Abstract
Tob (transducer of erbB2) is a member of antiproliferative family proteins and acts as a bone morphogenic protein inhibitor as well as a suppressor of proliferation in T cells, which have been implicated in postmenopausal bone loss. To determine the effect of Tob deficiency on estrogen deficiency-induced bone loss, we analyzed bone metabolism after ovariectomy or sham operation in Tob-deficient mice. Ovariectomy in WT mice decreased trabecular bone volume and bone mineral density (BMD) as expected. In Tob-deficient mice, ovariectomy reduced bone volume and BMD. However, even after ovariectomy, both trabecular bone volume and BMD levels in Tob-deficient bone were comparable to those in sham-operated WT bones. Bone formation parameters (mineral apposition rate and bone formation rate) in the ovariectomized Tob-deficient mice were significantly higher than those in the ovariectomized WT mice. In contrast, the ovariectomy-induced increase in the bone resorption parameters, osteoclast surface, and osteoclast number was similar between Tob-deficient mice and WT mice. Furthermore, in ex vivo nodule formation assay, ovariectomy-induced enhancement of nodule formation was significantly higher in the bone marrow cells from Tob-deficient mice than in the bone marrow cells from ovariectomized WT mice. Both Tob and estrogen signalings converge at bone morphogenic protein activation of alkaline phosphatase and GCCG-reporter gene expression in osteoblasts, revealing interaction between the two signals. These data indicate that Tob deficiency prevents ovariectomy-induced bone loss through the superenhancement of osteoblastic activities in bone and that this results in further augmentation in the bone formation rate and the mineral apposition rate after ovariectomy in vivo.
Bone metabolism is regulated by continuous remodeling through the maintenance in the reciprocal cycles of resorption and formation. This bone remodeling is based on the balanced actions between osteoclasts resorbing old bones and osteoblasts forming new bones (1). However, in postmenopausal women, bone resorption levels exceed those of bone formation, resulting in osteoporosis and the accompanying increases in bone fragility and susceptibility to fractures (2). Approximately 100 million people are estimated to suffer from this disease worldwide (2). Several drugs have been developed to treat osteoporosis, with many to inhibit bone resorption and only a few promoting bone formation (3). Treatment of osteoporosis by modulating of only one of the two processes, such as bone resorption and bone formation, in bone remodeling limits the efficacy of these drugs in restoring the remodeling balance and normal bone mass. Recent progress has identified certain genetics that lead to the loss of balance underlying pathogenesis of postmenopausal osteoporosis (4). However, molecules involved in the determination of the levels of reduction in bone due to estrogen deficiency are not yet fully understood.
Tob (transducer of ErbB2) is a member of the antiproliferative gene family consisting of BTG1, PC3/TIS21/BTG2, ANA/BTG3, PC3B, and Tob2 (5-13). The Tob gene encodes a 45-kDa protein that was identified by West-Western procedure by using ErbB2 as a probe (8). These proteins suppress cell growth when exogenously expressed in NIH 3T3 cells. However, in vivo roles of Tob, especially with respect to the pathological situation, are still to be elucidated. Investigation into the role of the Tob gene has just begun using knockout mice (14). Tob-deficient mice are born normally, grow similarly to WT mice, and are fertile (14). Although no apparent morphological changes were observed in their skeleton and other tissues until 2 months after birth, the bones in Tob-deficient mice were found to be osteosclerotic later in their lives (14). Tob-deficient mice reveal high levels of bone mass in adults due to an increase in bone formation without a major alteration in bone resorption (14). Furthermore, Tob acts as an inhibitor against bone morphogenic protein (BMP)-induced Smad signaling to activate transcription by means of association with Smads in vitro (14). In addition, Tob was reported to function as a negative regulator of IL-2 transcription and proliferation of T cells (15), which have been implicated in postmenopausal osteoporosis models (16).
Estrogen depletion enhances bone resorption, which subsequently stimulates bone formation. The levels of the enhancement of bone resorption exceed those of bone formation in the estrogen-depleted condition. This uncoupling results in a net reduction of bone mass (3). The mechanism underlying the uncoupling after estrogen depletion has been one of the major issues in research on bone metabolism (3). Estrogen depletion enhances IL-7 expression in bone marrow, and this cytokine in turn increases receptor activator of NF-κB (RANK) ligand expression to activate bone resorption while it suppresses bone formation, suggesting that IL-7 is one of the molecules involved in the uncoupling events (17). However, the full details for molecular events that lead to uncoupling are not yet understood.
Because Tob suppresses bone formation in the adult animals without altering bone resorption, it may also be involved in the uncoupling events in bone after estrogen depletion. To examine the possible role of Tob in estrogen depletion-induced bone loss, we ovariectomized Tob-deficient mice and compared the bone loss to that in ovariectomized (OVX) WT mice. Tob deficiency preserves bone mass even after the bone loss because of estrogen-induced enhancement in bone resorption, suggesting that Tob is involved in the determination of the levels of bone mass after estrogen depletion.
Materials and Methods
Certain methods (including mineralized nodule formation and osteoclast formation from bone marrow cells and RT-PCR analysis) can be found in Supporting Methods, which is published as supporting information on the PNAS web site.
Animals. Female WT and Tob-deficient mice in a 129/S3×C57BL/6F2 background derived from the original heterozygous crosses were maintained as separate colonies. Nine-week-old female Tob-deficient and WT mice (28 mice in total), 16-20 g in body weight, were randomly assigned in equal numbers into sham operation and ovariectomy groups.
Cell Culture. ROS17/2.8 cells were obtained from Gideon A. Rodan (Merck Research Laboratories, West Point, PA) and were grown in modified Ham's F-12 nutrient mixture supplemented with 10% FBS. After transfection, these cells were maintained in phenol red-free α-MEM supplemented with 10% charcoal stripped FBS. MC3T3E1 cells (obtained from Hiroaki Kodama, Ohu University, Koriyama, Japan) were grown in α-MEM supplemented with 10% FBS and maintained in phenol red-free α-MEM with 10% charcoal stripped FBS after transfection.
Ovariectomy Model. WT mice and Tob-deficient mice were ovariectomized or sham-operated, and all of the mice were killed by overdosing of anesthetics 2 weeks after the surgery. The mice were injected with calcein at 4 mg/kg, 4 and 2 days before killing, respectively, to obtain dynamic parameters for bone formation. Uteri of the mice were excised and weighed to evaluate the effects of ovariectomy.
Body Weight. Body weights of sham-operated and OVX mice recorded every day were not altered during the 2 weeks experiments in both genotypes (data not shown). This finding confirmed that stress could be considered minimal in our experiments.
Micro-X-Ray Computed Tomography (μCT) Analysis of Bone. For measurements of the bone volume [trabecular bone volume (BV/TV)], the bones (femora) were subjected to μCT analysis with Musashi (Nittetsu Elex, Osaka). The data were subsequently quantified by using a Luzex-F automated image analysis system (Nireco, Tokyo). The fractional bone volume (BV/TV) was measured in a square area of 1.47 mm2 with its closest and furthest edges at 0.2 and 2.3 mm, respectively, distal to the growth plate in the distal ends of the femur. Threshold for the measurements was set at 110 arbitrary units for the μCT analyses.
Bone Mineral Density (BMD). BMD of the right whole femora was measured based on dual-energy x-ray absorptiometry with PIXI (GE Lunar, Madison, WI).
Deoxypyridinoline (Dpyd) in Urine. Urinary Dpyd levels were measured on day 14 of the experiments by ELISA (Metra Biosystems, Mountain View, CA). Urine was collected from the mice in metabolic cages during the last 24 h (on day 14 after surgery), and independent samples from each group were analyzed.
Histomorphometric Analysis of Bone. For decalcified sections, serial 5-μm-thick sagittal sections of the tibiae were made by using a microtome and were stained for tartrate-resistant acid phosphatase (TRAP) followed by staining with toluidine blue. Histomorphometry was conducted to quantify the number of osteoclasts (N.Oc/BS) and osteoclast surface (Oc.S/BS) as defined by Parfitt et al. (18). TRAP-positive multinucleated cells attached to bone were counted as osteoclasts. Measurements were made within an area with its closest and furthest edges at 0.35 and 0.60 mm, respectively, distal to the growth plate of the proximal ends of the tibiae.
For undecalcified sections of the tibiae, serial 3-μm-thick frontal sections were made by using a microtome. The metaphyseal cancellous bone fraction was measured in an area with its closest and furthest edges 0.35 and 0.60 mm distal to the growth plate. Dynamic histomorphometric analyses were conducted within this area.
Alkaline Phosphatase (ALP) Assay. ROS17/2.8 cells were transfected with Smad1 expression vector (for the cells treated with BMP2), estrogen receptor α expression vector (for the cells treated with estrogen; kindly provided by Benita Katzenellenbogen, University of Illinois, Urbana), or Tob expression vector using FuGENE6 (Roche, Indianapolis) and treated with vehicle, 17β-estradiol (10-7 M), or BMP2 (50 ng/ml). After 72 h, the cells were lysed and scraped into 0.25 ml of a buffer containing 10 mM Tris·HCl (pH 7.5), 0.5 mM MgCl2, and 0.1% Triton X-100. These cell lysates were homogenized through freeze-and-thaw. ALP activity in the samples was assayed by using Na2 p-nitrophenyl phosphate as substrate. Protein contents in each sample were determined according to the Coomassie blue G method.
Luciferase Assay. MC3T3-E1 cells were cultured in phenol red free α-MEM supplemented with 10% charcoal-stripped FBS. The cells were transfected with a luciferase reporter linked to BMP response element (GCCGX12) and Smad1 expression vector (for the cells treated with BMP2), estrogen receptor α expression vector (for the cells treated with estrogen), or Tob expression vector using Fu-GENE6 and treated with vehicle, 17β-estradiol (10-7 M), or BMP2 (200 ng/ml). The cells were harvested 72 h after treatment and lysed. Luciferase activities were assayed by using Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions, and the firefly luciferase activities were normalized against those of Renilla luciferase.
Statistical Evaluations. The results are presented as mean values ± SEM. Statistical analysis was performed based on the Mann-Whitney U test. P values <0.05 were considered statistically significant.
Results
Ovariectomy in Tob-Deficient Mice. Tob-deficient mice and WT mice were comparable in body weight and showed no significant difference in body weight gain during the 2-week experimental period, although the OVX mice tended to gain more weight compared with the respective sham group mice (data not shown).
To confirm the estrogen status in the OVX or sham-operated mice, the uterine wet weight was measured 2 weeks after ovariectomy. Ovariectomy significantly decreased by ≈80% the uterine weight of both Tob-deficient mice and WT mice. Tob deficiency per se had no effect on the basal levels or reduced levels of uterus weight (Fig. 1).
Fig. 1.
Uterine weight after ovariectomy in Tob-deficient mice. Shown is uterine weight in sham-operated (SHAM) and OVX WT mice and Tob-deficient (TOB KO) mice. Wet uterus was weighed at the end of the experiments. Ovariectomy significantly decreased the uterine weight in both WT mice and Tob-deficient mice. The reduction levels in uterus weight after OVX were comparable between WT mice and Tob-deficient mice. Asterisks indicate statistical significance.
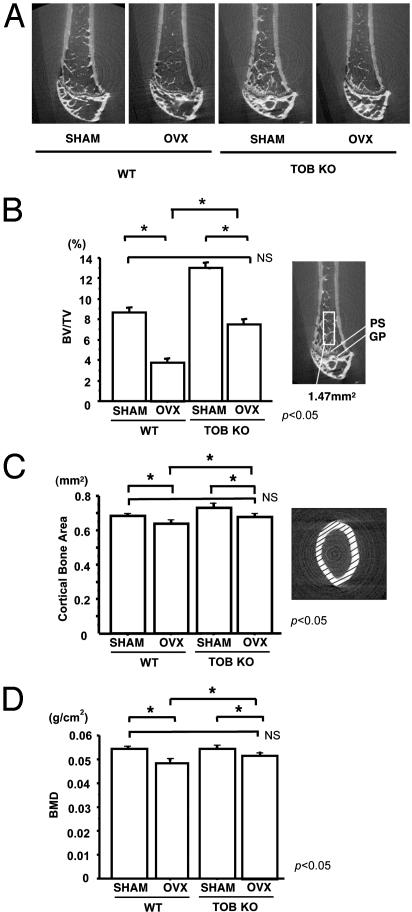
Tob Deficiency Preserves Bone Mass After Ovariectomy. Quantification of the two-dimensional BV/TV in the distal end of the femur was conducted by using μCT and an automated image analyzer (Fig. 2A). BV/TV in the WT mice was significantly reduced 2 weeks after ovariectomy compared with that in the sham-operated WT mice (3% vs. 8%, respectively; P < 0.05) (Fig. 2B). In contrast, although bone volume in Tob-deficient mice was reduced after ovariectomy (7%) compared with sham-operated Tob-deficient mice (12%), the final levels were still comparable to the sham-operated WT mice (7% vs. 8%) (Fig. 2B). Similar to cancellous bone volume, the cortical bone area in OVX WT mice was less than that of sham-operated mice. Ovariectomy reduced the levels of cortical bone area in Tob-deficient mice. However, the levels of the cortical bone area after ovariectomy in Tob-deficient mice were comparable to those in sham-operated WT mice (Fig. 2C).
Fig. 2.
Tob deficiency preserves bone volume and BMD even after ovariectomy-induced reduction. (A) μCT analysis of the femora of mice. WT or Tob-deficient (TOB KO) mice were either ovariectomized (OVX) or sham-operated (SHAM). Trabecular bone patterns in both WT mice and Tob-deficient mice were altered to be more sparse after ovariectomy. (B) μCT-based quantification of the BV/TV in sham-operated (SHAM) or OVX WT and Tob-deficient (TOB KO) mice. BV/TV within the area indicated by a rectangle was quantified as described in Materials and Methods. WT mice and Tob-deficient mice were reduced after ovariectomy compared with that in the sham-operated mice. Although bone volume in Tob-deficient mice was reduced after ovariectomy compared with sham-operated Tob-deficient mice, the final levels were still comparable to those in the sham-operated WT mice. (C) μCT-based quantification of the cortical bone volume in sham-operated (SHAM) or OVX WT and Tob-deficient (TOB KO) mice. The cortical bone area was measured by using μCT sections in a plane perpendicular to the long axis within the mid-diaphysis. (D) BMD of the whole femora of sham-operated (SHAM) or OVX WT and Tob-deficient (TOB KO) mice was measured by using a small animal dual-energy x-ray absorptiometry apparatus. Similar to BV/TV, BMD levels in OVX Tob-deficient mice were comparable to those in sham-operated WT mice and were higher than those in OVX WT mice.
We further examined BMD of the whole femur. The BMD of femur of WT mice after ovariectomy was less than that in sham-operated WT mice, and such reduction was also observed after ovariectomy in Tob-deficient mice (Fig. 2D). However, even after ovariectomy, BMD levels in Tob-deficient bone were comparable to those in sham-operated WT bones and were higher than those in OVX WT bones (OVX WT mice, 48.5 mg/cm2; OVX Tob-deficient mice, 50.8 mg/cm2) (Fig. 2D). These data indicate that Tob deficiency preserves bone mass even after bone loss due to ovariectomy.
Tob Deficiency Preserves Bone Volume by Means of Elevated Levels of Bone Formation. In Tob-deficient mice, basal levels of mineral apposition rate (MAR) in the sham-operated group were higher than those in WT mice as reported in ref. 14. These elevated levels were comparable to the enhanced levels of MAR in OVX WT mice (Fig. 3A). Interestingly, ovariectomy in Tob-deficient mice further enhanced MAR significantly compared with the sham-operated group in Tob-deficient mice (Fig. 3A). Similarly, the bone formation rate (BFR) was enhanced by ovariectomy in WT mice, and the basal levels of BFR in Tob-deficient mice in the sham-operated group were comparable to those in OVX WT mice (Fig. 3B). Ovariectomy further enhanced BFR compared with the sham-operated mice in the Tob-deficient background (Fig. 3B). These results indicated that superenhancement in bone formation was involved in Tob deficiency-induced preservation in bone volume even after ovariectomy.
Fig. 3.
Tob deficiency further enhances the ovariectomy-induced increase in bone formation in vivo. Bone formation parameters were measured in histomorphometric analysis of tibia. MAR (A) and BFR (B) were determined based on calcein labeling in sham-operated (SHAM) or OVX WT and Tob-deficient (TOB KO) mice. Calcein was injected at 4 mg/kg 4 and 2 days before killing. Measurement was conducted in the secondary spongiosa in tibiae. MAR and BFR were enhanced by ovariectomy in WT mice. Basal levels of MAR and BFR in Tob-deficient mice were comparable to those in OVX WT mice. However, ovariectomy further enhanced MAR and BFR levels in Tob-deficient mice compared to WT mice. *, P < 0.05.
Baseline levels of bone resorption parameters were similar between WT and Tob-deficient mice (Fig. 4 A and B). Ovariectomy increased the levels of Oc.S/BS and the N.Oc/BS in WT mice. In contrast to the superenhancement in the levels of bone formation parameters, ovariectomy enhanced the levels of bone resorption parameters in Tob-deficient mice to a degree similar to that observed in WT mice (Fig. 4 A and B).
Fig. 4.
Tob deficiency did not alter the ovariectomy-induced increase in the levels of bone resorption parameters. Bone resorption parameters were obtained based on histomorphometric analysis of bone. Oc.S/BS (A) and N.Oc/BS (B) in sham-operated (SHAM) or OVX WT and Tob-deficient (TOB KO) mice were examined. Oc.S/BS and N.Oc/BS levels were increased in WT mice and Tob-deficient mice after ovariectomy, and these levels were similar between WT mice and Tob-deficient mice. (C) Urinary Dpyd levels in sham-operated (SHAM) or OVX WT and Tob-deficient (TOB KO) mice. Similar to Oc.S/BS and N.Oc/BS levels, the levels of urinary Dpyd in OVX Tob-deficient mice were similar to those in OVX WT mice. *, P < 0.05.
To address Tob deficiency effects on systemic bone loss, we measured the levels of urinary Dpyd, a biochemical marker of bone resorption. Dpyd levels in OVX group were increased ≈2-fold compared with the sham-operated group in WT mice as expected (Fig. 4C). Similarly, ovariectomy enhanced significantly urinary Dpyd in Tob-deficient mice (Fig. 4C). However, enhancement in the Dpyd levels in OVX WT mice was similar to that in OVX Tob-deficient mice (Fig. 4C). These results indicate that Tob deficiency affects the levels of systemic bone formation but not those of bone resorption markers in vivo in OVX mice.
Nodule Formation and Osteoclast Formation from Bone Marrow Cells. To obtain insights into the cellular bases for the mechanism of Tob deficiency effects on estrogen deficiency-induced bone loss, we examined mineralized nodule formation and osteoclast formation in the cultures of bone marrow cells. An in vitro nodule formation assay in bone marrow cells obtained 14 days after ovariectomy revealed ≈5-fold enhancement in the nodule formation in the cells from OVX mice compared with sham-operated mice in the WT group (Fig. 5A). In the case of bone marrow cells derived from Tob-deficient mice, the levels of nodule formation in the sham-operated group were higher than those in the sham-operated WT group (Fig. 5A). Ovariectomy superenhanced nodule formation levels in the bone marrow cells from Tob-deficient mice compared with bone marrow cells from OVX WT mice (Fig. 5A).
Fig. 5.
Tob deficiency further enhances the ovariectomy-induced increase in nodule formation in vitro. Functional characterization of the bone marrow cells obtained from WT mice and Tob-deficient mice was conducted after OVX using cultures of these cells. (A) Mineralized nodule formation in the cultures of bone marrow cells from sham-operated (SHAM) or OVX WT mice and Tob-deficient (TOB KO) mice. The cells were cultured in presence of ascorbic acid and β-glycerophosphate for 21 days. OVX-induced enhancement in nodule formation was significantly more in the bone marrow cells from Tob-deficient mice than that in WT mice. (B) Osteoclast formation in the cultures of bone marrow cells from sham-operated (SHAM) or OVX WT mice and Tob-deficient (TOB KO) mice. The cells were treated with vitamin D and dexamethasone for 14 days. Enhancement in osteoclast development in cultures induced by ovariectomy was similar between Tob-deficient mice and WT mice. *, P < 0.05.
The number of osteoclasts developed in the bone marrow cultures treated with dexamethasone and vitamin D was enhanced by ≈80% in the OVX group compared with the sham-operated group in WT mice (Fig. 5B). In contrast to Tob deficiency-induced superenhancement in nodule formation, enhancement of osteoclast development induced by ovariectomy was similar between Tob-deficient mice and WT mice (Fig. 5B). These data indicate that Tob deficiency prevents ovariectomy-induced bone loss through the superenhancement in osteoblast differentiation in bone marrow environment, which would result in the augmentation in BFR and bone mass gain in vivo.
Tob Deficiency Alters Gene Expression in Bone After Ovariectomy in Vivo. To examine molecular events underlying the Tob deficiency-induced superenhancement in bone formation after ovariectomy, RNA was obtained from bone marrow in femora. In the bone marrow obtained from WT mice, ovariectomy up-regulated mRNA expression levels of bone formation-related osteogenic genes such as ALP, type I collagen, osteocalcin, Cbfa1, and osterix as well as those of bone resorption-related genes encoding RANK, RANK ligand, and osteoprotegerin (Fig. 6). Expression of bone resorption-related genes in the bone marrow of Tob-deficient mice was up-regulated by ovariectomy to the levels comparable to those in WT mice (Fig. 6B). In contrast, the levels of ovariectomy-induced enhancement in bone formation-related gene expression in bone marrow were significantly higher in Tob-deficient mice compared with those in OVX WT mice (Fig. 6A).
Fig. 6.
Tob deficiency further augments the ovariectomy-induced enhancement in the expression of genes encoding osteoblast phenotype-related proteins. mRNA expression of the genes encoding bone formation-related proteins and bone resorption-related proteins was examined in bone marrow tissue. (A) Ovariectomy-induced enhancement in the levels of mRNA expression of bone formation-related genes in the bone marrow in Tob-deficient mice was more than that in WT mice. (B) Ovariectomy enhanced mRNA expression levels of bone resorption-related genes encoding RANK, RANK ligand, and osteoprotegerin (OPG) in bone marrow. The enhancement levels were similar between WT mice and Tob-deficient mice.
These observations indicate that Tob deficiency preserves bone mass after ovariectomy through the superenhancement in the expression of bone formation-related genes in the bone marrow environment after ovariectomy.
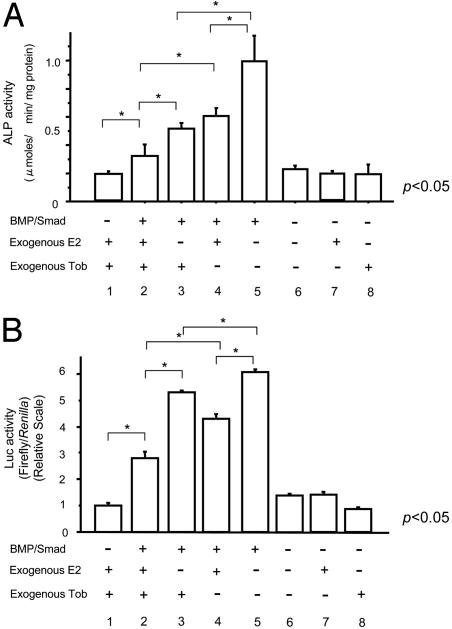
Tob and Estrogen Signalings Converge at the Regulation of BMP-Induced ALP Gene Expression in Osteoblasts. Finally, we addressed whether Tob and estrogen interact to regulate BMP signaling in osteoblasts. BMP treatment enhanced ALP activity levels in osteoblastic ROS17/2.8 cells even in the presence of exogenous estrogen and overexpression of Tob (Fig. 7A, lane 1 vs. lane 2, P < 0.05). Removal or exogenous estrogen (still in the presence of exogenous overexpressed Tob) enhanced the BMP-induced ALP activity in these osteoblasts (Fig. 7A, lane 2 vs. lane 3, P < 0.05). The presence or absence of estrogen did not alter the basal ALP levels in these cells (Fig. 7A, lanes 6-8). Removal of exogenous Tob per se could also enhance BMP-induced ALP expression levels (Fig. 7A, lane 2 vs. lane 4, P < 0.05). Removal of both exogenous estrogen and Tob further enhanced the ALP expression levels compared with the removal of estrogen alone (Fig. 7A, lane 3 vs. lane 5, P < 0.05) or the removal of Tob alone (Fig. 7A, lane 4 vs. lane 5, P < 0.05). Thus, estrogen and Tob signalings converge at the regulation of BMP-induced ALP expression in osteoblastic cells.
Fig. 7.
BMP signaling is enhanced by the absence of exogenous estrogen and Tob in osteoblasts. (A) Convergence of Tob and estrogen signaling in the regulation of phenotype (ALP) expression in osteoblasts. Smad1, estrogen receptor, and Tob expression vectors were cotransfected into ROS17/2.8 osteoblastic cells, which were then exposed to BMP2 or estrogen or were further transfected with Tob expression vector. ALP activity assay was conducted 3 days after the treatment. (B) Convergence of Tob and estrogen signaling at the transcriptional events. A luciferase reporter gene containing concatameric BMP response elements (GCCGx12) as well as Smad1, estrogen receptor, or Tob expression vectors were cotransfected into MC3T3E1 osteoblastic cells, which were then exposed to BMP2 and estrogen. Luciferase assay was conducted 3 days after the treatment.
To examine molecular events underlying such Tob and estrogen signalings observed in the regulation of BMP action on ALP expression, osteoblastic MC3T3E1 cells were transfected with a luciferase reporter gene that is linked to concatamerized BMP-response elements GCCGx12. Even in the presence of exogenous estrogen and Tob overexpression, BMP could enhance expression levels of the luciferase reporter gene in osteoblasts in culture (Fig. 7B, lane 1 vs. lane 2, P < 0.05). Removal of exogenous estrogen (still in the presence of exogenous Tob) enhanced the BMP-induced luciferase activity in these cells (Fig. 7B, lane 2 vs. lane 3, P < 0.05). Removal of exogenous Tob per se could also enhance luciferase reporter expression levels by ≈70% (Fig. 7A, lane 2 vs. lane 4, P < 0.05). Removal of both exogenous Tob and estrogen moderately but significantly enhanced the reporter gene expression compared with the removal of estrogen alone or Tob alone (Fig. 7B, lane 3 vs. lane 5, P < 0.05; lane 4 vs. lane 5, P < 0.05). These results indicate that Tob and estrogen interact to target BMP-induced transactivation through a common regulatory enhancer sequence. Such findings correspond to the observation that Tob and estrogen interact to modulate BMP-dependent enhancement of ALP expression in osteoblasts.
Discussion
We demonstrated that Tob deficiency preserves bone mass even after bone loss due to ovariectomy, and Tob deficiency effects were through further enhancement of osteoblastic activities in vivo and in vitro in addition to their activation by ovariectomy in bone marrow environment. We refer to these phenomena as superenhancement.
Bone Formation in Cancellous and Cortical Bone Envelope Is Enhanced in the Absence of Tob Even After Ovariectomy. In WT mice, bone resorption parameters and bone formation parameters were enhanced by ovariectomy, and bone resorption exceeded bone formation, resulting in reduction in bone volume. In Tob-deficient mice, the ovariectomy-induced increase in bone resorption parameters was comparable to the increase in these parameters in WT mice. However, ovariectomy-induced enhancement in bone formation parameters was further enhanced in Tob-deficient mice, resulting in preservation of bone mass even after ovariectomy. Such effects of Tob deficiency were observed not only in trabecular bone envelope but also in cortical bone envelope. This in vivo observation could be an indirect effect on bone through its influence on the other organs. However, mineralized nodule formation conducted to elucidate cellular mechanisms underlying the effects of Tob deficiency revealed that ovariectomy-induced enhancement in nodule formation was significantly higher in the bone marrow cells from Tob-deficient mice than in the bone marrow cells from OVX WT mice. In contrast, ovariectomy-induced enhancement of osteoclast development was similarly observed in the cultures of cells from Tob-deficient mice and WT mice. These data indicate that, although ovariectomy per se enhanced osteoblastic activity in bone marrow cells in culture, Tob deficiency further enhanced osteoblastic differentiation after ovariectomy.
Tob Deficiency and T Cells in Ovariectomy-Induced Bone Loss. One of the possible mechanisms of estrogen deficiency-induced bone loss in vivo is the enhanced tumor necrosis factor (TNF) production in T cells due to an increased bone marrow T cell number (17). In this regard, it is intriguing that Tob, which was reported to function as a negative regulator of T cell proliferation, is expressed in anergic T cells and suppresses IL-2 gene expression in association with cytokine transcription (15). If ovariectomy enhances T cell number in bone marrow cells, which results in increased total TNF production (17), Tob deficiency may further increase T cell proliferation in estrogen-deficient mice, and this may result in a further increase in TNF production compared with the situation in OVX WT mice. However, we observed that ovariectomy increased the levels of bone resorption parameters similarly in Tob-deficient and WT mice. Thus, Tob deficiency does not appear to alter the T cell-dependent phenomenon associated with ovariectomy such as TNF production in the Tob-deficient mice.
Tob Serves to Keep a Reservoir in the Capability of Bone Formation. Even after ovariectomy, the levels of bone formation parameters in Tob-deficient mice further increased to reach levels higher than the increased levels in OVX WT mice. Such further enhancement was specific to bone formation parameters because enhanced bone resorption parameters after ovariectomy in Tob-deficient mice stayed at levels similar to those in WT mice. These observations indicated the uncoupling between bone formation and resorption in these mice. Thus, Tob action can be interpreted to keep a certain fraction of bone formation activity in silent state. In other words, Tob may reserve a fraction of bone mass as a reservoir, which is independent from a fraction of bone mass subjected to coupling with bone resorption activity. This reservoir may stay away from normal remodeling activity. In the event that Tob function is inactivated, bone mass may be increased by using the reservoir. Thus, Tob could be an interesting target for the development of the therapeutic measures for osteoporosis.
Estrogen and Tob Signalings Converge at BMP-Induced Transcriptional Event. Our analyses indicate the convergence of the estrogen and Tob signals in the regulation of BMP-induced enhancement of ALP expression in osteoblasts. Interaction between Tob and estrogen was also observed at their regulation of BMP enhancement in ALP expression, because simultaneous removal of exogenous estrogen and Tob further enhanced BMP-induced ALP activity in osteoblasts compared with the removal of either one of the two. Finally, removal of exogenous Tob or estrogen signaling enhanced BMP/Smad-dependent activation of the GCCG-luciferase promoter. Thus, the two signaling pathways converge at GCCG sequence-dependent transcription. These observations on the interactions between Tob and estrogen on BMP/Smad-dependent transcription and ALP expression in vitro were in parallel to those on osteoblastic functions such as BFR and MAR seen in vivo.
It was reported that estrogen receptor α binds to Smad1 to inhibit transcription only in the presence of BMP in the cases of 293T and MCF7 cells in culture (19). Our observation indicated that not only estrogen but also Tob could suppress BMP/Smad-dependent transcriptional events through the GCCG sequence in osteoblasts in vitro. Because BMP activates Tob expression, it is intriguing to postulate that BMP-induced Tob may also play a role as a suppressive cofactor in estrogen-dependent suppression of Smad signaling in 293T and MCF7 cells. Interestingly, BMP4 (but not BMP2) and estrogen signaling may cooperate to stimulate proliferation of prolactinoma cells, suggesting that a certain cell background dependency may exist in modulation of the interactions between BMP and estrogen (20).
Interaction Between Estrogen Activity and Tob. 17β-estradiol has been reported to rapidly activate the mitogen-activated protein kinase pathway in osteoblasts (21). Activation of this pathway triggers cell proliferation and differentiation. Its activation by estrogen receptor ligands suggests their involvement in cell-cycle control. Recently, it has been reported that growth factor stimulation of the receptor tyrosine kinase/Ras/mitogen-activated protein kinase pathway resulted in phosphorylation of Tob (22). Tob phosphorylated by extracellular signal-regulated kinase 1/2 suppressed cyclin D1 expression, thus inhibiting cell proliferation (23), forming a negative feedback system. If this is the case in osteoblasts as well, Tob deficiency may lead to an increase in cell proliferation upon growth factor stimuli, and such action may compensate for the lack of estrogen-dependent proliferation by means of mitogen-activated protein kinase. This could serve as a possible alternative pathway to enhance bone formation in Tob-deficient mice in addition to the Tob regulation of Smad/BMP signaling.
In conclusion, Tob deficiency prevents ovariectomy-induced bone loss through the enhancement of osteoblastic activity in the bone marrow environment, which results in the augmentation of BFR and bone mass gain in vivo.
Supplementary Material
Acknowledgments
We thank Professor Shohei Kasugai for his support. This research was supported by the Japanese Ministry of Education [21st Century Center of Excellence (COE) Program for Frontier Research on Molecular Destruction and Reconstruction of Tooth and Bone Grants-in-Aid 15659352, 14207056, 14034214, and 14028022], grants from the Japan Space Forum, the National Space Development Agency of Japan, the Japan Society for the Promotion of Science (JSPS; the Research for the Future Program, Genome Science, and the Integrated Action Initiative, Core-to-Core Program) (to M.N.), and a JSPS research grant and postdoctoral fellowship (to M.U.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BMP, bone morphogenic protein; BV/TV, trabecular bone volume; BMD, bone mineral density; MAR, mineral apposition rate; BFR, bone formation rate; Oc.S/BS, osteoclast surface; N.Oc/BS, osteoclast number; μCT, micro-x-ray computed tomography; Dpyd, deoxypyridinoline; ALP, alkaline phosphatase; RANK, receptor activator of NF-κB; OVX, ovariectomized.
References
- 1.Manolagas, S. C. (2000) Endocr. Rev. 21, 115-137. [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis and Therapy (2001) J. Am. Med. Assoc. 285, 785-795. [DOI] [PubMed] [Google Scholar]
- 3.Rodan, G. A. & Martin, T. J. (2000) Science 289, 1508-1514. [DOI] [PubMed] [Google Scholar]
- 4.Karsenty, G. (1999) Genes Dev. 13, 3037-3051. [DOI] [PubMed] [Google Scholar]
- 5.Bradbury, A., Possenti, R., Shooter, E. M. & Tirone, F. (1991) Proc. Natl. Acad. Sci. USA 88, 3353-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher, B. S., Lim, R. W., Varnum, B. C., Kujubu, D. A., Koski, R. A. & Herschman, H. R. (1991) J. Biol. Chem. 266, 14511-14518. [PubMed] [Google Scholar]
- 7.Rouault, J. P., Rimokh, R., Tessa, C., Paranhos, G., Ffrench, M., Duret, L., Garoccio, M., Germain, D., Samarut, J. & Magaud, J. P. (1992) EMBO J. 11, 1663-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuda, S., Kawamura-Tsuzuku, J., Ohsugi, M., Yoshida, M., Emi, M., Nakamura, Y., Onda, M., Yoshida, Y., Nishiyama, A. & Yamamoto, T. (1996) Oncogene 12, 705-713. [PubMed] [Google Scholar]
- 9.Rouault, J. P., Falette, N., Guehenneux, F., Guillot, C., Rimokh, R., Wang, Q., Berthet, C., Moyret-Lalle, C., Savatier, P., Pain, B., et al. (1996) Nat. Genet. 14, 482-486. [DOI] [PubMed] [Google Scholar]
- 10.Guehenneux, F., Duret, L., Callanan, M. B., Bouhas, R., Hayette, S., Berthet, C., Samarut, C., Rimokh, R., Birot, A. M., Wang, Q., et al. (1997) Leukemia 11, 370-375. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida, Y., Matsuda, S., Ikematsu, N., Kawamura-Tsuzuku, J., Inazawa, J., Umemori, H. & Yamamoto, T. (1998) Oncogene 16, 2687-2693. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida, Y., Matsuda, S. & Yamamoto, T. (1997) Gene 191, 109-113. [DOI] [PubMed] [Google Scholar]
- 13.Ikematsu, N., Yoshida, Y., Kawamura-Tsuzuku, J., Ohsugi, M., Onda, M., Hirai, M., Fujimoto, J. & Yamamoto, T. (1999) Oncogene 18, 7432-7441. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida, Y., Tanaka, S., Umemori, H., Minowa, O., Usui, M., Ikematsu, N., Hosoda, E., Imamura, T., Kuno, J., Yamashita, T., et al. (2000) Cell 103, 1085-1097. [DOI] [PubMed] [Google Scholar]
- 15.Tzachanis, D., Freeman, G. J., Hirano, N., van Puijenbroek, A. A., Delfs, M. W., Berezovskaya, A., Nadler, L. M. & Boussiotis, V. A. (2001) Nat. Immunol. 2, 1174-1182. [DOI] [PubMed] [Google Scholar]
- 16.Roggia, C., Gao, Y., Cenci, S., Weitzmann, M. N., Toraldo, G., Isaia, G. & Pacifici, R. (2001) Proc. Natl. Acad. Sci. USA 20, 13960-13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weitzmann, M. N., Roggia, C., Toraldo, G., Weitzmann, L. & Pacifici, R. (2002) J. Clin. Invest. 110, 1643-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parfitt, A. M., Drezner, M. K., Glorieux, F. H., Kanis, J. A., Malluche, H., Meunier, P. J., Ott, S. M. & Recker, R. R. (1987) J. Bone Miner. Res. 2, 595-610. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto, T., Saatcioglu, F. & Matsuda, T. (2002) Endocrinology 143, 2635-2642. [DOI] [PubMed] [Google Scholar]
- 20.Paez-Pereda, M., Giacomini, D., Refojo, D., Nagashima, A. C., Hopfner, U., Grubler, Y., Chervin, A., Goldberg, V., Goya, R., Hentges, S. T., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 1034-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endoh, H., Sasaki, H., Maruyama, K., Takeyama, K., Waga, I., Shimizu, T., Kato, S. & Kawashima, H. (1997) Biochem. Biophys. Res. Commun. 235, 99-102. [DOI] [PubMed] [Google Scholar]
- 22.Maekawa, M., Nishida, E. & Tanoue, T. (2002) J. Biol. Chem. 277, 37783-37787. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki, T., K-Tsuzuku, J., Ajima, R., Nakamura, T., Yoshida, Y. & Yamamoto, T. (2002) Genes Dev. 16, 1356-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.