Abstract
Adiponectin is a circulating bioactive hormone secreted by adipocytes as oligomers ranging in size from 90 kDa trimers and 180 kDa hexamers to larger high molecular weight oligomers that may reach 18- or 36-mers in size. While total circulating adiponectin levels correlate well with metabolic health, it is the relative distribution of adiponectin complexes that is most clinically relevant to glucose sensitivity and inflammation. High molecular weight adiponectin best mirrors insulin sensitivity, while trimeric adiponectin dominates with insulin resistance and adipose tissue inflammation. Experimental animal and in vitro models have also linked the relative fraction of high molecular weight adiponectin to its positive effects. Quantitating adiponectin size distribution thus provides a window into metabolic health and can serve as a surrogate marker for adipose tissue fitness.
Here, we present a detailed protocol for isolating and quantitating adiponectin complexes in serum or plasma that has been extensively utilized for both human clinical samples and numerous animal models under various experimental conditions. Examples are presented of different adiponectin distributions and tips are provided for optimization using available equipment. Comparison of this rigorous approach to other available methods is also discussed. In total, this summary is a blueprint for the expanded quantitation and study of adiponectin complexes.
1. INTRODUCTION
Adipose tissue is at the center of the worldwide obesity epidemic as an important endocrine tissue that plays a literally expanding role in systemic metabolic health with weight gain. While most characterized adipocyte secretory proteins increase with increased adiposity and serve as signals of metabolic dysregulation or adipose tissue inflammation, adiponectin is unique in its positive correlation with metabolic health (Turer & Scherer, 2012). As adipose tissue expands, Body Mass Index (BMI) is increased, and insulin sensitivity is limited, and circulating levels of adiponectin are reduced (Kusminski & Scherer, 2009). Inversely, adiponectin levels are “normal” at lower BMIs and can be increased upon weight loss. These extensive clinical correlations have been confirmed in a host of animal models and in vitro studies (Turer&Scherer,2012).Thisrelationshipisnotmerelycorrelative,however, as adiponectin is a potent regulator of cellular glucose and lipid metabolism and has demonstrated numerous cytoprotective effects (Kusminski & Scherer, 2009).These findingshave mademeasuringadiponectin levelsacross a host of pathologies both clinically interesting and experimentally essential. Adiponectin circulates as multimeric complexes; its oligomerization state may reflect the degree of adiponectin's metabolic benefits.
Adiponectin is an approximately 30 kDa protein secreted from adipocytes as a highly stable 90 kDa trimer, two-trimer hexamers (sometimes referred to as low molecular weight, LMW), and multihexamers of 12, 18, or 36 total monomers in size (Pajvani et al., 2003). It is these high molecular weight (HMW) complexes that have demonstrated the most profound examples of adiponectin's prometabolic and anti-inflammatory potential (Pajvani et al., 2004). Measurements capable of differentiating HMW adiponectin, or its fractional distribution (as compared to circulating trimer abundance), have been the most well-correlated to metabolic health and general wellbeing (Baessler et al., 2011; Hamilton et al., 2011; Lo et al., 2011; Matsumoto, Toyomasu, Uchimura, & Ishitake, 2013). Thus, while total levels are important—and indeed reflective of adipose tissue health—the fractional amount of HMW may be the best biomarker of clinical and experimental model relevance.
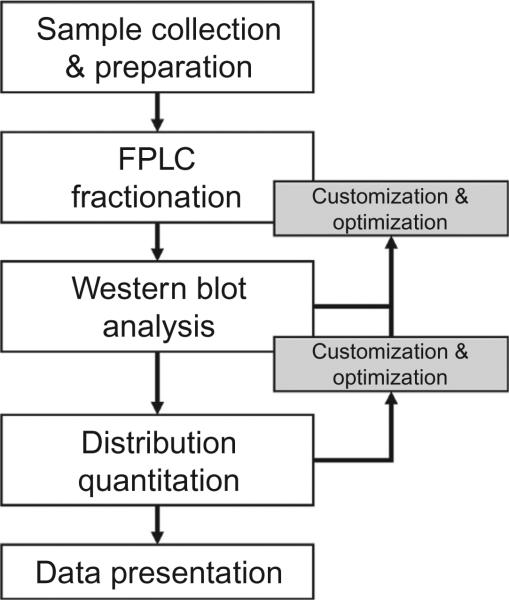
There have been more than 400 manuscripts in which the researchers have performed distribution measurements and then taken advantage of analyzed differences in adiponectin oligomer size when interpreting their results. Some early examples include Bobbert et al. (2005), Fisher et al. (2005), Kobayashi et al. (2004), Lara-Castro, Luo, Wallace, Klein, and Garvey (2006), and Pajvani et al. (2004); each highlighting the importance and contribution of adiponectin oligomers. The fast protein liquid chromatography (FPLC)-based protocol presented here has been utilized for many years by many users and researchers and has been refined continuously with column performance and sample volumes. This approach was originally established in Schraw, Wang, Halberg, Hawkins, and Scherer (2008). Figure 13.1 is a representative flowchart of the protocol that highlights the customization and optimization of the procedures as the methods are put into place locally and feedback is generated; potential changes to this outline are highlighted in each section.
Figure 13.1.
Flowchart of adiponectin complex separation, isolation, and quantitation utilizing FPLC and Western blot. At multiple steps during the suggested procedure there are opportunities for customization and optimization of individual equipment or sample needs.
2. SAMPLE COLLECTION AND PREPARATION
Adiponectin complexes are highly stable. Briefly, the structure and stability of adiponectin oligomers is based in the collagenous domain: numerous posttranslational modifications along the collagenous domain are necessary for trimer formation and mutations to this sequence result in adiponectin that is unable to be secreted (Wang, Lam, Yau, & Xu, 2008). This synthesis is a primary reason why recombinant production of full-length adiponectin complexes requires mammalian cells. Higher order adiponectin complex formation requires two trimers forming a hexamer by disulfide bond at cysteine 36 (C39 in mice); hexamer formation is requisite for the formation of subsequent higher molecular weight forms (Pajvani et al., 2003; Wang et al., 2008). The noncovalent bonding of HMW forms is thus only broken under low pH or reducing conditions.
The stability of adiponectin to light, temperature, and serum proteases allows for analysis of adiponectin complexes in samples that have not been optimally maintained (e.g., having experienced multiple freeze–thaw cycles). Adiponectin can be measured in samples that have been stored at —20 or —80 °C for many years, if not decades, provided they have not been dehydrated over years in storage. It is still recommended, however, that samples be carefully handled and maintained as any serum or plasma sample.
Clinical collections of serum or plasma using lithium heparin or EDTA have been tested and are recommended.
Complexes are, however, sensitive to even modest acidic conditions and become destabilized below pH 7. If samples are shipped on dry ice, care has to be taken to use tightly capped tubes. Alternatively, additional buffer (e.g., 50 mM HEPES, pH 7.5 final concentration) can be added to ensure that the pH stays above 7.0 upon the unintended exposure to CO2 in shipment.
Samples must be centrifuged before proceeding to fractionation. Even if care was taken in removing serum or plasma from the collected blood, it is essential for best practice that centrifugation at >10,000 × g for 5 min be utilized to ensure that no debris enters the FPLC system. Filtration of samples is strongly discouraged due to the potential exclusion of the large-sized HMW adiponectin complexes. Samples may then either be loaded directly to the FPLC or diluted in running buffer (Section 3.1) depending on loading method or capabilities of a given system (Table 13.1).
Table 13.1.
Suggested sample dilutions based on FPLC loading method and standard loop sizes
| Loading method | Volume sample (μL) | Volume buffer (μL) |
|---|---|---|
| Micro syringe | ||
| 25 μL sample loop | 25 | 10 |
| 50 μL sample loop | 25 | 35 |
| 200 μL sample loop | 25 | 200 |
| Standard syringea | ||
| 200 μL sample loop | 40 | 360 |
| Autosampler | Dead volume-dependent. Sample must be prepared such that actual injection volume contains 20–25 μL sample | |
Standard syringes are not recommended for smaller sample loops; this loading volume will affect elution time precision.
Volumes are based on 20 μL of sample reaching the column and no air potentially remaining in the sample loop. Samples low in adiponectin may require increased sample volumes (with reduced buffer volume).
3. GEL FRACTIONATION BY FPLC
To analyze abundance of adiponectin complexes, high-resolution molecular weight separation must be employed. While defined here as fractionation by FPLC, the actual type of pump system used is irrelevant because the end pressure and flow rate considerations are determined by the selected column performance capabilities. All of the work carried out in our laboratory has utilized anÁKTA FPLC system equipped with a single Superdex 200 10/300 GL column (both GE Healthcare Biosciences; Pittsburgh, PA). One of our units relies on anÁKTA HPLC pump, but the end settings in operation are essentially identical when the same column is employed.
The Superdex 200 10/300 GL column is ideal for the task of separating adiponectin complexes from serum samples because it offers a large bed volume (50 μL sample volume) and provides excellent separation from 10 to 600 kDa. Another column with similar separation characteristics may be utilized; the following procedure details are based on the Superdex 200 10/300 GL column. Optimization of the fractionation protocol (Section 3.3) may help when using another system or column to achieve comparable separation results to proceed to analysis. None of this analytical procedure or system needs to be performed in or placed into a 4 °C cooler.
3.1. Required materials
3.1.1 Separation system
The flow rates and volumetric settings described in the protocol are based on using the listed gel filtration column.
Superdex 200 10/300 GL column
- FPLC/HPLC system equipped with
- — 280 nm UV lamp or 214 nm for visualizing protein absorbance
- — an automatic fraction collector for collection into tubes or, ideally, 96-well plates
- — 96-well, nonbinding plates for sample collection
3.1.2 Solutions
All solutions utilized in the FPLC system should be vacuum filtered using a 0.22 mm filter and sufficiently degassed.
- Running buffer: HEPES/Ca2+, pH 7.4
- — 25 mM HEPES
- — 150 mM NaCl
- — 1 mM CaCl2
ddH2O for column rinsing
20% EtOH (prepared with ddH2O) for column storage
3.2. Technical procedure
During separation, the 280 nm UV display of the sample permits identification of adiponectin complexes. Each of the three primary complexes elutes before albumin with distinct protein peaks (Fig. 13.2A). Albumin concentrations do not vary across samples so immediately during the separation the relative abundance of each species may be discernable based on each peak's approximate mAUvalueonthey-axiscomparedtoalbumin.Lowersampleloadingvolumes (Fig. 13.2B) may make this unblinded initial assessment more difficult.
Figure 13.2.
Example FPLC gel filtration elution curves assessed at 280 nm detection. Samples are plotted as mAU (y-axis) versus volume (x-axis) with the red dashed box indicating the first 24 collected eluted fractions. (A) HMW, LMW, and trimeric adiponectin each elute as a distinct protein peak ahead of albumin. (B) Lower sample loading volumes result in more unique peaks (left), but too low of plasma volume coupled with low adiponectin makes discerning the peaks difficult (right). Here, this sample may contain limited HMW adiponectin. (C) Improper elution collection setting can result in adiponectin lost to the waste stream. The first 24 collected contain all of the serum albumin and fail to capture nearly half of the HMW adiponectin (inset, arrow).
Our protocol is designed to separate and collect nearly 100% of the adiponectin in a sample across 24 eluted fractions to maximize the size separation while allowing all of the adiponectin to be detected on one gel (Section 4). A stepwise protocol with flow rates and volumes is summarized in Table 13.2. Collected elution volumes and overall collection time/ volume should be adjusted so as not to lose significant amounts of adiponectin to the waste stream (Fig. 13.2C).
Table 13.2.
Suggested operating parameters for FPLC operation, gel filtration, and sample collection based on a GE Superdex 200 10/300 GL column
| Procedure step | Flow rate (mL/min) | Approx. pressure (MPa) | Volume (mL) | Column fractiona |
|---|---|---|---|---|
| Equilibration | 0.6 | 0.6–0.7 | 4.8 | 0.2 |
| Sample injection | 0.3 | 0.3–0.35 | 0.75 | 0.03 |
| Elution before collection | 0.3 | 0.3–0.35 | 6.24 | 0.26 |
| Fraction collection | 0.3 | 0.3–0.35 | 15.6 | 0.65 |
| Regeneration | 0.6 | 0.6–0.7 | 21.6 | 0.9 |
| Water rinse | 0.6 | 0.6 | 25 | 1.04 |
| Ethanol storage | 0.6 | 0.6–0.7 | 25 | 1.04 |
Specific to Superdex 200 10/300 GL column with 24 mL packed bed.
Sample system pressures are for a column of moderate age. New columns will operate at 0.1 MPa less; older columns should be cleaned once pressures exceed 0.75 MPa. Volumes and column fractions are given to provide protocol programming guidelines following the Section 3 methodology.
With our current FPLC-driven gel fractionation system, we strive to maintain column performance for maximum separation while making subsequent adiponectin assessment fast and easy. For these reasons, we operate at lower flow rates and operating pressure than necessary for the Superdex 200 10/300 GL column. Our protocol (Table 13.2) also includes an equilibration and regeneration step totaling one column volume to essentially wash the columns between samples. For increased speed of fractionation, these steps may be eliminated or reduced and flow rates may be increased to operate the column at 0.75 MPa (the manufacturer's suggested operating pressure) throughout. Rinsing with ddH2O and storage of the column, even for short periods (i.e., weekends), is suggested.
3.3. Customization and optimization
The numbers listed for this protocol in Table 13.2 are based on normal operation of a routinely maintained system. Even so, as columns age and in-line filters slowly clog, performance decreases. It is recommended that a regular column cleaning and filter replacement routine be utilized to ensure optimal system performance. The flow rates listed in the protocol limit the column pressure to reduce column matrix compaction, but heavy usage will inevitably reduce column separation performance. One can either replace the column or make slight adjustments to the protocol as follows. Every system and column may perform differently so it is necessary to adjust the collection protocol accordingly. Changes and adjustments should be applied to all samples equivalently, as separation performance and comparisons can be negatively affected when calculating distribution (Section 5.1).
Protocol adjustment is based on a feedback of multiple outputs and should be performed with a sample containing measurable HMW and trimeric species. The primary feedback is the 280 nm UV detector curve. As described in the technical procedure (Section 3.2), the start of fraction collection should be adjusted so as to begin collecting once initial protein elution occurs—the first peak is HMW adiponectin. With high protein loads or increased column compaction, this point may drift (Fig. 13.2C). We have designed our protocol to have the first appearance of adiponectin be in collected fraction 1 (plate well A1) and end to be by fraction 24 (plate well H3) (Fig. 13.2A), but a starting protocol could begin collection earlier. A wider collection window ensures that all adiponectin forms are collected, and the 24 fractions analyzed through Western blotting can be selected based on the 280 nm UV curve. The secondary feedback is following membrane imaging (Sections 4 and 5). Once the distribution of adiponectin is quantified, changes can be made such that minimum values are achieved in the first and last collected fractions. To calculate a change, multiply the current fraction volume (260 μL in our case) by the number of fractions adiponectin currently appears in or is believed to stretch into (e.g., 26 fractions). Divide this number by 24 to yield a new fraction collection volume (282 μL in the current example). This will optimize all subsequent runs such that all adiponectin is fractionated into 24 wells for easier Western blot quantitation.
Feedback from Western blot analysis may also suggest that loading more sample is necessary due to the dilution of adiponectin. The volumes provided in Table 13.1 should provide sufficient adiponectin to be analyzed if plasma concentrations are within a normal physiologic range. At times, however, patients or experimental animals may exhibit markedly low adiponectin. While reporting these low levels is important, the purpose of this protocol is to quantitate complex distribution, so sufficient sample must be loaded to later visualize. It is not recommended to exceed the Superdex 200 10/300 GL column's 50 μL loading limit of plasma. Even though adiponectin levels are low in these samples, other plasma proteins are not, so the increased protein load may affect complex peak separation and should be considered later in quantitation.
4. WESTERN BLOT ANALYSIS
Following optimized gel fractionation, the adiponectin complexes are effectively distributed throughout 24 collected elutions from the FPLC system. While the UV 280 nm curve of the system yields an approximation of adiponectin complex distribution to its three most abundant forms, Western blot analysis is necessary to complete quantitation of the collected fractions. This ensures that only adiponectin is included in the protein concentration analysis and provides a linear means by which to quantitate and present adiponectin complex distribution following immunoblotting. From the standard method presented here, it will be clear why quantitation works best and is most precise if the fractionation (Section 3) was optimized for maximal separation of adiponectin across the largest elution volume.
4.1. Required materials
Each laboratory likely has its own procedure for immunoblotting. The procedures for SDS-PAGE and membrane transfer are therefore intentionally short. We suggest a few antibodies for labeling adiponectin and list some helpful technical aspects based on experience and our in-house procedure. We utilize the LI-COR Odyssey Imaging System (LI-COR Biosciences; Lincoln, NE) for immunoblot imaging and quantification.
4.1.1 SDS-PAGE
We have found that the Bio-Rad Criterion system works well as 26-well gels for running all 24 fractions at once (with two protein ladder bookends) are offered. We have utilized both Criterion XT Bis–Tris and the newer Criterion TGX precast gels with no impact on the method (both Bio-Rad Laboratories; Hercules, CA).
The SDS-PAGE buffers for these two gels are different: MOPS and Tris/glycine/SDS, respectively, according to the manufacturer's instructions (both are available in easy-to-use concentrated forms).
– Bio-Rad Criterion XT Bis–Tris 26-well 4–12% gel (345-0125) or TGX 26-well 4–15% gel (567-1085)
– MOPS running buffer
– 5 × Laemmli sample buffer with dithiothreitol1 (DTT) added just before use
4.1.2 Immunoblot analysis
– Primary antibody against adiponectin. Do bear in mind that the primary antigen is frequently the amino-terminal variable domain, and hence, the antibodies offer limited reactivity across species. Millipore AB3784P and AB269P (both from EMD Millipore; Billerica, MA) have been tested against human and mouse samples, respectively.
– Secondary antibody labeled with an infrared dye emitting at 700 nm (Cy5, Alexa Fluor 680, LI-COR IRDye 700) or 800 nm (Cy7, Alexa Fluor 790, LI-COR IRDye 800).
– LI-COR Odyssey Imaging System.
– Nitrocellulose membrane (0.22 or 0.45 μm pore size) or Bio-Rad Midi Transfer Pack (170-4159).
4.2. Technical aspects
For this method, the gel percentage or gradient do not affect the ability to later identify adiponectin with a reasonably specific primary antibody; because gels may serve other purposes and serum may be run directly for native analysis without fractionation (Section 6), 4–12% or 4–15% gels are recommended to both permit migration of unreduced HMW and hexamer adiponectin at the top and sufficient separation of adiponectin monomers from IgG light chains at the bottom. Reduced adiponectin will run on the gel at approximately 29 kDa. Some protein molecular weight markers utilize a standard at 26 (or 29)kDa in size that runs at different speeds depending on the buffer system; check the compatibility so as to not lose adiponectin or mis-cut the immunoblot membrane.
Following fractionation, the adiponectin concentration in each fraction will be low, having been diluted by over 100-fold from that of the original plasma sample. Heat 50 μL of the first 24 fractions (or 24 fractions containing adiponectin based on the 280 nm curve) with 10 μL Laemmli buffer containing fresh 2 M DTT at 95 °C for 5 min. To maximize detection, 15 μL—the full well capacity—of each reduced fraction are then loaded onto the gel for SDS-PAGE separation. If adiponectin concentrations are known to be low and/or the 280 nm fractionation curve has protein peaks of under 5 mAU, a different gel system with larger well capacity may be necessary to load sufficient adiponectin for subsequent immunoblot labeling. Trichloroacetic acid (TCA) precipitation of each 260 μL fraction with direct resuspension in loading buffer may also aid in detection of low adiponectin fractions. End detection may also be limited by primary antibody sensitivity.
Membrane selection and secondary detection should be made based on the laboratory's imaging of choice. We have listed materials for use the with LI-COR Odyssey system that couples infrared detection with straightforward image analysis of the scanned membrane. With infrared detection, polyvinylidene difluoride (PVDF) membrane may yield higher background intensities with the LI-COR scanner, so this procedure uses nitrocellulose. Anecdotally, milk used as a blocking buffer or antibody diluent may also increase infrared background. If using the Bio-Rad Criterion 26-well gels, the Bio-Rad Trans-Blot Turbo packs provide a prepackaged system for protein transfer (midi size; used with the Mixed MW setting) to nitrocellulose. Secondary antibody concentrations should be kept low (1:5000) for best signal to noise. If adiponectin bands are barely detectable, or absent, more reduced sample may need to be loaded requiring a switch to a larger well gel system. Longer antibody incubations can be tried first on the initial membrane, then larger capacity gels, then TCA precipitation of collected fractions, and then finally (if possible) loading a larger amount of plasma to the FPLC for a new fractionation.
5. COMPLEX DISTRIBUTION QUANTITATION AND PRESENTATION
Quantification of adiponectin complex distribution from the Western blot iselementarywith image analysissoftware thatyieldsband intensities.We have utilized the LI-COR Odyssey system and software for several years in whichtheintegrated infraredintensityvalueislinear withconcentration.This system and analysis also permits precise quantification of adiponectin concentrations if a few known standards are run concurrently to create a standard curve covering the infrared intensity range. In the absence of running standards in parallel, all fraction intensities are plotted as measured or compared to each other to obtain a fractional distribution. Using the LI-COR Odyssey software, each integrated band intensity is measured using the rectangular region of interest (ROI) band tool, with the background measurements for the tool set to “All” (using Top and Bottom and Side to Side for normalization). Care should be taken to center the ROI on the band and the same size/shape ROI can be copied and pasted to maintain a consistent and normal area across all bands (as integrated intensity is area dependent).
5.1. The art of quantification
To designate each peak as HMW, hexamer, and trimer, the lowest intensity value between the apices is set as the division. This fraction cannot be necessarily standardized from sample to sample, however, (e.g., fraction 7 is the division between HMW and hexamer) as complete gel filtration will shift with protein concentration and should be noted (Section 3.3). A sample with very little HMW may have a discernable hexamer peak start before that of one enriched in HMW. Also, even with optimized fractionation there is never a zero value achieved between peaks. In the absence of a known adiponectin concentration standard, the intensity values for each fraction can be normalized by subtracting the lowest value from all intensities, thus yielding a fraction intensity curve.
5.2. Data presentation
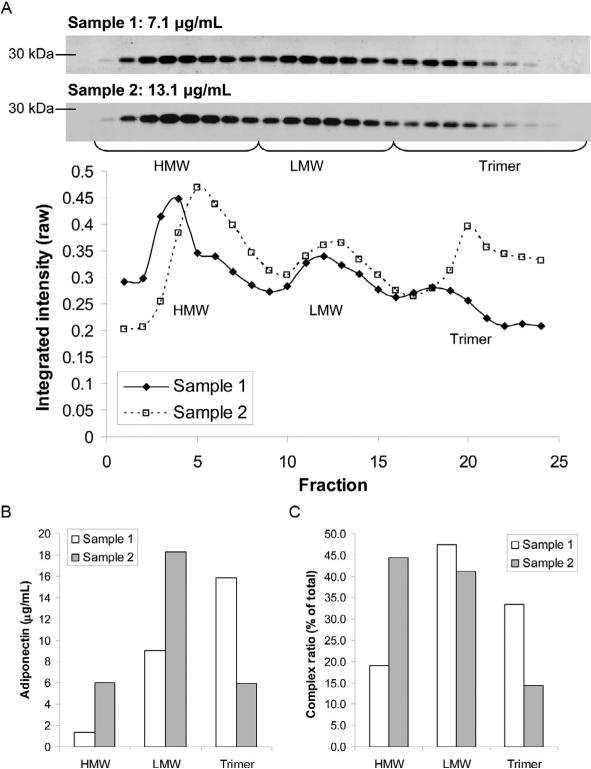
The resultant data from intensity quantifications can be expressed in several ways, each with its inherent pros and cons. The x-axis of fraction intensity/ concentration plots has no units, so a summation of intensities or calculated areas under the curves are equivalent in each option. None of these presentation methods allow data to be misrepresented and are only a personal visual choice.
Option 1 (Fig. 13.3A): The data are presented as the raw plots of two sample fraction intensities. Pros: the relative peak distribution is clear between groups; best for mutations in which one complex is absent. Cons: only represents one sample of each group rather than averages and may be difficult to interpret ratios of each complex to each other if total concentration makes the peaks of one sample much greater. Option 2 (Fig. 13.3B): Area under the curve calculations of each peak presented as concentrations. Pros: provides a relative value of each species; averages of multiple samples can be used for statistical analysis. Cons: ratios may be difficult to discern, subtle changes such as a HMW shoulder would disappear, nonzero values between peaks makes precise quantification impossible, and requires knowing the total concentration by another method.
Figure 13.3.
Presentation of adiponectin complex quantitative data can differ by choice and purpose. (A) From their immunoblots (top), raw quantified elution curves from two samples (bottom) highlight the peak distribution. The brackets demarcate the fractions identified as HMW, LMW, and trimeric adiponectin. (B) Expression of the summed peak intensities from (A) expressed as adiponectin concentrations based on comparison of fraction band intensities to the sample concentration from ELISA. (C) Expressed as each peaks value divided by the total can highlight changes in complex distribution across groups of experimental samples.
Option 3 (Fig. 13.3C): Percent distribution of each peak/complex using area under the curve. Pros: changes are clear on a bar graph and statistics from sample to sample readily applied. Cons: Nonzero values between peaks makes precise quantification impossible and subtle changes such as a HMW shoulder disappear.
6. COMPARISON TO OTHER TECHNIQUES
Utilizing an FPLC to fractionate every serum or plasma sample and then identify adiponectin in each fraction by running a full gel for each sample is time consuming for the number of samples processed. It does, however, provide a complete representation of adiponectin complex distribution and thus represents the best method for demonstrating changes in oligomer size. This is particularly powerful for experimental groups in which the changes may be subtle and clinical populations in which a shift toward trimer from LMW may represent a negative metabolic shift. Other less rigorous techniques are possible; we briefly assess these with their respective pros and cons.
6.1. SDS-PAGE fraction analysis
The FPLC fractionation protocol presented here relies on gel filtration/size exclusion chromatography to separate adiponectin complexes by molecular size. Gel electrophoresis provides essentially the same capability without the burden or cost of FPLC equipment and time. Using nonreducing conditions and limited detergents, adiponectin complexes can be reasonably identified by immunoblot following electrophoresis. Complexes can be further stabilized by chemical crosslinking (Scherer, Williams, Fogliano, Baldini, & Lodish, 1995). The Bio-Rad gels described in Section 4 can be used for this alternative method, though care must be taken to run the samples slowly on ice, at low power (setting the amperage low), and with no added reducing agents or detergents. While less labor-intensive than the FPLC-based method, there are two large problems with the technique. First, it is challenging to not have some complexes break down with heat or in using gels that may contain some reducing agents or detergents. Since it is unknown as to which complexes break down, the relative abundance is skewed and it cannot be assumed, for example, that monomers are solely from the trimer pool. Second, FPLC isolation of complexes followed by reduction to monomers allows for antibody detection to be linear with abundance. The band intensities of a native gel immunoblot will not be linear with adiponectin concentration due to the number of potential antigens present within each “fraction.” Distribution calculations are, therefore, skewed by the number of immunoglobulins per complex.
Pros: Relatively inexpensive and fast. Multiple samples can be assessed on the sample gel. Small sample volumes needed.
Cons: Difficult to perform without detergents or reduction so challenging to not have some complex breakdown. HMW complexes do not sufficiently enter gel for separation. Antibody detection of nonreduced forms not linear with concentration.
6.2. HMW adiponectin enzyme-linked immunosorbent assay analysis
In recent years, the desire to measure adiponectin complex distribution in a more rapid, high-throughput technique has led to the commercial development of enzyme-linked immunosorbent assays (ELISAs) specific to human HMW adiponectin. Some kits utilize monoclonal antibodies that are claimed to be specific to the HMW oligomer, while others utilize sample pretreatment targeted to eliminate LMW and trimeric adiponectin (Ebinuma et al., 2006; Komura et al., 2008; Sinha et al., 2007). Comparison of these methods to the gel fractionation FPLC protocol in our laboratory suggests that these kits generally yield a good representation of the sample, except perhaps at the extremes of very high and very low HMW levels (Sinha et al., 2007). To date, however, these are limited to human adiponectin analysis.
Pros: Fast and high throughput. Small sample volumes needed.
Cons: Cost per sample when run in triplicate. Must run both full and HMW ELISAs for distribution and still lack LMW or trimeric abundance. Sample collection may alter digestion when necessary. Species use limited to available kits.
7. CONCLUDING REMARKS
Adiponectin is a metabolically active adipokine that is increasingly appreciated as a powerful marker of glucose homeostasis and inflammation. More than 10,000 publications have been published on the topic. An increasing number of clinical studies and correlations have linked total adiponectin and the abundance of its HMW oligomers with a variety of pathological states (Aleksandrova et al., 2012; Baessler et al., 2011; Hamilton et al., 2011; Lo et al., 2011; Matsumoto et al., 2013; Mazaki-Tovi et al., 2009). This has made measuring adiponectin levels across a range of medical and experimental disciplines, from endocrinology and nephrology to reproduction and oncology, a noteworthy biomarker and disease effector. This protocol for quantitating adiponectin complexes presents the most reliable method for analysis of oligomer distribution and has been extensively used for clinical and experimental samples. While other methods to estimate this distribution exist, complex separation by FPLC isolation and fractionation is the most thorough in determining changes in the three most abundant species. Despite its advantages, this is a rather laborious technique, and its use for large-scale clinical studies with hundreds or thousands of samples is rather limited. Further individual customization, optimization and potential automatization of the protocol to maximize costs and time benefits hopefully makes this protocol operational for many laboratories interested in adiponectin.
ACKNOWLEDGMENTS
J. M. R. is supported by the American Heart Association Scientist Development Grant 12SDG12050287. This work was also supported by the National Institutes of Health (Grants R01-DK55758, R01-DK099110 and P01-DK088761 to P. E. S.).
Footnotes
Make sure you achieve maximal reducing power. Human adiponectin, in particular, is occasionally reduced incompletely and may be observed as a 60 kDa band.
REFERENCES
- Aleksandrova K, Boeing H, Jenab M, Bueno-de-Mesquita HB, Jansen E, van Duijnhoven FJ, et al. Total and high-molecular weight adiponectin and risk of colorectal cancer: The European Prospective Investigation into Cancer and Nutrition Study. Carcinogenesis. 2012;33(6):1211–1218. doi: 10.1093/carcin/bgs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baessler A, Schlossbauer S, Stark K, Strack C, Riegger G, Schunkert H, et al. Adiponectin multimeric forms but not total adiponectin levels are associated with myocardial infarction in non-diabetic men. Journal of Atherosclerosis and Thrombosis. 2011;18(7):616–627. doi: 10.5551/jat.8359. [DOI] [PubMed] [Google Scholar]
- Bobbert T, Rochlitz H, Wegewitz U, Akpulat S, Mai K, Weickert MO, et al. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 2005;54(9):2712–2719. doi: 10.2337/diabetes.54.9.2712. [DOI] [PubMed] [Google Scholar]
- Ebinuma H, Miyazaki O, Yago H, Hara K, Yamauchi T, Kadowaki T. A novel ELISA system for selective measurement of human adiponectin multimers by using proteases. Clinica Chimica Acta. 2006;372(1–2):47–53. doi: 10.1016/j.cca.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Fisher FM, Trujillo ME, Hanif W, Barnett AH, McTernan PG, Scherer PE, et al. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005;48(6):1084–1087. doi: 10.1007/s00125-005-1758-7. [DOI] [PubMed] [Google Scholar]
- Hamilton MP, Gore MO, Ayers CR, Xinyu W, McGuire DK, Scherer PE. Adiponectin and cardiovascular risk profile in patients with type 2 diabetes mellitus: Parameters associated with adiponectin complex distribution. Diabetes & Vascular Disease Research. 2011;8(3):190–194. doi: 10.1177/1479164111407784. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circulation Research. 2004;94(4):e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura N, Kihara S, Sonoda M, Kumada M, Fujita K, Hiuge A, et al. Clinical significance of high-molecular weight form of adiponectin in male patients with coronary artery disease. Circulation Journal. 2008;72(1):23–28. doi: 10.1253/circj.72.23. [DOI] [PubMed] [Google Scholar]
- Kusminski CM, Scherer PE. The road from discovery to clinic: Adiponectin as a biomarker of metabolic status. Clinical Pharmacology and Therapeutics. 2009;86(6):592–595. doi: 10.1038/clpt.2009.155. [DOI] [PubMed] [Google Scholar]
- Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55(1):249–259. [PubMed] [Google Scholar]
- Lo MM, Salisbury S, Scherer PE, Furth SL, Warady BA, Mitsnefes MM. Serum adiponectin complexes and cardiovascular risk in children with chronic kidney disease. Pediatric Nephrology. 2011;26(11):2009–2017. doi: 10.1007/s00467-011-1906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Toyomasu K, Uchimura N, Ishitake T. Low-molecular-weight adiponectin is more closely associated with episodes of asthma than high-molecular-weight adiponectin. Endocrine Journal. 2013;60(1):119–125. doi: 10.1507/endocrj.ej12-0277. [DOI] [PubMed] [Google Scholar]
- Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Erez O, Gotsch F, et al. Maternal serum adiponectin multimers in preeclampsia. Journal of Perinatal Medicine. 2009;37(4):349–363. doi: 10.1515/JPM.2009.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/ adiponectin. Implications fpr metabolic regulation and bioactivity. Journal of Biological Chemistry. 2003;278(11):9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. Journal of Biological Chemistry. 2004;279(13):12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. Journal of Biological Chemistry. 1995;270(45):26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology. 2008;149(5):2270–2282. doi: 10.1210/en.2007-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha MK, Songer T, Xiao Q, Sloan JH, Wang J, Ji S, et al. Analytical validation and biological evaluation of a high molecular-weight adiponectin ELISA. Clinical Chemistry. 2007;53(12):2144–2151. doi: 10.1373/clinchem.2007.090670. [DOI] [PubMed] [Google Scholar]
- Turer AT, Scherer PE. Adiponectin: Mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: Mechanisms and functional implications. Biochemical Journal. 2008;409(3):623–633. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]