Abstract
Background
Ovarian prostaglandins are critical in normal ovulation processes, thus their inhibition may provide contraceptive benefits. This study was performed to determine the effect of the cyclooxygenase-2 (COX2) inhibitor, celecoxib, on ovulation and luteal events in women.
Study design
Randomized double-blind crossover design. Ovulatory reproductive-aged women underwent ovarian ultrasound and serum hormone monitoring during four menstrual cycles (control cycle, treatment cycle 1, washout cycle, treatment cycle 2). Subjects received study drug (oral celecoxib 400 mg or placebo) either 1) once daily starting on cycle day 8 and continuing until follicle rupture or the onset of next menses if follicle rupture did not occur (pre-LH surge dosing) or 2) once daily beginning with the LH surge and continued for 6 days (post-LH surge dosing). Subjects were randomly assigned to one of the above treatment schemes and received the other in the subsequent treatment cycle. The main outcomes were evidence of ovulatory and luteal dysfunction as determined by inhibited/delayed follicle rupture and reduced luteal progesterone synthesis or lifespan, respectively.
Results
A total of 20 women enrolled and completed the study (Group 1 = 10, Group 2 = 10) with similar demographics between groups. Nineteen subjects exhibited normal ovulation in the control cycle (one had a blunted LH peak). In comparison to control cycles, treatment cycles resulted in a significant increase in ovulatory dysfunction [pre-LH treatment: 30% (6/20), p = 0.04; post-LH treatment: 25% (5/20), p = 0.04]. Peak progesterone, estradiol, and LH levels and luteal phase length did not differ significantly between control and either treatment cycles.
Conclusions
Although treatment with celecoxib before or after the LH surge increases the rate of ovulatory dysfunction, most women ovulate normally. Thus, this selective COX2 inhibitor appears to be of limited usefulness as a potential emergency contraceptive.
Keywords: COX 2 inhibitor, prostaglandin, emergency contraception, celecoxib, dysfunctional ovulation
Introduction
Currently available hormonal emergency contraception (EC), such as levonorgestrel or ulipristal acetate, work by inhibiting ovulation [1, 2]. Although levonorgestrel inhibits ovulation in 83% of menstrual cycles at a follicular measurement of 12–14 mm, it blocks ovulation in only about 12% of cycles when the follicle is larger (18–20 mm) (1–3). By contrast, ulipristal acetate prevents ovulation in 60% of cycles up to a follicular measurement of 18–20 mm. Thus, ulipristal can block follicle rupture when given at the time of the LH surge, which explains its greater efficacy and longer treatment window of up to 120 hours after unprotected intercourse [3]. Failures occur principally in women having unprotected sex after the peak of the LH surge, as they receive no benefits from either of these emergency therapies.
Ovarian prostaglandins (PGs) synthesized through the rate-limiting enzyme cyclooxygenase-2 (COX2; also known as prostaglandin-endoperoxide synthase-2, or PTGS2) play a critical role in ovulation and luteal development [4–14]. The inability to synthesize ovarian PGs in COX2 null mutant mice causes infertility by preventing ovulation or cumulus-ooycte expansion (C-OE) [13, 14]. The role of PGs in fertility appears to be dependent on PGE2 synthesis as the deletion of the PGE2 subtype 2 receptor (PTGER2) results in female sterility due to a failure of cumulus-oocyte complexes to undergo C-OE [13]. Moreover, the expression of genes encoding proteins involved in PGE2 synthesis and signaling are highly expressed after follicle rupture in primate and domesticated animal species [15, 16] suggesting a role for PGs in the development of the corpus luteum. Thus, a COX2-selective inhibitor may offer a much broader window of treatment as an emergency contraceptive through interfering with both ovulatory and luteal activities. Nonhuman primate and human studies using the COX2 inhibitor meloxicam (Glenmark Generics Inc, Mahwah, NJ) have demonstrated that the drug, creates ovulatory dysfunction when dosed before the LH surge (late follicular phase) and may enhance the inhibitory effects of the levonorgestrel-based emergency contraceptive [17–20]. Another COX2 inhibitor, rofecoxib, also demonstrated delayed follicle rupture when dosed before the LH surge but this formulation was voluntarily withdrawn from the market in 2004 [21].
Since meloxicam is only moderately selective for COX2, we hypothesized that treatment with the more highly selective COX2 inhibitor, celecoxib (Pfizer Inc, NY, NY) [22], would be a more effective agent in terms of causing ovulatory dysfunction [22]. Furthermore, since PG synthesis and action has been implicated in luteal development in animal models [15, 16], we hypothesized that treatment with celecoxib would adversely affect luteal function. Our prior pilot study [23] demonstrated that daily administration of celecoxib caused a delay in luteal phase events in some women but the study was not designed to monitor ovulation or to allow us to determine the timing of this effect. Therefore in the current study, we sought to further isolate celecoxib’s mechanism of action and its window of effectiveness by evaluating its effects specifically prior to and after the LH surge.
Materials and methods
A prospective randomized double-blind crossover study was conducted at Oregon Health & Science University (OHSU) in Portland, OR, from January 2010 to February 2011. The OHSU Institutional Review Board approved the study protocol, and subjects volunteered to participate after reviewing and signing a written informed consent.
Healthy reproductive-aged (18–35 years old) women with regular cycles (every 26–34 days), not currently using or needing hormonal contraception, were recruited. To ensure enrollment of ovulatory women, we required that subjects demonstrate a single progesterone (P) level of at least 3 ng/mL during the luteal phase (days 18–25) of the menstrual cycle prior to study entry. Additional exclusion criteria included allergy to or routine use of nonsteroidal anti-inflammatory drugs (e.g. aspirin, ibuprofen), known cardiac risk factors (i.e., personal history of hypertension, obesity, cardiac disease and/or diabetes) and pyrosis or gastroesophageal reflux.
A computer-generated randomized scheme was created by the OHSU research pharmacy. A unique consecutive study number was assigned to each participant. Study drug (celecoxib 400 mg po daily) and an identical placebo were obtained from the research pharmacy. Women were randomized into one of two dosing schedules using a crossover design for 4 menstrual cycles [control cycle, treatment 1, washout cycle, treatment 2]. In each treatment cycle, subjects received study medication for both “pre-LH” surge (initiated on cycle day 8 and continued until follicle rupture or until the onset of next menses if follicle rupture did not occur) and “post-LH” surge (initiated at the time of LH surge as determined by home urine LH testing and continued for a total of 6 days). If an LH surge was not detected, the “post-LH” surge treatment was not taken. An LH surge identified by home urine testing was verified with a serum LH assay at the end of the study.
In order to maintain allocation concealment, all study subjects received 2 bottles for each treatment cycle – one with study drug and the other with an identical placebo. They were instructed on when to initiate drug 1 and when to switch to drug 2 based on the dosing schedule above. The onset of next menses was defined as two consecutive days of spotting or bleeding. Group 1 received pre-LH surge dosing of celecoxib and post-LH dosing of placebo during treatment cycle 1 and pre-LH dosing of placebo with post-LH dosing of celecoxib during treatment cycle 2. Group 2 received the opposite order of active and placebo treatments during the two cycles. Women were seen for twice weekly visits (no more than 4 days apart) during the control and treatment cycles to obtain blood samples for pituitary (LH) and ovarian hormone levels [P and estradiol (E2)], and to perform transvaginal ultrasound (TVUS) to monitor ovarian activity. Starting on cycle day 8, subjects performed daily home urine LH testing (Clearview, Shared Services Center, Orlando, FL), and reported for daily clinic visits and TVUS starting within 24 hours of an LH surge. These daily visits were continued until follicle rupture was observed by ultrasound, or for up to four days. Visits then resumed twice weekly until menses.
LH, E2 and P4 assays were performed at the Endocrine Technology Services Laboratory (ETSL) at the Oregon National Primate Research Center (ONPRC, Beaverton, Oregon) using an automated Immulite 2000 chemiluminescent assay system (Siemens, Deerfield, IL 60015, USA). All assays were analyzed altogether at the completion of the study. Assay sensitivity of LH, E2 and P assays is 0.1 ng/mL, 20 pg/mL and 0.2 ng/mL, respectively. Three quality controls with low, median and high values for LH, E2 or P4 were routinely analyzed before each sample run, and the quality control (QC) values were confirmed within 10% of the mean values detected over the last 12 months (n=9). For LH, the intra-assay CV was 6.3% at 10.8 ng/ml, 6.9% at 24.2 ng/ml and 6.7% at 45.5 ng/ml. For E2, the intra-assay CV was 8.1% at 87.3 pg/ml, 7.4% at 179.5 pg/ml and 6.5% at 1159.8 pg/ml. For P4, the inter-assay CV was 9.8% at 1.4 ng/ml, 7.4% at 3.0 ng/ml, and 6.5% at 13.5 ng/ml. The intraassay variation for median and high value QCs was less than 6% for all three parameters.
The research team and study participants were blinded to group allocation. The randomization code was revealed to the investigators only after data analysis. Demographic information was gathered at study entry. Women filled out menstrual diaries to track menstrual cycle length, pill compliance and symptoms. Pill counts were performed at each study visit as an additional check to confirm compliance.
The primary outcome was “ovulatory dysfunction” (yes/no) as defined by Jesam and colleagues [18] as: (1) no follicle rupture or (2) follicle rupture that was (a) not preceded within 24–48 hours by an LH peak, (b) preceded by a blunted LH peak (<21 IU/L) and/or (c) followed by a luteal phase progesterone peak of < 3 ng/mL. Analysis for the primary outcome (yes/no) was performed using chi-squared testing for cycle comparisons for all participants and each group. P, E2, and LH levels were graphed based on cycle day (data not shown) and then also normalized to the day of the LH surge (day 0). Day of follicle rupture was independently interpreted by 3 of the investigators (ABE, CD, JTJ) who were blinded to treatment or control cycle. Follicular rupture was determined by a 50% reduction in follicular size or by complete disappearance of the follicular cyst [18]. Any discrepancies were reviewed jointly and agreed on by consensus. Length of the luteal phase, maximum P, E2, and LH serum levels, maximum follicle diameter and endometrial stripe measurement were compared between control and treatment cycles using ANOVA with Bonferroni post hoc testing. Descriptive statistics were used to compare demographics. Intent to treat analysis was utilized.
Based upon the results of Jesam [18], we anticipated that treatment with celecoxib would result in at least a 60% increase in ovulation dysfunction but powered the study to detect a difference of 20% between baseline (control) and either treatment. To detect a difference of this magnitude, a sample size of 10 subjects in each group was needed (89% power with an alpha of 0.05).
Results
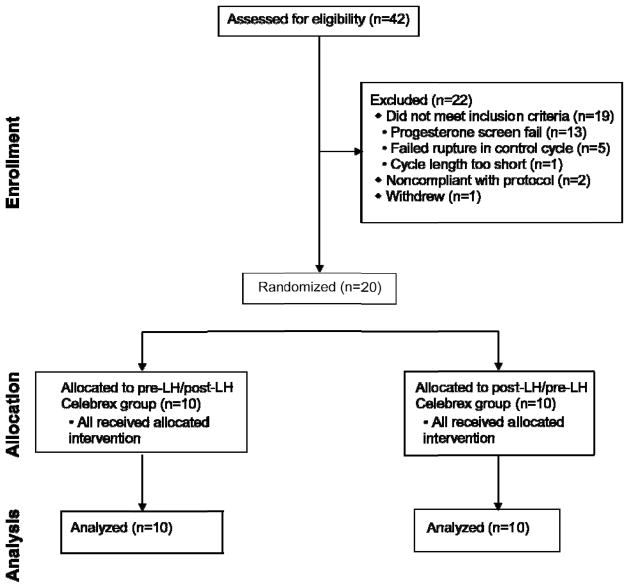
Forty-two subjects were screened for enrollment into the study with 20 randomized to treatment (Group 1, n=10; Group 2 n=10) (Figure 1). There were no clinically meaningful or significant demographic differences between groups (Table I). All 20 subjects completed the study.
Figure 1.
Study flow (CONSORT)
Table 1.
Demographics
| Groups | Pre-LH/Post-LH Celecoxib n=10 |
Post-LH/Pre-LH Celecoxib n=10 |
|---|---|---|
|
| ||
| Agea | 26.2 (5.9) | 28.5 (4.9) |
|
| ||
| Raceb | ||
| Non-Hispanic, Caucasian | 9 | 8 |
| Other | 1 | 2 |
|
| ||
| Paritya | 0 (0.4) | 0 (1.0) |
|
| ||
| Weighta (kg) | 62.8 (5.5) | 68 (7.5) |
|
| ||
| BMIa (kg/m2) | 23.1 (2.3) | 24.2 (2.7) |
|
| ||
| Self-reported cycle length prior to study entrya (days) | 30.2 (1.2) | 28.2 (1.5) |
|
| ||
| Control cycle lengtha (days) | 29.3 (2) | 27.9 (3.1) |
mean (SD);
absolute number
All but one subject demonstrated evidence of normal ovulation during the control cycle. The one subject meeting ovulatory dysfunction criteria in the control cycle was due to a blunted LH peak (8.7 ng/mL), with normal follicle rupture and P levels. She exhibited normal ovulation in both treatment cycles. In comparison to control cycles, treatment cycles resulted in a significant increase in ovulatory dysfunction [pre-LH celecoxib dosing: 30% (6/20), p = 0.04; post-LH celecoxib dosing: 25% (5/20), p= 0.04] (Table II). The timing of drug administration, pre- versus post-LH surge, did not appear to affect the rates of response to the drug. The baseline characteristics of those subjects that demonstrated ovulatory dysfunction in any cycle were not different from those with normal ovulatory processes (data not shown).
Table 2.
Proportion of cycles presenting with normal ovulation or ovulatory dysfunction without or without an unruptured follicle
| Cycle type | Normal ovulatory processes | Ovulatory dysfunction | ||||
|---|---|---|---|---|---|---|
| Total | No follicle rupture, blunted LH | No follicle rupture, normal LH | P < 3 ng/mL | Blunted LH | ||
| Control | 19/20 (95%) | 1/20 (5%) | 0 | 0 | 0 | 1 |
| Pre-LH surge celecoxib administration | 14/20 (70%) | 6/20 (30%) | 1 | 3 | 1 | 1 |
| Post-LH surge celecoxib administration | 15/20 (75%) | 5/20 (25%) | 1 | 1 | 1 | 2 |
Average maximum follicular diameter was not significantly different between any of the control and treatment cycles [control, 20.5 mm (SD 3.3); pre-LH celecoxib, 24.6 mm (SD 7.8); post-LH celecoxib, 21.5 mm (SD 6.3); ANOVA p = 0.09]. Maximum follicular diameter in treatment cycles with and without ovulatory dysfunction were not different [normal ovulation, 22 mm (SD 4.8); ovulatory dysfunction, 25 mm (SD 11); p = 0.28]. However, treatment cycles with ovulatory dysfunction due to unruptured follicles (n = 6) did have a significantly larger maximum follicular diameter [31.9 mm (SD 10.7)] as compared to treatment cycles with ovulatory dysfunction with follicular rupture [16.7 mm (SD 4.4)] or normal ovulation [22 mm (SD 4.8)] (ANOVA p < 0.001).
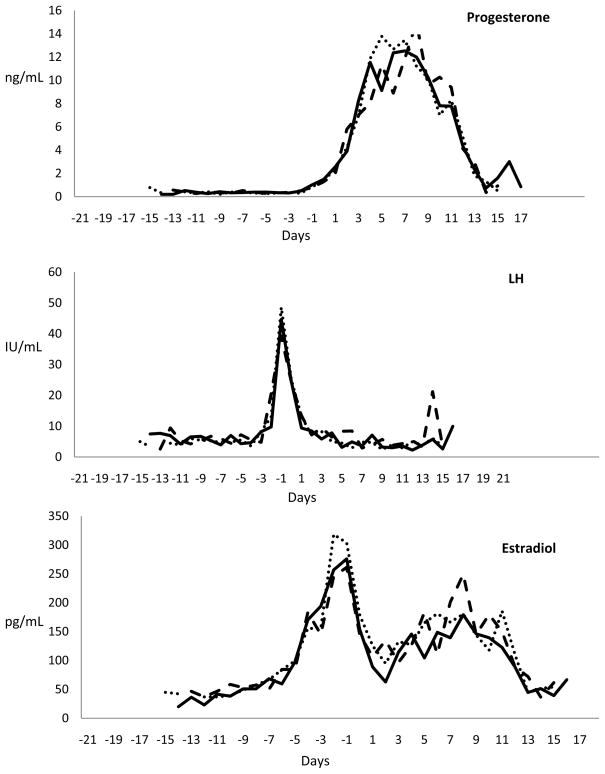
Overall, there were no differences in patterns or peak levels of LH [control 39.7 ng/mL (SD 25); pre-LH celecoxib, 46.2 ng/mL (SD 28.4); post-LH celecoxib, 42.6 ng/mL (SD 22.2)], E2 [control 274 pg/mL (SD 99.6); pre-LH celecoxib, 289.4 pg/mL (SD 124.5); post-LH celecoxib, 282.2 pg/mL (SD 106.7)] or P [control, 13.3 ng/mL (SD 3.3); pre-LH celecoxib, 13.5 ng/mL (SD 3.9); post-LH celecoxib, 12.3 ng/mL (SD 4.7)] even when normalized to the start of the luteal phase (Figure 2). As expected, peak levels of LH and P were significantly different in treatment cycles with and without ovulatory dysfunction as this was how we defined our primary outcome. Peak levels of E2 were similar between treatment cycles with and without ovulatory dysfunction [normal, 283.9 pg/mL (SD 88.5); ovulatory dysfunction, 230.8 pg/mL (SD 181.3); p = 0.3] and they remained similar even when dividing cycles with ovulatory dysfunction into those with or without follicular rupture (ANOVA p = 0.27).
Figure 2.
Average serum levels of progesterone, LH, and estradiol normalized to days of the luteal phase (line = control cycle, dots = pre-LH dosing treatment cycle, dashes = post-LH dosing treatment cycle).
Cycle and luteal phase length as well as maximum endometrial stripe did not differ between control and treatment cycles or between treatment cycles with or without ovulatory dysfunction. Only two women reported unscheduled bleeding at any time during the study; both episodes occurred during treatment cycles without ovulatory dysfunction (1 pre-LH celecoxib dosing, 1 post-LH celecoxib dosing) and only lasted 2 days.
No serious adverse events or minor or major side effects attributable to celecoxib occurred during the study.
Discussion
We found that celecoxib, a highly potent and selective COX2 inhibitor, disrupted ovulation. However, the effect was modest at best with ovulatory dysfunction demonstrated in only 25–30% of treatment cycles when dosed before and after LH surge.
The dosing strategy in our study differed from the previous research on the less selective COX2 inhibitor, meloxicam [17, 18], and a no longer available COX2 inhibitor, rofecoxib [21]. These protocol differences may account for the divergent rates of ovulatory dysfunction between the two studies; we dosed before or after LH surge, while dosing of meloxicam was started in the late follicular phase (follicle ≥ 18mm) and continued for 5 days [17, 18]. More importantly, however, our study confirmed ovulatory status prior to study entry and also during a control cycle whereas Jesam [18] only required women to have regular menstrual cycles. We excluded a total of 18 women who reported regular menstrual cycles but did not have P levels indicating ovulation during an eligibility cycle or who failed to demonstrate follicle rupture in the control cycle. The two inhibitors may also differ in their ovarian effects due to metabolism or pharmacokinetics [14, 24]. Additionally, more minor pathways, like PTGS1, may play a backup role in the absence of COX2 [22]. However, the differences between the studies of meloxicam and our results should be interpreted with caution, as the studies involved different investigators, and enrolled different populations with slightly different monitoring [17, 18, 23].
In accordance with the previously published work on prostaglandin inhibitors, we categorized our end-points into normal ovulatory function, no follicle rupture within 4 days or apparently normal follicle rupture but abnormal LH and/or P rise (ovulatory dysfunction). Of these, ovulatory dysfunction due to a poor LH rise is the weakest criterion, as either the LH peak could have been missed or perhaps even with a blunted LH rise, normal ovulation and fertilization are still possible. A study of cynomolgus monkeys treated with celecoxib during controlled ovulation cycles suggested that treatment impaired oocyte maturation in vivo and prevented fertilization in vitro, but it is unclear whether COX2 inhibition has the potential to affect fertility once follicle rupture occurs in vivo [25]. As no PG synthesis inhibitor has been shown to prevent follicle rupture in greater than 45% of human cycles, this category of drug appears to be of limited usefulness when given alone as an EC.
We selected our treatment and study design in an effort to provide the maximum inhibition of the COX2 enzyme and to determine if the timing of treatment - pre versus post-LH surge - would affect outcomes. Demonstration of a post-LH effect is critical to increasing the effectiveness of a peri-coital contraceptive. Strengths of our study include confirmation of ovulatory status in enrolled subjects, its randomized, blinded study design and blinded adjudicated categorization of the primary endpoints. We used the highest approved dose of celecoxib, and it is unlikely that an increase in dose would have altered our outcomes. Limitations of our approach include the infrequent assessment of LH during the pre-ovulatory interval. However, this is unlikely to have affected our main results, as few cycles were categorized as ovulatory dysfunction due to LH alone.
Results with meloxicam have led to considerable interest in developing COX2 inhibitors as novel EC agents. Our results with the more selective COX2 inhibitor, celecoxib, suggest that this enthusiasm may be somewhat premature. Directly comparing the two drugs and further investigating metabolic and end-organ differences that may affect the treatment effect of highly-selective and moderately-selective COX2 inhibitors may ultimately inform the development of an EC with a broader window of action. Further research investigating targets that can impair follicle rupture after the LH surge leading to an EC with a broad window of action is urgently needed.
Acknowledgments
Funding: Society of Family Planning (ABE), Eunice Kennedy Shriver NICHD U54 Contraceptive Development and Research Center Grant HD055744 (JTJ, JDH), NICHD R01 HD42000 (JDH), and NCRR RR000163 (JDH).
Footnotes
Clinical Trials Registration Number: NCT01129245
References
- 1.Croxatto HB, Brache V, Pavez M, et al. Pituitary-ovarian function following the standard levonorgestrel emergency contraceptive dose or a single 0. 75-mg dose given on the days preceding ovulation. Contraception. 2004;70:442–50. doi: 10.1016/j.contraception.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555–62. doi: 10.1016/S0140-6736(10)60101-8. [DOI] [PubMed] [Google Scholar]
- 3.Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25:2256–63. doi: 10.1093/humrep/deq157. [DOI] [PubMed] [Google Scholar]
- 4.Baird DD, Weinberg CR, McConnaughey DR, Wilcox AJ. Rescue of the corpus luteum in human pregnancy. Biol Reprod. 2003;68:448–56. doi: 10.1095/biolreprod.102.008425. [DOI] [PubMed] [Google Scholar]
- 5.Bogan RL, Murphy MJ, Stouffer RL, Hennebold JD. Systematic determination of differential gene expression in the primate corpus luteum during the luteal phase of the menstrual cycle. Mol Endocrinol. 2008;22:1260–73. doi: 10.1210/me.2007-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennefors BL, Sjogren A, Hamberger L. Progesterone and adenosine 3′,5′-monophosphate formation by isolated human corpora lutea of different ages: influences of human chorionic gonadotropin and prostaglandins. J Clin Endocrinol Metab. 1982;55:102–7. doi: 10.1210/jcem-55-1-102. [DOI] [PubMed] [Google Scholar]
- 7.Hahlin M, Dennefors B, Johanson C, Hamberger L. Luteotropic effects of prostaglandin E2 on the human corpus luteum of the menstrual cycle and early pregnancy. J Clin Endocrinol Metab. 1988;66:909–14. doi: 10.1210/jcem-66-5-909. [DOI] [PubMed] [Google Scholar]
- 8.Hodgen GDIJ. Recognition and maintenance of pregnancy. In: Knobil ENJ, editor. The Physiology of Reproduction. New York: Raven; 1988. [Google Scholar]
- 9.Sargent EL, Baughman WL, Novy MJ, Stouffer RL. Intraluteal infusion of a prostaglandin synthesis inhibitor, sodium meclofenamate, causes premature luteolysis in rhesus monkeys. Endocrinology. 1988;123:2261–9. doi: 10.1210/endo-123-5-2261. [DOI] [PubMed] [Google Scholar]
- 10.Zelinski-Wooten MB, Stouffer RL. Intraluteal infusions of prostaglandins of the E, D, I, and A series prevent PGF2 alpha-induced, but not spontaneous, luteal regression in rhesus monkeys. Biol Reprod. 1990;43:507–16. doi: 10.1095/biolreprod43.3.507. [DOI] [PubMed] [Google Scholar]
- 11.Stouffer R. Structure, function, and regulation of the corpus luteum. In: Knobil ENJ, editor. The Physiology of Reproduction. New York: Raven; 1988. [Google Scholar]
- 12.Stouffer RL, Nixon WE, Hodgen GD. Disparate effects of prostaglandins on basal and gonadotropin-stimulated progesterone production by luteal cells isolated from rhesus monkeys during the menstrual cycle and pregnancy. Biol Reprod. 1979;20:897–903. doi: 10.1095/biolreprod20.4.897. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi T, Morrow JD, Wang H, Dey SK. Cyclooxygenase-2-derived prostaglandin E(2) directs oocyte maturation by differentially influencing multiple signaling pathways. J Biol Chem. 2006;281:37117–29. doi: 10.1074/jbc.M608202200. [DOI] [PubMed] [Google Scholar]
- 14.Dinchuk JE, Car BD, Focht RJ, et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–9. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- 15.Bogan RL, Murphy MJ, Stouffer RL, Hennebold JD. Prostaglandin synthesis, metabolism, and signaling potential in the rhesus macaque corpus luteum throughout the luteal phase of the menstrual cycle. Endocrinology. 2008;149:5861–71. doi: 10.1210/en.2008-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arosh JA, Banu SK, Chapdelaine P, Madore E, Sirois J, Fortier MA. Prostaglandin biosynthesis, transport, and signaling in corpus luteum: a basis for autoregulation of luteal function. Endocrinology. 2004;145:2551–60. doi: 10.1210/en.2003-1607. [DOI] [PubMed] [Google Scholar]
- 17.Bata MS, Al-Ramahi M, Salhab AS, Gharaibeh MN, Schwartz J. Delay of ovulation by meloxicam in healthy cycling volunteers: A placebo-controlled, double-blind, crossover study. J Clin Pharmacol. 2006;46:925–32. doi: 10.1177/0091270006289483. [DOI] [PubMed] [Google Scholar]
- 18.Jesam C, Salvatierra AM, Schwartz JL, Croxatto HB. Suppression of follicular rupture with meloxicam, a cyclooxygenase-2 inhibitor: potential for emergency contraception. Hum Reprod. 2010;25:368–73. doi: 10.1093/humrep/dep392. [DOI] [PubMed] [Google Scholar]
- 19.Massai MR, Forcelledo ML, Brache V, et al. Does meloxicam increase the incidence of anovulation induced by single administration of levonorgestrel in emergency contraception? A pilot study. Hum Reprod. 2007;22:434–9. doi: 10.1093/humrep/del369. [DOI] [PubMed] [Google Scholar]
- 20.Hester KE, Harper MJ, Duffy DM. Oral administration of the cyclooxygenase-2 (COX-2) inhibitor meloxicam blocks ovulation in non-human primates when administered to simulate emergency contraception. Hum Reprod. 2010;25:360–7. doi: 10.1093/humrep/dep424. [DOI] [PubMed] [Google Scholar]
- 21.Pall M, Friden BE, Brannstrom M. Induction of delayed follicular rupture in the human by the selective COX-2 inhibitor rofecoxib: a randomized double-blind study. Hum Reprod. 2001;16:1323–8. doi: 10.1093/humrep/16.7.1323. [DOI] [PubMed] [Google Scholar]
- 22.Lemke TWD. Table 36.5. Foyle’s principles of medicinal chemistry. Baltimore: Lippincott, Williams, Wilkins; 2008. [Google Scholar]
- 23.Edelman AB, Jensen JT, Hennebold JD. A nonhormonal model for emergency contraception: prostaglandin synthesis inhibitor effects on luteal function and lifespan, a pilot study. Contraception. 2010;81:496–500. doi: 10.1016/j.contraception.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues AD. Impact of CYP2C9 genotype on pharmacokinetics: are all cyclooxygenase inhibitors the same? Drug Metab Dispos. 2005;33:1567–75. doi: 10.1124/dmd.105.006452. [DOI] [PubMed] [Google Scholar]
- 25.Duffy DM, Stouffer RL. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum Reprod. 2002;17:2825–31. doi: 10.1093/humrep/17.11.2825. [DOI] [PubMed] [Google Scholar]