Abstract
Environmental toxicants such as polychlorinated biphenyls (PCBs) have been implicated in the promotion of multiple inflammatory disorders including cardiovascular disease, but information regarding mechanisms of toxicity and cross-talk between relevant cell signaling pathways is lacking. To examine the hypothesis that cross-talk between membrane domains called caveolae and nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathways alter PCB-induced inflammation, caveolin-1 was silenced in vascular endothelial cells, resulting in a decreased PCB-induced inflammatory response. Cav-1 silencing (siRNA treatment) also increased levels of Nrf2-ARE transcriptional binding, resulting in higher mRNA levels of the antioxidant genes glutathione s-transferase and NADPH dehydrogenase quinone-1 in both vehicle and PCB-treated systems. Along with this upregulated antioxidant response, Cav-1 siRNA treated cells exhibited decreased mRNA levels of the Nrf2 inhibitory protein Keap1 in both vehicle and PCB-treated samples. Silencing Cav-1 also decreased protein levels of Nrf2 inhibitory proteins Keap1 and Fyn kinase, especially in PCB-treated cells. Further, endothelial cells from wildtype and Cav-1−/− mice were isolated and treated with PCB to better elucidate the role of functional caveolae in PCB-induced endothelial inflammation. Cav-1−/− endothelial cells were protected from PCB-induced cellular dysfunction as evidenced by decreased vascular cell adhesion molecule (VCAM-1) protein induction. Compared to wildtype cells, Cav-1−/− endothelial cells also allowed for a more effective antioxidant response, as observed by higher levels of the antioxidant genes. These data demonstrate novel cross-talk mechanisms between Cav-1 and Nrf2 and implicate the reduction of Cav-1 as a protective mechanism for PCB-induced cellular dysfunction and inflammation.
Keywords: caveolin-1, Nrf2, antioxidant response, polychlorinated biphenyl, oxidative stress, endothelial cell dysfunction
Introduction
Exposure to persistent environmental pollutants such as polychlorinated biphenyls has been linked to the induction and/or exacerbation of multiple human pathologies including diabetes and atherosclerosis (Gustavsson and Hogstedt, 1997; Goncharov et al., 2008; Goncharov et al., 2010; Carpenter, 2011; Uemura, 2012). Specifically, coplanar polychlorinated biphenyls (PCBs) have been shown to initiate the earliest stages of atherosclerotic plaque formation, e.g., endothelial cell dysfunction and inflammation (Lim et al., 2008; Majkova et al., 2009; Majkova et al., 2010).
Once utilized extensively for their productive thermal and electrical capabilities, the production of PCBs and PCB mixtures, such as aroclors, was banned in the 1970s due to further data highlighting their substantive public health concerns (ATSDR, 2000; Faroon et al., 2003). However, PCBs continue to impact ecosystems and human health due to their environmental prevalence and persistence (chemical stability) (Petriello et al., 2013). Additionally, the lipophilic nature of coplanar PCBs allows for their interaction with lipid membranes and lipid storage depots, leading to bioaccumulation and biomagnification through the food chain. Even today, PCB-containing products continue to be used in developing nations, further exposing humans to high occupational levels of these harmful pollutants (Schettgen et al., 2012). In countries such as the United States, the primary sources of exposure stem from air pollution and contaminated food (ATSDR, 2000). Although multiple categories of PCBs have been developed, dioxin-like, non-ortho-substituted coplanar PCBs such as PCB 77 and PCB 126 exhibit the highest levels of in vitro and in vivo toxicity and pro-inflammatory properties, especially in the vasculature (Giesy et al., 2000).
Once believed to be an innate barrier, endothelial cells now appear to play an extremely important role in the initiation and progression of atherosclerosis (Libby, 2001; Libby, 2002; Libby, 2012). Coplanar PCBs can further promote endothelial cell inflammation and dysfunction through caveolae lipid micro-domains (Layne et al., 2011; Petriello et al., 2013). Endothelial cell activation can lead to an upregulation of adhesion molecules such as Vascular Cell Adhesion Molecule-1 (VCAM-1) which promotes pro-inflammatory leukocyte infiltration and chemokine production that left unchecked can lead to the formation of foam cells and subsequent arterial blockage (Croce and Libby, 2007). Coplanar PCBs can induce oxidative stress in endothelial cells and in turn cause the upregulation of pro-inflammatory proteins through an NFκB-mediated signaling cascade (Lim et al., 2008). Interestingly, it has been shown that PCBs may also be pro-atherogenic by activating other pro-inflammatory pathways such as the lipid signaling domain caveolae (Majkova et al., 2010).
Lipid raft microdomains known as caveolae are flask-shaped invaginations found at the lipid membrane and have been shown to play important roles in endocytosis, atherosclerosis, and environmental pollutant toxicity (Majkova et al., 2009; Pavlides et al., 2012). Caveolin-1 (Cav-1), the major structural and signaling protein involved in the caveolae pathway, has been shown through its “Cav-1 binding domain” (CBD) to interact and bind multiple other proteins, many of which are involved in inflammation and atherosclerosis (Frank et al., 2004; Majkova et al., 2010; Layne et al., 2011; Panneerselvam et al., 2012; Pavlides et al., 2012; Sowa, 2012). Coplanar PCBs preferentially sequester in caveolae cellular fractions, and exposure to coplanar PCBs upregulates Cav-1 protein expression and caveolae formation (Lim et al., 2008). Silencing Cav-1 via siRNA technology prevents PCB-induced cytochrome P450-mediated superoxide production and subsequent endothelial activation and dysfunction (Lim et al., 2008). Importantly, it has been shown that aortic endothelial cells isolated from mice that lack the Cav-1 gene are protected from toxicant-induced cellular dysfunction, but the mechanism of this protection has yet to be elucidated (Han et al., 2010).
Physiological systems have evolved multiple signaling pathways to limit the toxicity of xenobiotics such as PCBs. The most significant regulator of redox status and homeostasis, the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) antioxidant pathway, has been shown to be critical in protecting endothelial cells from PCB toxicity (Han et al., 2012). Nrf2 is primarily regulated by its major inhibitory protein, iNrf2 or Keap1, which promotes Nrf2’s ubiquitination and maintains basal levels of Nrf2 activation. Nrf2 also can be inhibited by Fyn kinase, which can promote Nrf2 nuclear exit and degradation (Niture et al., 2013). Nrf2 also can be directly activated via phosphorylation by kinases such as Akt and PKC delta (Wang et al., 2008a; Niture et al., 2013). Nrf2 can become activated by xenobiotic electrophiles, reactive oxygen species (ROS), and bioactive phytochemicals found in healthful nutrition (Kim et al., 2010; Baird and Dinkova-Kostova, 2011; Higgins and Hayes, 2011). Multiple electrophilic bioactive nutrients including components of ginseng, green tea and vegetables such as broccoli have been shown to activate Nrf2, but interestingly, different nutrients may induce Nrf2 through differing mechanisms (e.g., disruption of Keap1/Nrf2 interaction or increased phosphorylation via relevant kinases (Newsome et al.; Park et al., 2010; Han et al., 2012; Miao et al., 2012). Nrf2 has been shown to be regulated through the cross-talk of multiple signaling cascade pathways such as the aryl hydrocarbon receptor (AhR) and NFκB pathways (Hayes et al., 2009; Yeager et al., 2009; Wakabayashi et al., 2010). Many xenobiotics such as dioxins and coplanar PCBs can activate both AhR and Nrf2 simultaneously, and in fact, this concordant upregulation can be evolutionarily explained since the gene promoter for AhR contains multiple Nrf2 binding elements (AREs) and the promoter for Nrf2 contains AhR binding sites (xenobiotic response elements) (Hayes et al., 2009). Evidence for direct cross-talk between Nrf2 and NFκB is not as well understood, but multiple studies have shown that activation of Nrf2 leads to a diminished pro-inflammatory NFκB response (Bellezza et al., 2012; Yang et al., 2013). Interestingly, we have previously shown that the AhR is a binding partner of Cav-1 (Lim et al., 2008) which has led us to hypothesize that novel cross-talk between Cav-1 and Nrf2 could exist and that this cross-talk may help to explain mechanistically the protection from coplanar PCBs observed in Cav-1 −/− animals.
Thus, the current study has been designed to investigate mechanistically how the cross-talk between Cav-1 and Nrf2 can modulate PCB-induced cellular dysfunction. Our data provide strong evidence that there are multiple levels of Cav-1/Nrf2 cross-talk and that Cav-1 inhibits the Nrf2 antioxidant response. Thus, reduction or downregulation of endothelial Cav-1 may lead to an upregulated antioxidant response regulated by Nrf2, which could better prime a physiological system prior to toxicological insult.
2. Materials and Methods
Materials and Chemicals
3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) was obtained from AccuStandard Inc. (New Haven, CT). VCAM-1 and Keap1 primary antibodies and horseradish peroxidase-conjugated goat secondary antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). β-actin and GST primary antibodies were purchased from Sigma (St. Louis, MO). The GST antibody used recognized native as well as denatured-reduced forms of GST protein. NQO1 primary antibody was purchased from Abcam (Cambridge, MA). Fyn kinase, P-Akt, Akt, P-PKC delta and Nrf2 primary antibodies and horseradish peroxidase-conjugated rabbit secondary antibody were purchased from Cell Signaling Technologies (Danvers, MA).
Cell culture and experimental media
Primary vascular endothelial cells were isolated from porcine pulmonary arteries (Han et al., 2010). Cells were cultured in M199 (Gibco, Grand Island, NY), supplemented with fetal bovine serum (FBS; Gibco). EAhy.926 human endothelial cells were cultured as described previously (Lim et al., 2007). Endothelial cells were grown to confluence, followed by incubation overnight in medium containing 1% FBS prior to cell treatment. Stock solutions of PCB 126 were prepared in DMSO; control cultures were treated with DMSO vehicle. The levels of DMSO in experimental media were 0.05%. Porcine and human endothelial cells were treated with PCB 126 at 0.25 µM for 16 h and mouse pulmonary endothelial cells were treated with 2.5 µM for 24 h, which are established concentrations used previously in our laboratory (Han et al., 2010; Han et al., 2012). Porcine primary endothelial cells, Eahy.926 human endothelial cells, and isolated mouse endothelial cells contained Cav-1 protein levels (wildtype) and exhibited VCAM-1 induction due to PCB 126 treatment, as previously reported (Lim et al., 2007; Lim et al., 2008; Han et al., 2012). Endothelial cells isolated from wildtype and Cav-1 −/− mice were identified by morphology, platelet endothelial cell adhesion molecule-1 (PECAM1) bead pull-down, and Von Willebrand Factor (VWF) protein expression.
Mouse endothelial cell isolation
Endothelial cells were isolated from Cav-1 deficient (Cav-1−/−) and wildtype mice (both genotypes were purchased from Jackson Laboratory, Bar Harbor, ME). All animals were housed in Association for Assessment and Accreditation of Laboratory Animal Care-certified animal facilities at the University of Kentucky. Age matched C57BL/6 mice were used as controls because Cav-1-deficient mice are backcrossed onto C57BL/6 mice. Endothelial cells were isolated and cultured as described previously (Salous et al., 2013). Briefly, whole lungs were homogenized in culture media containing type II collagenase and dispase. Cells were added to gelatin-coated tissue culture plates in DMEM media containing 20% FBS, heparin, antibiotics, and endothelial cell growth supplement. Endothelial cells were preferentially selected by using an antibody-coated magnetic bead mix (Invitrogen, Carlsbad, CA. Briefly, sheep anti-rat IgG beads were prepared and mixed with rat anti-mouse CD31 PECAM antibodies overnight (BD Biosciences, San Jose, CA). Bead/antibody conjugates were collected via magnetic separation and subsequently were incubated for 1 h with cells isolated from lungs. Cells were then trypsinized, transferred to a magnetic separator, and magnetically bound cells were seeded in 35mm plates. Endothelial cell isolation was confirmed by cobblestone morphology and the presence of VWF endothelial cell marker.
Real-time PCR
The levels of mRNA expression were assessed by real-time PCR using a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA) and SYBR Green master mix (Applied Biosystems) as described earlier (Han et al., 2010). Sequences were designed using the Primer Express Software 3.0 for real-time PCR (Applied Biosystems) and synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). Sequences for porcine VCAM-1 and β-actin were described in earlier articles published from our laboratory (Ramadass et al., 2003; Majkova et al., 2009). Porcine NQO1 sequences were: sense, 5′-CCCGGGAACTTTCAGTATCCT-3′; and antisense, 5′-CTGCGGCTTCCACCTTCTT-3′; porcine GST-omega 1 sequences were: sense, 5′-GCTGAGCCAAGTGGGAGACA-3′; and antisense, 5′CCTCGGCCATTGAAATAGTGA-3′; Porcine Keap1 sequences were: sense, 5’ AGCTGGGATGCCTCAGTGTT-3’; and antisense, 5’-AGGCAAGTTCTCCCAGACATTC-3’; porcine GPx1 sequences were: sense, 5′-TGCCTTCAATCCGGAGGTAA-3′; and antisense, 5′GAATGGGATGACCTGGAAGTTAGT-3′; porcine MRP1 sequences were: sense, 5′-GTCCTGTTTGCTGCCTGTT-3′; and antisense, 5′AGTATGCGGTGATCTGCAGTGA-3′;
Cav-1 siRNA and transfection studies
Double stranded small interfering RNA targeting Cav-1 was synthesized as described previously (Repetto et al., 2005; Lim et al., 2007; Zheng et al., 2012). Porcine endothelial cells and human endothelial cells were transfected with control or Cav-1 siRNA at a final concentration of 80 nM using GeneSilencer transfection reagent (Genlantis, SanDiego, CA) in OptiMEM serum free media. Cells were incubated with the transfection mixture for 4 h, followed by the addition of 10% FBS to the cell media. Cells were used for treatments after 48 h incubation.
Immunoblotting
Western blot analyses for VCAM-1, β-actin, NQO1, Keap1, Fyn kinase, GST, Nrf2, P-PKC delta and P-Akt were performed as described previously (Han et al., 2010). Briefly, cells were lysed in RIPA buffer, centrifuged and the protein concentrations were determined via Bradford Assay. Proteins were separated via 10% SDS-PAGE, transferred to nitrocellulose membranes, and blocked in 5% non-fat milk or BSA. Primary antibodies were added at a concentration of 1:1000 overnight and appropriate secondary antibodies subsequently were added for 2 h at a concentration of 1:4000.
Preparation of nuclear extracts and electrophoretic mobility shift assay (EMSA)
Nuclear extracts of endothelial cells were prepared using NE-PER nuclear extraction reagents (Thermo, Rockford, IL) according to the manufacturer’s protocol. Nuclear extract concentrations were determined using Bradford reagent (Bio-Rad, Richmond, CA). DNA binding activities of Nrf2 were determined using a LightShift Chemiluminescent EMSA kit (Pierce, Rockford, IL) according to the manufacturer’s protocol. DNA-binding reactions were performed with a final volume of 20 µL buffer containing 5 µg of nuclear extract, 50 ng/µL Poly (dI•dC), and biotin end-labeled oligonucleotides. Synthetic 5’-biotinylated complementary oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA). Oligonucleotides containing the antioxidant response element (ARE) sequence from the porcine NQO1 promoter (5’-TAGTCACAGTGACTCAGCGAGATTC-3’) were used as described previously (Zheng et al., 2012).
Statistical analysis
Data were analyzed using SigmaStat software (Systat Software, Point Richmond, CA). Comparisons between treatments were made by two-way ANOVA with post-hoc comparisons of the means. To elucidate trends within groups, multiple comparison procedures were completed for tests that displayed overall significance (p≤0.05–0.1). Groups were considered significantly different with a determined p value of p<0.05.
3. Results
Cav-1 silencing prevents PCB-induced endothelial cell dysfunction by increasing antioxidant gene expression
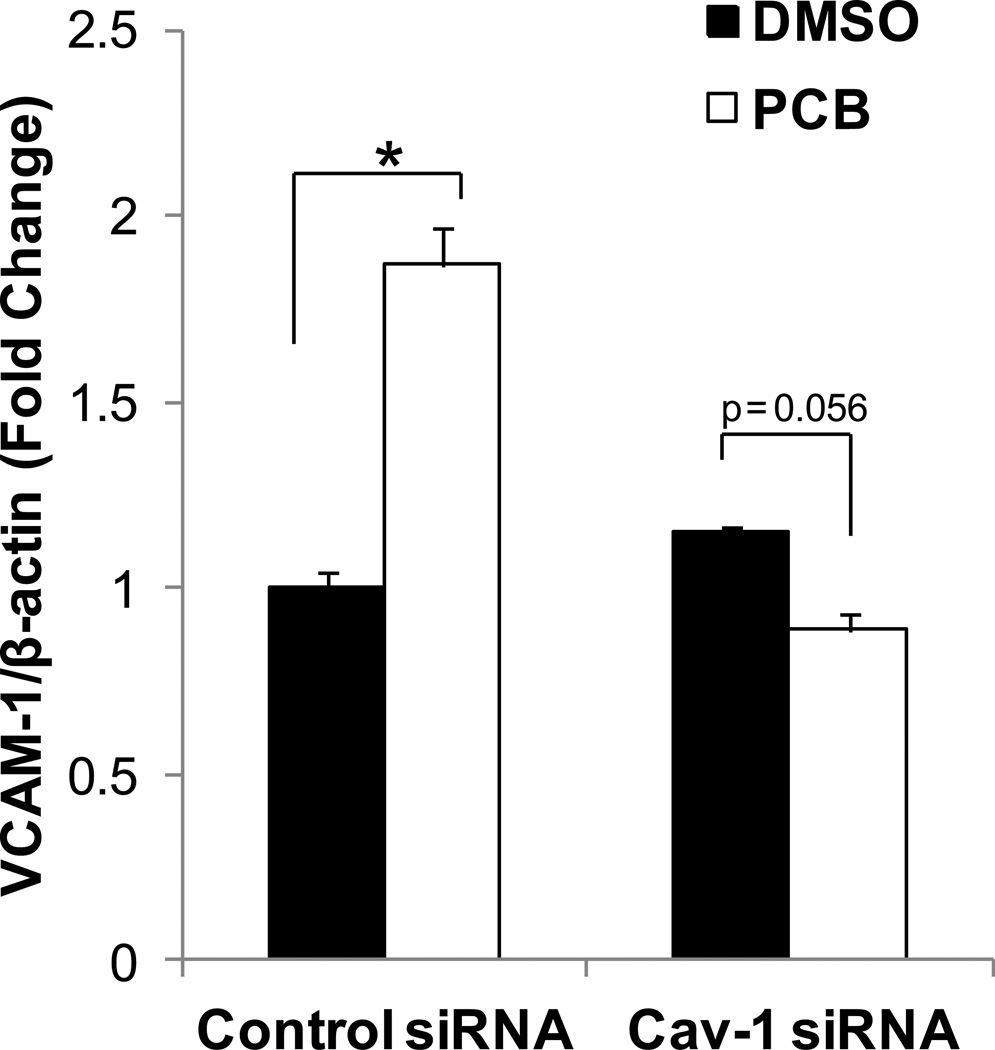
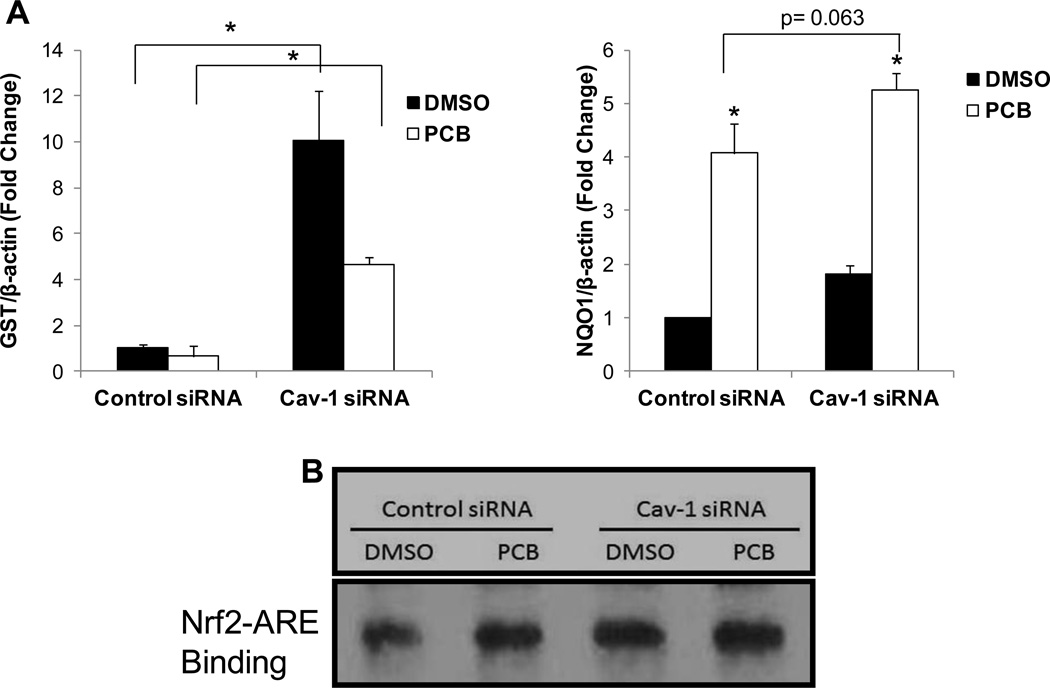
Previously, we have shown that coplanar PCBs can initiate cellular dysfunction, as evidenced by an increase in VCAM-1 protein levels, in endothelial cells that contain functional caveolae (Lim et al., 2008; Han et al., 2010). Here, we treated primary porcine vascular endothelial cells with a single low dose (0.25 µM) of PCB 126 for 16 h and saw a statistically significant upregulation of VCAM-1 mRNA expression in control siRNA treated cells (Fig. 1). However, this upregulation was not observed in cells treated with Cav-1 siRNA. We have previously shown that the antioxidant controller Nrf2 is critical for cellular defense against PCB toxicity, thus we hypothesized that Cav-1 silenced cells were protected through a Cav-1/Nrf2 cross-talk mechanism. Cav-1 siRNA treated cells displayed increased Nrf2-antioxidant response element (ARE) transcriptional binding (EMSA) which was most evident when comparing between baseline levels of vehicle DMSO treated groups (Fig. 2B). We then investigated, via real time PCR (RT-PCR), mRNA expression levels of two major Nrf2 target genes GST and NQO1 and saw statistically significant increases of expression for both genes in cells treated with Cav-1 siRNA compared to control scrambled siRNA (Fig. 2A). As with the EMSA results, the most drastic and significant differences between Cav-1 and control siRNA treated cells were seen in DMSO vehicle groups. Basal levels of GST expression were approximately 10 fold higher in Cav-1 siRNA treated cells but only approximately 5 fold higher in Cav-1 siRNA treated cells after exposure to PCB. NQO1 levels were also significantly higher in cells silenced for Cav-1 but to much less of a degree (Fig. 2A). Additionally, NQO1 basal protein levels were significantly increased in Cav-1 transfected cells after 48 h compared to control transfected cells (Supplemental. Fig. 1A). Finally, basal mRNA levels of two additional Nrf2 targets, glutathione peroxidase-1 (GPX1) and multi-drug resistance protein-1 (MRP1) were compared between Cav-1 and control transfected cells, and it was determined via RT-PCR that even after just 24 h of Cav-1 transfection, expression levels of both of these cytoprotective genes were doubled in cells silenced for Cav-1 (Supplemental Fig. 1B).
Fig. 1.
Expression of VCAM-1 mRNA in porcine vascular endothelial cells exposed to coplanar PCB 126. Cells were transfected with either scrambled control or Cav-1 targeted siRNA for 48 h and subsequently exposed to PCB 126 at a concentration of 0.25 µM for 16 h. mRNA levels were measured using real-time PCR. Results were normalized to β-actin and are depicted as fold change compared to control siRNA DMSO treatments. Results represent the mean± SEM (n=2 for each treatment group). PCB 126 increases VCAM-1 mRNA levels only in endothelial cells with Cav-1 (*p<0.05).
Fig. 2.
Expression of antioxidant enzymes and Nrf2 transcriptional binding in cells silenced for Cav-1. Porcine endothelial cells were transfected with either scrambled control or Cav-1 targeted siRNA for 48 h and subsequently exposed to PCB 126 at a concentration of 0.25 µM for 16 h. mRNA levels of GST and NQO1 were measured using real-time PCR. Results were normalized to β-actin and graphed as fold change compared to control siRNA DMSO treatments. Results represent the mean± SEM (n=2 for each treatment group). (A) Cells silenced for Cav-1 display increased mRNA levels of GST in both vehicle and PCB treated groups compared to control siRNA transfected cells (*p<0.05). GST levels were diminished in Cav-1 siRNA transfected cells when exposed to PCB, but these levels were still statistically significantly higher than control siRNA transfected cells exposed to PCB (*p<0.05). Also, PCB treatment increased the expression of NQO1 in both Cav-1 siRNA and control siRNA groups (*p<0.05), and there was a significant trend of increased expression in cells silenced for Cav-1 (p=0.063). (B) Cells silenced for Cav-1 display increased levels of Nrf2 – ARE binding as determined by EMSA compared with control siRNA transfected cells.
Decreased Cav-1 expression promotes Nrf2 activity by decreasing levels of multiple Nrf2 inhibitory proteins
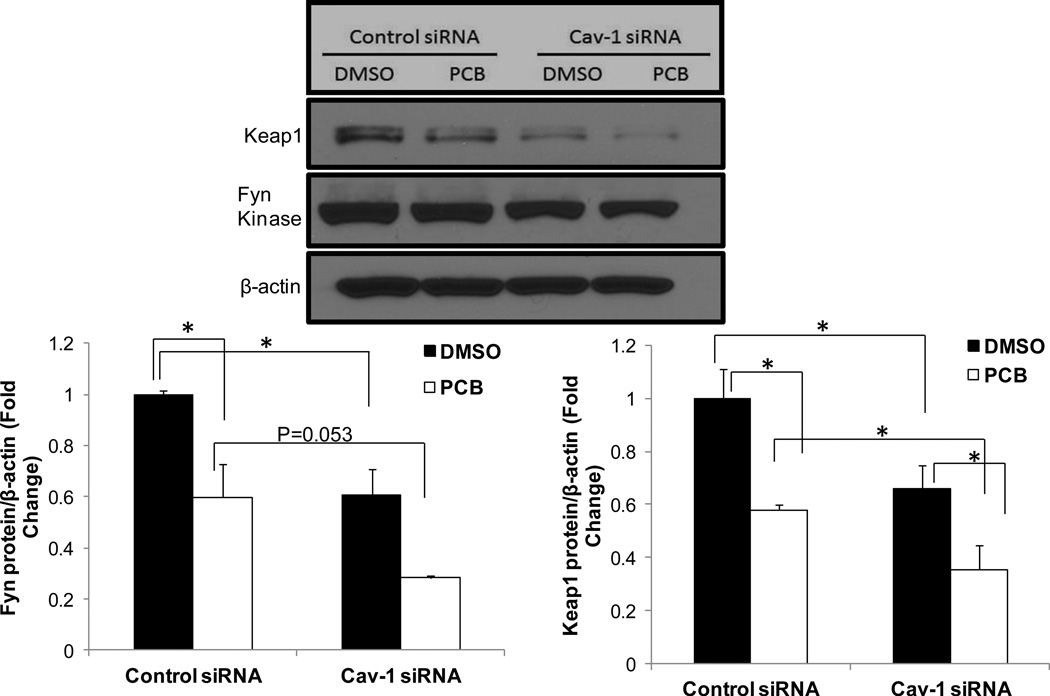
Nrf2 is inhibited by multiple factors including Keap1 and Fyn kinase; therefore we investigated levels of these proteins in an attempt to elucidate further novel mechanisms of Cav-1/Nrf2 cross-talk (Fig. 3). To explain the upregulated Nrf2 activity in Cav-1 depleted cells, we hypothesized that levels of these inhibitory proteins necessarily would be diminished early on during PCB exposure. Thus, we treated human endothelial cells (HUVEC fusion) with control or Cav-1 targeted siRNAs and subsequently exposed the HUVEC-based cell line to 0.25 µM PCB 126 for a minimal time duration (4 h). As expected, PCB exposure caused a decrease in protein expression of the Nrf2 inhibitory proteins Keap1 and Fyn kinase in both Cav-1 and control siRNA treated cells. More importantly, at basal DMSO conditions, cells silenced for Cav-1 also displayed significantly lower levels of Keap1 and Fyn compared to DMSO-treated control siRNA cells. Exposing PCB to these Cav-1 silenced cells resulted in a statistically significant decrease in Fyn and Keap1 compared to Cav-1 silenced cells treated with DMSO. Although antibodies for Fyn and Keap1 used by our laboratory did not work considerably well in our mouse and porcine models, we did examine, via RT-PCR, Keap1 mRNA levels in Cav-1 silenced porcine cells and observed similarly significant trends as with human endothelial cell protein data (Supplemental Fig. 2). Finally, whole livers were harvested from wildtype and Cav-1 −/− mice, and via RT-PCR it was determined that Cav-1 −/− mice expressed approximately 75% mRNA expression levels of Keap1 in the liver compared to age matched wildtype controls (n=4 for each genotype, data not shown). This observed decrease in Keap1 in whole livers of Cav-1 −/− mice did not quite reach the diminished levels observed in endothelial cells from porcine or human silenced for Cav-1(compare to Fig. 3. or Supp. Fig. 1., but this may be due to genetic variability in the mice or multiple cell types found in whole liver lysates.
Fig. 3.
Expression of Nrf2 inhibitory proteins is decreased in cells silenced for Cav-1. Human endothelial cells were transfected with either scrambled control or Cav-1 targeted siRNA for 48 h and subsequently exposed to PCB 126 at a concentration of 0.25 µM for 4 h. Protein levels of Keap1 and Fyn kinase were measured via western blot. Results were normalized to β-actin and graphed as fold change compared to control siRNA DMSO treatments. Results represent the mean±SEM (Fyn: n=2, Keap1: n=3 for each treatment group). Cells silenced for Cav-1 display decreased protein levels of Keap1 and Fyn in both vehicle and PCB treated groups compared to control siRNA transfected cells (*p<0.05). PCB treatment also significantly decreased Fyn and Keap1 levels within transfection groups (*p<0.05). Western blot above shows representative sample of visual results.
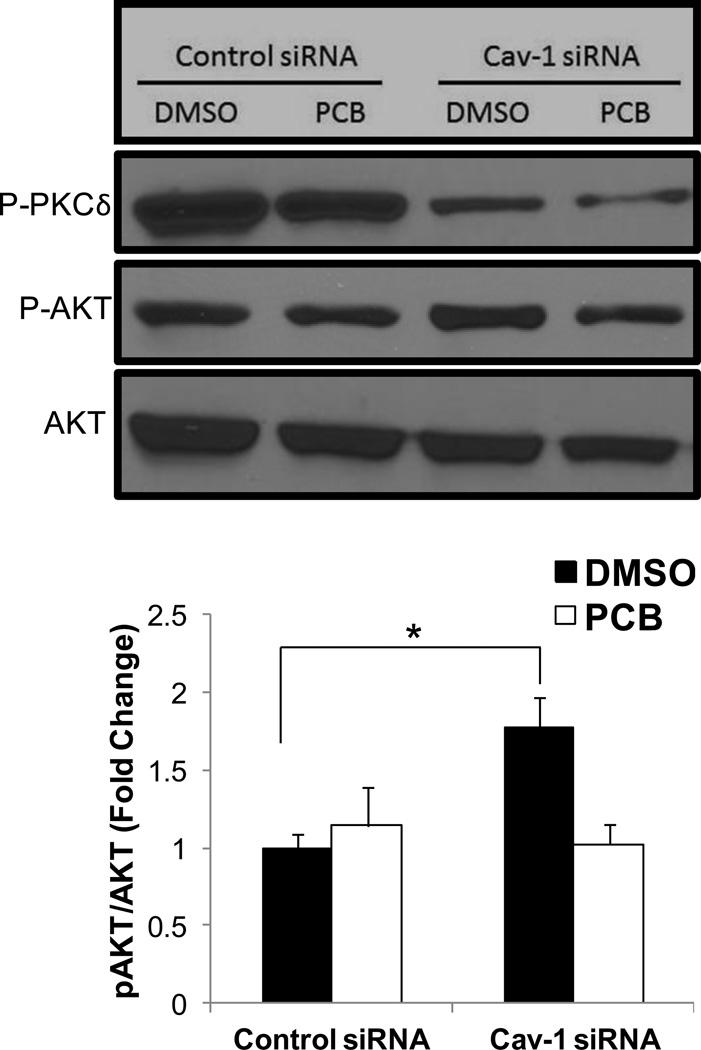
Decreased Cav-1 expression promotes the phosphorylation of Akt but not phosphorylation of PKC delta
Alternative mechanisms of Nrf2 activation have been investigated including direct phosphorylation of Nrf2 by PKC delta and Akt. To investigate the ability of Cav-1 silencing to alter either of these Nrf2 effectors, human endothelial cells were transfected with either scrambled or Cav-1 targeting siRNAs and subsequently treated with 0.25 µM PCB 126 for 4 h. Interestingly, Cav-1 silencing decreased levels of phosphorylated PKC delta in both DMSO and PCB treatment groups, although significantly increased levels of phosphorylated Akt were seen in baseline vehicle treatment groups but not when treated with PCB (Fig. 4).
Fig. 4.
Expression of phosphorylated Akt and PKC delta proteins in human endothelial cells exposed to coplanar PCB 126. Cells were transfected with either scrambled control or Cav-1 targeted siRNA for 48 h and subsequently exposed to PCB 126 at a concentration of 0.25 µM for 4 h. Protein levels of P-Akt and P-PKC delta were measured via Western blot. Results were normalized to β-actin and depicted as fold change compared to control siRNA DMSO treatments. Results represent the mean±SEM (n=3 for each treatment group). Cells silenced for Cav-1 display decreased protein levels of P-PKC delta in both vehicle and PCB treated groups. Cells targeted with Cav-1 siRNA and treated with DMSO vehicle had significantly increased levels of P-Akt compared to control transfected cells also treated with DMSO (*p<0.05). Western blot above shows representative sample of visual results.
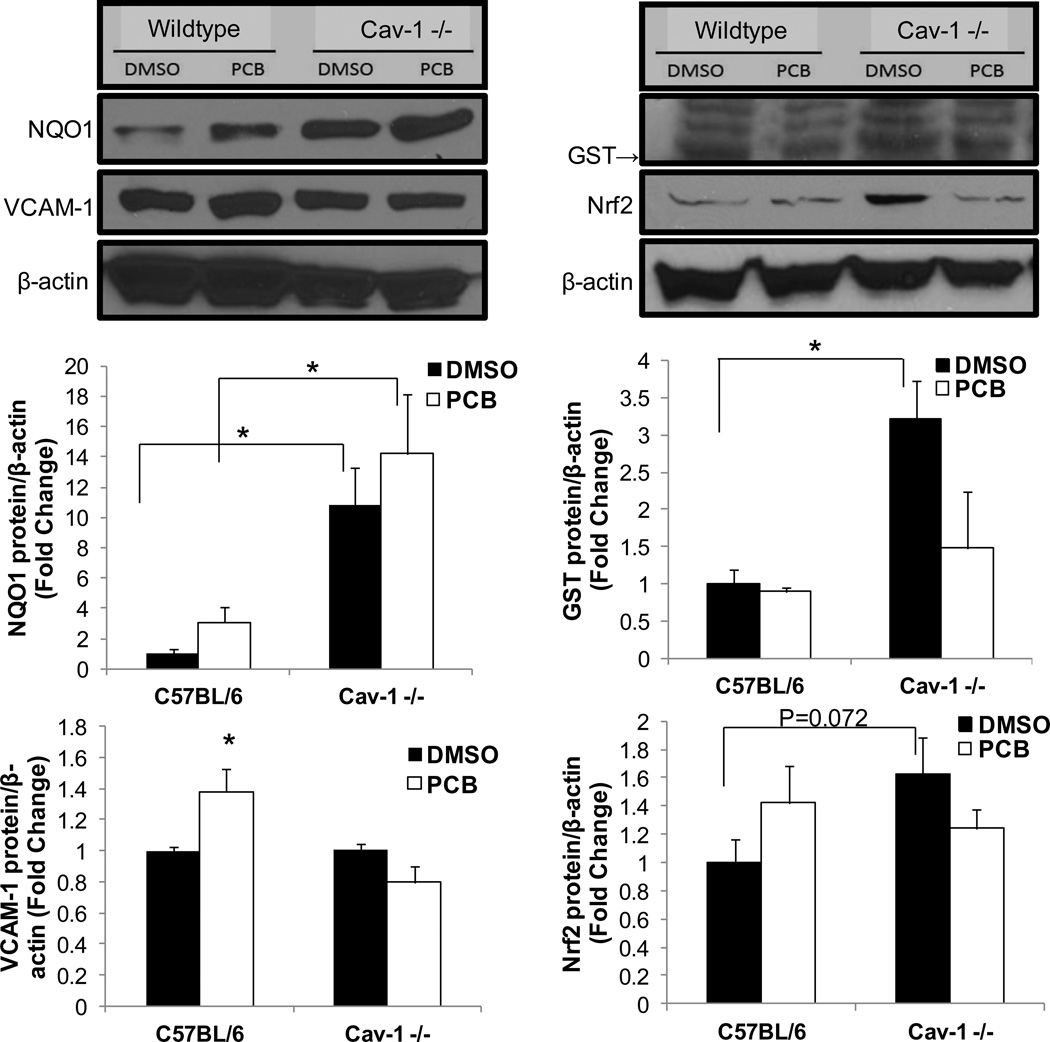
Cells isolated from Cav-1 −/− mice are protected from PCB-induced inflammation via upregulated Nrf2 activity
Lung endothelial cells were next isolated from Cav-1 deficient and wildtype mice of the same background and subsequently exposed to coplanar PCB 126 to confirm the importance of Cav-1/Nrf2 cross-talk in PCB-induced toxicity. VCAM-1 protein levels were measured via Western blot and quantified via densitometry. As mirrored in the siRNA experiments utilizing porcine cells, PCB 126 only increased cellular dysfunction (VCAM-1 induction) in cells isolated from C57BL/6 mice and not Cav-1 −/− mice (Fig. 5). We attribute this protection in Cav-1 −/− mice to the considerably higher protein levels of antioxidant enzymes such as NQO1 and GST. Cells isolated from Cav-1 −/− mice displayed approximately 10 fold and 3 fold increases in NQO1 and GST, respectively, in vehicle treated groups when compared to cells isolated from C57Bl/6 control mice (Fig. 5). Treating mouse endothelial cells with PCB resulted in the same trends observed in the previous porcine siRNA experiments (e.g. decreased levels of GST and higher levels of NQO1 compared to DMSO vehicle). Importantly, although an overall interactive effect was not seen, we did see a trend toward significance (P=0.072) for increased Nrf2 protein levels in cells isolated from Cav-1 −/− mice when comparing baseline DMSO groups between mouse genotypes (Fig. 5). Combining this finding with the EMSA results displayed in Fig. 2B, together, help to show that a decrease in cellular Cav-1 protein promotes upregulated Nrf2 activity that is evident in multiple species.
Fig. 5.
Confirmation of Cav-1 Nrf2 cross-talk in cells isolated from Cav-1 −/− mice. Endothelial cells from Cav-1 −/− and age matched wildtype control mice were isolated and subsequently exposed to PCB 126 at a concentration of 2.5 µM for 24 h. Protein levels of NQO1, VCAM-1, GST and Nrf2 were measured via Western blot. Results were normalized to β-actin and depicted as fold change compared to control siRNA DMSO treatments. Results represent the mean±SEM (NQO1, GST, Nrf2: n=3, VCAM-1: n=2 for each treatment group). Cells isolated from Cav-1 −/− mice did not display PCB-induced VCAM-1 upregulation and showed significantly increased levels of NQO1 (*p<0.05). Cells isolated from Cav-1 −/− mice displayed significantly higher levels of GST under DMSO vehicle conditions compared to wildtype cells treated with DMSO (*p<0.05). Also, cells isolated from Cav-1 −/− mice displayed a trend toward being significantly increased compared to vehicle treated control cells. Western blots above show representative samples of visual results.
4. Discussion
There is a growing body of evidence that implicates human exposure to persistent organic pollutants (POPs) such as polychlorinated biphenyls with increased risk of inflammatory related diseases such as atherosclerosis. The endothelium is a major target of PCB toxicity due to, e.g., constant low dose release from adipose fat stores caused by dynamic equilibrium and/or weight loss. Understanding the multiple signaling pathways involved and their cross-talk is a critical step in designing preventative and therapeutic methods to counteract pollutant-induced endothelial cell dysfunction and inflammation.
The importance of caveolae, lipid rafts, and Cav-1 in inflammation and heart disease has become a source of much research, but until recently, cross-talk between caveolae-related proteins and other factors has not been elucidated. Now, evidence points to interactions between Cav-1, AhR, and Nrf2, all proteins involved in inflammation and toxicant-induced disease (Lim et al., 2008; Li et al., 2012; Volonte et al., 2013). This work shows that a low dose of coplanar PCB 126 is sufficient to induce a pro-inflammatory endothelial cell environment and that loss of Cav-1 is protective and results in increased Nrf2 activity via multiple novel cross-talk mechanisms.
Coplanar PCBs have been shown to induce early stages of atherosclerosis via altering the cellular redox balance. Treatment with coplanar PCBs such as PCB 77 or PCB 126 has been shown in endothelial cells to increase oxidative stress, and inflammatory markers such as MCP-1 expression, and monocyte adhesion, but protection is seen in cells and mice deficient for the Cav-1 gene (Lim et al., 2008; Majkova et al., 2009; Han et al., 2010). The pro-inflammatory effects of PCBs may be linked to their uptake via caveolae, and the work described here suggests that Cav-1 deficiency protects against PCB-induced cellular dysfunction by allowing for a more effective Nrf2-controlled antioxidant response. Previously it was believed that lack of Cav-1 protected these cells and animals from PCB toxicity through an impaired PCB uptake mechanism. Previous work in our laboratory further has shown that PCBs preferentially sequester in the caveolae fraction of endothelial cells and PCB treatment increases the formation of functional caveolae at the lipid membrane (Lim et al., 2008). Disruption of functional caveolae and/or downregulation of Cav-1 may protect against toxicant-induced cytotoxicity via multiple mechanisms including altering the uptake of contaminants, altering toxicity kinetics and, as described in this work, inducing an upregulated Nrf2 antioxidant response. However, specific uptake mechanisms of PCBs may vary in different cell types and among congener of PCBs which may implicate the importance of the upregulated Nrf2 response over other mechanisms of protection in certain cell types and cellular conditions (Bourez et al., 2012; Bourez et al., 2013) Loss of Cav-1 has been shown by our laboratory and others to cause an upregulated Nrf2 antioxidant response (Zheng et al., 2012), but until recently, mechanisms of this cross-talk had not been elucidated. Two groups have now shown that Nrf2 can be added to a growing list of proteins that can be inhibited by Cav-1 via direct binding within the cell (Li et al., 2012; Volonte et al., 2013). These studies, however, did not highlight other possible mechanisms of cross-talk such as decreased Keap1 or Fyn kinase levels in Cav-1 diminished cells. Here, we show that protein levels of Keap1 and Fyn are diminished in cells silenced for Cav-1, which may help to mechanistically explain the observed upregulated Nrf2 response. Keap1 has been indicated as the major inhibitor of Nrf2 by promoting ubiquitination and subsequent proteosomal degradation. Very little is known however about cellular regulation of Keap1 expression levels, especially during transcription. Studies have shown that Keap1 levels can be decreased by epigenetic mechanisms as well as endogenous microRNAs, but future work is required to clarify the mechanism connecting diminished Cav-1 levels with decreased Keap1 expression (Eades et al., 2011). It is important to mention that some groups have shown that loss of Cav-1 in certain cell types, especially in a cancer environment, promotes oxidative stress and can drastically alter tumor recurrence (Pavlides et al., 2010). In our endothelial cell models, though, we have not observed an increase in ROS levels with Cav-1 siRNA treatment and have only observed PCB-induced cellular dysfunction in mice and endothelial cells that have wildtype levels of Cav-1 (Lim et al., 2008). It will be important to create endothelial specific Cav-1 −/− mice in the future to better understand important connections between endothelial Cav-1, oxidative stress levels, Nrf2, and protection from xenobiotic-induced toxicity.
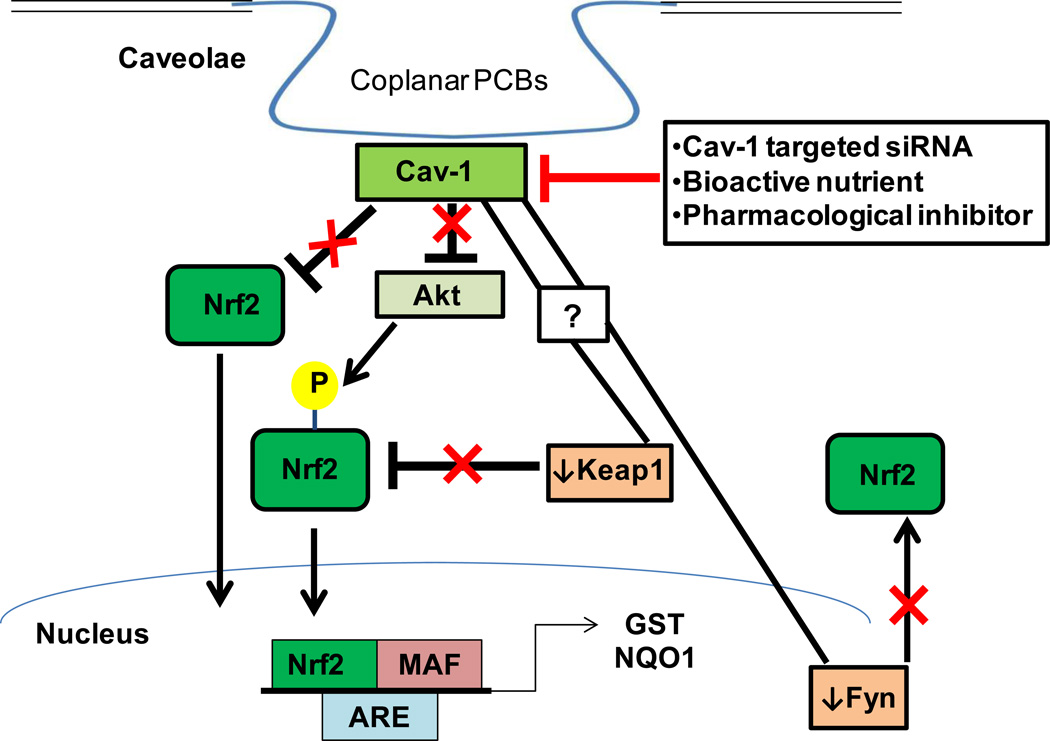
Recently, alternative mechanisms of Nrf2 activation have been described including direct phosphorylation by PKC delta and Akt. Articles have implicated Cav-1 as a direct inhibitor of multiple PKCs, and loss of Cav-1 has resulted in increased PKC auto-phosphorylation and activation (Oka et al., 1997). Interestingly, we did not observe this trend with the PKC isotype that has been shown to directly activate Nrf2 (Fig. 4). Some groups have shown, however, that activation of PKC isoform delta can be detrimental to the vasculature and can play an important role in the promotion of cardiovascular diseases, indicating that an increase in activated PKC delta may not be all positive (Das Evcimen and King, 2007). Activated Akt, however, was significantly increased in cells treated with Cav-1 siRNA. An interaction between Cav-1 and Akt has been implicated previously, but more work is necessary to determine if this observed increase in Akt can be correlated with Nrf2 activation (Feng et al., 2010). Future experiments should also investigate Cav-1’s role in other cellular Nrf2-related kinases such as phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), ER-localized pancreatic endoplasmic reticulum kinase (PERK), and protein kinase CK2 (Pi et al., 2007). Here, we add to a growing body of evidence that cross-talk between Cav-1 and Nrf2 exists and that multiple mechanisms of cross-talk may be evident (Fig. 6).
Fig. 6.
Exposure to coplanar PCBs is regulated through the cross-talk of Cav-1 and Nrf2. Coplanar PCBs preferentially sequester within caveolae fractions in endothelial cells and exposure to coplanar PCBs increases Cav-1 expression. Nrf2 can protect against PCB-induced toxicity, however, Cav-1 effectively inhibits the Nrf2 antioxidant response via multiple mechanisms: (1) Cav-1 directly inhibits Nrf2 through “Cav-1 binding domain” interaction. (2) Cav-1 inhibits Akt-directed phosphorylation of Nrf2. (3) Cav-1 regulates the levels of Nrf2 inhibitory proteins Keap1 and Fyn kinase. Downregulation of Cav-1 by targeted siRNA, bioactive nutrients such as polyphenols, and/or pharmacological inhibitors lead to an upregulated Nrf2 response via multiple mechanisms. (1) Decreased Nrf2 inhibition through “Cav-1 binding domain” interaction. (2) Increased Akt-directed phosphorylation of Nrf2. (3) Decreased levels of Nrf2 inhibitory proteins Keap1 and Fyn kinase. Downregulation of Cav-1 may result in a more profound Nrf2 antioxidant response which may better protect against PCB and related toxicological insults.
Downregulation of endothelial Cav-1 may prove to be an effective modulator of toxicant-induced disease as well as other pathologies hallmarked by chronic inflammation. We have shown previously that healthful nutrition, which is high in bioactive food components such as polyphenols and omega-3 polyunsaturated fatty acids, can protect against PCB-induced cellular dysfunction (Majkova et al., 2011; Han et al., 2012; Newsome et al., 2013). Laboratories have shown that these nutrients may protect through multiple mechanisms including disruption of functional caveolae, increased Nrf2 activity, and decreased NFκB activation (Ramadass et al., 2003; Watkins et al., 2007; Wang et al., 2008b; Han et al., 2012; Zheng et al., 2012; Newsome et al., 2013). It is plausible to hypothesize that the cross-talk between Cav-1 and Nrf2 pathways is critical for nutritional and/or pharmacological modulation of toxicant-induced disease. For example, statin treatment (simvastatin) can downregulate Cav-1 protein levels and activate endothelial nitric oxide synthase (eNOS) (Naoum et al., 2004). Also, eNOS upregulation via statins was not as profound in Cav-1 deficient human endothelial cells (Meda et al., 2010). To create more targeted and effective nutritional and pharmacological therapeutics, more work is needed to better elucidate the impact on the increasingly complex cross-talk between caveolae and Nrf2 (Fig. 6).
Our data suggest that cross-talk between Cav-1 and Nrf2 exists and may be more complex than described previously. Cav-1 inhibits Nrf2 activation, and decreasing Cav-1 levels increases the expression of Nrf2 target genes. This increased induction of protective genes may help to explain why cells and mice lacking Cav-1 are protected against pro-inflammatory polychlorinated biphenyl exposure. Our studies provide concordant findings from experiments using cellular models from three different mammalian species, i.e., pig, mouse, and human, suggesting some relevance of our findings to human health and risk assessments. Caveolae and Nrf2-related proteins may prove to be important targets for effective nutritional and/or pharmacological protection against the toxicity of pro-inflammatory xenobiotics.
Supplementary Material
Highlights.
Reduction of caveolin-1 protein protects against polychlorinated biphenyl toxicity
Decreasing caveolin-1 levels increases the Nrf2 antioxidant response
Reducing caveolin-1 levels decreases expression of Nrf2 inhibitory proteins
Caveolin-1/Nrf2 cross-talk is evident in mouse, human, and porcine endothelial cells
Acknowledgments
This research was supported by grants from NIEHS/NIH (P42ES007380), the University of Kentucky Agricultural Experiment Station, and American Heart Association. The authors would also like to thank the laboratories of Drs. Susan Smyth and Andrew Morris of the University of Kentucky for their assistance with mouse endothelial cell isolations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- ATSDR. Health DO, Services AH, editors. Polychlorinated Biphenyls (PCB) Toxicity Exposure Pathways. 2000 http://www.atsdr.cdc.gov/csem/csem.asp?csem=22&po=4, pp.
- Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Archives of toxicology. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- Bellezza I, Tucci A, Galli F, Grottelli S, Mierla AL, Pilolli F, Minelli A. Inhibition of NF-kappaB nuclear translocation via HO-1 activation underlies alpha-tocopheryl succinate toxicity. The Journal of nutritional biochemistry. 2012;23:1583–1591. doi: 10.1016/j.jnutbio.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Bourez S, Joly A, Covaci A, Remacle C, Larondelle Y, Schneider YJ, Debier C. Accumulation capacity of primary cultures of adipocytes for PCB-126: influence of cell differentiation stage and triglyceride levels. Toxicology letters. 2012;214:243–250. doi: 10.1016/j.toxlet.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Bourez S, Van den Daelen C, Le Lay S, Poupaert J, Larondelle Y, Thome JP, Schneider YJ, Dugail I, Debier C. The dynamics of accumulation of PCBs in cultured adipocytes vary with the cell lipid content and the lipophilicity of the congener. Toxicology letters. 2013;216:40–46. doi: 10.1016/j.toxlet.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Carpenter D. Exposure to Polychlorinated Biphenyls Is Associated With an Increased Risk of Hypertension and Cardiovascular Disease. Epidemiology. 2011;22:S147–S147. [Google Scholar]
- Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Current opinion in hematology. 2007;14:55–61. doi: 10.1097/00062752-200701000-00011. [DOI] [PubMed] [Google Scholar]
- Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacological research : the official journal of the Italian Pharmacological Society. 2007;55:498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Eades G, Yang M, Yao Y, Zhang Y, Zhou Q. miR-200a regulates Nrf2 activation by targeting Keap1 mRNA in breast cancer cells. The Journal of biological chemistry. 2011;286:40725–40733. doi: 10.1074/jbc.M111.275495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faroon O, Samuel Keith L, Smith-Simon C, De Rosa C. In: Polychlorinated biphenyls:human health aspects. Organization WH, editor. Geneva; 2003. pp. 1–64. [Google Scholar]
- Feng S, Wang Y, Wang X, Wang Z, Cui Y, Liu J, Zhao C, Jin M, Zou W. Caveolin-1 gene silencing promotes the activation of PI3K/AKT dependent on Eralpha36 and the transformation of MCF10ACE. Science China. Life sciences. 2010;53:598–605. doi: 10.1007/s11427-010-0100-x. [DOI] [PubMed] [Google Scholar]
- Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- Giesy JP, Kannan K, Blankenship AL, Jones PD, Hilscherova K. Dioxin-like and non-dioxin-like toxic effects of polychlorinated biphenyls (PCBs): implications for risk assessment. Central European journal of public health. 2000;(8 Suppl):43–45. [PubMed] [Google Scholar]
- Goncharov A, Bloom M, Pavuk M, Birman I, Carpenter DO. Blood pressure and hypertension in relation to levels of serum polychlorinated biphenyls in residents of Anniston, Alabama. J Hypertens. 2010;28:2053–2060. doi: 10.1097/HJH.0b013e32833c5f3e. [DOI] [PubMed] [Google Scholar]
- Goncharov A, Haase RF, Santiago-Rivera A, Morse G, McCaffrey RJ, Rej R, Carpenter DO. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environmental research. 2008;106:226–239. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson P, Hogstedt C. A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) American journal of industrial medicine. 1997;32:234–239. doi: 10.1002/(sici)1097-0274(199709)32:3<234::aid-ajim8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Han SG, Eum SY, Toborek M, Smart E, Hennig B. Polychlorinated biphenyl-induced VCAM-1 expression is attenuated in aortic endothelial cells isolated from caveolin-1 deficient mice. Toxicology and applied pharmacology. 2010;246:74–82. doi: 10.1016/j.taap.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SG, Han SS, Toborek M, Hennig B. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicology and applied pharmacology. 2012;261:181–188. doi: 10.1016/j.taap.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Dinkova-Kostova AT, McMahon M. Cross-talk between transcription factors AhR and Nrf2: lessons for cancer chemoprevention from dioxin. Toxicological sciences : an official journal of the Society of Toxicology. 2009;111:199–201. doi: 10.1093/toxsci/kfp168. [DOI] [PubMed] [Google Scholar]
- Higgins LG, Hayes JD. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug metabolism reviews. 2011;43:92–137. doi: 10.3109/03602532.2011.567391. [DOI] [PubMed] [Google Scholar]
- Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutation research. 2010;690:12–23. doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Layne J, Majkova Z, Smart EJ, Toborek M, Hennig B. Caveolae: a regulatory platform for nutritional modulation of inflammatory diseases. The Journal of nutritional biochemistry. 2011;22:807–811. doi: 10.1016/j.jnutbio.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Liu H, Zhou JS, Cao JF, Zhou XB, Choi AM, Chen ZH, Shen HH. Caveolin-1 inhibits expression of antioxidant enzymes through direct interaction with nuclear erythroid 2 p45-related factor-2 (Nrf2) The Journal of biological chemistry. 2012;287:20922–20930. doi: 10.1074/jbc.M112.352336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. What have we learned about the biology of atherosclerosis? The role of inflammation. Am J Cardiol. 2001;88:3J–6J. doi: 10.1016/s0002-9149(01)01879-3. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EJ, Majkova Z, Xu S, Bachas L, Arzuaga X, Smart E, Tseng MT, Toborek M, Hennig B. Coplanar polychlorinated biphenyl-induced CYP1A1 is regulated through caveolae signaling in vascular endothelial cells. Chemico-biological interactions. 2008;176:71–78. doi: 10.1016/j.cbi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EJ, Smart EJ, Toborek M, Hennig B. The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. American journal of physiology. Heart and circulatory physiology. 2007;293:H3340–H3347. doi: 10.1152/ajpheart.00921.2007. [DOI] [PubMed] [Google Scholar]
- Majkova Z, Layne J, Sunkara M, Morris AJ, Toborek M, Hennig B. Omega-3 fatty acid oxidation products prevent vascular endothelial cell activation by coplanar polychlorinated biphenyls. Toxicology and applied pharmacology. 2011;251:41–49. doi: 10.1016/j.taap.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkova Z, Smart E, Toborek M, Hennig B. Up-regulation of endothelial monocyte chemoattractant protein-1 by coplanar PCB77 is caveolin-1-dependent. Toxicology and applied pharmacology. 2009;237:1–7. doi: 10.1016/j.taap.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkova Z, Toborek M, Hennig B. The role of caveolae in endothelial cell dysfunction with a focus on nutrition and environmental toxicants. Journal of cellular and molecular medicine. 2010;14:2359–2370. doi: 10.1111/j.1582-4934.2010.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda C, Plank C, Mykhaylyk O, Schmidt K, Mayer B. Effects of statins on nitric oxide/cGMP signaling in human umbilical vein endothelial cells. Pharmacology reports. 2010;62(1):100–112. doi: 10.1016/s1734-1140(10)70247-4. [DOI] [PubMed] [Google Scholar]
- Miao X, Bai Y, Su W, Cui W, Xin Y, Wang Y, Tan Y, Miao L, Fu Y, Su G, Cai L. Sulforaphane prevention of diabetes-induced aortic damage was associated with the up-regulation of Nrf2 and its down-stream antioxidants. Nutrition & metabolism. 2012;9:84. doi: 10.1186/1743-7075-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naoum JJ, Zhang S, Woodside KJ, Song W, Guo Q, Belalcazar LM, Hunter GC. Aortic eNOS expression and phosphorylation in Apo-E knockout mice: differing effects of rapamycin and simvastatin. Surgery. 2004;136(2):323–328. doi: 10.1016/j.surg.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Newsome B, Petriello M, Han S, Murphy M, Eske K, Sunkara M, Morris A, Hennig B. Green tea diet decreases PCB 126-induced oxidative stress in mice by upregulating antioxidant enzymes. Journal of nutritional biochemistry. 2013 doi: 10.1016/j.jnutbio.2013.10.003. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2-an update. Free radical biology & medicine. 2013 doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka N, Yamamoto M, Schwencke C, Kawabe J, Ebina T, Ohno S, Couet J, Lisanti MP, Ishikawa Y. Caveolin interaction with protein kinase C. Isoenzyme-dependent regulation of kinase activity by the caveolin scaffolding domain peptide. The Journal of biological chemistry. 1997;272:33416–33421. doi: 10.1074/jbc.272.52.33416. [DOI] [PubMed] [Google Scholar]
- Panneerselvam M, Patel HH, Roth DM. Caveolins and heart diseases. Advances in experimental medicine and biology. 2012;729:145–156. doi: 10.1007/978-1-4614-1222-9_10. [DOI] [PubMed] [Google Scholar]
- Park SH, Jang JH, Chen CY, Na HK, Surh YJ. A formulated red ginseng extract rescues PC12 cells from PCB-induced oxidative cell death through Nrf2-mediated upregulation of heme oxygenase-1 and glutamate cysteine ligase. Toxicology. 2010;278:131–139. doi: 10.1016/j.tox.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Pavlides S, Gutierrez-Pajares JL, Danilo C, Lisanti MP, Frank PG. Atherosclerosis, caveolae and caveolin-1. Advances in experimental medicine and biology. 2012;729:127–144. doi: 10.1007/978-1-4614-1222-9_9. [DOI] [PubMed] [Google Scholar]
- Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the "reverse Warburg effect": a transcriptional informatics analysis with validation. Cell cycle. 2010;9:2201–2219. doi: 10.4161/cc.9.11.11848. [DOI] [PubMed] [Google Scholar]
- Petriello MC, Newsome B, Hennig B. Influence of nutrition in PCB-induced vascular inflammation. Environmental science and pollution research international. 2013 doi: 10.1007/s11356-013-1549-5. PMID:23417440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J, Bai Y, Reece JM, Williams J, Liu D, Freeman ML, Fahl WE, Shugar D, Liu J, Qu W, Collins S, Waalkes MP. Molecular mechanism of human Nrf2 activation and degradation: role of sequential phosphorylation by protein kinase CK2. Free radical biology & medicine. 2007;42:1797–1806. doi: 10.1016/j.freeradbiomed.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadass P, Meerarani P, Toborek M, Robertson LW, Hennig B. Dietary flavonoids modulate PCB-induced oxidative stress, CYP1A1 induction, and AhR-DNA binding activity in vascular endothelial cells. Toxicological sciences : an official journal of the Society of Toxicology. 2003;76:212–219. doi: 10.1093/toxsci/kfg227. [DOI] [PubMed] [Google Scholar]
- Repetto S, Salani B, Maggi D, Cordera R. Insulin and IGF-I phosphorylate eNOS in HUVECs by a caveolin-1 dependent mechanism. Biochemical and biophysical research communications. 2005;337:849–852. doi: 10.1016/j.bbrc.2005.09.125. [DOI] [PubMed] [Google Scholar]
- Salous AK, Panchatcharam M, Sunkara M, Mueller P, Dong A, Wang Y, Graf GA, Smyth SS, Morris AJ. Mechanism of rapid elimination of lysophosphatidic acid and related lipids from the circulation of mice. Journal of lipid research. 2013;54:2775–2784. doi: 10.1194/jlr.M039685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettgen T, Gube M, Esser A, Alt A, Kraus T. Plasma polychlorinated biphenyls (PCB) levels of workers in a transformer recycling company, their family members, and employees of surrounding companies. Journal of toxicology and environmental health. Part A. 2012;75:414–422. doi: 10.1080/15287394.2012.674905. [DOI] [PubMed] [Google Scholar]
- Sowa G. Caveolae, caveolins, cavins, and endothelial cell function: new insights. Frontiers in physiology. 2012;2:120. doi: 10.3389/fphys.2011.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura H. [Associations of exposure to dioxins and polychlorinated biphenyls with diabetes: based on epidemiological findings]. Nihon eiseigaku zasshi. Japanese journal of hygiene. 2012;67:363–374. doi: 10.1265/jjh.67.363. [DOI] [PubMed] [Google Scholar]
- Volonte D, Liu Z, Musille PM, Stoppani E, Wakabayashi N, Di YP, Lisanti MP, Kensler TW, Galbiati F. Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence. Molecular biology of the cell. 2013;24:1852–1862. doi: 10.1091/mbc.E12-09-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who's listening? Antioxidants & redox signaling. 2010;13:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chen Y, Sternberg P, Cai J. Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Investigative ophthalmology & visual science. 2008a;49:1671–1678. doi: 10.1167/iovs.07-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lim EJ, Toborek M, Hennig B. The role of fatty acids and caveolin-1 in tumor necrosis factor alpha-induced endothelial cell activation. Metabolism: clinical and experimental. 2008b;57:1328–1339. doi: 10.1016/j.metabol.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins BA, Hannon K, Ferruzzi M, Li Y. Dietary PUFA and flavonoids as deterrents for environmental pollutants. The Journal of nutritional biochemistry. 2007;18:196–205. doi: 10.1016/j.jnutbio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Yang YC, Lii CK, Wei YL, Li CC, Lu CY, Liu KL, Chen HW. Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-kappaB pathways. The Journal of nutritional biochemistry. 2013;24:204–212. doi: 10.1016/j.jnutbio.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Yeager RL, Reisman SA, Aleksunes LM, Klaassen CD. Introducing the "TCDD-inducible AhR-Nrf2 gene battery". Toxicological sciences : an official journal of the Society of Toxicology. 2009;111:238–246. doi: 10.1093/toxsci/kfp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Morris A, Sunkara M, Layne J, Toborek M, Hennig B. Epigallocatechin-gallate stimulates NF-E2-related factor and heme oxygenase-1 via caveolin-1 displacement. The Journal of nutritional biochemistry. 2012;2:163–168. doi: 10.1016/j.jnutbio.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.