Abstract
Context
Alzheimer disease (AD) imposes a severe burden upon patients and their caregivers. Severity of psychiatric symptoms and behavioral disturbances are important determinants of caregivers’ experience of burden. These symptoms may be improved with atypical antipsychotic treatment.
Objective
In this study we use data from the CATIE-AD trial to evaluate the effect of atypical antipsychotics as compared to placebo on the experiences of caregivers of outpatients with Alzheimer disease.
Design
We compared the effect of atypical antipsychotic drugs (olanzapine, risperidone or quetiapine) considered together as a group, to placebo, on experiences of caregivers of AD outpatients. We also evaluated whether improvement in patients’ psychiatric and behavioral symptoms mediated the relationship between drug treatment and caregiver burden.
Setting
CATIE-AD included outpatients in usual care settings, and assessed treatment effectiveness over a nine-month period.
Participants
Data from CATIE-AD participants who had at least one post-baseline outcome assessment, and from their caregivers, were examined in an intention-to-treat analysis (ITT) (N=361), and then in a phase 1 only analysis including only observations while on the initially randomized drug (N=153).
Measures
The Burden Interview, Beck Depression Inventory, and the NPI Caregiver Distress Scale were used to evaluate caregiver burden.
Results
In both ITT and phase 1-only analyses, caregivers of patients treated with second generation antipsychotics (SGAs) scored significantly lower than those on placebo on both the Burden Interview (p = 0.009) and the NPI Caregiver Distress Scale’s scores (p = 0.0209). These differences appeared to have been mediated by lower levels of agitation, hostility, and psychotic distortions.
Conclusion
In AD patients with symptoms of psychosis, agitation or aggressive behavior, medications can have a small but significant impact on caregiver burden.
Keywords: Caregivers Burden, Alzheimer Disease, Antipsychotic
Alzheimer disease (AD), is a costly and debilitating illness.1. By mid century, 81 millions cases of dementia are expected worldwide2, and the cost of their care will approache 200 billion dollars per year in the USA alone. AD imposes a severe burden on patients and their relatives, particularly those directly responsible for their care.
Caregivers of AD patients are often subject to enormous stress3–7 and are at high risk for depression8–13, increased utilization of health services14, 15 and psychotropic medications16, 17. Adverse effects of caregiving are especially pronounced among those who care for patients with dementia18, and they appear to have a higher than expected mortality19.
Psychiatric and behavioral symptoms are common in patients with AD20–22 and severity of psychiatric symptoms and behavioral disturbances have been reported as the main determinants of caregivers experiences of burden23–25.
The National Institute of Mental Health (NIMH) Clinical Antipsychotic Trials of Intervention Effectiveness –Alzheimer Disease (CATIE-AD) study, a large NIMH-funded, randomized controlled trial was designed to compare the effectiveness of antipsychotic medications and placebo in patients with AD and psychosis or agitated/aggressive behavior26. In contrast to the usual efficacy trial, CATIE-AD included outpatients in usual care settings, and assessed treatment effectiveness on several clinical outcome measures over a nine-month intervention period. A recent report using the CATIE-AD data found that clinical symptoms such as anger, aggression, and paranoid ideas improved with atypical antipsychotic treatment although no differences were found among the different antipsychotic drugs on most clinical outcome measures27. Aditional recent analyses of data from CATIE-AD showed that severity of such psychiatric symptoms and behavioral disturbances are among the strongest clinical correlates of caregivers’ experience of burden25. We thus hypothesize that since treatment with atypical antipsychotics alleviates these symptoms for patients they may also reduce caregiver burden.
Methods
Study Design
The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE)-AD study was a large NIMH-funded, randomized controlled trial designed to compare the effectiveness of 3 antipsychotic medications and placebo over 9 months in outpatients with AD with psychotic symptoms and/or agitated/aggressive behavior at 42 U.S. sites. Participants were initially randomly assigned to receive olanzapine, quetiapine, risperidone or placebo under double-blind conditions in a 2:2:2:3 allocation ratio (phase 1). Those whose initial, assigned treatments were discontinued (end of phase 1) could be randomly and double-blindly assigned to receive treatment with one of the two SGAs that they were not initially assigned to or with citalopram (phase 2). Participants receiving placebo in phase 1 received citalopram or one of the 3 SGAs in a 3:1:1:1 ratio in phase 2. Participants whose phase 2 treatments were discontinued could then be randomly assigned to open label treatment with one of the active agents not yet received (phase 3). Patients could be shifted at any time to open treatment with physician’s choice of medication and continue data collection. We present data on both patients in the entire intention- to-treat sample (i.e., those who received at least one follow-up assessment regardless of actual treatment received) (N=361) as well as those assessed during treatment with the initially randomized drug (phase 1 only, N = 153).
The trial was designed to encourage prescribing as close as possible to typical clinical practices. Study physicians adjusted dosages based on their clinical judgments and participants’ responses to treatment28.
The study was reviewed and approved by an Institutional Review Board (IRB) at each site. Written informed consent was obtained from the patients or their legally authorized representatives and from the partners or caregivers who participated with the patients. Details of the study design and entry criteria have been presented elsewhere26, 28. The current study relies on data collected at baseline, and 3, 6 and 9 months classified according to the original randomized group assignment, as well as limiting analysis to observations while patients were on their phase 1 drug.
Measures
Caregiver burden
The Burden Interview29 is a widely used 22-item assessment tool for measuring caregivers’ perceived burden from providing care in areas such as physical health, psychological well being, finances, and their interactions with the patient. Items are answered on a five-point scale ranging from 0= never to 4 = nearly always. Scores are added to give total score ranges from zero to 88, with higher scores implying greater perceived caregiver burden.
Caregiver Depression
The Beck Depression Inventory30 includes 21 questions, with each response scored on a scale from 0 to 3. Higher total scores indicate more severe depressive symptoms.
Caregiver Distress
The Caregiver Distress Scale, is a composite of the scores based on the distress items of the Neuropsychiatric Inventory (NPI) described in greater detail below31.
Psychiatric and behavioral symptoms in the patient were assessed with the Brief Psychiatric Rating Scale (BPRS;32, 33), in which a 7 point Likert scale is used to measure the severity of 18 psychiatric and behavioral symptoms and includes five-factor subscales: Agitation; Hostile Suspiciousness; Psychosis; Withdrawn Depression; and Cognitive Dysfunction. Symptoms were also measured with the NPI31, a measure of the frequency and severity of 12 psychiatric symptoms over the previous month. The items assess delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation, apathy/indifference, disinhibition, irritability/lability, aberrant motor behavior, sleep disturbance, and appetite and eating disorder. Caregivers rate each symptom, in terms of both frequency (1 to 4) and severity (1 to 3) indicating their distress from each symptom on a scale from 0 (not distressing at all) to 5 (extremely distressing). The NPI symptom score is calculated by multiplying the scores for severity and frequency (range=0 if absent and 1–12 if present).
The Cornell Scale for Depression in Dementia (CSDD)34 was used to measure patient mood symptoms, ideational disturbances of depression, and neurovegetative signs.
Cognitive functioning
Cognitive functioning was assessed with: 1) The Mini Mental State Examination (MMSE)35, a brief 30-item measure of global cognitive ability and 2) the AD Assessment Scale –Cognitive Subscale (ADAS-Cog), an 11-item assessment of memory, language, visuoconstructive skill, and ideational praxis36.
Activities of daily living
The AD Cooperative Study –Activities of Daily Living Scale (ADCS-ADL)37 is an inventory of basic and instrumental functional skills and abilities.
Quality of life
The Alzheimer Disease Related Quality of Life (ADRQL)38 measures health-related quality of life in patients with AD. Items assess behaviors that reflect social interaction, maintenance of interests, participation in activities, cheerfulness, and freedom from distress.
Level of needed care
The Dependence Scale39 is a measure of the amount of caregiver assistance needed by the patient to accomplish daily activities. Based on an interview with the caregiver, the patient’s Equivalent Institutional Care (EIC) level is derived as follows: Level 1= Limited home care (needs some help with activities such as shopping or housekeeping); Level 2= Supervised adult home care (supervised setting with constant companionship and regular help with cooking and housekeeping); Level 3= Health-related facility (24-hour supervision for personal care and safety). The Caregiver Activity Scale40, represents the total time that the caregiver spends providing assistance for a patient over the past 24 hours in five care-need domains.
Data Analyses
First we compared baseline data between ITT groups who had at least one follow-up assessment (N = 361), and those with none (N= 60). using an analysis of variance (ANOVA). Then to evaluate the randomization within this ITT sub sample, we used ANOVA to compare patients who were randomized to placebo (N = 124) to those randomized to a SGA (N = 237) on the same baseline variables.
Next we compared baseline data on patients in the Phase I only sample, i.e. patients who had at least one follow-up visit while on the randomly assigned treatment (N = 153) with study participants, who did not have follow-up data while on randomly assigned drug. (N = 268). In addition, to evaluate the randomization within the Phase I only sub-sample, we compared baseline characteristics of patients in this sub sample who were assign placebo (N = 45) to those assigned a SGA (N = 108).
The primary analysis compared the average differences in the three burden measures across all time points between patients who received antipsychotic or placebo treatment. All available follow-up data were used in both the intention to treat and the Phase 1-only analyses. We used longitudinal mixed models which adjusted for the correlatedness of observations from the same individuals with unique patient identifier modeled as a random intercept, and controlled for time and for the baseline value of each dependant variable. Because of small sample sizes of burden data on individual drugs we did not attempt to compare the effects of individual drugs against placebo.
For burden measures on which there was a significant treatment effect, we conducted further analyses to determine whether improvement in psychiatric and behavioral symptoms among the patients mediated the relationship between drug treatment and caregiver burden. In these analyses, we repeated the mixed model analyses described above but added the NPI and BPRS patient scores as time-varying covariates since they appeared to have improved with antipsychotic treatment in previously published analyses of CATIE AD data27. If the variable representing treatment was no longer significant after the inclusion of these covariates, we inferred that the added covariates mediated the relationship between medication treatment and burden. We then examined two further the models covarying for the BPRS and the NPI scores separately to test if either of these measures was an independent mediator of the relationship between medication and burden. If treatment became non significant in the model in which BPRS was entered alone, we further repeated the model to include each of the BPRS subsocres separately.
Results
Patient characteristics and treatment
Mean age of participants was 77.9 (SD 7.5) years; 56% were female; 21% were non-white. Overall, 77–85% of patients in each treatment group discontinued the Phase 1 medication treatment prior to the end of the 36-week study period. As reported in prior previously28, the median duration of Phase 1 treatment was 7.1 weeks and did not differ significantly across treatment groups (median duration ranged from 5.3 to 8.1 weeks in the four groups). Data on patient participation and outcomes werewere reported previously27, 28, 41.
Subgroup Characteristics at baseline
Comparisons of baseline data on patients in the ITT sample for whom we have follow up data with the rest of the sample showed that caregivers of patients who had follow-up data were significantly older and more likely to be spouses than children of their caregivers (p = 0.05) (Table 1). Patients included in the analyses also had significantly less severe general psychiatric symptoms and a higher quality of life at baseline. In the ITT sample baseline caregiver burden and distress scores were higher for patients assigned to placebo compared to those assigned to SGA although these differences were small in magnitude (data available from first author).
Table 1.
Comparison of baseline characteristics of the ITT sample with follow up data and the remainder of trial participants without follow up data.
| Included | Excluded | |||||
|---|---|---|---|---|---|---|
| N | % | Mean (SD) | N | % | Mean (SD) | |
| Patients Sociodemographic Characteristics | 361 | 280 (77.6%) | 60 | 51 (85.0%) | ||
| White Race | 361 | 280 (77.6%) | 60 | 51 (85.0%) | ||
| Age | 361 | 77.8 (7.4) | 60 | 78.2 (7.5) | ||
| Female | 361 | 198 (54.8%) | 60 | 37 (61.7%) | ||
| Race/Ethnicity | ||||||
| White Race | 361 | 280 (77.6%) | 60 | 51 (85.0%) | ||
| Black Race | 361 | 67 (18.6%) | 60 | 8 (13.3%) | ||
| Marital Status | ||||||
| Married | 361 | 219 (60.7%) | 60 | 30 (50.0) | ||
| Education | 348 | 12.3 (3.3) | 57 | 11.8 (3.3) | ||
| Caregivers Sociodemographic Characteristics | ||||||
| Age* | 233 | 63.7 (15.0) | 38 | 58.0 (17.6) | ||
| Female Sex | 270 | 194 (71.9%) | 49 | 32 (65.3%) | ||
| Relationship with Patient | ||||||
| Spouse* | 317 | 150 (56.)%) | 49 | 16 (32.7%) | ||
| Child* | 268 | 80 (29.9%) | 49 | 24 (49.0%) | ||
| Psychiatric and Behavioral Symptoms | ||||||
| Neuropsychiatric Inventory (NPI) | 358 | 36.3 (18.0) | 56 | 40.4 (20.1) | ||
| Brief Psychiatric Rating Scale (BPRS)** | 359 | 27.1 (12.1) | 60 | 31.6 (13.0) | ||
| Cornell Scale for Depression in Dementia (CSDD) | 357 | 9.8 (5.4) | 59 | 10.4 (5.9) | ||
| Cognitive Skills | ||||||
| Mini Mental State Examination (MMSE) | 359 | 15.1 (5.6) | 57 | 14.5 (6.9) | ||
| AD Assessment Scale –Cognitive Subscale (ADAS-Cog) | 334 | 34.5 (13.1) | 47 | 35.4 (14.6) | ||
| Functional Abilities | ||||||
| AD Cooperative Study –Activities of Daily Living Scale (ADCS-ADL) | 357 | 39.7 (16.8) | 56 | 35.0 (19.4) | ||
| Quality of Life | ||||||
| AD-Related Quality of Life* | 358 | 68.0 (14.0) | 58 | 63.3 (16.8) | ||
| Care Needs | ||||||
| Dependence Scale | 357 | 3.3 (1.0) | 55 | 3.4 (1.1) | ||
| Equivalent Institutional Care | 357 | 1.91 (0.64) | 55 | 1.89 (0.71) | ||
| Caregivers Activity Scale | 355 | 16.1 (11.7) | 54 | 17.9 (13.2) | ||
| Caregivers Burden | ||||||
| Burden Interview | 356 | 33.9 (15.9) | 53 | 37.6 (16.7) | ||
| NPI Distress Score | 358 | 16.27 (8.5) | 56 | 17.8 (9.0) | ||
| Beck Depression Inventory | 356 | 8.2 (7.2) | 54 | 9.7 (8.1) |
P = 0.05;
p = 0.001
Comparisons of patients in the phase 1-only sample for whom we have follow-up data with those for whom no follow up data were available showed that significantly more patients who had follow-up assessments were married (65.7% vs. 47.7%); fewer were females (50.7% vs. 64.7%); more of their caregivers were spouses (61.7% vs. 35.1%) and fewer were children (25.7 vs. 45.9%). No significant differences in clinical variables were noted (Table 2). Among patients in the phase 1-only group, those who were randomized to placebo were significantly more likely to be black than those assigned to SGAs (13.0% vs. 28.9%) (Table 3.).
Table 2.
Baseline Characteristics of Patients With Versus Without Follow-Up Caregiver Burden Data While Receiving Phase 1 Drug Treatment
| Variable | N | Follow-Up Caregiver Burden Data Obtained | N | No Follow-Up Caregiver Burden Data Obtained |
|---|---|---|---|---|
| Patient sociodemographic characteristics | ||||
| Age, Mean (SD), y | 268 | 77.5 (7.3) | 153 | 78.6 (7.6) |
| Female sex, n (%)** | 268 | 136 (50.7) | 153 | 99 (64.7) |
| Race/ethnicity, n (%) | ||||
| White | 268 | 212 (79.1) | 153 | 119 (77.8) |
| Black | 268 | 48 (17.9) | 153 | 27 (17.6) |
| Marital Status, married, n (%)*** | 268 | 176 (65.7) | 153 | 73 (47.7) |
| Caregiver sociodemographic characteristics | ||||
| Age, Mean (SD), y | 180 | 63.8 (15.4) | 91 | 61.0 (15.7) |
| Female sex, n (%) | 207 | 147 (71.0) | 112 | 79 (70.5) |
| Education, mean (SD), y | 260 | 12.4 (3.4) | 145 | 11.9 (3.4) |
| Caregiver relationship with patient, n (%) | ||||
| Spouse*** | 206 | 127 (61.7) | 111 | 39 (35.1) |
| Child*** | 206 | 53 (25.7) | 111 | 51 (45.9) |
| Patient psychiatric and behavioral symptom scores, mean (SD) | ||||
| Neuropsychiatric Inventory | 265 | 38.0 (17.6) | 149 | 35.0 (19.0) |
| Brief Psychiatric Rating Scale | 267 | 27.3 (11.5) | 152 | 28.6 (13.6) |
| Cornell Scale for Depression in Dementia | 265 | 10.2 (5.2) | 151 | 9.5 (6.0) |
| Patient cognitive skills scores, mean (SD) | ||||
| Mini-Mental State Examination | 267 | 15.2 (5.8) | 149 | 14.7 (5.8) |
| Alzheimer’s Disease Cooperative Study – Activities of Daily Living scale | 252 | 34.8 (13.1) | 129 | 13.7 (1.2) |
| Patient functional abilities score, mean (SD) | ||||
| Alzheimer’s Disease Cooperative Study-Activities of Daily Living Scale | 266 | 39.3 (16.6) | 147 | 38.6 (18.4) |
| Patient quality-of-life score, mean (SD) | ||||
| Alzheimer’s Disease Related Quality of Life scale | 266 | 67.0 (13.6) | 150 | 67.9 (16.4) |
| Patient care-needs scores, mean (SD) | ||||
| Dependence Scale | 265 | 3.3 (1.0) | 147 | 3.3 (1.0) |
| Equivalent Institutional Care level* | 265 | 2.0 (0.6) | 147 | 7.8 (0.7) |
| Caregiver Activity Survey | 263 | 16.5 (11.8) | 146 | 16.0 (12.1) |
| Caregiver burden scores, mean (SD) | ||||
| Burden Interview | 265 | 35.1 (15.6) | 144 | 33.2 (16.6) |
| Neuropsychiatric Inventory Caregiver Distress Scale | 265 | 16.9 (8.5) | 149 | 15.7 (8.6) |
| Beck Depression Inventory | 255 | 8.6 (7.2) | 145 | 8.0 (7.5) |
P<.05, t test comparing the 2 groups
P<.001, t test comparing the 2 groups
P<.0001, t test comparing the 2 groups
Table 3.
Baseline Characteristics of Patients Included in the Phase I Sample Randomized to Medication vs. vs those Randomized to Placebo
| Drugs | Placebo | |||
|---|---|---|---|---|
| Patients Sociodemographic Characteristics | ||||
| White Race | ||||
| Age | 108 | 78.3 (7.9) | 45 | 79.1 (7.0) |
| Female | 108 | 69 (63.9%) | 45 | 30 (66.7%) |
| Race/Ethnicity | ||||
| White Race | 108 | 88 (81.5%) | 45 | 31 (68.9%) |
| Black Race* | 108 | 14 (13.0%) | 45 | 13 (28.9%) |
| Marital Status | ||||
| Married | 108 | 51 (47.2%) | 45 | 22 (48.9%) |
| Education | 101 | 12.0 (3.6) | 44 | 11.7 (2.8) |
| Caregivers Sociodemographic Characteristics | ||||
| Age | 62 | 59.4 (16.0) | 29 | 64.5 (14.6) |
| Female Sex | 79 | 54 (68.4%) | 33 | 25 (75.8%) |
| Education | 101 | 12.0 (3.4) | 44 | 11.7 (2.8) |
| Relationship with Patient | ||||
| Spouse | 78 | 27 (34.6%) | 33 | 12 (36.4%) |
| Child | 78 | 38 (48.7%) | 33 | 13 (39.4%) |
| Psychiatric and Behavioral Symptoms | ||||
| Neuropsychiatric Inventory (NPI) | 104 | 35.8 (19.2) | 45 | 33.1 (19.6) |
| Brief Psychiatric Rating Scale (BPRS) | 107 | 28.4 (13.4) | 45 | 28.9 (14.2) |
| Cornell Scale for Depression in Dementia (CSDD) | 106 | 9.5 (5.9) | 45 | 9.4 (6.2) |
| Cognitive Skills | ||||
| Mini Mental State Examination (MMSE) | 106 | 14.9 (5.9) | 43 | 14.3 (5.8) |
| AD Assessment Scale – Cognitive Subscale (ADAS-Cog) | 90 | 33.7 (14.2) | 39 | 35.2 (12.6) |
| Functional Abilities | ||||
| AD Cooperative Study – Activities of Daily Living Scale (ADCS-ADL) | 105 | 39.0 (19.3) | 42 | 37.7 (16.2) |
| Quality of Life | ||||
| AD-Related Quality of Life | 108 | 68.3 (16.5) | 42 | 66.8 (16.2) |
| Care Needs | ||||
| Dependence Scale | 105 | 3.3 (1.0) | 42 | 3.2 (0.8) |
| Equivalent Institutional Care | 105 | 1.8 (0.7) | 42 | 1.8 (0.6) |
| Caregivers Activity Scale | 104 | 15.7 (12.4) | 42 | 16.6 (11.2) |
| Hospitalization for at least 2 weeks | ||||
| Caregivers Burden | ||||
| Burden Interview | 102 | 33.5 (16.4) | 42 | 32.5 (17.5) |
| NPI Distress Score | 104 | 16.1 (8.4) | 45 | 14.7 (9.2) |
| Beck Depression Inventory | 101 | 7.6 (7.4) | 44 | 8.8 (7.9) |
P = 0.05
Burden outcome in the ITT and phase I only groups
Caregivers of patients in the ITT sample randomized to SGAs scored significantly lower on the Burden Interview (less burden) score (p = 0.009) and the NPI distress scale (less distress) (p = 0.0209) than those assigned to placebo. Effect sizes were small with 0.18 standard deviation unit differences for both measures. The differences in the caregiver depression mean scores, in contrast, were not significant (Table 4).
Table 4.
Outcome of ITT Drugs vs. Placebo Groups
| Placebo LMS |
Drugs LSM |
t | P | |
|---|---|---|---|---|
| Caregivers Burden | ||||
| Burden Interview | 33.0 | 30.0 | 6.86 | 0.0090 |
| NPI Distress Score | 10.6 | 9.0 | 5.36 | 0.0209 |
| Beck Depression Inventory | 8.1 | 7.8 | 0.24 | 0.5185 |
LMS = Lease Square Mean values across all follow up data points adjusted for the basline value of the dependant vaiable.
Burden outcome in the phase 1-only groups
The same pattern was observed in the phase 1-only sample. Caregivers of patients in the SGAs group scored significantly lower on both the burden interview score (p = 0.0264) and the NPI distress scale (p = 0.0467). Effect sizes appeared larger than in the ITT analysis but were still rather modest at 0.26 standard deviation units for the Burden Measure and 0.25 on the NPI distress scale. As in the ITT analysis the differences in depression scores were not significant (Table 5 and Figure 1).
Table 5.
Outcome of Phase I Drugs vs. Placebo Groups
| Placebo LMS |
Drugs LMS |
t | P | |
|---|---|---|---|---|
| Caregivers Burden | ||||
| Burden Interview | 31.6 | 27.5 | 5.03 | 0.0264 |
| NPI Distress Score | 9.7 | 7.6 | 4.0 | 0.0467 |
| Beck Depression Inventory | 7.1 | 7.0 | 0.02 | 0.8826 |
LMS = Lease Square Mean values across all follow up data points adjusted for the basline value of the dependant vaiable.
Figure 1.
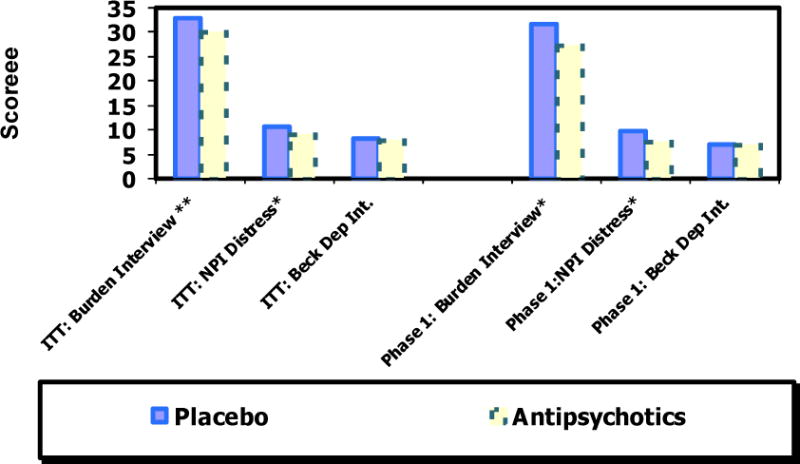
Least square means comaprison of placebo and antipsychotics: Intention to treat (ITT) and Phase 1 only
To examine the mediating effect of the BPRS and the NPI on the relationship of treatment to the burden interview score in the phase 1-only sample, we first entered both the BPRS and the NPI into the model including the burden interview score. In this model, treatment was no longer significant (p = 0.096). We then entered the NPI score alone and treatment was again no longer significant (p = 0.114). In the model in which only the BPRS was entered, treatment remained significant (p = 0.041). Thus, reductions in NPI scores appears to have mediated the relationship between treatment and the measure of burden.
When we entered both the BPRS and the NPI in the model with the NPI distress scale as outcome, treatment was no longer significant (p = 0.137). We then entered the NPI only and treatment was again non-significant (p = 0.130). We also entered the BPRS only and treatment was again not significant (p = 0.112). We therefore entered each of the BPRS five subscales separately. In the models including BPRS agitation (p = 0.154), hostile suspiciousness (p = 0.218), and psychotic distortion (p = 0.152) subscores treatment was not significant while in the models including withdrawn depression (p = 0.017) and cognitive dysfunction (p = 0.047), treatment remained significant.
Discussion
In this study both the ITT analysis, and the Phase 1 –only analysis, caregivers of patients assigned to take antipsychotic medications had lower burden scores than those randomized to placebo. Effect sizes were statistically significant but small, at about 0.18 at the ITT analysis to 0.25 on the Phase-I only analysis.
These findings are encouraging since the original analyses from CATIE-AD found no overall benefit for antipsychotics as compared to placebo on either the primary study outcome, time to all cause medication discontinuation28 or on a measure of Quality Adjusted Life Years used in the Cost Effectiveness analysis42. However, a recent publication on more specific clinical outcomes ratings in CATIE-AD27 showed small benefits for medication over placebo on two measures of psychiatric symptoms, the BPRS, the NPI, as well as the clinician-rated Clinical Global Impression of Change during phase 1 of the trial. Taken together with the results of this study there appears to be some clinical benefit for both patients and caregivers favoring the medications, especially early in treatment.
The effect of SGAs on caregiver measures seems to have been mediated by improvement in psychiatric symptoms, more specifically agitation, hostile suspiciousness, and psychotic distortion. Hence, this study thus suggests that improvements in these symptoms may lead to reduced burden and distress for caregivers, although not to reduced depression.
Previous studies have also clearly shown that severity of psychiatric symptoms and behavioral disturbances are the main correlates of caregivers’ experience of burden23, 25 and we thus hypothesized that treatment with atypical antipsychotics might both alleviate these symptoms for patients and reduce caregivers burden. Our data showing significant, if small, reduction of burden indicators even in the presence of small degrees of clinical improvement and suggest a high sensitivity of caregiver burden to even small changes in patient clinical status.
The lack of medication effects on caregivers’ depression is not surprising in light of earlier findings of weaker correlations between behavioral disturbances and caregivers depression23, 25. This also supports the notion that depression in caregivers might be distinct from burden and implies the need for different treatment. Despite the correlation of caregiver depression with psychiatric symptoms in AD patients, alleviation of these symptoms does not seem to have a direct effect on depression.
While antipsychotics have been shown to have a positive impact on behavioral symptoms in some clinical trials, their overall efficacy may be offset by adverse events that require medication discontinuation28, a phenomenon that was clearly evident in the short treatment durations observed in the CATIE AD trial. Additionally, studies have shown that that caregivers consider improvement in their relatives’ quality of life, an outcome not affected by medication in the CATIE AD trial, to be as important as prolonging the patient’s life, and that improvement in quality of life is more important to caregivers than either lengthening survival time or delaying admission to a nursing home43. A measure of Alzheimer disease related quality of life addressing issues such as social interaction, maintaining interests, and participating in activities was shown to be a robust predictor of reduced caregiver burden (Mohamed, Rosenheck et al. under review). Since antipsychotic medications were not beneficial in improving quality of life in phase 1 of CATIE AD27, psychosocial interventions designed to improve patients’ quality of life, perhaps through increased socialization and social interactions, may prove more powerful in reducing caregiver burden44–46.
Two notable methodological limitations require comment. Since some measures of patient symptoms were based on the caregiver reports, it is possible that caregiver ratings of the severity of these symptoms reflect, at least in part, their own emotional state. However, the use of proxy reporting is unavoidable in AD research and the use of multiple measures of both patients symptoms and caregivers distress reduces the impact of this limitation. A second limitation is the small sample size, especially in the placebo comparison group. However a clear and statistically significant signal of reduced burden in association with antipsychotic therapy was detected in this study.
We conclude that among AD patients with symptoms of psychosis, agitation or aggressive behavior, atypical antipsychotic medications may reduce agitation, suspiciousness, and psychosis enough to have a small but significant impact on caregivers’ experience of burden.
Acknowledgments
This work was supported by grant NO1 MH9001 from the NIMH, NIH N01 MH9001 (supported the conduction of the study) and USC Alzheimer’s Disease Research center NIH P50 AG05142 (supported part of Dr. Schneider’s effort in writing this manuscript). The manuscript was partially supported by a contract with Wyeth Pharmaceuticals (which partially supported Dr. Mohamed’s efforts in writing the manuscript). Dr. Lyketsos was supported by PO1-AGO5146 (Johns Hopkins Alzheimer’s Disease Research Center which supported Dr. Lykestos effort in writing the manuscript).
Dr. Rosenheck has received research support from Eli Lilly, Janssen Pharmaceutica, Astra-Zeneca and Wyeth Pharmaceuticals. He has been a consultant to GlaxoSmithKline, Bristol Myers Squibb, Organon and Janssen Pharmaceutica. He provided expert testimony for the plaintiffs in UFCW Local 1776 and Participating Employers Health and Welfare Fund, et al. v. Eli Lilly and Company; for the respondent in Eli Lilly Canada Inc vs Novapharm Ltd and Minister of Health, respondent; for the Patent Medicines Prices Review Board Canada, in the matter of Janssen Ortho Inc. and “Risperdal Consta” and testifying expert in Jones ex rel. the State of Texas v. Janssen Phamaceutica et al.; Dr Schneider has received consulting fees from AstraZeneca, Bristol Myers Squibb, Eli Lilly, Forest Laboratories, GlaxoSmithKline, Novartis, Johnson & Johnson, and Pfizer; and research grant support from Novartis and Pfizer; Dr. Sultzer has received research support from Eli Lilly and Forest Research Intitute; and Dr Lyketsos has received Grant support (research or CME) from NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, Glaxo-Smith-Kline, Eisai, Pfizer, Astra-Zeneca, Lilly, Ortho-McNeil, Bristol-Myers, Novartis, National Football League, and Elan; consulting fees from Astra-Zeneca, Glaxo-Smith Kline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Genentech, NFL Players Association, and NFL and a speaking honorarium or travel support from Pfizer, Forest, Glaxo-Smith Kline, and Health Monitor.
Footnotes
Note: None of the sponsers assisted in the preparation, review, or approval of the manuscript.
References
- 1.Manca A, Davies L, Burns A. Cost-effectiveness of therapeutics for Alzheimer’s Disease. In: Davis K, Charney D, Coyle J, editors. Neuropsychopharmacology: The Fifth Generation of Progress. New York, NY: Lippincott Williams & Wilkins; 2002. pp. 1267–1280. [Google Scholar]
- 2.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005 Dec 17;:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. Sect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donaldson C, Tarrier N, Burns A. The impact of the symptoms of dementia on caregivers. Br J Psychiatry. 1997 Jan;170:62–68. doi: 10.1192/bjp.170.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Haley WE. The family caregiver’s role in Alzheimer’s disease. Neurology. 1997 May;48(5 Suppl 6):S25–29. doi: 10.1212/wnl.48.5_suppl_6.25s. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson C, Tarrier N, Burns A. Determinants of carer stress in Alzheimer’s disease. Int J Geriatr Psychiatry. 1998 Apr;13(4):248–256. doi: 10.1002/(sici)1099-1166(199804)13:4<248::aid-gps770>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Aguglia E, Onor ML, Trevisiol M, Negro C, Saina M, Maso E. Stress in the caregivers of Alzheimer’s patients: an experimental investigation in Italy. Am J Alzheimers Dis Other Demen. 2004 Jul-Aug;19(4):248–252. doi: 10.1177/153331750401900403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amirkhanyan AA, Wolf DA. Caregiver stress and noncaregiver stress: exploring the pathways of psychiatric morbidity. Gerontologist. 2003 Dec;43(6):817–827. doi: 10.1093/geront/43.6.817. [DOI] [PubMed] [Google Scholar]
- 8.Cattanach L, Tebes JK. The nature of elder impairment and its impact on family caregivers’ health and psychosocial functioning. Gerontologist. 1991 Apr;31(2):246–255. doi: 10.1093/geront/31.2.246. [DOI] [PubMed] [Google Scholar]
- 9.Schulz R, Williamson GM. A 2-year longitudinal study of depression among Alzheimer’s caregivers. Psychol Aging. 1991 Dec;6(4):569–578. doi: 10.1037//0882-7974.6.4.569. [DOI] [PubMed] [Google Scholar]
- 10.Williamson GM, Schulz R. Coping with specific stressors in Alzheimer’s disease caregiving. Gerontologist. 1993 Dec;33(6):747–755. doi: 10.1093/geront/33.6.747. [DOI] [PubMed] [Google Scholar]
- 11.Gallant MP, Connell CM. Predictors of decreased self-care among spouse caregivers of older adults with dementing illnesses. J Aging Health. 1997 Aug;9(3):373–395. doi: 10.1177/089826439700900306. [DOI] [PubMed] [Google Scholar]
- 12.Song LY, Biegel DE, Milligan SE. Predictors of depressive symptomatology among lower social class caregivers of persons with chronic mental illness. Community Ment Health J. 1997 Aug;33(4):269–286. doi: 10.1023/a:1025090906696. [DOI] [PubMed] [Google Scholar]
- 13.Schulz R, Boerner K, Shear K, Zhang S, Gitlin LN. Predictors of complicated grief among dementia caregivers: a prospective study of bereavement. Am J Geriatr Psychiatry. 2006 Aug;14(8):650–658. doi: 10.1097/01.JGP.0000203178.44894.db. [DOI] [PubMed] [Google Scholar]
- 14.Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosom Med. 1991 Jul-Aug;53(4):345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Draper BM, Poulos CJ, Cole AM, Poulos RG, Ehrlich F. A comparison of caregivers for elderly stroke and dementia victims. J Am Geriatr Soc. 1992 Sep;40(9):896–901. doi: 10.1111/j.1532-5415.1992.tb01986.x. [DOI] [PubMed] [Google Scholar]
- 16.Baumgarten M, Battista RN, Infante-Rivard C, Hanley JA, Becker R, Gauthier S. The psychological and physical health of family members caring for an elderly person with dementia. J Clin Epidemiol. 1992 Jan;45(1):61–70. doi: 10.1016/0895-4356(92)90189-t. [DOI] [PubMed] [Google Scholar]
- 17.Grafstrom M, Fratiglioni L, Sandman PO, Winblad B. Health and social consequences for relatives of demented and non-demented elderly. A population-based study. J Clin Epidemiol. 1992 Aug;45(8):861–870. doi: 10.1016/0895-4356(92)90069-y. [DOI] [PubMed] [Google Scholar]
- 18.Ory MG, Hoffman RR, 3rd, Yee JL, Tennstedt S, Schulz R. Prevalence and impact of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999 Apr;39(2):177–185. doi: 10.1093/geront/39.2.177. [DOI] [PubMed] [Google Scholar]
- 19.Christakis NA, Allison PD. Mortality after the hospitalization of a spouse. N Engl J Med. 2006 Feb 16;354(7):719–730. doi: 10.1056/NEJMsa050196. [DOI] [PubMed] [Google Scholar]
- 20.Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000 May;157(5):708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- 21.Lopez OL, Becker JT, Sweet RA, et al. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2003 Summer;15(3):346–353. doi: 10.1176/jnp.15.3.346. [DOI] [PubMed] [Google Scholar]
- 22.Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry. 2005 Nov;162(11):2022–2030. doi: 10.1176/appi.ajp.162.11.2022. [DOI] [PubMed] [Google Scholar]
- 23.Coen RF, Swanwick GR, O’Boyle CA, Coakley D. Behaviour disturbance and other predictors of carer burden in Alzheimer’s disease. Int J Geriatr Psychiatry. 1997 Mar;12(3):331–336. [PubMed] [Google Scholar]
- 24.Cummings JL, Schneider L, Tariot PN, Kershaw PR, Yuan W. Reduction of behavioral disturbances and caregiver distress by galantamine in patients with Alzheimer’s disease. Am J Psychiatry. 2004 Mar;161(3):532–538. doi: 10.1176/appi.ajp.161.3.532. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed S, Rosenheck R, Sneider L. Caregiver Burden in Alzheimer’s disease. Am J Geriatr Psychiatry under review. doi: 10.1097/JGP.0b013e3181d5745d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider LS, Ismail MS, Dagerman K, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer’s disease trial. Schizophr Bull. 2003;29(1):57–72. doi: 10.1093/oxfordjournals.schbul.a006991. [DOI] [PubMed] [Google Scholar]
- 27.Sultzer DL, Davis SM, Tariot PN, et al. Clinical symptom responses to atypical antipsychotic medications in Alzheimer’s disease: phase 1 outcomes from the CATIE-AD effectiveness trial. Am J Psychiatry. 2008 Jul;165(7):844–854. doi: 10.1176/appi.ajp.2008.07111779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006 Oct 12;355(15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 29.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980 Dec;20(6):649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]
- 30.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961 Jun;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 31.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994 Dec;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 32.Beller SA, Overall JE. The Brief Psychiatric Rating Scale (BPRS) in geropsychiatric research: II. Representative profile patterns. J Gerontol. 1984 Mar;39(2):194–200. doi: 10.1093/geronj/39.2.194. [DOI] [PubMed] [Google Scholar]
- 33.Overall JE, Gorham DR. Introduction-the Brief Psychiatric Rating Scale (BPRS): Recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97–99. [PubMed] [Google Scholar]
- 34.Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988 Feb 1;23(3):271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 35.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 36.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984 Nov;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 37.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S33–39. [PubMed] [Google Scholar]
- 38.Rabins P, Kasper J, Kleinman L. Concepts and methods in the development of the ADRQL: an instrument for assessing health-related quality of life in persons with Alzheimer’s Disease. Journal of Mental Health Aging. 1999;5:33–48. [Google Scholar]
- 39.Stern Y, Albert SM, Sano M, et al. Assessing patient dependence in Alzheimer’s disease. J Gerontol. 1994 Sep;49(5):M216–222. doi: 10.1093/geronj/49.5.m216. [DOI] [PubMed] [Google Scholar]
- 40.Davis KL, Marin DB, Kane R, et al. The Caregiver Activity Survey (CAS): development and validation of a new measure for caregivers of persons with Alzheimer’s disease. Int J Geriatr Psychiatry. 1997 Oct;12(10):978–988. doi: 10.1002/(sici)1099-1166(199710)12:10<978::aid-gps659>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 41.Ismail MS, Dagerman K, Tariot PN, Abbott S, Kavanagh S, Schneider LS. National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness – Alzheimer’s Disease (CATIE-AD): baseline characteristics. Curr Alzheimer Res. 2007 Jul;4(3):325–335. doi: 10.2174/156720507781077214. [DOI] [PubMed] [Google Scholar]
- 42.Rosenheck RA, Leslie DL, Sindelar JL, et al. Cost-benefit analysis of second-generation antipsychotics and placebo in a randomized trial of the treatment of psychosis and aggression in Alzheimer disease. Arch Gen Psychiatry. 2007 Nov;64(11):1259–1268. doi: 10.1001/archpsyc.64.11.1259. [DOI] [PubMed] [Google Scholar]
- 43.Karlawish JH, Klocinski JL, Merz J, Clark CM, Asch DA. Caregivers’ preferences for the treatment of patients with Alzheimer’s disease. Neurology. 2000 Oct 10;55(7):1008–1014. doi: 10.1212/wnl.55.7.1008. [DOI] [PubMed] [Google Scholar]
- 44.Gallagher-Thompson D, Coon DW. Evidence-based psychological treatments for distress in family caregivers of older adults. Psychol Aging. 2007 Mar;22(1):37–51. doi: 10.1037/0882-7974.22.1.37. [DOI] [PubMed] [Google Scholar]
- 45.Teri L, Gibbons LE, McCurry SM, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA. 2003 Oct 15;290(15):2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- 46.Pinquart M, Sorensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects? Int Psychogeriatr. 2006 Dec;18(4):577–595. doi: 10.1017/S1041610206003462. [DOI] [PubMed] [Google Scholar]


