Abstract
The impact of pediatric chronic kidney disease (CKD) on acquisition of volumetric bone mineral density (BMD) and cortical dimensions is lacking. To address this issue we obtained tibia quantitative computed tomography scans from 103 patients age 5-21 years with CKD (26 on dialysis) at baseline and 12 months later. Gender, ethnicity, tibia length and/or age-specific Z-scores were generated for trabecular and cortical BMD, cortical area, periosteal and endosteal circumference, and muscle area based on over 700 reference subjects. Muscle area, cortical area, and periosteal and endosteal Z-scores were significantly lower at baseline compared to the reference cohort. Cortical BMD, cortical area and periosteal Z-scores all exhibited a significant further decrease over 12 months. Higher parathyroid hormone levels were associated with significantly greater increases in trabecular BMD and decreases in cortical BMD in younger patients (significant interaction terms for trabecular BMD and cortical BMD). The estimated GFR was not associated with changes in BMD Z-scores independent of parathyroid hormone. Changes in muscle and cortical area were significantly and positively associated in control subjects but not in CKD patients. Thus, children and adolescents with CKD have progressive cortical bone deficits related to secondary hyperparathyroidism and potential impairment of the functional muscle-bone unit. Interventions are needed to enhance bone accrual in childhood-onset CKD.
Introduction
Children with chronic kidney disease (CKD) have multiple risk factors for impaired bone accrual, including poor growth, delayed maturation, muscle deficits, decreased physical activity, abnormal mineral metabolism and secondary hyperparathyroidism. We recently reported that childhood-onset CKD was associated with significant deficits in cortical volumetric bone mineral density (BMD), cortical dimensions and muscle area, as measured by peripheral quantitative computed tomography (pQCT).1, 2 CKD was also associated with elevated trabecular BMD in younger participants only. The cross-sectional design limited the assessment of determinants of bone abnormalities and associations between bone and muscle outcomes.
To our knowledge, longitudinal studies of bone accrual in childhood CKD, in the absence of intervening renal transplantation, are limited to series of 7-18 participants.3-6 These studies were further limited by the use of dual energy x-ray absorptiometry (DXA) measures of areal BMD. DXA is a two-dimensional projection technique that obscures distinct CKD effects on trabecular and cortical bone,7 and underestimates volumetric BMD in children with growth failure.8
The objectives of this prospective cohort study were: 1) to assess changes in trabecular and cortical volumetric BMD and cortical dimensions over a one year interval in children and adolescents with mild to severe CKD, 2) to identify correlates of changes in pQCT parameters including CKD progression, intact parathyroid hormone (iPTH) levels, and medications, and 3) to assess the relations between changes in muscle area and bone dimensions (the functional muscle-bone unit) compared with longitudinal data in healthy reference participants.
Results
Participant Characteristics
This report describes 103 CKD participants with two pQCT scans, a median 12.5 months apart [interquartile range (IQR) 12.1, 13.2], including 83 from the prior cross-sectional study.2 The focus of this study is determinants of changes in bone, therefore, this cohort includes an additional 20 participants that were ineligible for the prior study due to a history of solid organ transplantation. All prior renal transplant recipients in this study were on dialysis; median interval since transplantation of 5.1 (IQR 3.9, 7.2) years and a median interval since starting dialysis of 11 (IQR 2, 56) months. Baseline characteristics are summarized in Table 1. The reference participants have been described.2, 9, 10
Table 1. Baseline Characteristics in Chronic Kidney Disease Participants.
| Non-Dialysis | Dialysis | |
|---|---|---|
| N | 77 | 26 |
| Age, yr | 13.2 (9.5, 16.7) | 18.4 (13.6, 19.3) |
| Male, n (%) | 50 (65) | 15 (58) |
| Race, n (%) | ||
| White | 62 (81) | 12 (46) |
| Black | 14 (18) | 11 (42) |
| Other | 1 (1) | 3 (12) |
| Tanner stage 1-2, n (%) | 35 (46) | 4 (15) |
| Height Z-score | -0.57 ± 1.37 | −1.19 ± 1.15 |
| BMI Z-score | 0.34 ± 1.24 | 0.25 ± 1.39 |
| Underlying renal diagnosis, n (%) | ||
| CAKUT | 52 (68) | 9 (35) |
| Focal segmental glomerulosclerosis | 7 (9) | 12 (46) |
| Systemic Inflammatory | 2 (3) | 1 (4) |
| Other | 16 (20) | 4 (16) |
| Age at CKD diagnosis, yr | 2.5 (birth,8.4) | 8.8 (1.5, 12.9) |
| Interval since CKD diagnosis, yr | 7.1 (5.1,12.1) | 8.5 (3.1,13.6) |
| Hemodialysis, n (%) | 16 (62) | |
| Duration of dialysis, months | 4.5 (1.0, 23.8) | |
| History of prior renal transplantation, n (%) | 14 (54) | |
| History of prior cardiac or liver transplantation, n (%) | 5 (6) | 1 (4) |
Results are presented as mean ± SD, median (interquartile range), or n (%).
CAKUT: Congenital anomalies of the kidney and urinary tract; FSGS: Focal segmental glomerulosclerosis
Compared with the non-dialysis CKD, dialysis participants were significantly older, more likely to be of black race and more likely to have focal segmental glomerulosclerosis (FSGS).
Clinical Course
Laboratory results, estimated glomerular filtration rate (eGFR) and medications are summarized in Table 2. There was a significant decline in renal function over the study period in non-dialysis CKD participants with a median decrease of 2 (IQR -7 to 1) ml/min/1.73m2. Two participants initiated dialysis. Serum iPTH and phosphorus levels increased significantly in the non-dialysis population. The proportion of non-dialysis participants above the normal iPTH range (>65 pg/ml) increased from 39 to 52% over the study. The mean iPTH was above the pediatric Kidney Disease Outcome Quality Initiative (KDOQI) CKD stage-specific target range in 32 (42%) and 13 (50%) of non-dialysis and dialysis participants, respectively, and above the lower European target ranges in 38 (49%) and 18 (69%), respectively.11, 12
Table 2. Laboratory Parameters at Baseline and Follow-up and Medication Exposure over the Study Interval.
| Non-Dialysis | Dialysis | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p value for change | Baseline | Follow-up | p value for change | |
| Laboratory Results | ||||||
| eGFR ml/min/1.73m2 | 41 ± 14 | 37 ± 16 | < 0.001 | |||
| iPTH (pg/ml) | 49 (27,84) | 61 (30,119) | < 0.01 | 146 (85,479) | 183 (62,504) | 0.85 |
| Phosphorus (mg/dL) | 4.4 (3.8,5.0) | 4.5 (4.0,5.5) | < 0.01 | 5.4 (3.9,6.5) | 6.5 (4.9,7.7) | < 0.05 |
| Corrected Calcium (mg/dL) | 9.6 (9.3,10.0) | 9.5 (9.2,10.0) | 0.67 | 9.4 (8.6,9.6) | 9.5 (8.9,10.1) | 0.16 |
| Bicarbonate (mmol/L) | 24 ± 3 | 24 ± 3 | 0.56 | 24 ± 4 | 25 ± 5 | 0.52 |
| Concurrent Medications, n (%) | ||||||
| Calcitriol | 34 (44) | 25 (96) | ||||
| Growth Hormone | 7 (9) | 2 (8) | ||||
| Calcineurin Inhibitor | 0 | 9 (33) | ||||
| Glucocorticoid | 4 (5) | 8 (31) | ||||
| Phosphate Binder | 25 (32) | 26 (100) | ||||
Results are presented as mean ± SD if normal distribution or as median and (interquartile range IQR)
These results are limited to those who have baseline and follow-up results for each parameter.
Calcium was corrected for those with an albumin ≤ 3.5 using Calcium + [0.8*(4.0 − albumin)]
Phosphate binders include both calcium and non-calcium contanining.
Among the 20 participants with a history of prior transplantation, 7 received glucocorticoids and 9 received calcineurin inhibitors during the study period. The remainder of participants treated with glucocorticoids had a diagnosis of FSGS, systemic inflammatory disease or IgA nephropathy.
Six CKD participants sustained a total of 7 fractures (1 tibia/fibula, 1 radius/ulna and 5 foot/toe) during the study interval (57/1,000 patient-years).
Peripheral QCT Outcomes
Table 3 summarizes pQCT Z-scores in CKD participants.
Table 3. pQCT and Anthropometric Z-scores at Baseline and Follow-up.
| Non-Dialysis | Dialysis | |||||
|---|---|---|---|---|---|---|
| Z-score | Baseline (n) | Follow-up | p Value | Baseline (n) | Follow-up | p value |
| Trabecular BMD | 0.05 ± 1.22 (65) | 0.00 ± 1.24 | 0.12 | -0.63 ± 2.52 (21) | -0.42 ± 2.36 | 0.27 |
| Cortical BMD | 0.29 ± 1.15 (74) | 0.15 ± 1.33 | 0.07 | -0.23 ± 1.70 (24) | -0.81 ± 1.76 | 0.04 |
| Section Modulus | -0.57 ± 1.11 (71) | -0.59 ± 1.09 | 0.60 | -0.47 ± 1.37 (26) | -0.61 ± 1.42 | 0.05 |
| Cortical Area | -0.43 ± 1.19 (69) | -0.53 ± 1.18 | 0.01 | -0.67 ± 1.46 (26) | -0.94 ± 1.53 | 0.01 |
| Endosteal Circumference | -0.37 ± 1.10 (71) | -0.34 ± 1.07 | 0.30 | 0.07 ± 1.01 (25) | 0.17 ± 0.97 | 0.09 |
| Periosteal Circumference | -0.52 ± 1.06 (69) | -0.60 ± 1.05 | 0.02 | -0.37 ± 1.37 (26) | -0.45 ± 1.43 | 0.19 |
| Muscle Area | -0.05 ± 1.05 (67) | -0.16 ± 1.09 | 0.009 | -1.03 ± 1.35 (26) | -1.07 ± 1.06 | 0.71 |
| Fat Area | −0.03 ± 1.31 (67) | -0.11 ± 1.40 | 0.66 | 0.17 ± 1.22 (26) | 0.05 ± 1.09 | 0.59 |
| Muscle Force | -0.47 ± 0.98 (43) | -0.65 ± 1.07 | 0.48 | -1.30 ± 1.15 (15) | -1.30 ± 1.58 | 0.96 |
| Physical Activity (hours/week) | ||||||
| Total | 13.2 (8.1,19.4) | 10.3 (5.7,17.4) | 0.07 | 7.9 (2.0,12.9) | 2.4(0.7,10.2) | 0.03 |
| High Impact | 5.9 (2.7,10.1) | 4.4 (1.8,8.2) | 0.15 | 2.0 (0.7,3.3) | 0.7 (0,2.2) | 0.02 |
| Height | −0.57 ± 1.37 (77) | -0.62 ± 1.34 | 0.15 | −1.19 ± 1.17 (25) | -1.24 ± 1.11 | 0.27 |
| BMI | 0.34 ± 1.24 (76) | 0.35 ± 1.23 | 0.65 | 0.22 ± 1.41 (25) | 0.13 ± 1.36 | 0.53 |
All values are expressed mean ±SD
Results at baseline and follow-up are limited to participants with data at both visits. Measures of muscle strength were obtained in the Children's Hospital of Philadelphia participants only.
Trabecular BMD
Overall, trabecular BMD Z-scores did not change significantly in all participants combined, or within the non-dialysis and dialysis groups. The multivariate regression model for changes in trabecular BMD Z-score demonstrated that a greater mean iPTH level was associated with a significantly greater change in trabecular BMD Z-score (p<0.001) and this effect was limited to the younger participants (interaction, p=0.001), adjusted for baseline trabecular BMD Z-score. These results are illustrated in Figure 1, according to age < or ≥ the median of 14 years. Changes in trabecular BMD Z-scores were significantly greater in the 11 participants that had elevated iPTH levels by European targets only, compared to the 47 without elevated iPTH by either target (p=0.04). Similar results were obtained in the 45 participants above both targets.
Figure 1. Association between changes in Trabecular BMD Z-Scores according to mean iPTH level.
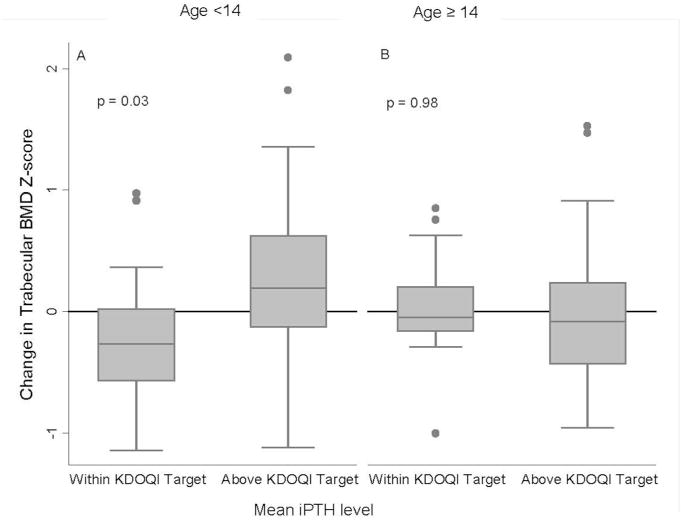
A. Changes in trabecular BMD Z scores were significantly greater in younger CKD participants (less than median age of 14 years) with a mean iPTH level above the KDOQI target range, compared to those with mean iPTH levels within or below the KDOQI target (p=0.03).
B. This association was not found among older (≥ 14 years of age) CKD participants (p=0.99).
Lower baseline eGFR was associated with greater increases in trabecular BMD Z-score (p<0.03) and analyses stratified on median age suggested this effect was more pronounced in the younger participants. The association between change in trabecular BMD Z-score and baseline eGFR was eliminated when the models were adjusted for mean iPTH levels.
Changes in trabecular BMD-Z scores were not associated with change in eGFR, etiology of CKD, interval since CKD diagnosis, concurrent recombinant growth hormone (rhGH), glucocorticoid, calcitriol or phosphate binder treatment, or physical activity in univariate or multivariate models.
Cortical BMD
At baseline, cortical BMD Z-scores were not significantly lower in CKD participants vs. reference participants overall (p=0.06). Baseline cortical BMD Z-scores were inversely associated with baseline iPTH concentrations in CKD participants (r=-0.25, p=0.01). Cortical BMD Z-scores decreased significantly within all participants combined (p=0.02). In the multivariate regression model of changes in cortical BMD Z-score, higher mean iPTH levels were associated with greater declines in cortical BMD Z-score (p=0.02) and this association was marginally greater in the younger participants (interaction p=0.05). Among participants < 14 years of age, a mean iPTH level greater than the KDOQI target range was associated with a 0.29 greater decline in cortical BMD Z-score compared with those within or below the target range. Declines in cortical BMD Z-scores were significantly greater in the 11 participants that had elevated iPTH levels by European targets only, compared to the 47 without elevated iPTH by either target (p=0.005). Similar results were obtained in the 45 participants above both targets.
Changes in cortical BMD Z-scores were not associated with baseline eGFR, change in eGFR, underlying renal disease, interval since CKD diagnosis, medications (concurrent rhGh, glucocorticoid, calcitriol or phosphate binders) or physical activity.
Cortical Dimensions
At baseline, section modulus (a summary measure of cortical dimensions and strength), cortical area, and periosteal and endosteal circumference Z-scores were significantly lower in the CKD participants (all p<0.01), vs. reference participants. At baseline, iPTH levels were positively associated with endosteal circumference Z-scores (r=0.30, p=0.003) and negatively with cortical area Z-score (r=-0.25, p=0.01) but were not associated with periosteal circumference or section modulus Z-scores (p>0.4).
Over the follow-up interval, cortical area Z-scores decreased significantly within non-dialysis CKD and dialysis participants. Periosteal circumference Z-scores decreased significantly in all participants combined (p<0.009).
In the multivariable model for changes in cortical area Z-score, baseline eGFR, change in eGFR, mean iPTH levels, disease characteristics and medications were not significant. The multivariable models for changes in endosteal and periosteal circumference Z-scores both demonstrated a positive association with higher mean iPTH levels (p=0.05 and p=0.017). None of the changes in cortical dimensions was associated with baseline or changes in renal function, diagnostic category, physical activity, or glucocorticoid, calcitriol or phosphate binder therapy.
The analyses of rhGH associations with bone outcomes were limited to the 58 participants < 16 years of age, including 8 treated with rhGH for a minimum of 6 months over the study interval. Concurrent rhGH therapy was associated with greater increases in section modulus [0.51 (95% C.I. 0.22, 0.79, p=0.001)] and cortical area Z-scores [0.27 (-0.05, 0.60), p=0.10)]; however, the results were significant for section modulus only. Concurrent rhGH was not associated with significant changes in periosteal [0.10 (-0.15, 0.35)] or endosteal [0.15 (-0.10, 0.40)] circumference Z-scores.
Muscle and Fat Cross-Sectional Area Z-scores
At the baseline visit, mean muscle area Z-scores in CKD participants were lower vs. reference participants (p=0.001). Over the follow-up period, there was a significant decrease in muscle area Z-scores in non-dialysis CKD participants and a non-significant decrease in dialysis participants. In the multivariable model for changes in muscle area Z-score, none of the participant, disease or treatment characteristics were significant.
At the baseline visit, mean fat area Z-scores in CKD participants were not significantly different vs. reference participants and the small decline was not significant. In the multivariable regression model for changes, concurrent rhGH therapy was associated with a decrease in fat area Z-scores (p=0.002).
Muscle Force and Physical Activity
Table 3 summarizes muscle force Z-scores and physical activity results. At baseline, CKD participants had significantly lower muscle force Z-scores vs. reference participants (p<0.001). Muscle force adjusted for muscle CSA was 11.9% (95% CI: 4.7, 18.5; p=0.002) lower in non-dialysis CKD participants and 16.2% (5.1, 26, p=0.006) lower in dialysis CKD participants, compared with the reference participants. Muscle force did not change significantly over the study interval.
At baseline, dialysis participants reported significantly fewer hours per week of total and moderate-to-high impact physical activity, vs. non-dialysis CKD participants and vs. reference participants (all comparisons p<0.01). Reference participants reported a median of 12.7 (IQR 8.1, 20.6) total hours per week and 5.7 (IQR 3.0, 9.7) moderate-to-high impact hours per week. In both CKD groups physical activity declined over the study interval.
Assessment of the Functional Muscle Bone Unit
In order to determine if changes in muscle area Z-scores in CKD participants were associated with the expected changes in cortical Z-scores, i.e. the “functional muscle-bone unit”,13 the 12 month changes in cortical area Z-scores were compared in the CKD participants and the 269 reference participants enrolled in the longitudinal substudy. These analyses were adjusted for baseline muscle area and baseline cortical area Z-scores, age and tibia growth. Changes in cortical area Z-scores were positively associated with changes in muscle area Z-scores in the reference participants (p<0.001); however, this association was absent in CKD participants (interaction p<0.001) (Figure 2).
Figure 2. Change in Cortical Area and Muscle Area Z-scores.
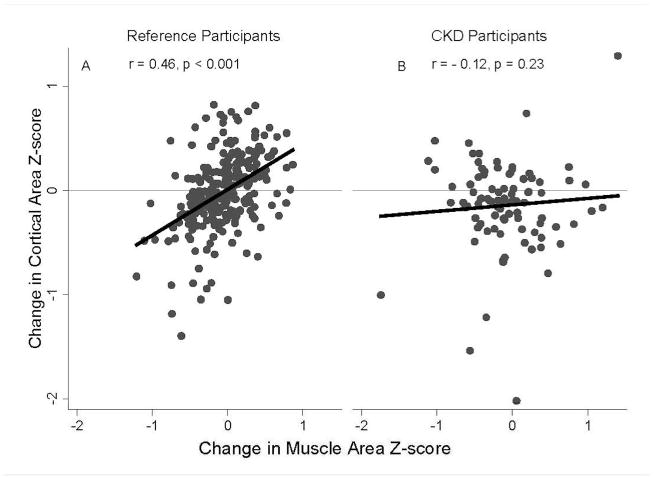
A. Over the follow-up period, greater increases in muscle area Z-scores were associated with greater increases in cortical area Z-scores in the reference participants, r = 0.46, p < 0.001.
B. In contrast, this association was absent in the CKD participants. r= -0.12, p=0.24.
Discussion
This is the first study to examine changes in trabecular and cortical volumetric BMD, cortical dimensions, muscle area and muscle strength in childhood CKD. Secondary hyperparathyroidism in CKD was associated with greater increases in trabecular BMD Z-scores and greater declines in cortical BMD Z-scores in younger participants. Cortical BMD, cortical area, and periosteal circumference Z-scores decreased significantly over one year. The positive association between changes in muscle and cortical area Z-scores observed in reference participants was absent in CKD.
Prior longitudinal bone imaging studies in children with CKD were limited to DXA and quantitative ultrasound methods.3-6 These studies were further limited by small sample sizes and inadequate reference data. Longitudinal studies using pQCT in CKD are limited to two radius studies in older adults: Obatake et al reported decreases in trabecular and cortical BMD over one year in 53 pre-dialysis patients (mean age 61 years).14 Fujimore et al reported decreases in cortical BMD and cortical bone area over two years in 53 hemodialysis patients (mean age 67 years).15 Given the absence of reference participants, it is not known if these changes were greater than expected with aging.
The association between hyperparathyroidism and increased trabecular BMD in younger children with CKD has been described in prior cross-sectional studies,2, 10, 16-19 and confirmed in this longitudinal study. We hypothesized that the elevated trabecular BMD in the younger children was due to iPTH and CKD effects at the metaphysis, with impaired resorption of the dense metaphyseal spongiosa.20
Our prior cross-sectional study demonstrated an inverse association between iPTH levels and cortical volumetric BMD across all ages; however, this longitudinal study only detected this association in younger participants. The study by Obatake et al failed to show an association between iPTH levels and changes in cortical BMD while the study by Fujimore et al detected an inverse association in males only.14, 15 Periosteal and endosteal circumference both increased with higher mean levels of iPTH in our study. The increase in periosteal circumference is consistent with prior human and animal studies of PTH therapy.21, 22 The increase in endosteal circumference is consistent with a study of bone biopsies in adult dialysis patients, demonstrating that hyperparathyroidism was associated with endocortical resorption.23
The European guidelines advise lower target PTH levels, compared with KDOQI. The observation that PTH levels above the European target (but within the KDOQI target range) were associated with changes in trabecular and cortical BMD, compared with participants that were not above the European target provides preliminary support for these lower targets.12 Prior studies have shown variable results with different PTH assay methods; 24 however, our study used the same assay throughout and confirmed the results using the bioactive 1-84 PTH level.
This study demonstrated that concurrent rhGH therapy was associated with preservation of section modulus Z-scores and greater declines in fat area Z-scores. However, the results must be interpreted with caution given the small number of participants treated with rhGH and our failure to detect significant associations with changes in periosteal and endosteal circumference or muscle area Z-scores. To our knowledge pediatric CKD BMD studies of growth hormone therapy are limited to DXA imaging. 5, 6, 25-27 These studies yielded conflicting results, likely due to variable reference data, and adjustments for short stature and intercurrent growth. None of these studies examined volumetric BMD or cortical dimensions. In contrast, a radius pQCT study by Schweizer et al28, in prepubertal children with growth hormone deficiency demonstrated that cortical total area (a measure of periosteal dimensions) increased significantly relative to height age; marrow area (a measure of endosteal dimensions) and cortical area did not change significantly after one year of rhGH therapy. This is consistent with our observation that rhGH was associated with improvements in cortical dimensions. Larger controlled studies are needed to establish rhGH effects on bone accrual in CKD.
The longitudinal data in our pediatric CKD cohort demonstrated significant and worsening cortical area deficits over one year. Given the strong association between cortical bone strength and muscle mass during normal growth (Figure 2A), we examined the associations between cortical bone and muscle area. The positive association was absent in CKD (Figure 2B). This may be explained by lower muscle force relative to muscle area in CKD 13, 29 and less physical activity.30, 31 Prior studies have demonstrated muscle abnormalities including muscle atrophy, myopathy, and reduced physical functioning in pediatric dialysis patients.19, 32
Prior pQCT studies in adults with pre-dialysis CKD33, and those on maintenance hemodialysis34 demonstrated that decreased cortical thickness and BMD were significantly associated with greater odds of fracture. While the sample size is inadequate to address age, sex, and skeletal site specific fracture rates, it is noteworthy that 6 of 103 CKD participants had a fracture during the study interval. This rate is higher than observed in healthy pediatric population-based studies at any age in males or females.35
The wide spectrum of underlying renal disease and duration, and differences in participant treatment characteristics is a significant limitation of this study. The study included participants with conditions and medications that can impact bone health, including glucocorticoids, calcineurin inhibitors and rhGH. Furthermore, the majority of dialysis participants had a history of prior allograft failure. The subgroup analyses had limited power and we were unable to define associations between disease characteristics and changes in bone outcomes. Therefore, the results should be considered with caution and used to generate hypotheses for future studies.
A second limitation is the lack of bone biopsy data, precluding measures of trabecular bone microarchitecture, turnover or mineralization. The term CKD-Mineral and Bone Disorder (CKD-MBD) describes a broad systemic disorder of mineral and bone metabolism, as well as vascular and soft-tissue calcification.36 We were also unable to address extra-skeletal calcification. A final limitation is the lack of measures of FGF-23. However, a recent bone biopsy study reported that FGF-23 levels were not associated with defective mineralization.37 Given that a recent biopsy study in 52 children with pre-dialysis CKD reported evidence of defective mineralization in 29% of patients with stage 2, 42% with stage 3, and 79% with stage 4/5 CKD, future studies comparing QCT scans and bone biopsies are needed to determine the sensitivity of QCT to identify mineralization defects.37
The study design has multiple important strengths. It is the largest longitudinal bone study of childhood onset CKD and the first to use pQCT to quantify changes in bone and muscle. It included a large robust longitudinal reference sample permitting adjustment for age, sex, race, tibia length and muscle area in the assessment of bone outcomes. Furthermore the use of Z-scores for volumetric BMD, cortical dimensions, and muscle deficits provide the first evidence of the clinical magnitude of the deficits.
We recently compared DXA and pQCT results in a cross-sectional analyses of 88 participants.38 While pQCT measures of trabecular BMD were associated with DXA measures of lumbar spine BMD (R2=0.13) and pQCT measures of cortical bone mineral content were associated with DXA whole body bone mineral content (R2=0.41), DXA measures were not associated with iPTH levels. Therefore, the clinical utility of DXA remains unproven.
In summary, our data demonstrated that children and adolescents with CKD have progressive declines in cortical bone accrual at one year follow-up, partially related to hyperparathyroidism and muscle deficits. Future studies are needed to assess the fracture implications of these findings, and to identify therapies to enhance bone quality in children with CKD.
Materials and Methods
Study participants
The study cohort consisted of children and adolescents aged 5-21 years with CKD (baseline eGFR < 90 ml/min/1.73m2) enrolled in a larger study of bone health in CKD at Children's Hospital of Philadelphia (CHOP) and Cincinnati Children's Hospital and Medical Center (CCHMC).1, 2, 38-40 Patients were ineligible for this study if they had a history of chronic conditions unrelated to CKD that may impact bone health or had a functioning renal allograft.
This study population was compared to a reference population of 903 healthy children and adolescents from CHOP recruited from general pediatric practices in the greater Philadelphia area.2, 10 Reference participants were excluded for medical history of illness or medications that could affect growth and development. A total of 269 completed a 12 month visit in a longitudinal substudy.10, 41
Study approval was obtained from the Institutional Review Boards at CHOP and CCHMC. Informed consent was obtained directly from study patients ≥ 18 years of age, and assent as well as parental consent in those < 18 years of age.
Anthropometry, Sexual Maturity and Race
Height was measured using a stadiometer (Holtain, Crymych, UK) and weight with a digital scale (Scaletronix, White Plains, New York). Tibia length was measured with a segmometer. Pubertal development was assessed using a self-assessment questionnaire and classified according to Tanner stage.42, 43 Participant's race was categorized by parents or the participant according to the National Institutes of Health categories.
Physical Activity Questionnaire
The physical activity questionnaire was designed to assess biomechanical impact.10, 44, 45 The questionnaire was introduced mid-study in the reference participants and completed in 401 participants.
CKD Disease Characteristics and Medications
Medical charts were reviewed for the date of diagnosis, cause of underlying renal disease, fracture history, medications, and dialysis and transplant history. Underlying renal disease was categorized as congenital anomalies of the kidney and urinary tract (CAKUT), FSGS, systemic inflammatory disease and other. All fracture events during the study were confirmed by review of radiology reports.
Peripheral Quantitative Computed Tomography
Bone, muscle and fat measures were obtained in the left tibia using a Stratec XCT2000 pQCT device (Orthometrix, White Plains, NY). Scan parameters, software and calibration procedures have been described.2, 10 Bone measurements were obtained at the 3% metaphyseal site for trabecular volumetric BMD (mg/cm3) and at the 38% diaphyseal site for cortical volumetric BMD, (mg/cm3), periosteal and endosteal circumference (mm), cortical cross sectional area (mm2), and polar section modulus (mm3). Fat and muscle area (mm3) were assessed 66% proximal to the distal physis. The manufacturer's hydroxyapatite phantom was scanned daily. In our laboratory, the coefficient of variation (CV) ranged from 0.5 to 1.6% for pQCT outcomes in children and adolescents.
Measurement of Muscle Force
Muscle force in ankle dorsiflexion was assessed using isometric dynamometry (Biodex Medical Systems, Inc, Shirley, NY).46 The Biodex measures were performed in 280 reference participants and in the CKD participants at the baseline and 12 month follow-up visit at CHOP only.
Laboratory Measurements
Non-fasting blood chemistries and iPTH were measured at baseline, 6 and 12 months in non-dialysis participants and baseline, 3, 6, and 12 months in dialysis participants.
Serum bicarbonate (mmol/L), calcium (mg/dL), phosphorus (mg/dL), and albumin (g/dL) concentrations were measured in clinical laboratories using standard methods and calcium levels were adjusted for albumin levels.47 Plasma PTH (pg/ml) was measured using two different immunoradiometric assays (IRMA) with I125I- labeled tracer (Scantibodies Clinical Laboratory, Santee, CA, USA). The traditional second generation IRMA measured iPTH. The third generation assay used a specific polyclonal PTH antibody directed against the most N-terminal PTH region as tracer antibody in order to measure the bioactive 1-84 PTH molecule. The intra-assay CV for the intact and bioactive PTH assays were 3 to 5% and 3 to 10% respectively. 48
Serum creatinine (mg/dL) was measured by spectrophotometric enzymatic assay (Vitros, Johnson & Johnson Co, Rochester, NY) with a CV of 1% to 5%. Estimated GFR (ml/min/1.73m2) was calculated based on height and serum creatinine using the pediatric estimating equation from the Chronic Kidney Disease in Children prospective cohort study 49. CKD stage was classified according to National Kidney Foundation guidelines.50 Dialysis patients were assigned an eGFR of 7.5 ml/min/1.73m2 for models examining baseline eGFR as a continuous variable, and for calculating change in eGFR in the two participants that started dialysis during the study.
Statistical Analysis
Analysis were performed using Stata 11.0 (Stata Corp., College Station, TX, USA). A p value < 0.05 was considered statistically significant and two sided tests of hypotheses were used throughout. Continuous variables were expressed as means ± SD or median (IQR) if not normally distributed. Group differences were assessed using Student's t test or the Wilcoxon rank sum test. Changes within the CKD patients were tested using the paired t-test or the Wilcoxon signed rank test. Differences in proportions were tested using the chi-square test. Correlations between continuous variables were assessed by Pearson or Spearman's correlations. Although this study included multiple comparison, we did not apply a Bonferroni correction because this assumes the multiple outcomes are independent.51 The majority of bone and muscle Z-score were highly correlated. Rather, we evaluated the results for internal consistency and interpreted isolated results with caution.
Age- and sex- specific height and BMI Z-scores were calculated using national data. 52 All PTH levels were log transformed and a mean value created for each individual over the study interval, and used in the longitudinal models. The pQCT outcomes were converted to race and sex specific Z-scores using the LMS method based on the reference participants, as described.2, 10, 53, 54 This method accounts for the non-linearity, heteroscedasticity and skew of bone data during growth.55, 56 Trabecular and cortical BMD were assessed relative to age. Cortical geometry, muscle and fat outcomes were correlated with tibia length (all p<0.0001); therefore, the Z-scores were generated relative to tibia length and further adjusted for age and tibia length using linear regression analyses.
Changes in pQCT Z-scores within the CKD participants were annualized (change per 12 months) and assessed using multivariate regression models. Given the differences in disease and participant characteristics in the non-dialysis and dialysis participants, we stratified the annualized changes in bone outcomes (Table 3). However, the associations between changes in bone outcomes and exposure variables did not differ between the non-dialysis and dialysis participants. Therefore, multivariate regression models of changes examined the entire cohort together. The following covariates were tested for each pQCT outcome- baseline outcome Z-score, study location, age, sex, race, Tanner stage, disease characteristics, concurrent medications, and baseline and change values for eGFR and physical activity. Bone models also included mean iPTH levels. All of the models that included the iPTH covariate were repeated using 1-84 or 7-84 PTH levels, yielding the same results (data not shown). The correlations between iPTH, 1-84 PTH and 7-84 PTH exceeded 0.98; therefore it was not possible to assess independent associations. The 1-84/7-84 ratio was not significant in any longitudinal models.
Secondary analyses were performed comparing pQCT Z-scores in participants that were above the European iPTH target but within KDOQI target range, vs. those without elevated iPTH by either target.
The associations between changes in muscle and cortical area Z-scores were compared in the CKD participants and the longitudinal subset of reference participants using multivariable regression analysis. Models for peak muscle torque were compared in the CKD and reference participants adjusted for age, sex, race, tibia length and muscle area.57
Acknowledgments
Funding Sources: NIH R01-DK060030, R01-HD040714, K24-DK076808, UL1-RR-024134 and UL1-RR-026314 and the University of Ottawa Faculty of Medicine
Footnotes
Disclosures: None.
References
- 1.Foster BJ, Kalkwarf HJ, Shults J, et al. Association of chronic kidney disease with muscle deficits in children. J Am Soc Nephrol. 2011;22:377–386. doi: 10.1681/ASN.2010060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wetzsteon RJ, Kalkwarf HJ, Shults J, et al. Volumetric bone mineral density and bone structure in childhood chronic kidney disease. J Bone Miner Res. 2011;26:2235–2244. doi: 10.1002/jbmr.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pluskiewicz W, Adamczyk P, Drozdzowska B, et al. Skeletal status in adolescents with end-stage renal failure: a longitudinal study. Osteoporos Int. 2005;16:289–295. doi: 10.1007/s00198-004-1672-8. [DOI] [PubMed] [Google Scholar]
- 4.Swolin-Eide D, Magnusson P, Hansson S. Bone mass, biochemical markers and growth in children with chronic kidney disease: a 1-year prospective study. Acta Paediatr. 2007;96:720–725. doi: 10.1111/j.1651-2227.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson VL, Wang J, Kaskel FJ, et al. Changes in body composition of children with chronic renal failure on growth hormone. Pediatr Nephrol. 2000;14:695–700. doi: 10.1007/s004670000342. [DOI] [PubMed] [Google Scholar]
- 6.Van Dyck M, Gyssels A, Proesmans W, et al. Growth hormone treatment enhances bone mineralisation in children with chronic renal failure. Eur J Pediatr. 2001;160:359–363. doi: 10.1007/s004310100734. [DOI] [PubMed] [Google Scholar]
- 7.Parfitt AM. A structural approach to renal bone disease. J Bone Miner Res. 1998;13:1213–1220. doi: 10.1359/jbmr.1998.13.8.1213. [DOI] [PubMed] [Google Scholar]
- 8.Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leonard MB, Elmi A, Mostoufi-Moab S, et al. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terpstra AM, Kalkwarf HJ, Shults J, et al. Bone Density and Cortical Structure after Pediatric Renal Transplantation. J Am Soc Nephrol. 2012 doi: 10.1681/ASN.2011050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.K/DOQI Clinical Practice guidelines for bone metabolism and disease in children with chronic kidney disease. Am J Kidney Dis. 2005;46(Suppl 1):1–103. [Google Scholar]
- 12.Klaus G, Watson A, Edefonti A, et al. Prevention and treatment of renal osteodystrophy in children on chronic renal failure: European guidelines. Pediatr Nephrol. 2006;21:151–159. doi: 10.1007/s00467-005-2082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoenau E, Neu CM, Beck B, et al. Bone mineral content per muscle cross-sectional area as an index of the functional muscle-bone unit. J Bone Miner Res. 2002;17:1095–1101. doi: 10.1359/jbmr.2002.17.6.1095. [DOI] [PubMed] [Google Scholar]
- 14.Obatake N, Ishimura E, Tsuchida T, et al. Annual change in bone mineral density in predialysis patients with chronic renal failure: significance of a decrease in serum 1,25-dihydroxy-vitamin D. J Bone Miner Metab. 2007;25:74–79. doi: 10.1007/s00774-006-0730-z. [DOI] [PubMed] [Google Scholar]
- 15.Fujimori A, Okada S, Sakai M, et al. Relationship between biochemical markers and radial cortical bone changes in hemodialysis patients. Nephron Clin Pract. 2011;118:c375–379. doi: 10.1159/000323669. [DOI] [PubMed] [Google Scholar]
- 16.Behnke B, Altrogge H, Delling G, et al. Bone mineral density in pediatric patients after renal transplantation. Clin Nephrol. 1996;46:24–29. [PubMed] [Google Scholar]
- 17.Behnke B, Kemper MJ, Kruse HP, et al. Bone mineral density in children with primary hyperoxaluria type I. Nephrol Dial Transplant. 2001;16:2236–2239. doi: 10.1093/ndt/16.11.2236. [DOI] [PubMed] [Google Scholar]
- 18.Lima EM, Goodman WG, Kuizon BD, et al. Bone density measurements in pediatric patients with renal osteodystrophy. Pediatr Nephrol. 2003;18:554–559. doi: 10.1007/s00467-002-1041-9. [DOI] [PubMed] [Google Scholar]
- 19.Tenbrock K, Kruppa S, Mokov E, et al. Analysis of muscle strength and bone structure in children with renal disease. Pediatr Nephrol. 2000;14:669–672. doi: 10.1007/s004670000360. [DOI] [PubMed] [Google Scholar]
- 20.Mehls O, Ritz E, Krempien B, et al. Roentgenological signs in the skeleton of uremic children. An analysis of the anatomical principles underlying the roentgenological changes. Pediatr Radiol. 1973;1:183–190. doi: 10.1007/BF00974065. [DOI] [PubMed] [Google Scholar]
- 21.Fox J, Miller MA, Newman MK, et al. Effects of daily treatment with parathyroid hormone 1-84 for 16 months on density, architecture and biomechanical properties of cortical bone in adult ovariectomized rhesus monkeys. Bone. 2007;41:321–330. doi: 10.1016/j.bone.2007.04.197. [DOI] [PubMed] [Google Scholar]
- 22.Ma YL, Marin F, Stepan J, et al. Comparative effects of teriparatide and strontium ranelate in the periosteum of iliac crest biopsies in postmenopausal women with osteoporosis. Bone. 2011;48:972–978. doi: 10.1016/j.bone.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Schober HC, Han ZH, Foldes AJ, et al. Mineralized bone loss at different sites in dialysis patients: implications for prevention. J Am Soc Nephrol. 1998;9:1225–1233. doi: 10.1681/ASN.V971225. [DOI] [PubMed] [Google Scholar]
- 24.Martin KJ, Gonzalez EA. Parathyroid hormone assay: problems and opportunities. Pediatr Nephrol. 2007;22:1651–1654. doi: 10.1007/s00467-007-0508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boot AM, Nauta J, de Jong MC, et al. Bone mineral density, bone metabolism and body composition of children with chronic renal failure, with and without growth hormone treatment. Clin Endocrinol (Oxf) 1998;49:665–672. doi: 10.1046/j.1365-2265.1998.00593.x. [DOI] [PubMed] [Google Scholar]
- 26.van der Sluis IM, Boot AM, Nauta J, et al. Bone density and body composition in chronic renal failure: effects of growth hormone treatment. Pediatr Nephrol. 2000;15:221–228. doi: 10.1007/s004670000470. [DOI] [PubMed] [Google Scholar]
- 27.Lanes R, Gunczler P, Orta N, et al. Changes in bone mineral density, growth velocity and renal function of prepubertal uremic children during growth hormone treatment. Horm Res. 1996;46:263–268. doi: 10.1159/000185098. [DOI] [PubMed] [Google Scholar]
- 28.Schweizer R, Martin DD, Schwarze CP, et al. Cortical bone density is normal in prepubertal children with growth hormone (GH) deficiency, but initially decreases during GH replacement due to early bone remodeling. J Clin Endocrinol Metab. 2003;88:5266–5272. doi: 10.1210/jc.2003-030432. [DOI] [PubMed] [Google Scholar]
- 29.Ruth EM, Weber LT, Schoenau E, et al. Analysis of the functional muscle-bone unit of the forearm in pediatric renal transplant recipients. Kidney Int. 2004;66:1694–1706. doi: 10.1111/j.1523-1755.2004.00937.x. [DOI] [PubMed] [Google Scholar]
- 30.Clapp EL, Bevington A, Smith AC. Exercise for children with chronic kidney disease and end-stage renal disease. Pediatr Nephrol. 2011 doi: 10.1007/s00467-010-1753-1. [DOI] [PubMed] [Google Scholar]
- 31.Weaver DJ, Jr, Kimball TR, Knilans T, et al. Decreased maximal aerobic capacity in pediatric chronic kidney disease. J Am Soc Nephrol. 2008;19:624–630. doi: 10.1681/ASN.2007070773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alayli G, Ozkaya O, Bek K, et al. Physical function, muscle strength and muscle mass in children on peritoneal dialysis. Pediatr Nephrol. 2008;23:639–644. doi: 10.1007/s00467-007-0711-z. [DOI] [PubMed] [Google Scholar]
- 33.Jamal SA, Cheung AM, West SL, et al. Bone mineral density by DXA and HR pQCT can discriminate fracture status in men and women with stages 3 to 5 chronic kidney disease. Osteoporos Int. 2012 doi: 10.1007/s00198-012-1908-y. [DOI] [PubMed] [Google Scholar]
- 34.Jamal SA, Gilbert J, Gordon C, et al. Cortical pQCT measures are associated with fractures in dialysis patients. J Bone Miner Res. 2006;21:543–548. doi: 10.1359/jbmr.060105. [DOI] [PubMed] [Google Scholar]
- 35.Cooper C, Dennison EM, Leufkens HG, et al. Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res. 2004;19:1976–1981. doi: 10.1359/JBMR.040902. [DOI] [PubMed] [Google Scholar]
- 36.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 37.Wesseling-Perry K, Pereira RC, Tseng CH, et al. Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin J Am Soc Nephrol. 2012;7:146–152. doi: 10.2215/CJN.05940611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffin LM, Kalkwarf HJ, Zemel BS, et al. Assessment of dual-energy x-ray absorptiometry measures of bone health in pediatric chronic kidney disease. Pediatr Nephrol. 2012 doi: 10.1007/s00467-012-2116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalkwarf H, Denburg M, Strife C, et al. Vitamin D Status in Children and Adolescents with Chronic Kidney Disease. 2011 doi: 10.1038/ki.2011.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuchman S, Thayu M, Shults J, et al. Interpretation of biomarkers of bone metabolism in children: impact of growth velocity and body size in healthy children and chronic disease. J Pediatr. 2008;153:484–490. doi: 10.1016/j.jpeds.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thayu M, Denson LA, Shults J, et al. Determinants of changes in linear growth and body composition in incident pediatric Crohn's disease. Gastroenterology. 2010;139:430–438. doi: 10.1053/j.gastro.2010.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanner J, Whitehouse R, Marshall W, et al. Assessment of Skeletal Maturity and Prediction of Adult Height (TW2 Method) Academic Press; London England: 1975. [Google Scholar]
- 43.Morris N, Udry J. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980 doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 44.Aaron DJ, Kriska AM, Dearwater SR, et al. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol. 1995;142:191–201. doi: 10.1093/oxfordjournals.aje.a117618. [DOI] [PubMed] [Google Scholar]
- 45.Kemper HC, Bakker I, Twisk JW, et al. Validation of a physical activity questionnaire to measure the effect of mechanical strain on bone mass. Bone. 2002;30:799–804. doi: 10.1016/s8756-3282(02)00709-3. [DOI] [PubMed] [Google Scholar]
- 46.Wetzsteon RJ, Zemel BS, Shults J, et al. Mechanical loads and cortical bone geometry in healthy children and young adults. Bone. 2011;48:1103–1108. doi: 10.1016/j.bone.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serum-Calcium. Lancet. 1979;1:858–859. [PubMed] [Google Scholar]
- 48.Gao P, Scheibel S, D'Amour P, et al. Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1-84: implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res. 2001;16:605–614. doi: 10.1359/jbmr.2001.16.4.605. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 51.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 53.Dubner SE, Shults J, Baldassano RN, et al. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn's disease. Gastroenterology. 2009;136:123–130. doi: 10.1053/j.gastro.2008.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wetzsteon RJ, Shults J, Zemel BS, et al. Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephrotic syndrome. J Bone Miner Res. 2009;24:503–513. doi: 10.1359/JBMR.081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- 56.Cole T, Green P. LMS Chartmaker Pro. 2006 [Google Scholar]
- 57.Wetzsteon R, Zemel B, Shults J, et al. Mechanical loads and cortical bone geometry in healthy children and young adults. Bone. 2011;48:1103. doi: 10.1016/j.bone.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


