by damage to them, is considered in turn. Figure 1 represents a schematic summary and conceptual model of the brain regions in which alcoholic and nonalcoholic groups have been found to differ with regard to cognition inclusive of working memory, vigilance, and proactive interference. Although based upon neuroimaging studies, the regions highlighted in Fig. 1 lack neuroanatomical precision, but they reflect the findings reported in this review, with one exception: We collapsed findings from the two cerebral hemispheres. In viewing Fig. 1, therefore, it is important to note that the differences between alcoholic and nonalcoholic groups are exclusive of brain laterality effects; these effects are sensitive to stimulus materials (e.g., verbal versus visuospatial) and task demands (e.g., attention, perception, motor response, etc.; Oscar-Berman and Schendan 2000; Oscar-Berman et al. 1997). Thus, because alcoholics commonly display a pattern of deficits that includes visuospatial, attentional, and emotional abnormalities characteristic of patients with right hemisphere damage, some investigators have suggested that the right hemisphere may be more vulnerable to the effects of alcoholism than the left hemisphere (Ellis and Oscar-Berman 1989; Oscar-Berman and Bowirrat 2005). As evidence accumulates, it may favor the view that group differences between the hemispheres may be greater for the right hemisphere than for the left (Harris et al. 2007; Makris et al. 2007).
Fig. 1.
A schematic summary and conceptual model of the brain regions in which alcoholic and nonalcoholic groups have been found to differ with regard to cognitive functioning. The model is based, in large part, upon fMRI evidence comparing abstinent alcoholic patients and healthy controls during cognitive probing of spatial and verbal working memory (Desmond et al. 2003; Pfefferbaum et al. 2001a; Tapert et al. 2001), vigilance (Tapert et al. 2001), and proactive interference (De Rosa et al. 2004). Studies using alcohol cue-exposure and emotional tasks are not included. The figure lacks anatomical precision in terms of activation extent and laterality differences. Even though more studies are needed to confirm, refine, and extend these findings, the observation of decreased BOLD (blood oxygen level dependent) activation in lateral and medial prefrontal and also parietal areas in alcoholics seems robust. This evidence is in overall agreement with studies showing decrease in global cortical metabolism and blood flow, especially in frontal areas, using PET (Adams et al. 1993; Dao-Castellana et al. 1998; Volkow et al. 1992; Wang et al. 1993), perfusion-weighted MRI (Clark et al. 2007a), and SPECT (Gansler et al. 2000) methodologies. Furthermore, extensive evidence from structural neuroimaging and neuropathology studies confirms that the frontal lobes are particularly vulnerable to alcohol-related brain damage (Harper and Matsumoto 2005; Oscar-Berman and Marinkovic 2003; Sullivan and Pfefferbaum 2005). However, the activation decrease is not uniform as alcoholics show increased activity especially in a fronto–parieto–cerebellar network. This system may be engaged in order to compensate for deficient dorsolateral prefrontal and parietal contributions and maintain the accuracy of behavioral performance (Sullivan and Pfefferbaum 2005). More studies are needed to investigate interactions between structural abnormalities, behavioral deficits, and functional brain activations as modulated by cognitively challenging tasks. Alc. Alcohol group; Cont. control group
Frontal lobe structure and function
Although alcoholism-related cortical changes have been documented throughout the brain, many studies consistently have found the frontal lobes to be more vulnerable to alcohol-related brain damage than other cerebral regions (Dirksen et al. 2006; Gansler et al. 2000; Gilman et al. 1996; Oscar-Berman et al. 2004; Pfefferbaum et al. 1997; Ratti et al. 2002). Neuropathological studies performed on the brains of deceased patients have revealed decreased neuron density in the frontal cortex of alcoholics (Harper and Matsumoto 2005). Harper (1998) and his collaborators established that 15–23% of cortical neurons are selectively lost from the frontal association cortex following chronic alcohol consumption. Structural MRI studies have shown frontal lobe volume losses in alcoholic subjects (Pfefferbaum et al. 1997), and frontal abnormalities in alcoholics also have been identified with fMRI scans (Tapert et al. 2001), in addition to reduced regional blood flow measurements (Gansler et al. 2000; Melgaard et al. 1990), reduced amplitude of ERPs (Chen et al. 2007), and with measurements of lower glucose metabolism throughout the brain (including prefrontal cortex) during alcohol intoxication (Volkow et al. 1995). Frontal lobe blood flow (Nicolás et al. 1993) and metabolism (Volkow et al. 1992, 2002) may decrease in alcoholics before significant shrinkage or major cognitive problems become detectable (Nicolás et al. 1993; Wang et al. 1993).
The frontal lobes are connected with the other lobes of the brain, and through multiple interconnections, they receive and send fibers to numerous subcortical structures as well (Fuster 1997, 2006). The anterior region of the frontal lobes (prefrontal cortex) plays a kind of executive regulatory role within the brain (Goldberg 2001; Lichter and Cummings 2001). Executive functions (which depend upon many of our cognitive abilities, such as attention, perception, memory, and language) are defined differently by different theorists and researchers. Most agree, however, that executive functions are human qualities, including self-awareness, that allow us to be independent individuals with purpose and foresight about what we will do and how we behave. For example, executive abilities include judgment, problem solving, decision-making, planning, and social conduct, and they allow us to monitor and change behavior flexibly and in accord with internal goals and contextual demands.
Prefrontal neurobehavioral dysfunction has been frequently observed in alcoholics with and without the dense amnesia of Korsakoff’s syndrome (Dirksen et al. 2006; Gansler et al. 2000; Oscar-Berman and Evert 1997; Oscar-Berman et al. 2004). In two recent studies (Dirksen et al. 2006; Oscar-Berman et al. 2004), we administered a series of neuropsychological tasks sensitive to dysfunction of frontal brain systems to abstinent alcoholics, including groups of patients with Korsakoff’s syndrome. The Korsakoff patients were impaired on tests of memory, fluency, cognitive flexibility, and perseverative responding. Non-Korsakoff alcoholics showed some frontal system deficits as well, but these generally were mild compared to the Korsakoff patients. In non-Korsakoff alcoholics, factors contributing to cognitive performance were age, duration of abstinence, duration of alcoholism, and amount of alcohol consumed.
In addition to causing changes in cognitive functions, damage to frontal brain systems often leads to aberrations of emotion and personality. This is due in part to the interactions of frontal brain regions with limbic and paralimbic centers that are part of a circuitry involved in processing information about reward and aversion to produce optimized and balanced behavior that is critical for normal emotional functioning (LaBar and Cabeza 2006; Makris et al. 2007).
Emotional changes have direct social and interpersonal significance (Kornreich et al. 2002). Among the abnormalities are affective processing deficits such as a diminished ability to recognize facial expressions of emotion (Clark et al. 2007b; Foisy et al. 2005; Howard et al. 2003; Kornreich et al. 2002; Philippot et al. 1999; Townshend and Duka 2003) and to decipher affective prosody in spoken language (Monnot et al. 2002). The abnormalities in emotional perception have been attributed to a combination of underlying factors, e.g., visuospatial deficits, abnormal processing of social information, poor inhibitory control, and interpersonal stress (Moselhy et al. 2001; Philippot et al. 1999).
Frontal personality traits have been described in terms of “disinhibition” and impulsivity, including aggression and a lack of concern for the consequences of untoward behaviors (Dougherty et al. 1999; Laakso et al. 2002; Marinkovic et al. 2000; Raine et al. 2000; Stevens et al. 2003). A deficit in response inhibition is enhanced when the response to be suppressed is tied to alcohol-related information (Noel et al. 2007). Disinhibition and antisocial traits are associated with increased risk for early-onset alcoholism (Mazas et al. 2000), and sensation or novelty seeking is associated with increased drinking (Finn et al. 2000). Indeed, a cluster of traits termed “antisocial personality disorder,” inclusive of hyperactivity and impulsivity, correlates highly with early-onset alcoholism, increased drinking (Brown et al. 1996; Finn et al. 2000; Mazas et al. 2000), alcohol-induced motor disinhibition (Marinkovic et al. 2000), and chronic alcohol use and dependence (Hesselbrock et al. 1985; Regier et al. 1990). In a recent study that looked for a possible relationship between impulsivity and alcohol dependence (Chen et al. 2007), alcoholic subjects with high impulsivity scores also manifested prefrontal reductions in brain ERP (P3) amplitudes to visual targets. Other investigators have found that shared neurochemical markers may underlie the commonalities between alcohol abuse and traits associated with antisocial personality disorder (Virkkunen and Linnoila 1993). This may be suggestive of a preexisting neurochemical milieu in certain individuals that is associated with the impulsive, hyperactive, or aggressive behaviors and which, in turn, leads to alcoholism. Thus, impulsive behavior may be a premorbid trait predisposing individuals to a spectrum of disorders including alcohol dependence (Pihl et al. 1993).
There may be a genetic predisposition to dysfunctional frontal circuitry in families with a history of alcoholism. During performance of a classic, visual oddball task, high-risk children of alcoholics showed lower bilateral fMRI activation in frontoparietal regions than control children of nonalcoholic parents (Rangaswamy et al. 2004). Further evidence of a genetic component of frontal lobe dysfunction in alcoholism was provided by a dynamic contrast MRI study in nonalcoholic subjects with family history of alcoholism: Adult children of alcoholics had abnormal patterns of regional cerebral blood volume changes in inferior prefrontal regions, as well as greater mood enhancement with an alprazolam-induced challenge, than subjects without a familial history of alcoholism (Streeter et al. 1998).
Limbic system structure and function
The limbic system monitors internal homeostasis, mediates memory and learning, and contributes to emotional feelings and behaviors. The limbic system also drives important aspects of sexual behavior, motivation, and feeding behaviors. Primary areas of the limbic system include the hippocampus, amygdala, septal nuclei, hypothalamus, and anterior cingulate gyrus. For the purpose of this review, because numerous studies of alcoholics have reported abnormalities in the amygdala, hippocampus, and hypothalamus, the discussion is focused on those brain regions.
Amygdala
The amygdala is a small almond-shaped structure, deep inside the anteroinferior region of the temporal lobe. It is a heterogeneous brain area consisting of 13 nuclei and cortical regions and their subdivisions (Sah et al. 2003), with connections to prefrontal cortex, the hippocampus, the septal nuclei, and the medial dorsal nucleus of the thalamus. A number of studies have linked the amygdala to the processing of motivational significance of stimuli and to the mediation and control of major emotions such as love, fear, rage, anxiety, and general negative affective states (Aggleton 2000; Amaral et al. 2003; Breiter and Rosen 1999; Everitt et al. 2003; LeDoux 2003; Pitkänen et al. 2000; Rolls 2000). The amygdala, being important in identifying danger, appears fundamental for self-preservation.
Neuroimaging studies have shown that the amygdala responds to facial expressions of many emotions, especially those with negative affective qualities such as sadness, anger, and fear (Blair et al. 1999; Breiter and Rosen 1999; Wang et al. 2005; Winston et al. 2003), even in the absence of conscious awareness of their presentation to subjects (Whalen et al. 1998). Neuroimaging studies have shown that conditioned responses to both aversive and positive stimuli are processed and largely mediated by the amygdala, having connections to early sensory processing areas as well as to autonomic centers. (Davis and Whalen 2001).
The amygdala is partially controlled by the brain’s dopamine system (Delaveau et al. 2005), as an essential part of the brain-reward circuitry—the same system that responds to alcohol and produces feelings of pleasure when good things happen (Koob 2003). In a recent study using fMRI in our laboratory (Marinkovic et al. 2007), we observed clear evidence of differences between abstinent long-term alcoholics and nonalcoholic controls in amygdala activation to emotional materials. The subjects were scanned while viewing emotional words and emotional facial expressions. Faces with negative and positive emotional expressions evoked significantly stronger bilateral amygdala activity in the controls than in the alcoholics, whose activations were blunted. A similar lack of emotional differentiation to facial expressions by alcoholics also was observed in the hippocampus. The observation that alcoholics respond to emotionally-valenced stimuli in an undifferentiated manner is consistent with clinical evidence of their interpersonal difficulties (Kornreich et al. 2002), and may contribute to adverse societal repercussions for alcoholics. Moreover, that both the amygdala and hippocampus were hyporesponsive is not surprising, since encoding of emotional memories depends on the hippocampus in conjunction with the amygdala, as well as their interaction (LaBar and Cabeza 2006; Phelps 2004; Richardson et al. 2004).
Hippocampus
As part of the limbic system, the hippocampus is intimately involved in motivation and emotion, and it also plays a central role in the formation of memories (Cipolotti and Bird 2006; LaBar and Cabeza 2006). The hippocampus consists of the complex inter-folded layers of the dentate gyrus and Ammon’s horn, which are continuous with the subiculum, which in turn merges with the parahippocampal gyrus. Although the notion that the hippocampus may play a role in brain mechanisms underlying anxiety is not new (Bannerman et al. 2002; Gray and McNaughton 2000), there is mounting evidence that the ventral hippocampus is part of a brain system associated with fear and/or anxiety (Bannerman et al. 2004; Kjelstrup et al. 2002; McHugh et al. 2004). The anatomy of the hippocampus is closely associated with subcortical structures, which contribute to the hypothalamic–pituitary–adrenal axis (Kjelstrup et al. 2002).
Results of a nonhuman animal study have suggested that the deleterious effect of ethanol on the survival of newly-formed neurons in the adult rat hippocampus could result in impairment of hippocampal-dependent cognitive functions (Herrera et al. 2003). Neurogenesis is primarily a developmental process that involves the proliferation, migration, and differentiation into neurons of primordial stem cells of the central nervous system. Neurogenesis declines until it ceases in the young adult mammalian brain, with two exceptions: The olfactory bulb and the hippocampus produce new neurons throughout adult life. The ethanol-induced reductions in hippocampal neurogenesis can be attributed to two general mechanisms: an effect on cell proliferation or on cell survival. These changes in hippocampal structure could be part of the anatomical basis for cognitive deficits observed in alcoholism.
Structural neuroimaging studies have demonstrated a reduction of hippocampal volume in alcoholics (Agartz et al. 1999; Beresford et al. 2006; Kurth et al. 2004; Sullivan et al. 1995). The loss of hippocampal volume has been attributed to changes in white matter (Harding et al. 1997), but the incorporation of newly-formed neurons to the dentate gyrus could also be affected by alcohol. One MRI study measured hippocampus volume in late-onset alcoholics (Type I) and violent, early-onset alcoholics (Type II), compared to nonalcoholic controls (Laakso et al. 2000). The right, but not left, hippocampus was significantly smaller in both alcoholic groups. While there was no correlation between the hippocampal volumes with age in the control subjects, there was tendency toward decreased volumes with aging and also with the duration of alcoholism in the Type I alcoholics. Hippocampal volume reduction also was reported in heavy chronically-drinking, alcohol-dependent subjects compared with nonalcoholic controls (Beresford et al. 2006), with left hippocampal volume reduction being slightly greater than on the right. A study of teens (aged 15–17 years) with alcohol use disorders found reduced left—but not right—hippocampal volume compared to healthy age-equivalent controls (Nagel et al. 2005). The groups were equivalent in right hippocampal, intracranial gray and white matter volumes, and memory performance. The authors suggested that premorbid volumetric differences might account for some of the observed group differences in hippocampal volume. Reduction of hippocampal volume in alcoholics is reversible after short periods of abstinence (White et al. 2000). Similarly, hippocampal dependent cognitive functions have shown reversibility after comparable periods of abstinence (Bartels et al. 2006).
Hypothalamus
The hypothalamus literally means “under the thalamus.” It is a small structure nestled within the limbic system directly above the brainstem. The hypothalamus has connections with many other brain regions, and it is involved in learning and memory, as well as in regulatory functions such as eating and drinking, temperature control, hormone regulation, and emotion. Long-term alcoholism and concomitant dietary insufficiencies have been associated with damage to the mammillary bodies of the hypothalamus, and memory deficits (amnesia) often follow. Although damage to other regions of the brain in addition to the hypothalamus have also been implicated (e.g., basal forebrain, hippocampus, fornix, medial and anterior nuclei of the thalamus), when amnesia occurs as a consequence of chronic alcoholism, it is referred to as alcohol-induced persisting amnestic disorder (APA 1994), or alcoholic Korsakoff’s syndrome (Oscar-Berman and Evert 1997). The specific memory impairments include severe anterograde amnesia for newly learned information, and some retrograde amnesia, i.e., loss of memory for events that happened long ago (prior to the appearance of obvious symptomatology).
Amnesia, especially anterograde amnesia, or memory loss for recent events, is an intriguing and serious disorder. Patients with Korsakoff’s syndrome are permanently unable to remember new information for more than a few seconds. Because new events are forgotten a few seconds after they occur, virtually nothing new is learned, and patients with Korsakoff’s syndrome live perpetually in the past. However, in contrast to patients with alcoholic dementia, who have generalized cognitive decline (including widespread memory loss), patients with Korsakoff’s syndrome retain old memories formed prior to the onset of alcohol-related brain damage.
Although anterograde amnesia is the most obvious presenting symptom in Korsakoff patients, these individuals have additional cognitive and emotional impairments (Clark et al. 2007b; Dirksen et al. 2006). Like patients with bilateral prefrontal cortical lesions, Korsakoff patients are abnormally sensitive to distractions (proactive interference). This sensitivity may be due to alcoholism-related prefrontal dysfunction, which impairs the ability to counteract the effects of cognitive interruptions. In addition to their memory problems and their sensitivity to interference, Korsakoff patients also tend to repeat unnecessary behaviors (perseverative responding), have restricted attention, retarded perceptual processing abilities, ataxia, and decreased sensitivity to reward contingencies (Oscar-Berman and Evert 1997).
As a part of a wider array of interrelated abnormalities, it has been shown that the hypothalamic–pituitary–adrenocortical (HPA) function is hyporeactive in chronic alcoholics (Errico et al. 2002; Lovallo 2006). Cortisol, in turn, increases mesencephalic dopaminergic transmission that underlies the activation of alcohol-induced brain reward circuitry (Bowirrat and Oscar-Berman 2005; Gianoulakis 1998; Piazza et al. 1996), in which the amygdala plays an essential role (Koob 2003). These additional abnormalities reflect widespread cerebral atrophy accompanying sustained alcohol abuse. Thus, consideration should be given to sensory and cognitive deficits that may be integral to the disease process caused by chronic alcoholism.
Cerebellar structure and function
The cerebellum is a brain structure that coordinates movement of voluntary muscles, balance, and eye movements, and it also is essential to the neural circuitry subserving cognition and emotion (Schmahmann 2000, 1997). The cerebellum contains about half of the brain’s neurons, but the nerve cells are so small that the cerebellum accounts for only 10% of the brain’s total weight. The cerebellum consists mainly of two large, tightly folded lobes, joined at the middle by the vermis. Also located anteriorly are the small flocculonodular lobes (flocculi). The cerebellum connects with the other brain structures through the cerebellar peduncles, located to the anterior of the cerebellum. Five different nerve cell types make up the cerebellum: stellate, basket, Purkinje, Golgi, and granule cells. The Purkinje cells are the only ones to send axons out of the cerebellum.
Atrophy of the cerebellum is commonly associated with alcoholism. White matter volume of the cerebellar vermis is significantly reduced (Baker et al. 1999; Pentney et al. 2002; Sullivan et al. 2000), and cerebellar vermian atrophy occurs in 25–40% of all alcoholics. Vermal white matter volume was reduced in ataxic alcoholics by 42%. It occurs even more often in people with additional thiamine deficiency, with 35–50% of those individuals showing evidence of superior vermian degeneration (Victor 1992). Gross vermian atrophy is commonly seen post mortem in alcoholics (Phillips et al. 1987; Sullivan et al. 2003), and it also has been observed with in vivo neuroimaging techniques (Sullivan 2003).
Over the past two decades, careful study has expanded the purview of the cerebellum to include influence on functions classically associated with frontal lobe functioning (Schmahmann 2000; Sullivan et al. 2003). As noted in the previous section on frontal lobes, this part of the brain has executive control functions such as cognitive flexibility, speed in allocation of attentional resources, shifting ability, inhibition of perseverative errors, abstractive and planning skills, and suppression of irrelevant information. Together, these observations suggest a functional role for frontocerebellar circuitry (Schmahmann 1997). Further, cerebellar volume shrinkage in alcoholics has been shown to correlate with performance on tests of executive function, traditionally attributed to frontal pathology, thus revealing the importance of disrupted frontocerebellar circuitry in the constellation of alcoholism-related functional impairments (Chanraud et al. 2007; Harris et al. 1999; Sullivan 2003; Sullivan et al. 2003). Additionally, an fMRI study of alcoholic and nonalcoholic control subjects performing a verbal working memory task showed that despite comparable levels of task performance by the two groups, the alcoholic group exhibited greater fMRI activation than the control group in the right superior cerebellar and left frontal regions (Desmond et al. 2003). As Sullivan and her colleagues have suggested, it may be that increased demands on frontal brain regions may be incurred to overcome alcoholism-related impairments, and that the cerebellum provides supplementary compensation for maintaining information in a compromised brain system (Desmond et al. 2003; Harris et al. 1999; Sullivan 2003)
Alcoholics with Korsakoff’s syndrome have shown a significant decrease in Purkinje cell density in the cerebellar vermis and molecular layer volume (Baker et al. 1999). A 36% reduction in Purkinje cell numbers in the flocculi suggests disruption of vestibulocerebellar pathways. This is of particular interest given recent data showing the importance of cerebellum in the organization of higher order cerebral functions (Schmahmann 2000).
Right hemisphere structure and function
Impairments in cognitive functioning found to be associated with alcoholism are also prominent in patients with damage to the right hemisphere (RH) of the brain unrelated to alcoholism. This similarity led Jones and Parsons (1971) to propose that RH functions may be more vulnerable than left hemisphere (LH) functions to the effects of chronic alcoholism. A variety of studies have found evidence to support this proposition (Ellis and Oscar-Berman 1989; Oscar-Berman and Schendan 2000). This evidence comes from findings of impairments of cognitive functions for which the RH is generally more specialized than the LH. These include: greater impairments on performance (nonverbal) tasks than on verbal tasks of IQ tests (Parsons and Leber 1981); reduced visual-spatial and perceptual-motor performance (Bowden and McCarter 1993; Ellis and Oscar-Berman 1989; Parsons 1987); emotional abnormalities (Clark et al. 2007b; Oscar-Berman et al. 1990); atypical laterality patterns on dichotic listening tests (Drake et al. 1990), but only for the male alcoholics); impaired performance for nonverbal stimuli on visual search tasks (Bertera and Parsons 1978); and limited attentional resources (Evert and Oscar-Berman 2001; Smith and Oscar-Berman 1992). It is important to note, however, that while many studies have found evidence to support the “right hemisphere hypothesis” of alcoholism-related cognitive decline, others have not found such support (Cermak et al. 1989; Mungas et al. 1994).
Based on the many findings that suggest RH functional decline associated with alcoholism, one might expect to find neuropathological and neuroradiological evidence indicating greater morphological and physiological changes in the RH than in the LH. However, this level of analysis has been marked by inconsistent findings. In a post mortem study of the brains of alcoholics, no differences were identified between the right and left hemispheres (Harper et al. 1985). Several studies using CT imaging in living alcoholics also have not found significant differences in brain morphology between the two hemispheres (Lee et al. 1979; Wilkinson and Carlen 1980). In two CT studies that did find evidence of hemispheric differences in alcoholics, changes were more marked in the LH than in the RH (Gebhardt et al. 1984; Golden et al. 1981). By contrast, an MRI study by Makris et al. (2007) demonstrated that abstinent alcoholic subjects had volumetric deficits in the RH of the brain’s extended reward and oversight system (EROS). This system includes dorsolateral prefrontal, orbitofrontal, and anterior cingulate cortices, the anterior insula, hippocampus, amygdala, nucleus accumbens, and ventral diencephalon. This circuitry processes information about reward and aversion and is fundamental for the determination of goals optimizing normal emotional functioning and its malfunction. The regions having the most pronounced RH deficits were dorsolateral prefrontal cortex, anterior insula, and nucleus accumbens (Makris et al. 2007). In a companion study (Harris et al. 2007) we used DTI to assess functional anisotropy (a measure of white matter integrity), in the brains of abstinent alcoholics compared to matched controls. We found that alcoholics had diminished functional anisotropy in the right frontal lobe in superior longitudinal fascicles II and III, orbito-frontal cortex white matter, and cingulum bundle, but not in corresponding left hemisphere regions. These right frontal and cingulum white-matter regional functional anisotropy measures provided 97–100% correct group discrimination and classification.
Findings with respect to hemispheric differences in alcoholics with the use of functional brain imaging studies also have shown inconsistent results. There have been reports that cerebral blood flow of the RH is more affected than the LH (Berglund 1981). Other studies have found either no difference in cerebral blood flow and glucose metabolism between the halves of the brain (Adams et al. 1993; Gilman et al. 1990; Nicolás et al. 1993; Samson et al. 1986; Wang et al. 1993), or increased hypometabolism in the LH (Erbas et al. 1992). To complicate matters, Volkow et al. (1992) found that the right frontal cortex showed greater hypometabolism than the left, and the left parietal cortex showed greater hypometabolism than the right.
Perhaps the most consistent evidence of greater RH dysfunction has come from studies utilizing electrophysiological measures, although this observation has to be tempered by the poor spatial resolution of ERPs. In one study, ERP abnormalities in alcoholics were particularly evident in the right frontal area (Porjesz and Begleiter 1982). In addition, the alcoholics did not show normal asymmetrical ERP responses (i.e., the finding that RH amplitudes are normally greater than LH amplitudes; see also Zhang et al. 1997). Similarly, Kostandov et al. (1982) found abnormally long latencies and small amplitudes of the P300 component of the ERP in the RH of the alcoholics, whereas LH measures did not differ between the alcoholic and control groups.
Implications for Treatment and Recovery
Clinicians must consider a variety of treatment methods to promote cessation of drinking, maintenance of sobriety, and recovery of impaired functioning. In a comprehensive review of the pharmacogenomics of alcohol response and addiction, Enoch (2003) noted that treatment is complicated by the comorbidity of alcoholism with other disorders (e.g., anxiety, depression, antisocial personality disorder, smoking, and other addictions). Furthermore, specific environmental contexts such as stress and alcohol-related stimuli, can stimulate craving for alcohol. Of interest, Sinha and colleagues (Fox et al. 2007; Sinha and Li 2007) have evaluated the separate contributions of stress and alcohol cues to alcohol craving, anxiety, and bodily responses in recently detoxified alcoholic individuals. The researchers reported that although there is overlap in brain circuits for processing stress and alcohol cues, each produced a dissociable psychobiological state involving subjective emotional, cardiovascular, and cortisol responses. That is, stress-induced alcohol craving was related to increased systolic and diastolic blood pressure, as well as sadness, anger, and anxiety ratings, but alcohol cue-induced craving was associated with increased salivary cortisol, as well as anxiety and fear ratings. Thus, co-occurring conditions having disparate underlying psychobiological underpinnings need to be considered when planning rehabilitation and treatment strategies.
First-line therapeutic targets for alcoholism are neurotransmitter pathway genes implicated in alcohol use. Of particular interest are the reward pathways (serotonin, dopamine, GABA, glutamate, and beta endorphin) and the behavioral stress response system (corticotrophin-releasing factor and neuropeptide Y). Common functional polymorphisms in these genes are likely to be predictive (although each with small effect) of individualized pharmacological responses. As we have noted earlier, genetic studies, including case-control association studies and genome wide linkage studies, have identified associations between alcoholism and common functional polymorphisms in several candidate genes. Meanwhile, the current pharmacological therapies are only modestly effective in preventing relapse and dependence in alcoholics (Doggrell 2006; Kranzler and Van Kirk 2001; Mann 2004), prompting more research. Additionally, treating co-occurring disorders remains a challenge, and the use of creative approaches that would encompass individualized psychosocial support, as well as a combination of treatments, might be the most effective way to address this problem.
Because alcoholism is associated with diverse changes to the brain and behavior, treatment professionals might find it most helpful to use a combination of neuropsychological observations and structural and functional brain imaging results in developing predictors of abstinence versus relapse, with the purpose of tailoring treatment methods to each individual patient. For example, the development of effective medications for controlling alcoholism relies upon knowledge about the neuroanatomical origins of neurotransmitters involved in craving, intoxication, and addiction. Neuroimaging methods have already provided significant insight into the nature of brain damage caused by heavy alcohol use, and the integration of results from different methods of neuroimaging will spur further advances in the diagnosis and treatment of alcoholism-related damage. Clinicians also can use brain imaging techniques to monitor the course of treatment because these techniques can reveal structural, functional, and biochemical changes in patients across time as a result of abstinence, therapeutic interventions, withdrawal, or relapse. Neuroimaging research already has shown that abstinence of less than a month can result in an increase in cerebral metabolism, particularly in the frontal lobes, and that continued abstinence can lead to at least partial reversal in loss of brain structure and function (Bartsch et al. 2007; Ende et al. 2005; Fein and Chang 2006; Gansler et al. 2000; O’Neill et al. 2001). Thus, through the combined efforts of scientists and clinicians, important strides already have been made in the diagnosis, prevention, and treatment of alcoholism, and hopefully there will be continued advances in the future.
Conclusions
Alcoholics are a diverse group. They experience different subsets of symptoms, and the disease has different origins and modulating influences for different people. Therefore, to understand the effects of alcoholism, it is important to consider the influence of a wide range of variables on a particular behavior or set of behaviors. The underpinnings of alcohol-induced brain defects are multivariate; to date, the available literature does not support the assertion that any one variable can consistently and completely account for these impairments. Instead, the identification of the most salient variables is a primary focus of current research. In the search for answers, we recommend an integrative approach that recognizes the interconnectivity of the different functional systems to account for the heterogeneity of outcome variables associated with alcoholism-related impairments and recovery of functions. It is helpful to use as many kinds of tools as possible, keeping in mind that specific deficits can be observed only with certain methods, with rigorous paradigms, and with particular groups of people with distinct risk factors. Such confluence of information can provide evidence linking structural damage, functional alterations, and the specific behavioral and neuropsychological effects of alcoholism. These measures also can determine the degree to which abstinence and treatment result in the reversal of atrophy and dysfunction.
Acknowledgments
The writing of this paper was supported by the US Department of Health and Human Services, NIAAA (R01-AA07112, K05-AA00219, and K01-AA13402), the Medical Research Service of the US Department of Veterans Affairs, and the Alcoholic Beverage Medical Research Foundation.
Contributor Information
Marlene Oscar-Berman, Departments of Anatomy and Neurobiology, Psychiatry, and Neurology, Boston University School of Medicine, L-815, 715 Albany Street, Boston, MA 02118, USA; Psychology Research Service, VA Healthcare System, Boston Campus, Boston, MA, USA.
Ksenija Marinković, Radiology Department, Harvard Medical School, Boston, MA, USA; Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA, USA.
References
- Abel EL, Kruger ML, Friedl J. How do physicians define “light,” “moderate,” and “heavy” drinking? Alcoholism: Clinical and Experimental Research. 1999;22(5):979–984. doi: 10.1111/j.1530-0277.1998.tb03692.x. [DOI] [PubMed] [Google Scholar]
- Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, Dede D, et al. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcoholism: Clinical and Experimental Research. 1993;17(2):205–210. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Agartz I, Momenam R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Archives of General Psychiatry. 1999;56(4) doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, editor. The amygdala: A functional analysis. 2nd ed. Oxford University Press; Oxford: 2000. [Google Scholar]
- Amaral DG, Bauman MD, Capitanio JP, Lavenex P, Mason WA, Mauldin-Jourdain ML, et al. The amygdala: Is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41(4):517–522. doi: 10.1016/s0028-3932(02)00310-x. [DOI] [PubMed] [Google Scholar]
- APA . Diagnostic and statistical manual of mental disorders (DSM-IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Baker KG, Harding AJ, Halliday GM, Kril JJ, Harper CG. Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke’s encephalopathy. Neuroscience. 1999;19(2):429–438. doi: 10.1016/s0306-4522(98)90664-9. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RMJ, Yee BK, Feldon J, Rawlins JNP. Ventral hippocampal lesions affect anxiety but not spatial learning. Behavioural Brain Research. 2002;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus-memory and anxiety. Neuroscience and Bio-behavioral Reviews. 2004;28(3):273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bartels C, Kunert HJ, Stawicki S, Kroner-Herwig B, Ehrenreich H, Krampe H. Recovery of hippocampus-related functions in chronic alcoholics during monitored long-term abstinence. Alcohol and Alcoholism. 2006 doi: 10.1093/alcalc/agl104. [DOI] [PubMed] [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, et al. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcoholism: Clinical and Experimental Research. 1999;23(7):1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, et al. Hippocampus volume loss due to chronic heavy drinking. Alcoholism: Clinical and Experimental Research. 2006;30(11):1866–1870. doi: 10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Berglund M. Cerebral blood flow in chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1981;5:295–303. doi: 10.1111/j.1530-0277.1981.tb04903.x. [DOI] [PubMed] [Google Scholar]
- Bertera JH, Parsons OA. Impaired visual search in alcoholics. Alcoholism: Clinical and Experimental Research. 1978;2(1):9–14. doi: 10.1111/j.1530-0277.1978.tb04685.x. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Saccone NL, Rice JP, Goate A, Foroud T, Edenberg H, et al. Defining alcohol-related phenotypes in humans. The collaborative study on the genetics of alcoholism. Alcohol Research and Health. 2002;26(3):208–213. [PMC free article] [PubMed] [Google Scholar]
- Blair RJR, Morris JS, Frith CD, Perrettand DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, et al. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs. 2000;32(Suppl i-iv):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Bowden SC, McCarter RJ. Spatial memory in alcohol-dependent subjects: Using a push-button maze to test the principle of equiavailability. Brain and Cognition. 1993;22(1):51–62. doi: 10.1006/brcg.1993.1024. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2005;132B(1):29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. In: McGinty JF, editor. Advancing from the ventral striatum to the extended amygdala. Vol. 877. New York Academy of Sciences; New York: 1999. pp. 523–547. [DOI] [PubMed] [Google Scholar]
- Brown SA, Gleghorn A, Schuckit MA, Myers MG, Mott MA. Conduct disorder among adolescent alcohol and drug abusers. Journal of Studies on Alcohol. 1996;57(3):314–324. doi: 10.15288/jsa.1996.57.314. [DOI] [PubMed] [Google Scholar]
- Bushman BJ, Cooper HM. Effects of alcohol on human aggression: An integrative research review. Psychological Bulletin. 1990;107(3):341–354. doi: 10.1037/0033-2909.107.3.341. [DOI] [PubMed] [Google Scholar]
- Cermak LS, Verfaellie M, Letourneau L, Blackford S, Weiss S, Numan B. Verbal and nonverbal right hemisphere processing by chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1989;13(5):611–616. doi: 10.1111/j.1530-0277.1989.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32(2):429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Chen AC, Porjesz B, Rangaswamy M, Kamarajan C, Tang Y, Jones KA, et al. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcoholism: Clinical and Experimental Research. 2007;31(1):156–165. doi: 10.1111/j.1530-0277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Bird CM. Amnesia and the hippocampus. Current Opinion in Neurology. 2006;19(6):593–598. doi: 10.1097/01.wco.0000247608.42320.f9. [DOI] [PubMed] [Google Scholar]
- Clark CP, Brown GG, Eyler LT, Drummond SP, Braun DR, Tapert SF. Decreased perfusion in young alcohol-dependent women as compared with age-matched controls. American Journal of Drug and Alcohol Abuse. 2007a;33(1):13–19. doi: 10.1080/00952990601082605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Oscar-Berman M, Shagrin B, Pencina M. Alcoholism and judgments of affective stimuli. Neuropsychology. 2007b;21(3):346–362. doi: 10.1037/0894-4105.21.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Dietz G, Johnson JP, MacMurray JP. Association of the enkephalinase gene with low amplitude P300 waves. Neuroreport. 1999;10(11):2283–2285. doi: 10.1097/00001756-199908020-00011. [DOI] [PubMed] [Google Scholar]
- Connor JP, Young RM, Lawford BR, Ritchie TL, Noble EP. D(2) dopamine receptor (DRD2) polymorphism is associated with severity of alcohol dependence. European Psychiatry. 2002;17(1):17–23. doi: 10.1016/s0924-9338(02)00625-9. [DOI] [PubMed] [Google Scholar]
- Courville CB. Effects of alcohol on the nervous system of man. San Lucas Press; Los Angeles: 1966. [Google Scholar]
- Crews FT, Buckley T, Dodd PR, Ende G, Foley N, Harper C, et al. Alcoholic neurobiology: Changes in dependence and recovery. Alcoholism: Clinical and Experimental Research. 2005;29(8):1504–1513. doi: 10.1097/01.alc.0000175013.50644.61. [DOI] [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, et al. Frontal dysfunction in neurologically normal chronic alcoholic subjects: Metabolic and neuropsychological findings. Psychological Medicine. 1998;28(5):1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcoholism: Clinical and Experimental Research. 2005;29(5):844–854. doi: 10.1097/01.alc.0000164374.32229.a2. [DOI] [PubMed] [Google Scholar]
- De Rosa E, Desmond JE, Anderson AK, Pfefferbaum A, Sullivan EV. The human basal forebrain integrates the old and the new. Neuron. 2004;41(5):825–837. doi: 10.1016/s0896-6273(04)00080-7. [DOI] [PubMed] [Google Scholar]
- de Wit H, Metz J, Wagner N, Cooper M. Behavioral and subjective effects of ethanol: Relationship to cerebral metabolism using PET. Alcoholism: Clinical and Experimental Research. 1990;14(3):482–489. doi: 10.1111/j.1530-0277.1990.tb00508.x. [DOI] [PubMed] [Google Scholar]
- Deckel AW, Hesselbrock V, Bauer L. Antisocial personality disorder, childhood delinquency, and frontal brain functioning: EEG and neuropsychological findings. Journal of Clinical Psychology. 1996;52(6):639–650. doi: 10.1002/(SICI)1097-4679(199611)52:6<639::AID-JCLP6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Delaveau P, Salgado-Pineda P, Wicker B, Micallef-Roll J, Blin O. Effect of levodopa on healthy volunteers’ facial emotion perception: An FMRI study. Clinical Neuropharmacology. 2005;28(6):255–261. doi: 10.1097/01.wnf.0000186651.96351.2e. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: An fMRI study. Neuroimage. 2003;19(4):1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Ezekiel F, Meyerhoff DJ, MacKay S, Dillon WP, Weiner MW, et al. Brain atrophy and cognitive function in older abstinent alcoholic men. Alcoholism: Clinical and Experimental Research. 1995;19(5):1121–1126. doi: 10.1111/j.1530-0277.1995.tb01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sclafani V, Finn P, Fein G. Psychiatric comorbidity in long-term abstinent alcoholic individuals. Alcoholism: Clinical and Experimental Research. 2007;31(5):795–803. doi: 10.1111/j.1530-0277.2007.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Edenberg HJ, Xuei X, Goate A, Kuperman S, Schuckit M, et al. Association of GABRG3 with alcohol dependence. Alcoholism: Clinical and Experimental Research. 2004;28(1):4–9. doi: 10.1097/01.ALC.0000108645.54345.98. [DOI] [PubMed] [Google Scholar]
- Dick DM, Foroud T. Candidate genes for alcohol dependence: A review of genetic evidence from human studies. Alcoholism: Clinical and Experimental Research. 2003;27(5):868–879. doi: 10.1097/01.ALC.0000065436.24221.63. [DOI] [PubMed] [Google Scholar]
- Dickson PA, James MR, Heath AC, Montgomery GW, Martin NG, Whitfield JB, et al. Effects of variation at the ALDH2 locus on alcohol metabolism, sensitivity, consumption, and dependence in Europeans. Alcoholism: Clinical and Experimental Research. 2006;30(7):1093–1100. doi: 10.1111/j.1530-0277.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- Dirksen CL, Howard JA, Cronin-Golomb A, Oscar-Berman M. Patterns of prefrontal dysfunction in alcoholics with or without Korsakoff’s syndrome, patients with Parkinson’s disease, and patients with rupture and repair of the anterior communicating artery. Neuropsychiatric Disease and Treatment. 2006;23(3):327–339. doi: 10.2147/nedt.2006.2.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggrell SA. Which treatment for alcohol dependence: Naltrexone, acamprosate and/or behavioural intervention? Expert Opinion on Pharmacotherapy. 2006;7(15):2169–2173. doi: 10.1517/14656566.7.15.2169. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, Moeller FG, Chokshi RV, Rosen VC. Effects of moderate and high doses of alcohol on attention, impulsivity, discriminability, and response bias in immediate and delayed memory task performance. Alcoholism: Clinical and Experimental Research. 2000;24(11):1702–1711. [PubMed] [Google Scholar]
- Dougherty DM, Moeller FG, Steinberg JL, Marsh DM, Hines SE, Bjork JM. Alcohol increases commission error rates for a continuous performance test. Alcoholism: Clinical and Experimental Research. 1999;23(8):1342–1351. [PubMed] [Google Scholar]
- Drake AI, Hannay HJ, Gam J. Effects of chronic alcoholism on hemispheric functioning: An examination of gender differences for cognitive and dichotic listening tasks. Journal of Clinical and Experimental Neuropsychology. 1990;12(5):781–797. doi: 10.1080/01688639008401019. [DOI] [PubMed] [Google Scholar]
- Dufour M, Fuller RK. Alcohol in the elderly. Annual Review of Medicine. 1995;46:123–132. doi: 10.1146/annurev.med.46.1.123. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, Meyerhoff DJ. A comparison of neurocognitive function in nonsmoking and chronically smoking short-term abstinent alcoholics. Alcoholism. 2006;39(1):1–11. doi: 10.1016/j.alcohol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Foffman PL, et al. Effects of moderate alcohol consumption on the central nervous system. Alcoholism: Clinical and Experimental Research. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T. The genetics of alcoholism: Identifying specific genes through family studies. Addiction Biology. 2006;11(3-4):386–396. doi: 10.1111/j.1369-1600.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Oscar-Berman M. Alcoholism, aging, and functional cerebral asymmetries. Psychological Bulletin. 1989;106:128–147. doi: 10.1037/0033-2909.106.1.128. [DOI] [PubMed] [Google Scholar]
- Ende G, Welzel H, Walter S, Weber-Fahr W, Diehl A, Hermann D, et al. Monitoring the effects of chronic alcohol consumption and abstinence on brain metabolism: A longitudinal proton magnetic resonance spectroscopy study. Biological Psychiatry. 2005;58(12):974–980. doi: 10.1016/j.biopsych.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Enoch MA. Pharmacogenomics of alcohol response and addiction. American Journal of Pharmacogenomics. 2003;3(4):217–232. doi: 10.2165/00129785-200303040-00001. [DOI] [PubMed] [Google Scholar]
- Erbas B, Bekdik C, Erbengi G, Enunlu T, Aytac S, Kumbasar H, et al. Regional cerebral blood flow changes in chronic alcoholism using Tc-99m HMPAO SPECT. Comparison with CT parameters. Clinical Nuclear Medicine. 1992;17(2):123–127. doi: 10.1097/00003072-199202000-00012. [DOI] [PubMed] [Google Scholar]
- Errico AL, King AC, Lovallo WR, Parsons OA. Cortisol dysregulation and cognitive impairment in abstinent male alcoholics. Alcoholism: Clinical and Experimental Research. 2002;26(8):1198–1204. doi: 10.1097/01.ALC.0000025885.23192.FF. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning. Annals of the New York Academy of Science. 2003;985:233–250. [PubMed] [Google Scholar]
- Evert DL, Oscar-Berman M. Selective attentional processing and the right hemisphere: Effects of aging and alcoholism. Neuropsychology. 2001;15(4):452–461. [PubMed] [Google Scholar]
- Fein G, Chang M. Visual P300s in long-term abstinent chronic alcoholics. Alcoholism: Clinical and Experimental Research. 2006;30(12):2000–2007. doi: 10.1111/j.1530-0277.2006.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Finn P, Scheiner DL. Sub-diagnostic psychiatric comorbidity in alcoholics. Drug and Alcohol Dependence. 2007;87(2-3):139–145. doi: 10.1016/j.drugalcdep.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Klein L, Finn P. Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 2004;28(10):1487–1491. doi: 10.1097/01.alc.0000141642.39065.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, McGillivray S, Finn P. Normal performance on a simulated gambling task in treatment-naive alcohol-dependent individuals. Alcoholism: Clinical and Experimental Research. 2006;30(6):959–966. doi: 10.1111/j.1530-0277.2006.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcoholism: Clinical and Experimental Research. 2006;30(9):1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Weafer J. Alcohol impairment of behavior in men and women. Addiction. 2004;99(10):1237–1246. doi: 10.1111/j.1360-0443.2004.00805.x. [DOI] [PubMed] [Google Scholar]
- Finn PR. Motivation, working memory, and decision making: A cognitive-motivational theory of personality vulnerability to alcoholism. Cognitive Neuroscience Reviews. 2002;1(3):183–205. doi: 10.1177/1534582302001003001. [DOI] [PubMed] [Google Scholar]
- Finn PR, Sharkansky EJ, Brandt KM, Turcotte N. The effects of familial risk, personality, and expectancies on alcohol use and abuse. Journal of Abnormal Psychology. 2000;109(1):122–133. doi: 10.1037//0021-843x.109.1.122. [DOI] [PubMed] [Google Scholar]
- Foisy ML, Philippot P, Verbanck P, Pelc I, van der Straten G. Emotional facial expression decoding impairment in persons dependent on multiple substances: Impact of a history of alcohol dependence. Journal of Studies on Alcohol. 2005;66(5):673–681. doi: 10.15288/jsa.2005.66.673. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcoholism: Clinical and Experimental Research. 2007;31(3):395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. 3rd ed. Lippincott-Raven; New York: 1997. [Google Scholar]
- Fuster JM. The cognit: A network model of cortical representation. International Journal of Psychophysiology. 2006;60(2):125–132. doi: 10.1016/j.ijpsycho.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Gansler DA, Harris GJ, Oscar-Berman M, Streeter C, Lewis RF, Ahmed I, et al. Hypoperfusion of inferior frontal brain regions in abstinent alcoholics: A pilot SPECT study. Journal of Studies on Alcohol. 2000;61(1):32–37. doi: 10.15288/jsa.2000.61.32. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: Preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcoholism: Clinical and Experimental Research. 2005;29(8):1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Gebhardt CA, Naeser MA, Butters N. Computerized measures of CT scans of alcoholics: Thalamic region related to memory. Alcohol. 1984;1(2):133–140. doi: 10.1016/0741-8329(84)90069-7. [DOI] [PubMed] [Google Scholar]
- Giancola PR. Executive functioning and alcohol-related aggression. Journal of Abnormal Psychology. 2004;113(4):541–555. doi: 10.1037/0021-843X.113.4.541. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Alcohol-seeking behavior: The roles of the hypothalamic-pituitary-adrenal axis and the endogenous opioid system. Alcohol Health and Research World. 1998;22(3):202–210. [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Adams KM, Johnsongreene D, Koeppe RA, Junck L, Kluin KJ, et al. Effects of disulfiram on positron emission tomography and neuropsychological studies in severe chronic alcoholism. Alcoholism: Clinical and Experimental Research. 1996;20(8):1456–1461. doi: 10.1111/j.1530-0277.1996.tb01149.x. [DOI] [PubMed] [Google Scholar]
- Gilman S, Adams K, Koeppe RA, Berent S, Kluin KJ, Modell JG, et al. Cerebellar and frontal hypometabolism in alcoholic cerebellar degeneration studied with positron emission tomography. Annals of Neurology. 1990;28(6):775–785. doi: 10.1002/ana.410280608. [DOI] [PubMed] [Google Scholar]
- Goldberg E. The executive brain: Frontal lobes and the civilized mind. Oxford University Press; New York: 2001. [Google Scholar]
- Golden CJ, Graber B, Blose I, Berg R, Coffman J, Bloch S. Difference in brain densities between chronic alcoholic and normal control patients. Science. 1981;211(4481):508–510. doi: 10.1126/science.7455693. [DOI] [PubMed] [Google Scholar]
- Goldman D, Dean M, Brown GL, Bolos AM, Tokola R, Virkkunen M, et al. D2 dopamine receptor genotype and cerebrospinal fluid homovanillic acid, 5-hydroxyindoleacetic acid and 3-methoxy-4-hydroxyphenylglycol in alcoholics in Finland and the United States. Acta Psychiatrica Scandinavica. 1992;86(5):351–357. doi: 10.1111/j.1600-0447.1992.tb03279.x. [DOI] [PubMed] [Google Scholar]
- Grant SA, Millar K, Kenny GN. Blood alcohol concentration and psychomotor effects. British Journal of Anaesthesiology. 2000;85(3):401–406. doi: 10.1093/bja/85.3.401. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. 2nd ed Oxford University Press; Oxford, England: 2000. [Google Scholar]
- Grillon C, Sinha R, O’Malley SS. Effects of ethanol on the processing of low probability stimuli: An ERP study. Psychopharmacology (Berlin) 1995;119(4):455–465. doi: 10.1007/BF02245862. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Wong A, Svoboda M, Kril JJ, Halliday GM. Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus. 1997;7(10):78–87. doi: 10.1002/(SICI)1098-1063(1997)7:1<78::AID-HIPO8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Harper CG. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? Journal of Neuropathology and Experimental Neurology. 1998;57(2):101–110. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Holloway RL. Brain shrinkage in chronic alcoholics: A pathological study. British Medical Journal. 1985;290(6467):501–504. doi: 10.1136/bmj.290.6467.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Matsumoto I. Ethanol and brain damage. Current Opinion in Pharmacology. 2005;5:73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, et al. Right frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Biological Psychiatry. 2007 doi: 10.1111/j.1530-0277.2008.00661.x. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GJ, Oscar-Berman M, Gansler DA, Streeter C, Lewis RF, Ahmed I, et al. Hypoperfusion of cerebellum and aging effects on cerebral cortex blood flow in abstinent alcoholics: A SPECT study. Alcoholism: Clinical and Experimental Research. 1999;23(7):1219–1227. [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Mann K, Weinberger DR, Goldman D. Serotonergic dysfunction, negative mood states, and response to alcohol. Alcoholism: Clinical and Experimental Research. 2001;25(4):487–495. [PubMed] [Google Scholar]
- Helzer JE, Bucholz KK, Bierut LJ, Regier DA, Schuckit MA, Guth SE. Should DSM-V include dimensional diagnostic criteria for alcohol use disorders? Alcoholism: Clinical and Experimental Research. 2006;30(2):303–310. doi: 10.1111/j.1530-0277.2006.00028.x. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, et al. Selective impairment of hippocampal neurogenesis by chronic alcoholism: Protective effects of an antioxidant. Proceedings of the National Academy of Sciences. 2003;100(13):7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock VM, Hesselbrock MN, Stabenau JR. Alcoholism in men patients subtyped by family history and antisocial personality. Journal of Studies on Alcohol. 1985;46(1):59–64. doi: 10.15288/jsa.1985.46.59. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR. Event-related potentials in women at risk for alcoholism. Alcohol. 1993;10:349–354. doi: 10.1016/0741-8329(93)90019-k. [DOI] [PubMed] [Google Scholar]
- Hoaken PN, Stewart SH. Drugs of abuse and the elicitation of human aggressive behavior. Addictive Behaviors. 2003;28(9):1533–1554. doi: 10.1016/j.addbeh.2003.08.033. [DOI] [PubMed] [Google Scholar]
- Hommer DW, Momenan R, Kaiser E, Rawlings RR. Evidence for a gender-related effect of alcoholism on brain volumes. American Journal of Psychiatry. 2001;158(2):198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Hommer DW, Momenan R, Rawlings RR, Ragan P, Williams W, Rio D, et al. Decreased corpus callosum size among alcoholic women. Archives of Neurology. 1996;53(4):359–363. doi: 10.1001/archneur.1996.00550040099019. [DOI] [PubMed] [Google Scholar]
- Howard JA, Oscar-Berman M, Marinkovic K, O’Reilly C, Harris GJ. Affective and cognitive changes in alcoholism: Recognition of faces and words; Paper presented at the Society for Neuroscience Annual Meeting; Washington, DC. 2003. [Google Scholar]
- Jääskeläinen IP, Schroger E, Näätänen R. Electrophysiological indices of acute effects of ethanol on involuntary attention shifting. Psychopharmacology (Berlin) 1999;141(1):16–21. doi: 10.1007/s002130050801. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berlin) 1999;146(4):373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Johnson C, Drgon T, Liu QR, Walther D, Edenberg H, Rice J, et al. Pooled association genome scanning for alcohol dependence using 104,268 SNPs: Validation and use to identify alcoholism vulnerability loci in unrelated individuals from the collaborative study on the genetics of alcoholism. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2006;141(8):844–853. doi: 10.1002/ajmg.b.30346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Greene D, Adams KM, Gilman S. Effects of abstinence and relapse upon neuropsychological function and cerebral glucose metabolism in severe chronic alcoholism. Journal of Clinical and Experimental Neuropsychology. 1997;19(3):378–385. doi: 10.1080/01688639708403866. [DOI] [PubMed] [Google Scholar]
- Jones B, Parsons OA. Impaired abstracting ability in chronic alcoholics. Archives of General Psychiatry. 1971;24(1):71–75. doi: 10.1001/archpsyc.1971.01750070073010. [DOI] [PubMed] [Google Scholar]
- Kalant H, Gomberg E, Hegedius A, Zucker R. Pharmacological interactions of aging and alcohol. In: Gomberg E, Hegedius A, Zucker R, editors. Alcohol Problems and Aging. National Institutes of Health; Bethesda: 1998. pp. 99–116. vol. NIAAA Research Monograph no. 33 (Publication no. 98-4163) [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE, Merikangas KR. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proceedings of the National Academy of Sciences. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatsky AL. Alcohol, cardiovascular diseases and diabetes mellitus. Pharmacological Research. 2007:55. doi: 10.1016/j.phrs.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Koelega HS. Alcohol and vigilance performance: A review. Psychopharmacology (Berlin) 1995;118(3):233–249. doi: 10.1007/BF02245951. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: Allostasis and beyond. Alcoholism: Clinical and Experimental Research. 2003;27(2):232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Philippot P, Foisy ML, Blairy S, Raynaud E, Dan B, et al. Impaired emotional facial expression recognition is associated with interpersonal problems in alcoholism. Alcohol and Alcoholism. 2002;37(4):394–400. doi: 10.1093/alcalc/37.4.394. [DOI] [PubMed] [Google Scholar]
- Kostandov EA, Arsumanov YL, Genkina OA, Restchikova TN, Shostakovich GS. The effects of alcohol on hemispheric functional asymmetry. Journal of Studies on Alcohol. 1982;43(5):411–426. doi: 10.15288/jsa.1982.43.411. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: A meta-analysis. Alcoholism: Clinical and Experimental Research. 2001;25(9):1335–1341. [PubMed] [Google Scholar]
- Kroft CL, Gescuk B, Woods BT, Mello NK, Weiss RD, Mendelson JH. Brain ventricular size in female alcoholics: An MRI study. Alcoholism: Clinical and Experimental Research. 1991;8(1):31–34. doi: 10.1016/0741-8329(91)91200-l. [DOI] [PubMed] [Google Scholar]
- Kurth C, Wegerer V, Reulbach U, Lewczuk P, Kornhuber J, Steinhoff BJ, et al. Analysis of hippocampal atrophy in alcoholic patients by a Kohonen feature map. Neuroreport. 2004;15(2):367–371. doi: 10.1097/00001756-200402090-00031. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Gunning-Dixon F, Vaurio O, Repo-Tiihonen E, Soininen H, Tiihonen J. Prefrontal volumes in habitually violent subjects with antisocial personality disorder and type 2 alcoholism. Psychiatry Research. 2002;114(2):95–102. doi: 10.1016/s0925-4927(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Savolainen L, Repo E, Soininen H, Aronen HJ, et al. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behavioral Brain Research. 2000;109(2):177–186. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews: Neuroscience. 2006;7(1):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lancaster Gender differences in animal studies. Implications for the study of human alcoholism. Recent Developments in Alcoholism. 1995;12:209–215. [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain, fear, and the amygdala. Cell and Molecular Neurobiology. 2003;23(4-5):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Moller L, Hardt F, Haubek A, Jensen E. Alcohol-induced brain damage and liver damage in young males. Lancet. 1979;2(8146):759–761. doi: 10.1016/s0140-6736(79)92113-5. [DOI] [PubMed] [Google Scholar]
- Lichter DG, Cummings JL, editors. Frontal-subcortical circuits in psychiatric and neurological disorders. The Guilford Press; New York: 2001. [Google Scholar]
- Lovallo WR. The hypothalamic-pituitary-adrenocortical axis in addiction. International Journal of Psychophysiology. 2006;59(3):193–194. doi: 10.1016/j.ijpsycho.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyvers M. “Loss of control” in alcoholism and drug addiction: A neuroscientific interpretation. Experimental and Clinical Psychopharmacology. 2000;8(2):225–249. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, et al. Decreased volume of the brain reward system in alcoholism. Alcoholism: Clinical and Experimental Research. 2007 doi: 10.1016/j.biopsych.2008.01.018. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. Pharmacotherapy of alcohol dependence: A review of the clinical data. CNS Drugs. 2004;18(8):485–504. doi: 10.2165/00023210-200418080-00002. [DOI] [PubMed] [Google Scholar]
- Mann K, Batra A, Gunthner A, Schroth G. Do women develop alcoholic brain damage more readily than men? Alcoholism: Clinical and Experimental Research. 1992;16(6):1052–1056. doi: 10.1111/j.1530-0277.1992.tb00698.x. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Abroms BD, Van Selst M, Fillmore MT. Alcohol-induced impairment of behavioral control: Differential effects on engaging vs. disengaging responses. Psychopharmacology (Berlin) 2005;182(3):452–459. doi: 10.1007/s00213-005-0116-2. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Experimental and Clinical Psychopharmacology. 2003;11(1):110–117. doi: 10.1037//1064-1297.11.1.110. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Halgren E, Klopp J, Maltzman I. Alcohol effects on movement-related potentials: A measure of impulsivity? Journal of Studies on Alcohol. 2000;61(1):24–31. doi: 10.15288/jsa.2000.61.24. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Halgren E, Maltzman I. Arousal-related P3a to novel auditory stimuli is abolished by moderately low alcohol dose. Alcohol and Alcoholism. 2001;36(6):529–539. doi: 10.1093/alcalc/36.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Halgren E, Maltzman I. Effects of alcohol on verbal processing: An ERP study. Alcoholism: Clinical and Experimental Research. 2004;28(3):415–423. doi: 10.1097/01.alc.0000117828.88597.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O’Reilly CE, Howard JA, Sawyer K, et al. Decreased limbic activation to emotional paces in chronic alcoholics. 2007 doi: 10.1111/j.1530-0277.2009.01026.x. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR, Nimmerrichter A, Riddle WR, Welch LW, Willcott MR. Brain proton magnetic resonance spectroscopy studies in recently abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19(4):1078–1082. doi: 10.1111/j.1530-0277.1995.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early-onset alcoholism. Alcoholism: Clinical and Experimental Research. 2000;24(7):1036–1040. [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behavioral Neuroscience. 2004;118(1):63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- Melgaard B, Henriksen L, Ahlgren P, Danielsen UT, Sorensen H, Paulson OB. Regional cerebral blood flow in chronic alcoholics measured by single photon emission computerized tomography. Acta Neurologica Scandinavica. 1990;82(2):87–93. doi: 10.1111/j.1600-0404.1990.tb01594.x. [DOI] [PubMed] [Google Scholar]
- Monnot M, Lovallo WR, Nixon SJ, Ross E. Neurological basis of deficits in affective prosody comprehension among alcoholics and fetal alcohol-exposed adults. Journal of Neuropsychiatry and Clinical Neuroscience. 2002;14(3):321–328. doi: 10.1176/jnp.14.3.321. [DOI] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: A review of the literature. Alcohol and Alcoholism. 2001;36(5):357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- Mulvihill LE, Skilling TA, Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. Journal of Studies on Alcohol. 1997;58(6):600–605. doi: 10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- Mungas D, Ehlers CL, Wall TL. Effects of acute alcohol administration on verbal and spatial learning. Alcohol and Alcoholism. 1994;29(2):163–169. [PubMed] [Google Scholar]
- Murdoch D, Phil RO, Ross D. Alcohol and crimes of violence: present issues. International Journal of Addictions. 1990;25(9):1065–1081. doi: 10.3109/10826089009058873. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research: Neuroimaging. 2005;139(3):181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi CT, Wilson JR. Long-term repeatability of human alcohol metabolism, sensitivity and acute tolerance. Journal of Studies on Alcohol. 1989;50(2):162–169. doi: 10.15288/jsa.1989.50.162. [DOI] [PubMed] [Google Scholar]
- NIAAA . Ninth special report to the US Congress on alcohol and health. NIAAA; Bethesda, MD: 1997. [Google Scholar]
- Nicolás JM, Catafau AM, Estruch R, Lomeña FJ, Salamero M, Herranz R, et al. Regional cerebral blood flow-SPECT in chronic alcoholism: Relation to neuropsychological testing. Journal of Nuclear Medicine. 1993;34(9):1452–1459. [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: Roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16(3):287–295. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Noel X, Van der Linden M, d’Acremont M, Bechara A, Dan B, Hanak C, et al. Alcohol cues increase cognitive impulsivity in individuals with alcoholism. Psychopharmacology (Berlin) 2007 doi: 10.1007/s00213-006-0695-6. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Hilt L. Possible contributors to the gender differences in alcohol use and problems. Journal of General Psychology. 2006;133(4):357–374. doi: 10.3200/GENP.133.4.357-374. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ. Effects of abstinence on the brain: Quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcoholism: Clinical and Experimental Research. 2001;25(11):1673–1682. [PubMed] [Google Scholar]
- Oscar-Berman M, Bowirrat A. Genetic influences in emotional dysfunction and alcoholism-related brain damage. Neuropsychiatric Disease and Treatment. 2005;1(3):211–229. [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Evert DL. Alcoholic Korsakoff’s syndrome. In: Nussbaum PD, editor. Handbook of neuropsychology and aging. Plenum; New York: 1997. pp. 201–215. [Google Scholar]
- Oscar-Berman M, Hancock M, Mildworf B, Hutner N, Weber DA. Emotional perception and memory in alcoholism and aging. Alcoholism: Clinical and Experimental Research. 1990;14(3):383–393. doi: 10.1111/j.1530-0277.1990.tb00491.x. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Hutner N. Frontal lobe changes after chronic alcohol ingestion. In: Hunt WA, Nixon SJ, editors. Alcohol-induced brain damage. NIAAA; Rockville, MD: 1993. pp. 121–156. vol. Monograph No. 22. [Google Scholar]
- Oscar-Berman M, Kirkley SM, Gansler DA, Couture A. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcoholism: Clinical and Experimental Research. 2004;28(4):667–675. doi: 10.1097/01.alc.0000122761.09179.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcoholism and the brain: An overview. Alcohol Research and Health. 2003;27(2):125–133. [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Schendan HE. Asymmetries of brain function in alcoholism: Relationship to aging. In: Obler L, Connor LT, editors. Neurobehavior of language and cognition: Studies of normal aging and brain damage. Kluwer Academic Publishers; New York: 2000. pp. 213–240. [Google Scholar]
- Oscar-Berman M, Shagrin B, Evert DL, Epstein C. Impairments of brain and behavior. The neurological effects of alcohol. Alcohol Health and Research World. 1997;21(1):65–75. [PMC free article] [PubMed] [Google Scholar]
- Parsons OA. Neuropsychological consequences of alcohol abuse: Many questions—some answers. In: Parsons O, Butters N, Nathan P, editors. Neuropsychology of alcoholism. Guilford Press; New York: 1987. pp. 153–175. [Google Scholar]
- Parsons OA. Neuropsychological measures and event-related potentials in alcoholics: Interrelationships, long-term reliabilities, and prediction of resumption of drinking. Journal of Clinical Psychology. 1994;50(1):37–46. doi: 10.1002/1097-4679(199401)50:1<37::aid-jclp2270500105>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Parsons OA. Alcohol abuse and alcoholism. In: Adams RL, Parsons OA, Culbertson JL, Nixon SJ, editors. Neuropsychology for clinical practice. American Psychological Press; Washington, D.C.: 1996. pp. 175–201. [Google Scholar]
- Parsons OA, Leber WR. The relationship between cognitive dysfunction and brain damage in alcoholics: Causal, interactive, or epiphenomenal? Alcoholism: Clinical and Experimental Research. 1981;5(2):326–343. doi: 10.1111/j.1530-0277.1981.tb04906.x. [DOI] [PubMed] [Google Scholar]
- Pentney RJ, Mullan BA, Felong AM, Dlugos CA. The total numbers of cerebellar granule neurons in young and aged Fischer 344 and Wistar-Kyoto rats do not change as a result of lengthy ethanol treatment. Cerebellum. 2002;1(1):79–89. doi: 10.1080/147342202753203113. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Rothfleisch J, Zelazo PD, Pihl RO. Acute alcohol intoxication and cognitive functioning. Journal of Studies on Alcohol. 1990;51(2):114–122. doi: 10.15288/jsa.1990.51.114. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Gonzalez G, Rosenheck R, Krystal JH. Comorbidity of alcoholism and psychiatric disorders. Alcohol Research and Health. 2002;26(1):81–89. [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26(3):891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiology of Aging. 2006;27(7):994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: An fMRI study. NeuroImage. 2001;14(1 Pt 1):7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: A magnetic resonance imaging study. Alcoholism: Clinical and Experimental Research. 1996;20(4):752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan EV. Sex differences in the effects of alcohol on brain structure. American Journal of Psychiatry. 2001;158(2):188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Serventi KL, Sullivan EV. Corpus callosum, pons, and cortical white matter in alcoholic women. Alcoholism: Clinical and Experimental Research. 2002;26(3):400–406. [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. NeuroImage. 2002;15(3):708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: Evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30(2):423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1997;21(3):521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19(5):1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Archives of General Psychiatry. 1998;55(10):905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14(2):198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Philippot P, Kornreich C, Blairy S, Baert I, Den Dulk A, Le Bon O, et al. Alcoholics’ deficits in the decoding of emotional facial expression. Alcoholism: Clinical and Experimental Research. 1999;23(6):1031–1038. [PubMed] [Google Scholar]
- Phillips SC, Harper CG, Kril J. A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain. 1987;110:301–314. doi: 10.1093/brain/110.2.301. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proceedings of the National Academy of Sciences of the USA. 1996;93(16):8716–8720. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihl RO, Peterson JB, Lau MA. A biosocial model of the alcohol-aggression relationship. Journal of Studies on Alcohol. 1993;11(Suppl):128–139. doi: 10.15288/jsas.1993.s11.128. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampus formation, perirhinal cortex, and postrhinal cortex in rat. A review. In: Scharfman HE, Schwarcz R, Witter MP, editors. The parahippocampal region: Implications for neurological and psychiatric diseases. Vol. 911. Annals of the New York Academy of Sciences; New York: 2000. pp. 369–391. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Brick J. Activity of neurons in the locus coeruleus of the rat: Inhibition by ethanol. Brain Research. 1977;131(1):174–179. doi: 10.1016/0006-8993(77)90039-7. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Evoked brain potential deficits in alcoholism and aging. Alcoholism: Clinical and Experimental Research. 1982;6(1):53–63. doi: 10.1111/j.1530-0277.1982.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Human brain electrophysiology and alcoholism. In: Tarter RE, Van Thiel DH, editors. Alcohol and the brain. Plenum Press; New York: 1985. pp. 139–182. [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57(2):119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Ardekani BA, Choi SJ, Tanabe JL, Lim KO, et al. A functional MRI study of visual oddball: Evidence for frontoparietal dysfunction in subjects at risk for alcoholism. Neuroimage. 2004;21(1):329–339. doi: 10.1016/j.neuroimage.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Ratti MT, Bo P, Giardini A, Soragna D. Chronic alcoholism and the frontal lobe: Which executive functions are impaired? Acta Neurologica Scandinavica. 2002;105(4):276–281. doi: 10.1034/j.1600-0404.2002.0o315.x. [DOI] [PubMed] [Google Scholar]
- Reed TE. The myth of “the average alcohol response.”. Alcohol. 1985;2(3):515–519. doi: 10.1016/0741-8329(85)90126-0. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) study. Journal of the American Medical Association. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nature Neuroscience. 2004;7(3):278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, et al. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298(5601):2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Precis of the brain and emotion. Behavioral and Brain Sciences. 2000;23:177–234. doi: 10.1017/s0140525x00002429. [DOI] [PubMed] [Google Scholar]
- Rosenbloom M, Sullivan EV, Pfefferbaum A. Using magnetic resonance imaging and diffusion tensor imaging to assess brain damage in alcoholics. Alcohol Research and Health. 2003;27(2):146–152. [PMC free article] [PubMed] [Google Scholar]
- Rourke SB, Løberg T. The neurobehavioral correlates of alcoholism. In: Grant I, Nixon SJ, editors. Neuropsychological assessment of neuropsychiatric disorders. 2nd ed. Oxford University Press; New York: 1996. pp. 423–485. [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: Anatomy and physiology. Physiological Review. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Samson Y, Baron JC, Feline A, Bories J, Crouzel C. Local cerebral glucose utilisation in chronic alcoholics: A positron tomographic study. Journal of Neurology, Neurosurgery and Psychiatry. 1986;49(10):1165–1170. doi: 10.1136/jnnp.49.10.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Bradley RJ, Harris RA, Jenner P, editors. The cerebellum and cognition (vol. 41), International review of neurobiology. Academic Press; San Diego: 1997. [Google Scholar]
- Schmahmann JD. The role of the cerebellum in affect and psychosis. Journal of Neurolinguistics. 2000;13:189–214. [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J. The search for genes contributing to the low level of response to alcohol: Patterns of findings across studies. Alcoholism: Clinical and Experimental Research. 2004;28:1449–1458. doi: 10.1097/01.alc.0000141637.01925.f6. [DOI] [PubMed] [Google Scholar]
- Schulte T, Sullivan EV, Muller-Oehring EM, Adalsteinsson E, Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: A diffusion tensor imaging study. Cerebral Cortex. 2005;15(9):1384–1392. doi: 10.1093/cercor/bhi020. [DOI] [PubMed] [Google Scholar]
- Seitz D, Widmann U, Seeger U. Localized protein magnetic resonance spectroscopy of the cerebellum in detoxifying alcoholics. Alcoholism: Clinical and Experimental Research. 1999;23(1):158–163. [PubMed] [Google Scholar]
- Shear PK, Jernigan TL, Butters N. Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1994;18(1):172–176. doi: 10.1111/j.1530-0277.1994.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress-and cue-induced drug and alcohol craving: Association with relapse and clinical implications. Drug and Alcohol Review. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Smith ME, Oscar-Berman M. Resource-limited information processing in alcoholism. Journal of Studies on Alcohol. 1992;53(5):514–518. doi: 10.15288/jsa.1992.53.514. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kaplan RF, Hesselbrock VM. Executive-cognitive functioning in the development of antisocial personality disorder. Addictive Behaviors. 2003;28(2):285–300. doi: 10.1016/s0306-4603(01)00232-5. [DOI] [PubMed] [Google Scholar]
- Stranges S, Freudenheim JL, Muti P, Farinaro E, Russell M, Nochajski TH, et al. Differential effects of alcohol drinking pattern on liver enzymes in men and women. Alcoholism: Clinical and Experimental Research. 2004;28(6):949–956. doi: 10.1097/01.alc.0000128229.23396.42. [DOI] [PubMed] [Google Scholar]
- Stranges S, Wu T, Dorn JM, Freudenheim JL, Muti P, Farinaro E, et al. Relationship of alcohol drinking pattern to risk of hypertension: A population-based study. Hypertension. 2004;44(6):813–819. doi: 10.1161/01.HYP.0000146537.03103.f2. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Ciraulo DA, Harris GJ, Kaufman MJ, Lewis RF, Knapp CM, et al. Functional magnetic resonanceimaging of alprazolam-induced changes in humans with familial alcoholism. Psychiatry Research. 1998;82(2):69–82. doi: 10.1016/s0925-4927(98)00009-2. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. Neuropsychological vulnerability to alcoholism: Evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s neuroscience and behavioral research. NIAAA; Bethesda, MD: 2000. pp. 473–508. vol. Monograph No. 34. [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebellotha-lamocortical systems: Speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcoholism: Clinical and Experimental Research. 2003;27(9):1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16(1):74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, et al. Disruption of frontocerebellar circuitry and function in alcoholism. Alcoholism: Clinical and Experimental Research. 2003;27(2):301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19(1):110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology (Berlin) 2005;180(4):583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: Relationships to changes in brain structure. Neuropsychology. 2000;14(2):178–188. [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism: Clinical and Experimental Research. 2000;24(5):611–621. [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism: Clinical and Experimental Research. 2001;21(2):236–245. [PubMed] [Google Scholar]
- Townshend JM, Duka T. Mixed emotions: Alcoholics’ impairments in the recognition of specific emotional facial expressions. Neuropsychologia. 2003;41(7):773–782. doi: 10.1016/s0028-3932(02)00284-1. [DOI] [PubMed] [Google Scholar]
- Victor M. The effects of alcohol on the nervous system. In: Lieber CS, editor. Medical and nutritional complications of alcoholism: Mechanisms and management. Plenum; New York: 1992. pp. 413–457. [Google Scholar]
- Virkkunen M, Linnoila M. Brain serotonin, type II alcoholism and impulsive violence. Journal of Studies on Alcohol (Supplement) 1993;11:163–169. doi: 10.15288/jsas.1993.s11.163. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Burr G, Pascani K, et al. Decreased brain metabolism in neurologically intact healthy alcoholics. American Journal of Psychiatry. 1992;149(8):1016–1022. doi: 10.1176/ajp.149.8.1016. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Burr G, Pascani K, et al. Monitoring the brain’s response to alcohol with positron emission tomography. Alcohol Health and Research World. 1995;19:296–299. [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, et al. Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: A preliminary study. Psychiatry Research. 2002;116(3):163–172. doi: 10.1016/s0925-4927(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Wang L, McCarthy G, Song AW, Labar KS. Amygdala activation to sad pictures during high-field (4 tesla) functional magnetic resonance imaging. Emotion. 2005;5(1):12–22. doi: 10.1037/1528-3542.5.1.12. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Franceschi D, Wong CT, Pappas NR, et al. Alcohol intoxication induces greater reductions in brain metabolism in male than in female subjects. Alcoholism: Clinical and Experimental Research. 2003;27(6):909–917. doi: 10.1097/01.ALC.0000071740.56375.BA. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Pappas NR, Wong CT, Pascani K, et al. Regional cerebral metabolism in female alcoholics of moderate severity does not differ from that of controls. Alcoholism: Clinical and Experimental Research. 1998;22(8):1850–1854. [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Roque CT, Cestaro VL, Hitzemann RJ, Cantos EL, et al. Functional importance of ventricular enlargement and cortical atrophy in healthy subjects and alcoholics as assessed with PET, MR imaging, and neuropsychologic testing. Radiology. 1993;186(1):59–65. doi: 10.1148/radiology.186.1.8416587. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18(1):411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Matthews DB, Best PJ. Ethanol, memory, and hippocampal function: A review of recent findings. Hippocampus. 2000;10:88–93. doi: 10.1002/(SICI)1098-1063(2000)10:1<88::AID-HIPO10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Whitfield JB, Zhu G, Madden PA, Neale MC, Heath AC, Martin NG. The genetics of alcohol intake and of alcohol dependence. Alcoholism: Clinical and Experimental Research. 2004;28(8):1153–1160. doi: 10.1097/01.alc.0000134221.32773.69. [DOI] [PubMed] [Google Scholar]
- Wilkinson DA, Carlen PL. Relation of neuropsychological test performance in alcoholics to brain morphology measured by computed tomography. Advances in Experimental and Medical Biology. 1980;126:683–699. doi: 10.1007/978-1-4684-3632-7_51. [DOI] [PubMed] [Google Scholar]
- Wilkinson DA, Carlen PL. Morphological abnormalities in the brains of alcoholics: Relationship to age, psychological test scores and patient type. In: Wood WG, Elias MF, editors. Alcoholism and Aging: Advances in Research. CRC Press; Boca Raton, FL: 1982. pp. 61–77. [Google Scholar]
- Wilson GT, Niaura R. Alcohol and the disinhibition of sexual responsiveness. Journal of Studies on Alcohol. 1984;45(3):219–224. doi: 10.15288/jsa.1984.45.219. [DOI] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. Neuroimage. 2003;20(1):84–97. doi: 10.1016/s1053-8119(03)00303-3. [DOI] [PubMed] [Google Scholar]
- Wise RA. Psychomotor stimulant properties of addictive drugs. Annals of the New York Academy of Sciences. 1988;537:228–234. doi: 10.1111/j.1749-6632.1988.tb42109.x. [DOI] [PubMed] [Google Scholar]
- Wuethrich B. Does alcohol damage female brains more? Science. 2001;291(5511):2077–2079. doi: 10.1126/science.291.5511.2077. [DOI] [PubMed] [Google Scholar]
- Yonker JE, Nilsson LG, Herlitz A, Anthenelli RM. Sex differences in spatial visualization and episodic memory as a function of alcohol consumption. Alcohol and Alcoholism. 2005;40(3):201–207. doi: 10.1093/alcalc/agh141. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Begleiter H, Porjesz B. Is working memory intact in alcoholics? An ERP study. Psychiatry Research. 1997;75(2):75–89. [PubMed] [Google Scholar]