Abstract
Rationale
Myocardial infarction (MI) causes an imbalance between matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) and is associated with adverse LV remodeling. A uniform reduction in TIMP-4 post-MI has been observed.
Objective
Examine post-MI remodeling with cardiac restricted overexpression of TIMP-4, either through a transgenic or viral delivery approach.
Methods and Results
MI was induced in mice, then randomized to targeted injection of an adenoviral construct (10 uL; 8×109 pfu/mL) encoding green fluorescent protein (GFP) and the full length human TIMP-4 (Ad-GFP-TIMP4) or GFP. A transgenic construct with cardiac restricted overexpression TIMP-4 (hTIMP-4exp) was used in a parallel set of studies. LV end-diastolic volume, an index of LV remodeling, increased by over 60% from baseline at 5 days post-MI and by over 100% at 21 days post-MI in the Ad-GFP only group. However, LV dilation was reduced by approximately 50% in both the Ad-GFP-TIMP4 and hTIMP-4exp groups at these post-MI time points. LV ejection fraction was improved with either Ad-GFP-TIMP4 or hTIMP-4exp. Fibrillar collagen expression and content were increased within the MI region with both TIMP-4 interventions, suggestive of matrix stabilization.
Conclusion
This study is the first to demonstrate that selective myocardial targeting for TIMP-4 induction through either a viral or transgenic approach favorably altered the course of adverse LV remodeling post-MI. Thus, localized induction of endogenous MMP inhibitors, such as TIMP-4, holds promise as a means to interrupt the progression of post-MI remodeling.
Keywords: Myocardial infarction, ventricular function, tissue inhibitors of matrix metalloproteinases, extracellular matrix
INTRODUCTION
Myocardial infarction (MI) evokes changes within the architecture of the left ventricular (LV) wall leading to chamber dilation. The matrix metalloproteinases (MMPs) are a family of endopeptidases known to degrade all components of the myocardial extracellular matrix (ECM). In experimental models of MI, gene deletion of certain MMPs or pharmacological inhibition of MMP activity directly modifies the LV remodeling process post-MI.1–5 However, in clinical studies, pharmacological MMP inhibition has been associated with adverse systemic effects, which have resulted in limited clinical utility.6–8 As a result, a renewed interest in the endogenous inhibitors of MMPs, the tissue inhibitors of MMPs (TIMPs), has developed.9–13 There are four known TIMPs, and through gene deletion studies, it has been demonstrated that a loss of TIMP mediated MMP inhibition can accelerate and/or exacerbate LV remodeling, particularly post-MI.2,11,12 For example, TIMP-1 gene deletion in mice caused accelerated LV dilation and pump dysfunction following MI.2 One particular TIMP, TIMP-4, has been demonstrated to have a predominant expression profile to the cardiovascular system and may likely hold relevance to the myocardial remodeling process.12,14–17 Following MI induction in animal systems, TIMP-4 levels significantly fall within the MI and border regions.5,16,17 In clinical observational studies, relative plasma TIMP-4 levels were reduced for up to several months in patients following MI.15 It is now becoming recognized that each TIMP imparts unique biological effects with respect to cellular growth, proliferation, and viability with TIMP-4 being no exception.8–10,13,18,19 Thus, restoring the balance between myocardial levels of MMPs and TIMPs, such as TIMP-4, may provide a mechanism by which to reduce adverse LV remodeling post-MI. The present study tested the central hypothesis that myocardial over-expression either through targeted viral induction of the full length human TIMP-4 or through transgenic mediated cardiac overexpression of TIMP-4 would favorably modify the adverse LV remodeling process following MI.
METHODS
This study utilized coronary ligation in mice to produce a reproducible MI size and predictable time dependent changes in LV geometry and function.1–3,20 MI was induced in wild type (WT) adult mice with or without targeted regional overexpression of human TIMP-4 through an adenoviral delivery approach (hTIMP-4ad) or through transgenic cardiac restricted overexpression of human TIMP-4 (hTIMP-4exp). The main response variables were LV geometry and function at 5 and 21 days post-MI. These post-MI time points were chosen as these reflect the key phases of the early wound healing/remodeling process and are reflective of the more rapid trajectories for LV remodeling and dysfunction post-MI.3,6,15,17 The secondary set of measurements included indices of ECM remodeling (collagen expression and content), MMP and TIMP expression, indices of myocyte hypertrophy and apoptosis, and determinants of inflammation/fibrosis. All animals were treated and cared for in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (Eighth Edition. Washington, DC: 2011).
Adenoviral construct of human Timp-4 and myocardial transduction
The full length human TIMP-4 cDNA as well as that for green fluorescent protein (GFP) were cloned using an adenoviral (Ad) shuttle plasmid approach, followed by transfection and purification to a final titer of 8×109 pfu/mL using approaches described previously and fully detailed in the Supplemental Methods.21,22 Using a 27 gauge needle, 10 uL of the Ad-GFP-TIMP-4 or Ad-GFP only was placed in the mid-myocardium. This injection approach and initial validation studies are shown in the Supplemental Methods.
Transgenic myocardial overexpression of human TIMP-4 (hTIMP-4exp)
The full length sequence of the human TIMP-4 gene14 was ligated to the cardiac-specific murine alpha-myosin heavy chain promoter23 and established in a FVB strain as detailed in the Supplemental Methods. The hTIMP-4exp mice demonstrated no obvious phenotypic abnormalities, were fertile, and produced viable litters.
MI induction and protocol
For these studies, the mice were anesthetized using isoflurane anesthesia (3% in oxygen), and a baseline echocardiogram (40 MHz ultrasonic scan head, Vevo 770, VisualSonics, Toronto, Canada) was first obtained.3,20,23 Following these measurements, the left anterior descending artery was exposed and ligated. For the adenoviral portion of the study, mice (C57, 2 mos, equal gender distribution, n=91) were randomized in a blinded, 1:1 fashion to undergo myocardial injection of Ad-GFP-TIMP-4 or Ad-GFP within the MI region. The intra-operative mortality was 12% (hemodynamic compromise or arrhythmogenesis; n=10), and the post-MI mortality was 13% (sudden death or rupture; n=11) with no differences between the Ad-GFP-TIMP-4 and Ad-GFP groups. For the hTIMP-4exp studies, the mice (n=32) were treated in identical fashion. The intraoperative and post-MI survival was similar to the adenoviral studies (13% and 12% respectively). For additional comparative purposes, age/time matched FVB or hTIMP-4exp mice underwent identical LV function studies and myocardial measurements (n=6/time point). At designated post-MI time points, a second echocardiogram was performed, the LV then removed and sectioned for morphometric and biochemical measurements.
Myocardial morphometric and biochemical measurements
LV sections were stained with hematoxylin and eosin for measurement of MI size and myocyte cross sectional area.3,5 LV sections were also stained with picrosirius red to determine the percent area of collagen. 20,23 LV samples were also prepared for RNA isolation for real-time PCR, immunoassay and MMP zymography as detailed in the Supplemental Methods. The mRNA levels were quantified for TIMP-4 (both human and mouse), TIMP-1-3, collagen type I and III, transforming growth factor receptor-1 (TGF-BR1), discoidin domain receptor-2 (DDR2), MMP-14 (MT1-MMP), as well as an apoptotic mRNA array (Supplemental Methods). The results were computed as a normalized (18s signal) cycle times (Ct). Substrate zymography was performed in order to assess the relative content of the gelatinases, MMP-2, and MMP-9.2,3,16,17 LV myocardial extracts were also subjected to a cytokine multiplex array (Supplemental Methods).
Fibroblast growth and expression with TIMP-4 transduction
Since it has been established that TIMPs may directly affect fibroblast form and function beyond that of altering MMP activity,8,9,13 murine myocardial fibroblast preparations using an outgrowth technique were prepared,24 and Ad-GFP-TIMP-4 or Ad-GFP transduction performed (Supplemental Methods).
Data analysis
Response variables were compared between the groups initially using a multi-way analysis of variance (ANOVA) in which the main treatment effects were Ad-GFP-TIMP-4 injection or hTIMP-4exp and the presence or absence of MI. Individual group mean comparisons were performed using pair-wise Bonferroni corrections. In addition, the matched baseline and post-MI comparisons were performed using a paired t-test. Comparisons to reference values were performed by a separate t-test, and potential treatment effects examined by a 2-way ANOVA followed by a Bonferroni corrected t-test as appropriate. For the morphometric measurements, a Gaussian distribution was first developed to ensure adequate sampling and then subjected to ANOVA. For the apoptosis array measurements, the delta-delta Ct values were computed to a fold change from referent control values, and a greater than 2-fold change was considered statistically significant. For the fibroblast studies, comparisons between treatments were performed using a paired-t statistic. All statistical procedures were performed using Systat statistical software (SPSS; Chicago, IL). Results are presented as mean ± SEM. Values of p<0.05 were considered statistically significant.
RESULTS
Induction of TIMP-4 improves LV function and geometry post-MI
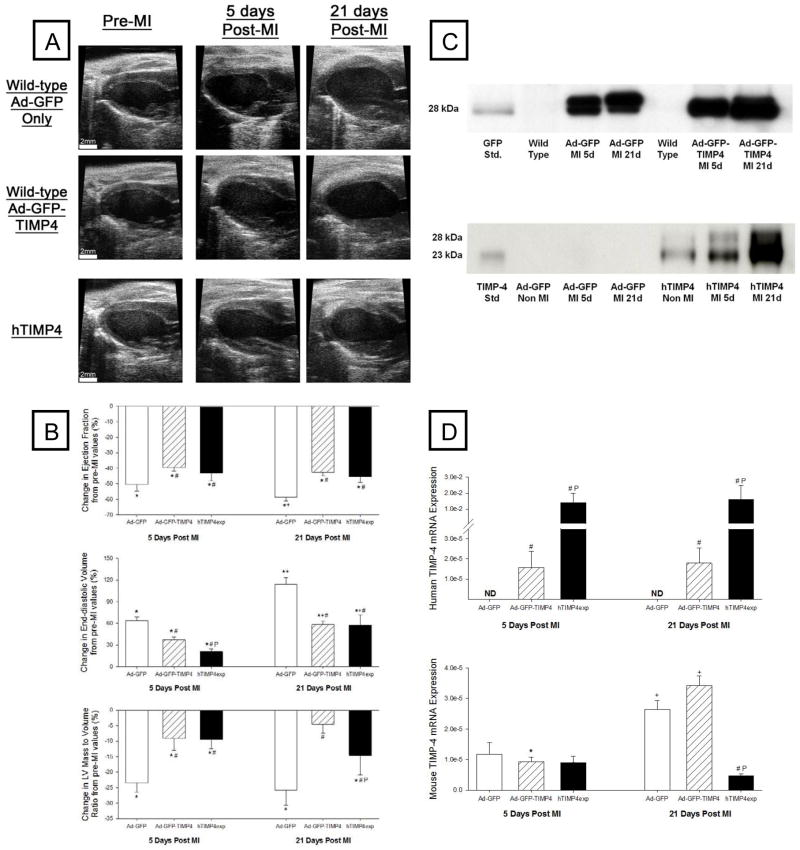
Representative LV long axis echocardiographic views at baseline and key post-MI time points following injection with Ad-GFP only or Ad-GFP-TIMP-4, as well as in hTIMP-4exp mice, are shown in Figure 1A. Significant LV dilation was evident in all post-MI groups but appeared attenuated with either adenoviral or transgenic overexpression of TIMP-4. LV geometry and function for non-MI values and at the post-MI time points along with sample sizes are summarized in Table 1. LV ejection fraction fell in all post-MI groups but was highest in the TIMP-4 induction groups. LV end-diastolic volume increased as a function of time post-MI but was reduced in the Ad-GFP-TIMP-4 group and reduced further in the hTIMP-4exp group. LV posterior wall thickness (at the site of the MI) and LV mass were higher in both TIMP-4 groups compared to Ad-GFP only values. An index of LV systolic function, LV velocity of shortening (Vcf), was higher in both TIMP-4 groups compared to Ad-GFP only values.
Figure 1.
(A) Representative echocardiograms of the LV long axis taken at end diastole at the baseline study (pre-MI time point) and in terminal studies performed at 5 or 21 days post-MI in mice injected with either Ad-GFP only (Top Panel) or the Ad-GFP-TIMP-4 construct (Middle Panel). In a second set of studies, mice with transgenic overexpression of human TIMP-4 (hTIMP-4exp) were treated in identical fashion (Bottom Panel). Significant LV dilation was observed in all groups at 5 and 21 days post-MI, but the relative degree of LV dilation appeared attenuated in both the Ad-GFP-TIMP4 and hTIMP-4exp groups. Summary data are presented in Table 1. (B) Key LV response variables as a function from baseline. LV ejection fraction significantly fell in all groups at 5 and 21 days post-MI, but this relative reduction was attenuated in the Ad-GFP-TIMP4 or hTIMP-4exp groups. LV end-diastolic volume and LV mass/volume ratio, both indices of post-MI remodeling, were significantly reduced in the Ad-GFP-TIMP-4 or hTIMP-4exp groups. (C) LV myocardial levels of GFP were expressed at equivalent levels in both adenoviral injection groups at both 5 and 21 days post-MI. In the hTIMP-4exp line, consistent human TIMP-4 levels were obtained in the non-MI referent control mice and these levels were higher at 5 and 21 days post MI. (D) LV myocardial mRNA levels for human TIMP-4 were detectable in the Ad-GFP-TIMP4 and hTIMP-4exp groups at 5 and 21 days post-MI. While endogenous mouse TIMP-4 was reduced at 5 days post-MI, expression levels were higher at 21 days post-MI with the notable exception in the hTIMP-4 group. [*p<0.05 compared to baseline (or referent non-MI values), # compared to Ad-GFP only group, Pp<0.05 vs Ad-GFP-TIMP-4 group].
Table 1.
LV geometry and function following 5 or 21 days of MI: Effects of adenovirus-mediated GFP (Ad-GFP), adenovirus-mediated GFP/TIMP-4 (Ad-GFP-TIMP4) induction, and transgenic overexpression of human TIMP-4 (hTIMP-4exp)
| Non-MI | Ad-GFP only 5 days post-MI | 21 days post-MI | Non-MI | Ad-GFP- TIMP4 5 days post-MI | 21 days post-MI | Non-MI | hTIMP- 4exp 5 days post- MI | 21 days post-MI | |
|---|---|---|---|---|---|---|---|---|---|
| Heart Rate (bpm) | 529±8 | 541±17 | 517±9 | 511±9 | 535±10 | 526±13 | 527±8 | 536±14 | 512±11 |
| Ejection fraction (%) | 61.4±1.0 | 30.9±2.9* | 26.5±1.5*+ | 61.3±0.9 | 38.2±1.7*# | 34.3±1.3*# | 62.9±0.9 | 41.3±2.7* | 36.0±2.5*# |
| End-diastolic volume (μL) | 52.8±1.6 | 87.5±4.1* | 98.4±5.8*+ | 53.6±1.8 | 70.7±3.9*# | 86.5±4.1*+# | 46.6±1.7 | 55.7±2.7 | 68.8±4.3*+# |
| Posterior wall thickness at end-diastole (mm) | 0.69±0.01 | 0.54±0.04* | 0.50±0.03* | 0.72±0.01 | 0.57±0.02* | 0.62±0.02*# | 0.79±0.01 | 0.64±0.02* | 0.61±0.01*# |
| LV mass (mg) | 80±2 | 107±5* | 116±5*+ | 83±3 | 108±4* | 122±5*+ | 87±2 | 94±3* | 109±3*+ |
| Vcf (s−1) | 7.00±0.33 | 2.61±0.78* | 2.43±0.22* | 6.84±0.45 | 3.34±0.34* | 3.78±0.34*# | 6.77±0.57 | 4.39±0.49* | 4.58±0.55*# |
| Sample size (n) | 29 | 15 | 20 | 34 | 20 | 14 | 22 | 14 | 19 |
Values presented as Mean ± SEM.
p<0.05 vs. respective pre-MI,
p<0.05 vs. respective 5 days post-MI,
p<0.05 vs. time-matched Ad-GFP only
The temporal changes in LV geometry and function following MI as a function of TIMP-4 induction are summarized in Figure 1B. This analysis further demonstrated that with either Ad-GFP-TIMP-4 injection or hTIMP-4exp, key indices of post-MI remodeling were attenuated.
A subset of studies were performed in identical fashion in FVB strain mice at baseline and at 5 and 21 days post-MI (n=6/time point; no Ad-GFP injections). Baseline, pre-MI LV ejection fraction, and end-diastolic volumes were equivalent to those shown in Table 1 (66±2%, 44±4 uL, respectively) and directionally changed to the same relative degree by 21 days post-MI (28±2%, 90±6 uL, respectively, both p<0.05 vs pre-MI values). Thus, the trajectory and magnitude of the changes in LV geometry and function in both the C57 and FVB strains at these defined post-MI time points were identical in the absence of human TIMP-4 induction.
Uniform TIMP-4 induction with adenoviral and transgenic approaches
LV myocardial samples from the targeted Ad injection region were subjected to GFP immunoblotting at 5 and 21 days and demonstrated a uniform and consistent signal for GFP (Figure 1C). Immunoblotting for human TIMP-4 was performed in the hTIMP-4exp at the targeted study endpoints (Figure 1C). A clear, specific, and uniform signal for human TIMP-4 was obtained in all non-MI hTIMP-4exp samples, which increased in the hTIMP-4exp myocardium following MI.
Human specific TIMP-4 and mouse specific TIMP-4 mRNA were quantified by PCR in LV myocardial samples taken at 5 or 21 days following injection and MI induction with Ad-GFP only, Ad-GFP-TIMP4, or in hTIMP-4exp mice (Figure 1D). In the Ad-GFP-TIMP-4 mice, mRNA levels significantly increased at 5 and 21 days post injection and post-MI. In the hTIMP-4exp mice, robust mRNA levels for human TIMP-4 were observed, which remained elevated at 5 and 21 days post-MI. Thus, a significant and persistent induction of specific human TIMP-4 was achieved within the targeted region with Ad injection and throughout the myocardium with transgenic TIMP-4 overexpression.
TIMP-4 induction does not alter MI size, but increases collagen content
MI size computed as a function of LV surface area was 37±3% with no difference between groups. Distribution plots for LV myocyte cross sectional area (CSA) for the remote region for all groups are shown in Figure 2A. CSA increased post-MI in all groups and was higher in the Ad-GFP-TIMP-4 group at 21 days post-MI. LV relative collagen content was computed in both the MI and remote regions (Figure 2B). While collagen content was increased within the MI region as well as in the remote region in all groups, these values were higher in both TIMP-4 induction groups.
Figure 2.
(A) Distribution plots for myocyte cross sectional area (CSA) for referent control and within the LV of the remote, viable myocardium at 5 and 21 days post-MI in the Ad-GFP, Ad-GFP-TIMP-4, and the hTIMP-4exp groups. A significant increase in myocyte CSA occurred post-MI in all groups, which appeared higher in the Ad-GFP-TIMP-4 group at 21 days post-MI. (B) LV relative collagen content was computed in both the MI and remote regions in all groups at 5 and 21 days post-MI using morphometric measurements on PSR stained sections. While collagen content was increased within the MI region as well as in the remote region in all groups, consistent with a post-MI fibrotic response, these values were higher in both TIMP-4 induction groups. [*p<0.05 compared to referent non-MI values, +p<0.05 vs respective 5 day values, # compared to Ad-GFP only group, $p<0.05 vs Ad-GFP-TIMP-4 group; sample sizes are n>10/group/time point].
TIMP-4 induction alters mRNA levels for other ECM regulatory proteins
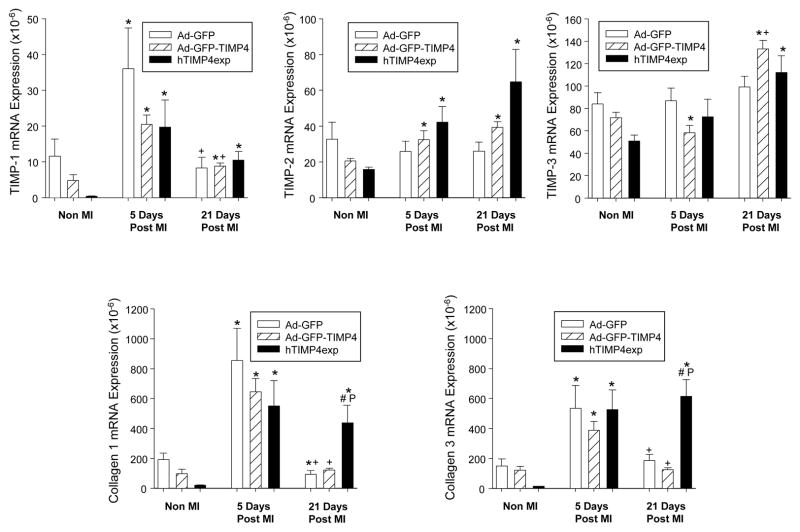
Relative myocardial mRNA levels for TIMP-1, TIMP-2, and TIMP-3 as well as the fibrillar collagens, collagen type I and III, are shown in Figure 3. TIMP-1 levels increased at 5 days post-MI and then fell at 21 days post-MI with no significant differences between groups. Relative TIMP-2 mRNA levels increased in the Ad-GFP-TIMP4 and hTIMP-4exp groups at 5 and 21 days post-MI. At 21 days post-MI, TIMP-3 mRNA levels were higher in the Ad-GFP-TIMP4 and hTIMP-4exp groups. At 5 days post-MI, increased mRNA levels for collagen I and III were equivalently increased in all groups. However, a persistent induction of collagen I and III occurred at 21 days post-MI in the hTIMP-4exp group.
Figure 3.
Relative myocardial mRNA levels for TIMP-1, TIMP-2, and TIMP-3, as well as for the fibrillar collagens, collagen type I and III, were computed in referent non-MI mice immediately following myocardial injection of Ad-GFP, Ad-GFP-TIMP4, or in hTIMP-4exp mice and at 5 and 21 days post-MI. TIMP-1 levels increased at 5 days post-MI and then fell at 21 days post-MI with no significant differences between groups. Relative TIMP-2 mRNA levels increased in the Ad-GFP-TIMP4 and hTIMP-4exp groups at 5 and 21 days post-MI. At 21 days post-MI, TIMP-3 mRNA levels were higher in the Ad-GFP-TIMP4 and hTIMP-4exp groups. At 5 days post-MI, increased mRNA levels for collagen I and III were equivalently increased in all groups. However, a persistent induction of collagen I and III occurred at 21 days post-MI in the hTIMP-4exp group. [*p<0.05 compared to referent non-MI values, +p<0.05 vs respective 5 day values, # compared to Ad-GFP only group, Pp<0.05 vs Ad-GFP-TIMP-4 group; sample sizes are n>10/group/time point].
LV myocardial mRNA levels for MT1-MMP, DDR2, and TGF-BR1 are summarized in Figure 4. MT1-MMP mRNA levels increased in all groups at 5 days post-MI and remained elevated at 21 days post-MI in the hTIMP-4exp group. Relative DDR2 mRNA levels appeared lower in the Ad-GFP-TIMP-4 group at 5 days post-MI, whereas DDR2 mRNA levels were increased in the hTIMP-4exp group at 21 days post-MI. TGF-BR1 mRNA levels increased in all post-MI time points in all groups but was highest in the hTIMP-4exp groups.
Figure 4.
(Left Panels) LV myocardial mRNA levels for MT1-MMP increased in all groups at 5 days post-MI and remained elevated at 21 days post-MI in the hTIMP-4exp group. Relative DDR2 mRNA levels appeared lower in the Ad-GFP-TIMP-4 group with no MI and at 5 days post-MI, whereas DDR2 mRNA levels were increased in the hTIMP-4exp group at 21 days post-MI. TGFbeta-R1 mRNA levels increased in all post-MI time points in all groups, but was highest in the hTIMP-4exp groups. (Middle Panels) LV myocardial IL-6 content increased in all groups at 5 days post-MI and reduced to referent control values by 21 days post-MI. However, IL-6 levels were lower in the hTIMP-4exp group at 5 days post-MI. LV myocardial IL-10 levels were detectable in referent control and Ad-GFP post-MI time points but were significantly reduced in the Ad-GFP-TIMP-4 group and non-detectable (ND) in the hTIMP-4exp group. MCP-1 was increased at 5 days post-MI in all groups but reduced in the hTIMP-4exp group. By 21 days post-MI, MCP-1 levels had fallen below referent control values. (Right Panels) LV myocardial expression levels for Bcl2 were higher at the non-MI and 21 day post-MI time point in the hTIMP-4exp group. A significant induction of Apaf1 and Ripk1 occurred at 5 days post-MI in the Ad-GFP group but not in either the Ad-GFP-TIMP-4 or hTIMP-4exp groups. [*p<0.05 compared to baseline (or referent non-MI values), +p<0.05 vs respective 5 day values, # compared to Ad-GFP only group, Pp<0.05 vs Ad-GFP-TIMP-4 group; sample sizes are n>6/group/time point].
TIMP-4 induction alters cytokine content and apoptotic mRNA levels post-MI
LV myocardial levels for specific cytokines occurred with TIMP-4 induction and are summarized in Figure 4. Specifically, IL-6 (interleukin-6) content increased in all groups at 5 days post-MI and reduced to referent control values by 21 days post-MI. However, IL-6 levels were lower in the hTIMP-4exp group at 5 days post-MI. LV myocardial IL-10 levels were significantly reduced in the Ad-GFP-TIMP-4 group and non-detectable in the hTIMP-4exp group. MCP-1 (monocyte chemoattractant protein 1) was increased at 5 days post-MI in all groups but reduced in the hTIMP-4exp group. LV myocardial expression levels for certain apoptotic genes were affected by MI and TIMP-4 induction and are shown in Figure 4. LV myocardial Bcl2 (B-cell leukemia/lymphoma 2) mRNA levels were higher at the non-MI and 21 day post-MI time point in the hTIMP-4exp group and were higher in the Ad-GFP-TIMP4 group at 21 days post-MI, but this did not reach predefined statistical significance. A significant induction of Apaf1 (apoptotic peptidase activating factor 1) and Ripk1 (receptor (TNRSF)-interacting serine-threonine kinase 1) occurred at 5 days post-MI in the Ad-GFP group but not in either the Ad-GFP-TIMP-4 or hTIMP-4exp groups.
TIMP-4 induction reduces MMP-9 and active MMP-2 levels post-MI
The latent form of MMP-9 (92 kDa) as well as the latent and active forms of MMP-2 (72/68 kDa, respectively) were identified and quantified by zymography/densitometry (Figure 5). In the Ad-GFP-TIMP4 and hTIMP-4exp groups, relative MMP-9 levels were lower post-MI. For MMP-2, the active form was reduced in both the Ad-GFP-TIMP4 and hTIMP-4exp groups post-MI.
Figure 5.
(Top) Representative myocardial zymograms performed in referent non-MI mice immediately following myocardial injection of Ad-GFP, Ad-GFP-TIMP4, or in hTIMP-4exp mice and at 5 and 21 days post-MI. The latent form of MMP-9 (92 kDa) and the latent and active forms of MMP-2 (72/68 kDa, respectively) were identified and quantified by densitometry. (Bottom) Summary results from zymography were computed as a percent of referent non-MI values, whereby referent values were set to 100% (dashed line). In the Ad-GFP group, MMP-9 values increased at 5 days post-MI and returned to within normal ranges by 21 days post-MI. In the Ad-GFP-TIMP4 and hTIMP-4exp group, relative MMP-9 levels did not increase at 5 days post-MI and were actually reduced at specific post-MI time points. For MMP-2, a robust increase in active MMP-2 occurred in the Ad-GFP group at 5 and 21 days post-MI, which was significantly blunted in both the Ad-GFP-TIMP4 and hTIMP-4exp groups. [*p<0.05 compared to referent non-MI values, +p<0.05 vs respective 5 day values, # compared to Ad-GFP only group; sample sizes are n>10/group/time point]
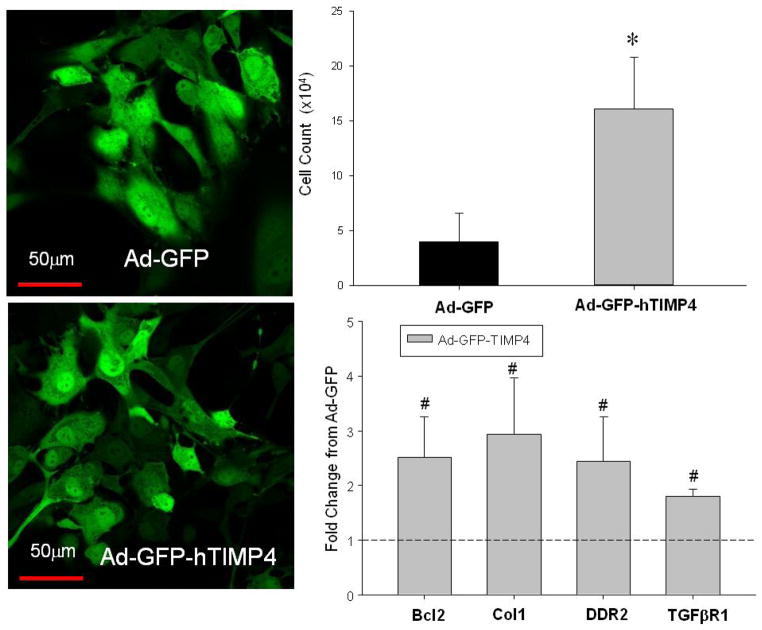
TIMP-4 induction in fibroblasts alters growth, and apoptotic and ECM mRNA levels
LV myocardial fibroblasts were transduced with either Ad-GFP or Ad-GFP-TIMP4 and yielded a uniform transduction efficiency of approximately 70% with both vectors (Figure 6). LV fibroblast cell density, indicative of cell proliferation, was higher at 24 hours post-transduction with TIMP-4. In addition, transduction of TIMP-4 in these LV fibroblast cultures resulted in significantly higher mRNA levels for Bcl2, collagen I, DDR2, and TGF-BR1 (Figure 6).
Figure 6.
(Left Panels) LV murine myocardial fibroblasts were transduced with either Ad-GFP or Ad-GFP-TIMP4, which yielded a uniform transduction efficiency of 70% with both vectors. LV fibroblasts were imaged for GFP fluorescence using confocal microscopy (Ziess LSM 510 Meta Confocal, Plan-Neofluar 43X, GFP excitation/emission 488/505–530 nm). (Right Panels) LV fibroblast cell density, indicative of cell proliferation, was higher at 24 hours post-transduction with TIMP-4. In addition, transduction of TIMP-4 in these LV fibroblast cultures resulted in significantly higher mRNA levels for Bcl2, collagen I, DDR2, and TGFbetaR1. [*p<0.05 compared to Ad-GFP only values, #p<0.05 fold change compared to Ad-GFP; n=3 with all studies performed in triplicate]
DISCUSSION
It has been clearly established in both animal models and clinical studies that an altered balance in matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) occurs following myocardial infarction (MI), which would favor myocardial extracellular matrix (ECM) degradation and facilitate adverse post-MI remodeling.1–5,12,15,18,17 While systemic pharmacological approaches to inhibit MMP activity demonstrated favorable effects on post-MI remodeling in animal studies,1–3,5 concerns regarding dosing strategies, systemic side-effects, and equivocal initial clinical results have limited progress on this approach.6–8 Thus, strategies to modulate MMP activity on a region and tissue specific manner in the context of post-MI remodeling may hold greater clinical translational potential. Under normal conditions, TIMP-4 is highly expressed within the cardiovascular system, particularly the myocardium.14,16,17 However, clinical and animal studies have demonstrated a relative reduction in TIMP-4 levels in the early post-MI period.15–17 The present study addressed this issue using two different but complementary strategies. First, myocardial injection of a replication adenoviral construct containing the full length human TIMP-4 sequence and a reporter protein green fluorescent protein at the time of MI induction in mice was performed and compared to mice with myocardial injection of the identical adenoviral construct but only encoding GFP. Second, MI was induced in transgenic mice with cardiac specific overexpression of human TIMP-4. The significant findings of this study were 3-fold. First, a robust expression of TIMP-4 could be achieved through targeted adenoviral injections at 5 and 21 days post-MI, which resulted in favorable effects on LV function and geometry in this post-MI period. Second, hTIMP-4exp provided similar protective effects in terms of reducing post-MI LV dilation and dysfunction. Third, augmenting myocardial TIMP-4 levels in the early post-MI period resulted in reduced elaboration of MMP-9 and reduced activational state of MMP-2. Taken together, this is the first study to demonstrate that myocardial augmentation of TIMP-4, either through an adenoviral delivery or through a transgenic approach, can alter the early course of post-MI remodeling.
TIMP-4 as a potential therapeutic strategy
Utilizing different transgenic constructs, past studies have demonstrated that the deletion of specific MMP/TIMP genes can alter the course of pathological LV remodeling.1–4,11,12 For example, MMP-2 gene deletion in mice caused a relative reduction in the degree of LV remodeling post MI and was associated with increased survival.1 Conversely, gene deletion of TIMPs, which bind to the active form of MMPs, have been demonstrated to exacerbate adverse LV remodeling.2,11,12 For example, TIMP-1 gene deletion accelerates LV dilation in mice post-MI,2 whereas TIMP-3 gene deletion causes LV dilation and dysfunction with aging or pressure overload.13 In addition to these transgenic studies, pharmacological MMP inhibition has also been demonstrated to alter the course of LV remodeling post-MI.1–3,5,17 However, while these transgenic and pharmacological approaches demonstrated a cause-effect relation between changes in the normal MMP-TIMP balance and adverse LV myocardial remodeling, these approaches entail affecting MMP activity throughout the entire organism. In the case of pharmacological MMP inhibition, concern for systemic side effects have tempered clinical development of this approach for treating adverse LV myocardial remodeling.6–8,25 The present study demonstrated that altering a determinant of MMP activity in a localized and time specific manner reduced the degree of LV dilation post-MI, reduced the degree of LV wall thinning within the MI region, and improved LV pump function. These favorable effects of either targeted gene delivery or by cardiac restricted overexpression of TIMP-4 on the post-MI remodeling process are similar to those achieved by either a global transgenic approach or through broad-spectrum, systemically delivered MMP inhibition.1–5,17 Thus, one of the important findings of this study is that localized modulation of the MMP/TIMP system can impart beneficial effects on the post-MI remodeling process and thereby provides a potential future therapeutic direction.
Rationale for targeting TIMP-4
A critical control point for net MMP activity is through forming non-covalent complexes with TIMPs.9,18,26 However, each TIMP may also have unique characteristics and biological functions that may be relevant to the myocardial remodeling process.8–13,18,19,26 First, it appears that TIMP-1 binds with less efficiency to the membrane type MMPs, which have been shown to be upregulated in human heart failure and with ischemia-reperfusion.18,26 Past studies from this laboratory have demonstrated that MT1-MMP induction can accelerate adverse post-MI remodeling.27 In kinetic studies, TIMP-4 binds with high affinity and thus is a potent inhibitor of this transmembrane matrix protease.18,19 It has been demonstrated that TIMP-2 forms a unique complex with proMMP-2 and MT1-MMP, which in turn can facilitate MMP-2 activation.18,26 Thus, induction of TIMP-4 as achieved in the present study would likely inhibit this activation cascade and reduce relative active MMP-2 levels. Clinical studies of post-MI remodeling and large animal studies have reported that TIMP-4 levels are notably reduced or actually extinguished.2,16,17 Finally, TIMP-4 gene deletion has been shown to exacerbate post-MI remodeling, providing further impetus for the studies executed herein.12
Cellular and extracellular effects of TIMP-4
It is unlikely that the relative improvement in LV function and geometry achieved by TIMP-4 augmentation was due to significant myocardial salvage or substantial differences in cardiac myocyte viability, as a uniform and consistent MI size was achieved in this permanent coronary occlusion model. However, TIMP-4 augmentation in this post-MI model did appear to increase myocardial growth of residual viable myocardium as measured by LV mass, wall thickness, and myocyte cross-sectional area. Furthermore, the anti-apoptotic gene Bcl2 was higher at a later post-MI time point with TIMP-4 induction. Indices of inflammation, such as IL-6, IL-10, and MCP-1, were increased post-MI but were suppressed to the greatest degree with transgenic TIMP-4 overexpression. The adenoviral injection approach may have induced a localized inflammatory response, which was not present with the transgenic approach for TIMP-4 overexpression. Taken together, these findings suggest that myocardial TIMP-4 augmentation did not adversely affect myocyte growth, viability, or local inflammatory pathways. The present study demonstrated that TIMP-4 induction altered MMP-9 levels post-MI, which are robustly synthesized and expressed by neutrophils and other inflammatory cells.18,26 This further supports the observations that TIMP-4 augmentation likely reduced local inflammatory pathways. Interestingly, myocardial TIMP-4 deletion has been reported to be associated with enhanced neutrophil infiltration,12 suggesting that TIMP-4 may serve to stabilize the myocardial matrix and thereby impede inflammatory cell migration with myocardial injury.
The potential effects of TIMP-4 augmentation on fibroblast growth and viability were addressed through both in-situ and in-vitro approaches. Myocardial expression of DDR2, which can be used as a surrogate marker for fibroblasts,28 was increased at 5 days post-MI in all groups but was further increased at 21 days post-MI with transgenic cardiac TIMP-4 overexpression. The transgenic model caused elevated TIMP-4 levels across the entire myocardium, whereas targeted adenoviral injections would only yield a regional increase specifically within the MI. Thus, the elevated DDR2 levels with transgenic overexpression of TIMP-4 likely drove fibroblast proliferation/transdifferentiation throughout both the MI and remote myocardial regions. The present study identified that coupled with the increased DDR2 mRNA levels, TGF-BR1 levels were also increased in the transgenic overexpression of TIMP-4 post-MI. Enhanced TGF signaling can induce fibroblast proliferation and transdifferentiation.28,29 Using murine cardiac fibroblast cultures, the present study demonstrated that transduction of TIMP-4 increased fibroblast proliferation, which was accompanied by changes in key determinants of apoptosis and ECM synthesis. These effects of TIMP-4 induction on fibroblast proliferation are in keeping with the findings reported by Lovelock et al.13 While extrapolation of these in-vitro studies to the in-vivo post-MI context must be done with caution, these observations support the postulate that myocardial induction of TIMP-4 affected fibroblast number and phenotype, which in turn may have played a contributory role in attenuating ECM turnover, increased ECM stability, and hence reduced adverse LV remodeling.
Several measures of ECM remodeling were undertaken in the present study. First myocardial fibrillar collagen expression increased markedly at 5 days post-MI, and then consistent with the wound healing response, fell to within normal limits at 21 days post-MI. While myocardial TIMP-4 induction through a targeted adenoviral approach did not alter fibrillar collagen expression, fibrillar collagen expression remained elevated with cardiac restricted overexpression of TIMP-4. However, collagen mRNA levels alone may not necessarily imply a net gain in collagen content. Morphometric measurements demonstrated that relative fibrillar collagen content was increased within both the MI and remote regions with either adenoviral mediated or by transgenic induction of TIMP-4. While the elevated myocardial collagen content did not appear to negatively affect LV geometry and function, the longer term consequences of higher collagen content on myocardial structure and function with TIMP-4 augmentation remains to be determined.
CONCLUSION
It must be recognized that the adenoviral injections were performed at the time of MI induction, and peak expression levels of TIMP-4 can be variable in the post-MI observation period. To address this limitation and to buttress the observations made by TIMP-4 adenoviral delivery, an entirely different approach and construct was integrated into the study design by the use of cardiac restricted overexpression of human TIMP-4. This provided a much more robust increase in TIMP-4 levels, and hence a more pronounced effect on LV remodeling and ECM structure was observed. In addition, this transgenic construct in turn has limitations in terms of a restricted pattern of expression to primarily cardiac myocytes and persistent expression rather than temporal specificity to the MI time point. Nevertheless, the similar directional changes in LV remodeling, function, and ECM structure observed in both forms of TIMP-4 augmentation provide clear support that selective myocardial induction of TIMP-4 was the primary mechanism for these effects post-MI. The myocardial induction of TIMP-4 either through adenoviral transduction or through transgenesis did appear to influence other endogenous TIMP levels. First, endogenous mouse levels of TIMP-4 were reduced, particularly in the cardiac restricted human TIMP-4 construct. This would suggest that some feedback mechanism occurred with persistent and significant overexpression of TIMP-4. Second, myocardial induction of TIMP-4 through either approach appeared to increase myocardial expression of TIMP-2 and TIMP-3, albeit to a modest degree, in the late post-MI period. Thus, whether and to what extent myocardial induction of TIMP-4 and the subsequent modification of other TIMP types may have played a role in the post-MI remodeling process remains to be established.
In conclusion, this study is the first to demonstrate that targeted and specific myocardial induction of TIMP-4 favorably altered post-MI remodeling. These findings provide a proof of concept that regional modulation of MMP/TIMP pathways, rather than through systemic or global approaches, can favorably affect LV form and function in the post-MI context.
Supplementary Material
Novelty and Significance.
What Is New?
Adverse left ventricular (LV) remodeling after myocardial infarction (MI) is often associated with LV dilation secondary to infarct expansion, in part due to continued extracellular matrix (ECM) turnover.
Continued extracellular matrix (ECM) turnover, particularly within the MI region is due to an imbalance between matrix metalloproteinases (MMPs) and endogenous tissue inhibitors of MMPs (TIMPs).
Transgenic and pharmacological modification of MMP activity has been found to diminish post-MI remodeling.
What New Information Does This Article Contribute?
Augmenting TIMP-4 within mouse myocardium following MI, significantly reduced adverse LV remodeling.
Regional induction of TIMP-4 within the MI region was sufficient to favorably alter the course of post-MI remodeling.
Localized induction of endogenous MMP inhibitors, such as TIMP-4, holds promise as a therapeutic target for post-MI remodeling.
ECM instability within the MI region has been identified as a contributory factor to adverse changes in LV geometry and function post-MI. MMPs, the ECM proteases, are induced following MI and can cause continued ECM turnover. Animal studies and clinical observational studies have identified an imbalance between MMP levels and TIMPs, notably that of TIMP-4. In the present study, we increased TIMP-4 levels within the myocardium following MI by either targeted viral delivery or transgenic myocardial overexpression. In both cases, TIMP-4 induction reduced LV dilation, and increased LV ejection fraction. These findings provide a proof of the concept that regional modulation of MMP/TIMP pathways, rather than through systemic pharmacological approaches, can favorably affect LV form and function post-MI.
Acknowledgments
SOURCES OF FUNDING
This study was supported by NIH grants HL059165, HL057952, HL095608, HL078825, and Merit Awards from the Veterans’ Affairs Health Administration.
Nonstandard Abbreviations and Acronyms
- Ad
Adenoviral
- ANOVA
Analysis of variance
- Apaf1
Apoptotic peptidase activating factor 1
- Bcl1
B-cell leukemia/lymphoma 2
- CSA
Cross sectional area
- Ct
Cycle time
- DDR2
Discoidin domain receptor-2
- ECM
Extracellular matrix
- GFP
Green fluorescent protein
- hTIMP-4ad
Human TIMP-4 through an adenoviral delivery approach
- hTIMP-4exp
Transgenic cardiac restricted overexpression of human TIMP-4
- IL
Interleukin
- LV
Left ventricular
- MCP-1
Monocyte chemoattractant protein 1
- MI
Myocardial infarction
- MMP
Matrix metalloproteinase
- mRNA
Messenger RNA
- PCR
Polymerase chain reaction
- Ripk1
Receptor (TNRSF)-interacting serine-threonine kinase 1
- RNA
Ribonucleic acid
- TGF-BR1
Transforming growth factor receptor-1
- TIMP
Tissue inhibitors of matrix metalloproteinase
- Vcf
Velocity of shortening
- WT
Wild type
Footnotes
DISCLOSURES
None.
References
- 1.Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J Clin Invest. 2005;115:599–609. doi: 10.1172/JCI22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikonomidis JS, Hendrick JW, Parkhurst AM, Herron AR, Escobar PG, Dowdy KB, Stroud RE, Hapke E, Zile MR, Spinale FG. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol. 2005;288:H149–58. doi: 10.1152/ajpheart.00370.2004. [DOI] [PubMed] [Google Scholar]
- 3.Spinale FG, Escobar GP, Hendrick JW, Clark LL, Camens SS, Mingoia JP, Squires CG, Stroud RE, Ikonomidis JS. Chronic matrix metalloproteinase inhibition following myocardial infarction in mice: differential effects on short and long-term survival. J Pharmacol Exp Ther. 2006;318:966–73. doi: 10.1124/jpet.106.104455. [DOI] [PubMed] [Google Scholar]
- 4.Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res. 2006;69:604–13. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Yarbrough WM, Mukherjee R, Escobar GP, Mingoia JT, Sample JA, Hendrick JW, Dowdy KB, McLean JE, Lowry AS, O’Neill TP, Spinale FG. Selective targeting and timing of matrix metalloproteinase inhibition in post-myocardial infarction remodeling. Circulation. 2003;108:1753–9. doi: 10.1161/01.CIR.0000091087.78630.79. [DOI] [PubMed] [Google Scholar]
- 6.Hudson MP, Armstrong PW, Ruzyllo W, Brum J, Cusmano L, Krzeski P, Lyon R, Quinones M, Theroux P, Sydlowski D, Kim HE, Garcia MJ, Jaber WA, Weaver WD. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol. 2006;48:15–20. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 7.Rizvi NA, Humphrey JS, Ness EA, Johnson MD, Gupta E, Williams K, Daly DJ, Sonnichsen D, Conway D, Marshall J, Hurwitz H. A phase I study of oral BMS-275291, a novel nonhydroxamate sheddase-sparing matrix metalloproteinase inhibitor, in patients with advanced or metastatic cancer. Clin Cancer Res. 2004;10:1963–70. doi: 10.1158/1078-0432.ccr-1183-02. [DOI] [PubMed] [Google Scholar]
- 8.Fingleton B. Matrix metalloproteinase inhibitors for cancer therapy: the current situation and future prospects. Expert Opin Ther Targets. 2003;7:385–97. doi: 10.1517/14728222.7.3.385. [DOI] [PubMed] [Google Scholar]
- 9.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20:161–8. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25:99–113. doi: 10.1007/s10555-006-7893-x. [DOI] [PubMed] [Google Scholar]
- 11.Kassiri Z, Oudit GY, Sanchez O, Dawood F, Mohammed FF, Nuttall RK, Edwards DR, Liu PP, Backx PH, Khokha R. Combination of tumor necrosis factor-alpha ablation and matrix metalloproteinase inhibition prevents heart failure after pressure overload in tissue inhibitor of metalloproteinase-3 knock-out mice. Circ Res. 2005;97:380–90. doi: 10.1161/01.RES.0000178789.16929.cf. [DOI] [PubMed] [Google Scholar]
- 12.Koskivirta I, Kassiri Z, Rahkonen O, Kiviranta R, Oudit GY, McKee TD, Kytö V, Saraste A, Jokinen E, Liu PP, Vuorio E, Khokha R. Mice with tissue inhibitor of metalloproteinases 4 (Timp4) deletion succumb to induced myocardial infarction but not to cardiac pressure overload. J Biol Chem. 2010;285:24487–93. doi: 10.1074/jbc.M110.136820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovelock JD, Baker AH, Gao F, Dong JF, Bergeron AL, McPheat W, Sivasubramanian N, Mann DL. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2005;288:H461–8. doi: 10.1152/ajpheart.00402.2004. [DOI] [PubMed] [Google Scholar]
- 14.Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996;271:30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- 15.Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation. 2006;114:1020–7. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 16.Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St John-Sutton MG, Gorman JH, Edmunds LH, Gorman RC, Spinale FG. Region and species specific induction of matrix metalloproteinases occurs with post-myocardial infarction remodeling. Circulation. 2003;107:2857–63. doi: 10.1161/01.CIR.0000068375.40887.FA. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation. 2003;107:618–25. doi: 10.1161/01.cir.0000046449.36178.00. [DOI] [PubMed] [Google Scholar]
- 18.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melendez-Zajgla J, Del Pozo L, Ceballos G, Maldonado V. Tissue inhibitor of metalloproteinases-4. The road less traveled. Mol Cancer. 2008;7:85. doi: 10.1186/1476-4598-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zile MR, Baicu CF, Stroud RE, Van Laer AO, Jones JA, Patel R, Mukherjee R, Spinale FG. Mechanistic relationship between MT1-MMP and the myocardial response to pressure-overload. Circ Heart Fail. 2014 doi: 10.1161/CIRCHEARTFAILURE.113.000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–14. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerald RD, Meidell RS. Adenovirus vectors. In: Hames BD, Glover D, editors. DNA Cloning – A Practical Approach: Mammalian Systems. Oxford University Press; Oxford: 1995. pp. 285–307. [Google Scholar]
- 23.Zile MR, Baicu CF, Stroud RE, Van Laer A, Arroyo J, Mukherjee R, Jones JA, Spinale FG. Pressure overload-dependent membrane type 1-matrix metalloproteinase induction: relationship to LV remodeling and fibrosis. Am J Physiol Heart Circ Physiol. 2012;302:H1429–37. doi: 10.1152/ajpheart.00580.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT, Sweterlitsch SE, Spinale FG. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res. 2005;66:410–9. doi: 10.1016/j.cardiores.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 25.Guo YH, Gao W, Li Q, Li PF, Yao PY, Chen K. Tissue inhibitor of metalloproteinases-4 suppresses vascular smooth muscle cell migration and induces cell apoptosis. Life Sci. 2004;75:2483–93. doi: 10.1016/j.lfs.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Zavadzkas JA, Mukherjee R, Rivers WT, Patel RK, Meyer EC, Black LE, McKinney RA, Oelsen JM, Stroud RE, Spinale FG. Direct regulation of membrane type 1 matrix metalloproteinase following myocardial infarction causes changes in survival, cardiac function, and remodeling. Am J Physiol Heart Circ Physiol. 2011;301:H1656–66. doi: 10.1152/ajpheart.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldsmith EC, Bradshaw AD, Spinale FG. Contributory pathways leading to myocardial fibrosis – moving beyond collagen expression. Am J Physiol Cell Physiol. 2013;304:C393–402. doi: 10.1152/ajpcell.00347.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.