Abstract
To investigate the potential clinical application of aptamers to prevention of HIV infection, single- stranded DNA (ssDNA) aptamers specific for CD4 were developed using the systematic evolution of ligands by exponential enrichment approach and next generation sequencing. In contrast to RNA-based aptamers, the developed ssDNA aptamers were stable in human serum up to 12 hr. Cell binding assays revealed that the aptamers specifically targeted CD4-expressing cells with high binding affinity (Kd=1.59 nM), a concentration within the range required for therapeutic application. Importantly, the aptamers selectively bound CD4 on human cells and disrupted the interaction of viral gp120 to CD4 receptors, which is a prerequisite step of HIV-1 infection. Functional studies showed that the aptamer polymers significantly blocked binding of viral gp120 to CD4-expressing cells by up to 70% inhibition. These findings provide a new approach to prevent HIV-1 transmission using oligonucleotide aptamers.
Keywords: ssDNA CD4 aptamer, gp120, HIV infection prevention, hybrid SELEX, specific blocking
1. Introduction
Aptamers are short, single-stranded (ss) oligonucleotides that have emerged as a new class of small molecule ligands with potential broad application (Hu and Zhang, 2013; Liu et al., 2011; Ni et al., 2011; Song et al., 2012; Strehlitz et al., 2012; Thiviyanathan and Gorenstein, 2012; Wang and Ray, 2012). As molecular ligands, oligonucleotide aptamers can specifically recognize and bind to a variety of targets with high affinity, including ions, toxins, drugs, low molecular weight molecules, peptides, proteins, cells, and tissues (Ni et al., 2011; Sefah et al., 2010; Shamah et al., 2008; Thiel et al., 2012; Tombelli and Mascini, 2010). Although aptamer applications have been investigated in biomedical research, the use of aptamers in clinical applications remains less explored (Cheng et al., 2013; Schmidt et al., 2004).
Recent advances in antiretroviral therapy have significantly reduced the morbidity and mortality associated with infection by the human immunodeficiency virus type 1 (HIV-1) (Andrieux-Meyer et al., 2012; Hatano et al., 2013; Scanlon and Vreeman, 2013). Despite these advances, elimination of the virus with antiretroviral therapy is not yet possible due to harmful side effects and the development of resistance (Fidler and Bock, 2013; Moss et al,, 2013). In addition, an effective vaccine for prevention of HIV-1 transmission is not yet available (Dhalla and Poole, 2013). Indeed, the continued emergence of drug-resistant HIV-1 strains and the high mutation rate of viral antigens necessitate the search for new therapeutics and, more importantly, better prevention approaches (Cahn and Wainberg, 2010; Peeters et al., 2010). One attractive and unique approach for prevention of transmission is to target and disrupt the viral entry mechanism into host cells (Teissier et al., 2010; Didigu and Doms, 2012; Gibson and Arts, 2012). Since HIV-1 transmission requires direct interaction of the viral gp120 and CD4 receptors on host cells, viral gp120 has been studies as a target for blocking HIV-1 transmission (Flores and Quesada, 2013). As the reverse transcriptase of HIV-1 is error prone, the hypervariable regions of gp120 mutate readily. Additionally, the hypervariable loops are highly glycosylated, forming what is often referred to as a ‘glycan shield’ that masks the conserved core binding regions (Castro et al., 2008). Thus, owing to its variability and shielded structure, the viral gp120 is a challenging target for prevention and/or therapy.
To overcome these technical obstacles and address clinical needs, we reasoned that an alternative entry inhibition strategy could be based on blockade of host CD4 receptor rather than viral gp120. To this end, ssDNA-based aptamers specific for cell-surface CD4 receptor were developed using a hybrid enrichment protocol (Hicke et al., 2001; Mann et al., 2010; Sefah et al., 2010). The developed aptamers significantly blocked the interaction between viral gp120 and CD4-expressing cells, suggesting a new approach to prevention of HIV-1 transmission.
2. Materials and methods
2.1. Reagents and cell lines
The CD4-positive cell line Karpas 299 was used to select ssDNA aptamers with high affinity for CD4-positive cells. CD4 recombinant protein (CD4-IgG2), obtained from the NIH AIDS Research and Reference Reagent Program (Germantown, MD), was used to further select DNA specific for the CD4 receptor at the protein level. Recombinant human CD4 protein with a polyhistidine tag at the C-terminus (Sino Biological Inc, Beijing, China) was used for aptamer affinity assays. The CD4-positive cell lines used included H9, SupT1, and U937 (ATCC, Manassas, VA). The CD4-negative cell lines tested were: B lymphoma cell lines CA46, JeKo-1, and Maver-1; breast cancer cell lines SKBR-3, MDA-MB-468, and T-47D; ovarian cancer cell lines HEY and A2780; and prostate cancer cell lines LN4, LNCaP, and PC3 (ATCC, Manassas, VA). All suspension cells were cultured with RPMI1640 medium with 10% FBS. All adherent cell lines were cultured with DMEM with 10% FBS.
2.2. Hybrid selection
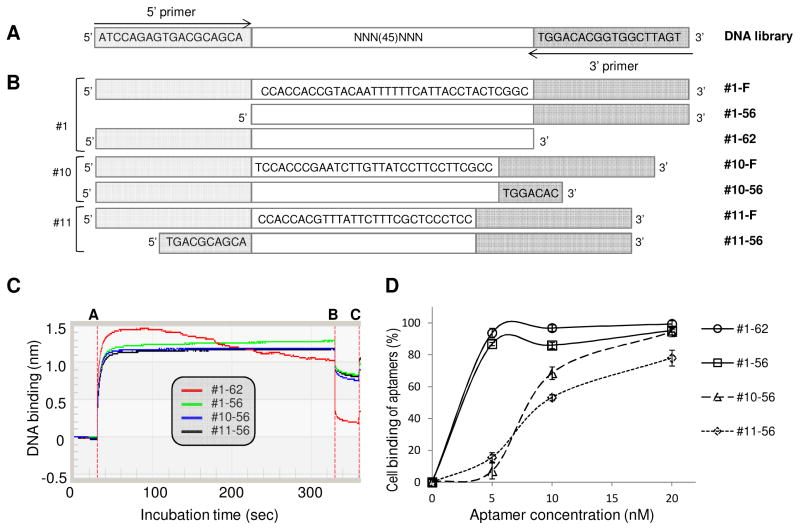
The DNA library used for aptamer selection consisted of a central, continuous stretch of 45 randomized sequences flanked by PCR primer sequences (5′-ATCCAGAGTGACGCAGCA -45N- TGGACACGGTGGCTTAGT 3′) (Fig. 2A) (Sefah et al. 2010). Cy5-labeled 5′ primer (5′-Cy5-ATCCAGAGTGACGC AGCA 3′) and biotinylated 3′ primer (5′-biotin- ACTAAGCCACCGTGTCCA 3′) were used in the initial PCR. DNA library and primers were purchased from Integrated DNA Technologies (Coralville, IA).
Fig. 2. DNA library design and sequences of developed full-length and truncated aptamers.
(A) Schematic of the sequences comprising the DNA library and (B) CD4-specific aptamers isolated in this study. “F” denotes the full-length sequence and the shortened sequences are depicted below. (C) Aptamers #1-62, #1-56, #10-56, and #11-56 were tested in an affinity binding assay with recombinant CD4 protein with a C-terminal polyhistidine (His) tag. First, the anti-His tag biosensor was bound with CD4 protein and incubated with 10nM ssDNA aptamer. During aptamer incubation, the instrument recorded the kinetic binding ability of aptamer. Between line A to B corresponds to the association phase and line B to C corresponds to the dissociation phase in all the sensorgrams. Aptamer #1-62 had the highest Kd value. (D) Cell-binding ability of the four aptamers using a flow cytometry-based assay with CD4-positive cells (Karpas 299). All aptamers had high binding affinity for CD4-positive cells at concentrations of 20 nM. Aptamer #1-62 bound nearly 100% of CD4-positive cells at 5 nM.
The first phase of aptamer development began with cell-based enrichment performed on the CD4-positive cell line Karpas 299 as previously described (Sefah et al., 2010). After 7 rounds of positive selection and 3 rounds of counter selection with the CD4-negative cell line CA46, the enriched DNA pool was used in sequential protein-based selection by incubating with CD4-IgG2 protein. The entire protein selection process was repeated five times. After a total of 15 rounds of cell- and protein-based selection, binding affinity and specificity of the selected pool was determined by flow cytometry (LSRII, BD Biosciences, San Jose, CA). The final selected pool was sequenced using second-generation sequencing (LC Sciences, Houston, TX).
2.3. Protein binding assay
To identify which aptamers had higher affinity for CD4 protein, we used the BLItz system (ForteBio, Inc. Menlo Park, CA) to test aptamer binding ability. Prior to starting the experiment, the anti-His biosensor was equilibrated in PBS buffer with 0.5% BSA for 10 minutes. The biosensor was then incubated with 50 μg/ml CD4 recombinant proteins (Sino Biological Inc, Beijing, China) for 10 minutes. After washing with PBS for 5 minutes, 10 nM CD4 DNA aptamers #1, #10 and #11 were incubated with the biosensor for another 5 minutes while the instrument recorded the kinetic binding ability of the aptamers.
2.4. Cell binding assay
Flow cytometry was used to monitor the enrichment of pools during the selection and to determine the binding affinity and specificity of the synthesized aptamers. Cell staining by aptamers and detection by flow cytometry were carried out as previously reported (Zhao et al, 2013). Based on fluorescent intensity, the binding affinity of aptamers was calculated by the reference method (Meng et al, 2012). The equilibrium dissociation constant (Kd) of the fluorescent ligand was obtained by fitting a plot of the specific intensity (Y) versus the aptamer concentration (X) to the equation Y=B max X/(Kd +X) using SigmaPlot (Jandel, San Rafael, CA).
To further detect specificity of synthesized DNA aptamers, SupT1 (CD4-positive) and CA46 (CD4-negative) cell lines were mixed at a ratio of 1:1. The mixture was incubated for 30 minutes at RT with 10 nM aptamer #1-62 and phycoerythrin (PE)-CD4 antibody. After washing with 500 μl PBS, CD4 staining was evaluated by flow cytometry. Binding sensitivity of aptamers was assessed by mixing 1×105 Sup-T1 cells with 0.5 μl peripheral whole blood. This mixture was stained for 30 minutes at RT with 10 nM Cy5-labeled aptamer #1-62 and PE-CD4 antibody. After washing once with 500 μl PBS, the CD4-positive population was detected by checking fluorescent PE and Alexa Fluor 700.
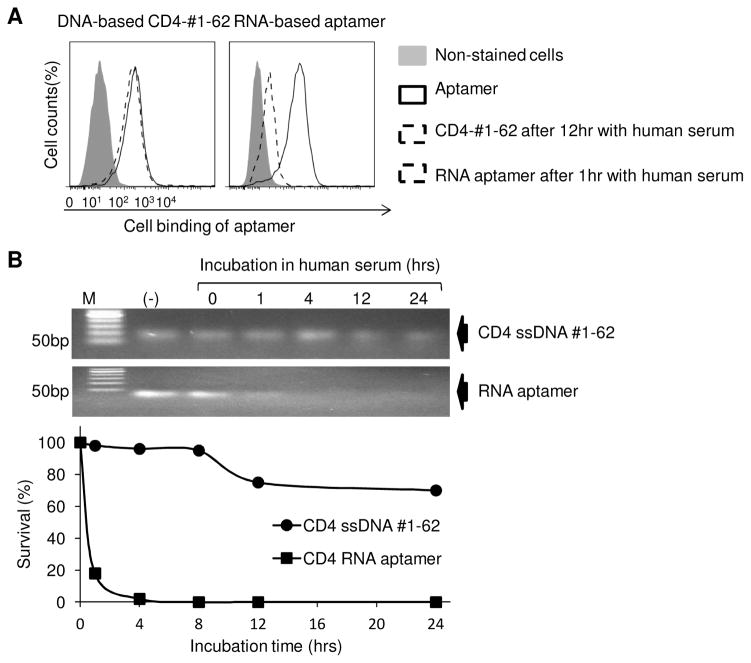
2.5. DNA-based ssDNA CD4-#1-62 stability in human serum
A total of 1 μg CD4-#1-62 aptamer or RNA-based CD4 aptamer (Davis et al, 1998; Zhang et al, 2010) was incubated at 37°C in 100 μl of 100% human serum (Atlanta Biological, Lawrenceville, GA, USA). At the time points shown in Figure 5B, residual CD4 ssDNA #1-62 and CD4 RNA aptamer were purified as previous reported (Zhao et al, 2011a), and analyzed on 3% agarose gels followed by ethidium bromide staining. The bands of DNA or RNA products were quantified using TotalLab software (FotoDyne, Hartland, WI, USA) and plotted using Kaleidagraph (Synergy Software, Reading, PA, USA). CD4 ssDNA #1-62 and RNA-based aptamer CD4 stability in human serum also was confirmed by flow cytometry to check aptamers binding ability with CD4 positive cell, 1 hour post-incubation in human serum.
Fig. 5. ssDNA CD4-#1-62 is stable in human serum.
(A) CD4 ssDNA aptamer #1-62 and the CD4 RNA-based aptamer were incubated in human serum and cell binding ability determined by flow cytometry. The ssDNA CD4-#1-62 retained nearly 100% binding capacity to K299 cells, even after 12 hrs of incubation. The RNA aptamer lost almost all ability to bind CD4-expressing cells after 1 hr incubation. (B) Gel electrophoresis showed CD4 ssDNA #1-62 to be stable in serum for 12 hrs, while RNA-based aptamer CD4 is almost completely degraded at 1 hr incubation in human serum.
2.6. Formulation of aptamer polymers
Streptavidin, a homotetrameric protein, can bind to four molecules of biotin with extraordinarily high affinity. Aptamers were labeled with biotin (Integrated DNA Technologies, Coralville, IA) at the 5′ terminus. Biotinylated aptamers were mixed with streptavidin (Sigma, St. Louis, MO) at a ratio of 4:1. After 1 hr of incubation, the aptamer-streptavidin conjugate was purified by ssDNA/RNA Clean & Concentrator (Zymo Research Corp, Irvine, CA) to remove free streptavidin. The concentration of the purified tetrameric aptamers was checked using a microplate reader (Biotek, Winooski, VT). Similarly, dimer and trimer forms of aptamers were also generated by using biotinized aptamer sequences to conjugate to streptavidin at the ratios of 2:1 or 3:1, respectively.
2.7. Blocking the interaction of viral gp120 and CD4-expressing cells
To assess the inhibition of gp120-CD4 binding by the CD4 ssDNA aptamer, synthesized aptamers #1-56, #1-62, #10-56, and #11-56 were pre-incubated with the CD4-expressing cell line Karpas 299 at a concentration of 10 μM. After 30 minutes, FITC-labeled gp120 (Immune Diagnosis, Inc. Woburn, MA) was added and cells were incubated for another 30 minutes. Cells were washed once with PBS, and bound FITC-labeled gp120 was determined by flow cytometry. For comparison, dimer, trimer, and tetramer forms of aptamers were generated and the inhibitory effect of these poly-aptamers was also quantified as describe above. The dose response for tetrameric aptamer was performed at concentrations ranging from 1 μM to 20 μM as shown in Figure 5D. All experiments were performed in triplicate at a minimum.
2.8. Effect of CD4-#1-62 aptamer on T cell surface biomarker expression and cell growth
To rule out the adverse effects on surface biomarker expression, Karpas 299 cells treated with aptamers or DNA library for 12 hrs were stained with antibodies specific for CD4, CD3, and CD45 and expression was quantified by flow cytometry. For cytotoxicity assays, cells were treated with 10 μM aptamer #1-62 or DNA library for 12 hrs, and apoptotic cells were visualized by using Annexin V-APC staining (BD Bioscience, San Jose, CA) and flow cytometry. Cells were treated with Doxorubicin (0.5 μM) for 12 hrs to induce apoptosis as a positive control.
3. Results
3.1. Development and characterization of CD4-specific aptamer sequences
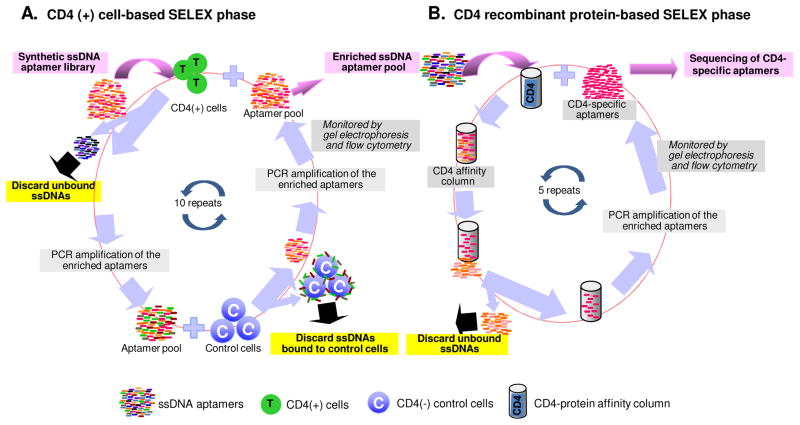
In this study, we employed a hybrid SELEX approach that combined cell-based enrichment with CD4-positive cells (Karpas 299) and protein-based selection with CD4-IgG2(Fig. 1). Because the gp120 binding site on CD4 is located within the V1 and V2 domains of CD4 extracellular region (Wang et al., 1990; Copon et al., 1991 and Ulrich et al., 1992), recombinant protein CD4-IgG2, which only contains the V1 and V2 domains, was used for selection to increase the probability of getting functional ssDNA. Next generation sequencing of the final CD4 aptamer pool detected a total of 535,100 reads and revealed 12 unique ssDNA sequences with dominant copy number (Table S1). Of the 12 sequences, three aptamers, #1, #10, and #11 (Fig. 2A), had more predicted stem-loop structure when analyzed with Oligoanalyzer software (Integrated DNA Technologies, Coralville, IA) (Fig. S1). It has been reported that oligonucleotide sequences with more stem-loop secondary structure have higher target binding affinity (Horn, et al., 2004). The minimum aptamer length required to maintain their secondary structure was then determined, and sequences that did not contribute to the predicted 2-dimensional core structure were deleted (Fig. 2B and Fig. S1). Therefore, aptamers #1-56, #1-62, #10-56, and #11-56 derived from three different sequences were synthesized for further evaluation.
Fig. 1. Hybrid SELEX approaches to develop CD4-specific aptamers.
The hybrid SELEX protocol included a two-step enrichment process that combines CD4-positive T-cell-based enrichment with biomarker-based selection. (A) CD4-binding aptamers were selected from a synthetic single strand DNA library with CD4-positive T cells. After PCR amplification of the enriched aptamers, negative selection, using CD4-negative control cells, was carried out and aptamers that non-specifically bind to cellular components were discarded. Seven rounds of positive and three negative selections with PCR amplification were performed. (B) Aptamers able to bind CD4-postive cells were then subjected to further enrichment with protein-based selection. Aptamers were added to a CD4 affinity column and unbound ssDNAs are discarded. PCR amplification of the bound aptamers was then performed. Protein-based selection was repeated 5 times. The final aptamer pool was amplified, sequenced and consists of CD4-specific aptamers.
Protein binding assays demonstrated that the synthetic aptamers had high binding affinity to recombinant CD4 protein (Fig. 2C), with aptamer #1-62 having the highest binding affinity. For cell binding assays, the aptamer sequences were labeled with Cy5 fluorescent reporter. Cultured CD4-expressing cells (Karpas 299) were treated with individual Cy5-labeled aptamers at room temperature. After incubating for 30 minutes, cells were washed with 500 μl PBS buffer. The resultant binding was quantified by flow cytometry. As shown in Fig. 2D, all cells stained by aptamers #1-62 and #1-56 completely separated from non-stained cells at final concentrations as low as 5 nM, indicating that they had high binding affinity. Aptamers #10-56 and #11-56 achieved cells completely shift from control cells at 20 nM. To determine the Kd of aptamer #1-62, more detailed cell binding assays were conducted. Briefly, cell binding ability of aptamer was detected under low dose of aptamers as Fig. S2 indicated. Based on fluorescent intensity of bound cells, the Kd calculated by the reference method (Meng et al., 2012) was determined to be 1.59 nM. Since aptamer #1-62 had the highest binding affinity for both CD4 proteins and CD4-expressing cells (Fig. 2C and 2D), it was selected for further functional evaluation.
3.2. Specific targeting of CD4-expressing cells by the aptamers
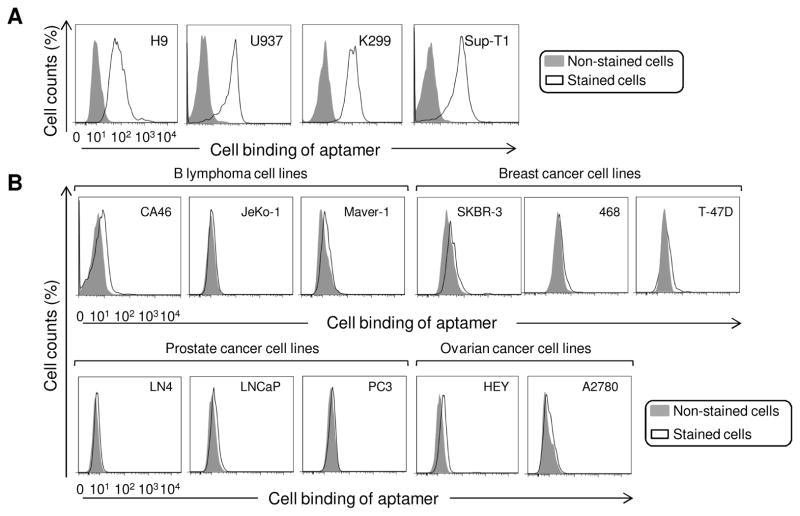
First, the aptamer #1-62 was tested against multiple CD4-expressing cell lines (Fig. 3A) and CD4-negative control cells, including B lymphoma, breast cancer, ovarian cancer, and prostate cancer cell lines (Fig. 3B). Cells were treated with aptamer #1-62 at 10 nM final concentration as described above and cell binding was examined by flow cytometry. Aptamer #1-62 specifically bound to all tested CD4-expressing cells and did not react to CD4-negative cells.
Fig. 3. Aptamer #1-62 specifically binds to CD4-expressing cell lines.
(A) The specificity of aptamer #1-62 (10 nM) was confirmed by testing additional CD4-positive cell lines: H9, U937, Karpas 299, and SupT1; (B) Aptamer #1-62 (10 nM) did not bind various CD4-negative cell lines. Tested cell lines included: B lymphoma cell lines: CA46, JeKo-1 and Maver-1; breast cancer cell lines: SKBR-3, 468 and T-47D; ovarian cancer cell lines: HEY and A2780; and prostate cancer cell lines: LN4, LNCaP and PC3.
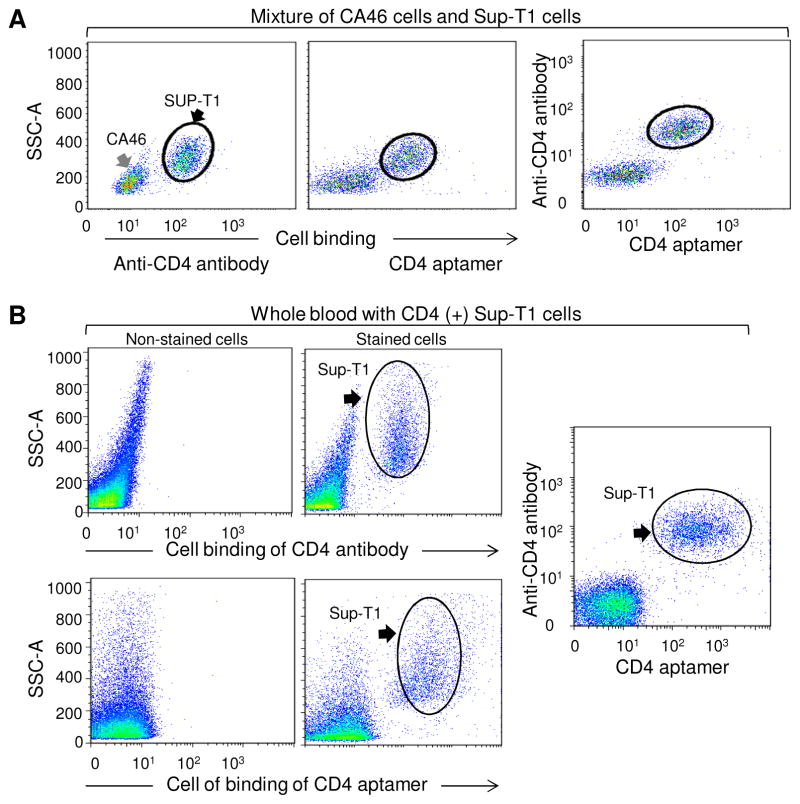
Currently, immunostaining with antibody probe is the gold standard for detection of CD4-expressing cells in the clinical laboratory. Therefore, we compared cell staining by anti-CD4 antibody and aptmer #1-62. For validation purposes, a 1:1 mixture of CD4-expressing SupT1 cells and CD4-negative CA46 cells was simultaneously stained with PE-labeled anti-CD4 antibody or Cy5-conjugated aptamer #1-62. As shown in Fig. 4A, two populations of cells were distinguished by antibody or aptamer staining. Notably, double staining of the cell mixture revealed that both antibody and aptamer targeted the same population of CD4-expressing cells with no interference to each other, suggesting that they recognize different epitopes on CD4 receptors (Fig. 4A, far right). Moreover, to determine whether the aptamer can be used for clinical applications, CD4-expressing cells (SupT1) were spiked into whole blood from a healthy donor and the blood-cell mixture was stained with aptamer #1-62 or anti-CD4 antibody. As shown in Fig. 4B, the spiked CD4-expressing cells were identified and separated from blood cells by both aptamer and antibody. Similarly, double staining of the blood mixture indicated that both aptamer #1-62 and anti-CD4 antibody targeted the same cells simultaneously, with no non-specific binding to background blood cells (Fig. 4B, far right).
Fig. 4. Aptamer #1-62 specifically recognizes CD4-positive cells in a cell mixture.
(A) Immunostaining of a mixture of CA46 (CD4-negative) and Sup-T1 (CD4-positive) cells with PE-labeled CD4 antibody was compared with Cy5-labeled aptamer #1-62. Staining with aptamer #1-62 was very similar to immunostaining with the CD4 antibody. (B) SupT1 cells (1 × 105) were mixed with 0.5 μL whole human peripheral blood, and the mixture was double stained with PE-CD4 antibody and cy5-labeled aptamer #1-62. Aptamer #1-62 readily bound to CD4-positive cells and was nearly indistinguishable from CD4 antibody staining.
3.3. High stability of the ssDNA aptamers in human serum
Although an RNA-based CD4 aptamer has been developed (Davis et al., 1998; Zhang et al, 2010), the high susceptibility of RNA sequences to environmental nucleases limited its potential clinical application. To compare biostability of the reported RNA-aptamers and our ssDNA-based aptamers, the ssDNA aptamer #1-62 or the RNA-based CD4 aptamers were added to human serum to mimic in vivo physiologic conditions. After incubation for 12 hr at 37°C, the CD4-expressing Karpas 299 tumor cells were added to the aptamer-serum mixture, and residual cell binding capacity of aptamers was assessed by flow cytometry. Fig. 5A demonstrated that ssDNA aptamer #1-62 retained almost 100% cell binding capacity. In contrast, under the same conditions, the RNA aptamers lost almost all ability to bind to tumor cells after 1 hr incubation in serum (Fig. 5A, right). For further confirmation, the residual products of aptamers were recovered from serum at different time points as indicated in Figure 5B, and visualized by gel electrophoresis. The ssDNA aptamer #1-62 had minimal change at 24 hrs, while the RNA aptamers were almost completely digested within 1 hr in serum. These findings indicate that the developed ssDNA aptamers are stable in human serum, a biological and physiological condition that is a requisite for in vivo use.
3.4. Blocking the interaction of HIV gp120 and CD4-expressing T cells by the synthetic aptamers
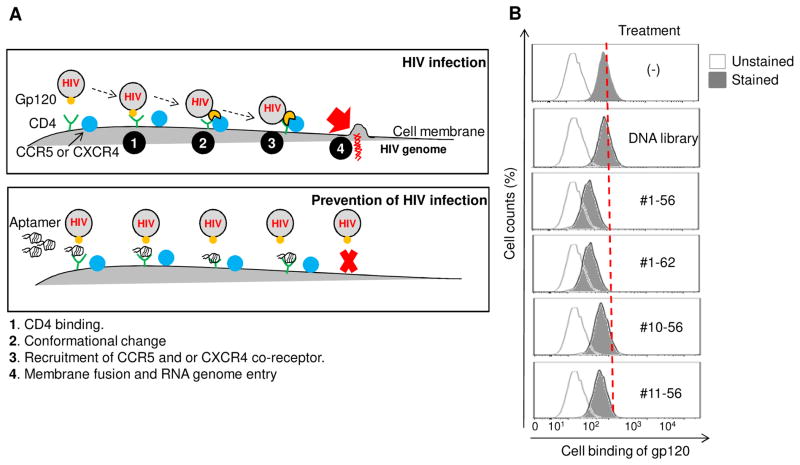
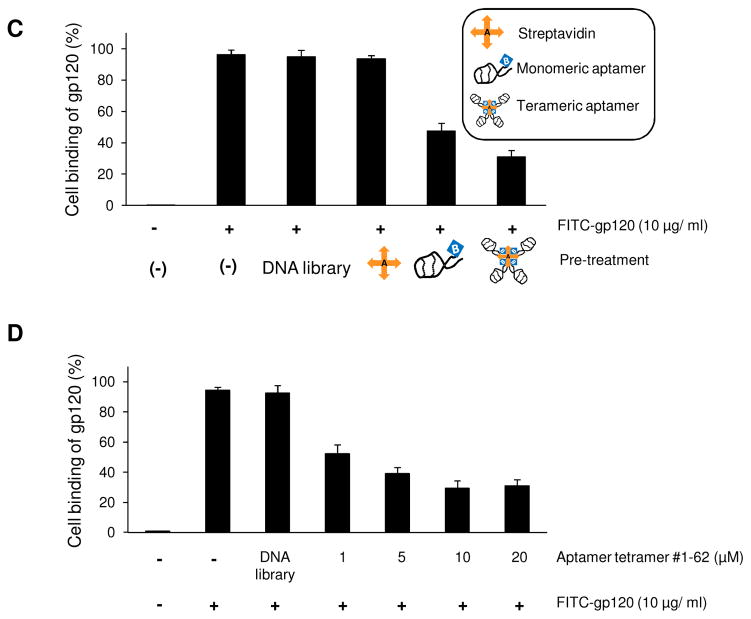
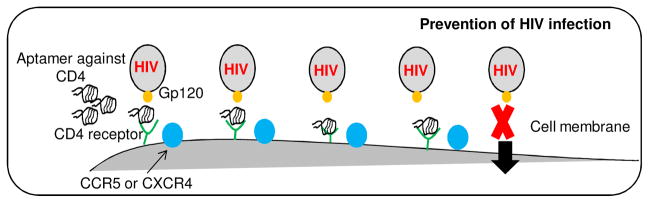
Since the developed aptamers specifically bind to CD4 proteins and CD4-expressing cells, we next wanted to test whether our aptamer was able to disrupt the interaction of viral gp120 and CD4 receptor on the cell surface, and thus potentially prevent HIV-1 infection of cells (Fig. 6A). To test our hypothesis, the CD4-expressing cells were incubated first with different aptamers for 30 minutes and FITC-labeled viral gp120 was then added. The resultant cell binding of viral gp120 was quantified by flow cytometry. As shown in Figure 6B, the presence of aptamers significantly inhibited binding of viral gp120 to CD4-expressing cells, resulting in a 20–50% reduction. Notably, aptamer #1-62 had the highest inhibitory effect. Since the small size of the aptamers may be inadequate to completely block gp120 binding, tetrameric aptamer #1-62 was formed and tested along with monomeric aptamers. Quantitative flow cytometric analysis revealed that the presence of monomeric aptamer resulted in 49% inhibition of gp120 binding to CD4-expressing cells. Under the same conditions, the tetrameric aptamer resulted in 65% inhibition, 16% more effective than its monomeric counterpart (Fig. 6C). In contrast, streptavidin treatment had no effect. Further validation studies revealed that the inhibitory effects of the tetrameric aptamers was dose-dependent and reached maximal inhibition (70% reduction) at a final concentration of 10 μM (Fig. 6D). Moreover, to determine whether the addition of aptamers can disrupt established gp120-CD4 binding, cells were first incubated with the FITC-labeled gp120 for 30 minutes and then treated with the tetrameric aptamer at different concentrations as indicated in Figure 6E. Flow cytometry analysis showed that the formed gp120-CD4 binding was disrupted as aptamer concentration increased (Fig. 6E), indicating that the aptamers competed with gp120 for CD4 binding on targeted cells.
Fig. 6. Inhibition of the gp120-CD4 interaction with CD4-specific aptamer.

(A) The viral gp120 binding to the CD4 receptor on the host T cells, initiates a cascade of events including Conformational change, recruitment of CCR5 and/or CXCR4 co-receptor that results in the fusion of HIV-1 host cell membrane and subsequent entry of HIV-1 RNA genome into host cells. Blockage of the gp120-CD4 interaction by CD4 aptamer may inhibit HIV infection. (B) Karpas 299 cells were pre-incubated with aptamers at a concentration of 10 μM for 30 min. Cells were washed and subsequently incubated with FITC-labeled gp120 for 30 mins, and bound gp120 was detected by flow cytometry. All four CD4-binding DNA aptamers reduced gp120 binding to CD4-positive cells. The DNA library was included as a negative control. (C) Karpas 299 cells were incubated with 10 μM streptavidin-conjugated aptamer #1-62 polymers or 10 μM monomeric aptamer #1-62 and tested in our gp120 binding assay. Whereas monomeric aptamer #1-62 reduced gp120 to 47.4% of control levels, gp120 binding was reduced to 31.1% in the presence of tetrameric aptamer #1-62. (D) Karpas 299 cells were incubated with increasing concentrations (1 to 20 μM) of tetrameric aptamer #1-62 and FITC-labeled gp120 was added at 30 min post. At 10 μM, only 30% of cells bound gp120. (E) Karpas 299 cells were incubated first with gp120-FITC for 30 minutes and then the indicated concentrations of tetrameric aptamer #1-62 were added. Gp120 binding was disrupted in a dose-dependent manner.
In addition to tetramer, dimer and trimer forms of the aptamers were also formulated by using biotinized aptamers to conjugate to streptavidin at the ratios of 2:1, and 3:1, respectively. Cell binding assays showed that polymer forms of aptamers induced higher inhibition of gp120 cell binding than that observed by monomeric aptamer. However, there was no statistical difference in the blocking effect among dimer, trimer, and tetramer forms (Fig. S3). We chose tetrameric aptamers for further study based on the fact that tetrameric aptamers had similar binding ability as monomers (Fig. S4). Finally, the potential effect of increasing concentrations of viral gp120 on the ability of aptamers to block binding was examined. In the absence of aptamer, gp120 cell binding (%) increased with increasing gp120 concentrations until reaching a maximal level at 10 μg/ml (Fig. S5). Interestingly, the presence of 10 μM tetrameric aptamers significantly inhibited the gp120 cell binding (>60% reduction). These finding indicate that the aptamers blocked CD4 receptors on T cells, but did not directly interact with gp120 and were not affected by gp120 concentrations.
3.5. No side effect of CD4 aptamers on T cell growth and surface biomarker expression
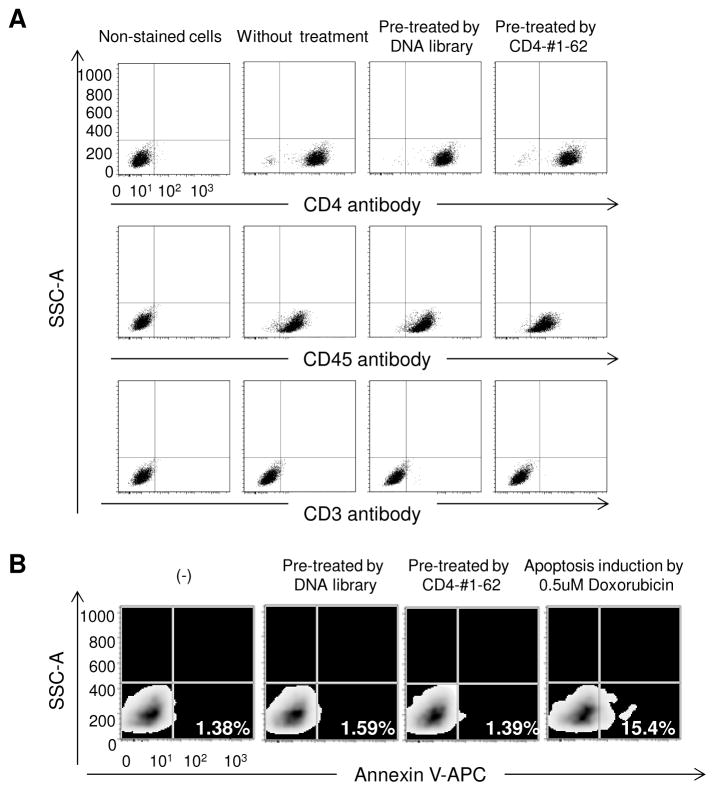
As CD4 is expressed on many cell types and needed for proper immune system function, we tested whether the presence of aptamers had any adverse effects on cell function. For this purpose, changes in T cell surface marker expression and cytotoxicity were evaluated following aptamer addition. As showed in Figure 7A, there was no change in the expression of CD4, CD3 and CD45 on cells treated with the aptamers compared to untreated cells or cells treated with the ssDNA library. In addition, apoptosis was measured by Annexin V-APC staining and aptamer treatment had no effect on cell apoptosis (Fig. 7B). These findings indicate that that aptamers were not cytotoxic.
Fig. 7. Addition of the CD4-#1-62 aptamer has no significant effect on T cell surface biomarker expression or cell death.
CD4-expressing K299 cells were treated with 10 μM CD4-#1-62 or the original DNA library as a negative control. After 12 hours cells were either stained with fluorescently-labeled antibodies against the standard T cell surface markers CD4, CD3, or CD45, or annexin V-APC to detect apoptotic cells. For the apoptosis assay, 0.5 μM doxorubicin treatments were used to induce apoptosis. (A) Detection of CD4, CD3 and CD45 expression in untreated or treated cells by aptamer or DNA library. (B) Apoptosis assay for untreated and treated cells.
4. Discussion
As short oligonucleotides, aptamers are non-immunogenic in vivo, and thus suitable for clinical applications (Kang and Lee, 2013; Song et al., 2012). In this study, we developed a biostable ssDNA aptamer specific for CD4. The presence of the aptamers significantly inhibited the interaction of gp120 of HIV-1 with human cells, suggesting a new approach to prevent viral transmission (Fig. 6A). However, the blockage of gp120 binding to CD4 receptor by the aptamers was not complete, possibly due to the small size of the aptamers and/or they did not bind to the core region of CD4 receptor responsible for gp120 association. Thus, we hypothesize that size modifications of the aptamers may achieve increased binding inhibition. In comparison to the aptamer monomer, polymer forms of aptamers showed a higher inhibitory effect, although there was no statistacal difference in blocking effect among dimer, trimer and tetramer forms (Fig. S3). Other strategies to increase physical size include chemical modifications, such as polyethylene (PEG) conjugation, which may further optimize bioavailability of aptamers and increase the efficacy of binding inhibition (Pasut and Veronese, 2009a; Pasut and Veronese, 2009b). Moreover, since the developed aptamers may target different epitopes on the CD4 receptor, cross-linking different aptamers might enhance their blocking potential.
Notably, the developed ssDNA aptamers were much more stable than the RNA-based aptamers (Fig. 5), and did not affect cell growth and T cell surface marker expression (Fig. 7), suggesting that the ssDNA aptamers may be suitable for clinical use. It is reasonable to hypothesize that the addition of the optimized ssDNA aptamers into vaginal gel could prevent HIV-1 transmission by directly blocking the interaction of gp120 to CD4-expressing cells (Fig. 6A). Importantly, the aptamers specifically target CD4, which is a stable host cell receptor, instead of viral gp120. Using this approach, one can overcome the major obstacle for prevention of HIV-1 transmission, which is the high mutation rate of virus (Cabrera and Clotet, 2005; Holtz and Mansky, 2013; Stray et al., 2013; Wainberg et al., 2012). The aptamers may also be useful for emergency treatment of patients that have accidental exposure of HIV-1 via needlstick or bodily fluid transfer by temporarily blocking CD4 receptors on host blood cells. Finally, as a molecular ligand, the CD4-specific aptamer could be a powerful tool for targeted therapy to CD4-expressing T cell lymphoma/leukemia when combined with a nanotechnology drug delivery system (Chang et al., 2011; Guo et al., 2011; Sundaram et al., 2012; Zhao et al., 2011a,b & 2013).
Supplementary Material
Highlights.
A protein-cell hybrid SELEX enrichment approach was used to develop ssDNA aptamers that are stable in serum and specific for CD4-expressing cells
The developed ssDNA aptamers significantly inhibit the interaction between HIV gp120 and CD4-expressing human cells
As a biomaterial, aptamers could be a powerful approach to prevent HIV-1 transmission, which is currently a major clinical obstacle.
Acknowledgments
This project was supported in part by NIH grants R01CA151955 (Y.Z.), R33CA173382 (Y.Z.), 5P50CA126752 (Y.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrieux-Meyer I, Calmy A, Cahn P, Clayden P, Raguin G, Katlama C, Vitoria M, Levin A, Lynch S, Goemaere E, et al. Preferred antiretroviral drugs for the next decade of scale up. J Int AIDS Soc. 2012;15:17986. doi: 10.7448/IAS.15.2.17986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera C, Clotet B. Mutations resulting in resistence of antiretroviral drugs in HIV-infected patients. Med Clin (Barc) 2005;124:618–619. doi: 10.1157/13074392. [DOI] [PubMed] [Google Scholar]
- Cahn P, Wainberg MA. Resistance profile of the new nucleoside reverse transcriptase inhibitor apricitabine. J Antimicrob Chemother. 2010;65:213–217. doi: 10.1093/jac/dkp422. [DOI] [PubMed] [Google Scholar]
- Castro E, Belair M, Rizzardi GP, Bart PA, Pantaleo G, Graziosi C. Independent evolution of hypervariable regions of HIV-1 gp120: V4 as a swarm of N-Linked glycosylation variants. AIDS Res Hum Retroviruses. 2008;24:106–113. doi: 10.1089/aid.2007.0139. [DOI] [PubMed] [Google Scholar]
- Chang M, Yang CS, Huang DM. Aptamer-conjugated DNA icosahedral nanoparticles as a carrier of doxorubicin for cancer therapy. ACS Nano. 2011;5:6156–6163. doi: 10.1021/nn200693a. [DOI] [PubMed] [Google Scholar]
- Cheng C, Chen YH, Lennox KA, Behlke MA, Davidson BL. In vivo SELEX for Identification of Brain-penetrating Aptamers. Mol Ther Nucleic Acids. 2013;2:e67. doi: 10.1038/mtna.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copon DJ, Ward RH. The CD4-gp120 interaction and AIDS pathogenesis. Annu Rev Immunol. 1991;9:649–678. doi: 10.1146/annurev.iy.09.040191.003245. [DOI] [PubMed] [Google Scholar]
- Davis KA, Abrams B, Lin Y, Jayasena SD. Staining of cell surface human CD4 with 2′-F-pyrimidine-containing RNA aptamers for flow cytometry. Nucleic acids research. 1998;26 (17):3915–3924. doi: 10.1093/nar/26.17.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalla S, Poole G. Barriers to Participation in Actual HIV Vaccine Trials. Curr HIV Res. 2013 Apr;11(3):238–45. doi: 10.2174/1570162x11311030009. [DOI] [PubMed] [Google Scholar]
- Didigu CA, Doms RW. Novel approaches to inhibit HIV entry. Viruses. 2012;4:309–324. doi: 10.3390/v4020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler S, Bock P. Prophylactic antiretroviral HIV therapy prevents infection in heterosexual men and women. Evid Based Med. 2013 Oct;18(5):184–5. doi: 10.1136/eb-2012-101034. [DOI] [PubMed] [Google Scholar]
- Flores A, Quesada E. Entry Inhibitors Directed Towards Glycoprotein gp120: An Overview on a Promising Target for HIV-1 Therapy. Curr Med Chem. 2013;20:751–771. [PubMed] [Google Scholar]
- Gibson RM, Arts EJ. Past, present, and future of entry inhibitors as HIV microbicides. Curr HIV Res. 2012;10:19–26. doi: 10.2174/157016212799304616. [DOI] [PubMed] [Google Scholar]
- Guo J, Gao X, Su L, Xia H, Gu G, Pang Z, Jiang X, Yao L, Chen J, Chen H. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32:8010–8020. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Hatano H. Immune activation and HIV persistence: considerations for novel therapeutic interventions. Curr Opin HIV AIDS. 2013 May;8(3):211–6. doi: 10.1097/COH.0b013e32835f9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke BJ, Marion C, Chang YF, Gould T, Lynott CK, Parma D, Schmidt PG, Warren S. Tenascin-C aptamers are generated using tumor cells and purified protein. J Biol Chem. 2001;276(52):48644–45654. doi: 10.1074/jbc.M104651200. [DOI] [PubMed] [Google Scholar]
- Holtz CM, Mansky LM. Variation of HIV-1 Mutation Spectra among Cell Types. J Virol. 2013;87:5296–5299. doi: 10.1128/JVI.03576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn WT, Convery MA, Stonehouse NJ, Adams CJ, Liljas L, Phillips SV, Stockley PG. The crystal structure of a high affinity RNA stem-loop complexed with the bacteriophage MS2 capsid: further challenges in the modeling of ligand-RNA interactions. RNA. 2004;10(11):1776–1782. doi: 10.1261/rna.7710304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Zhang K. The application of aptamers in cancer research: an up-to-date review. Future Oncol. 2013;9:369–376. doi: 10.2217/fon.12.201. [DOI] [PubMed] [Google Scholar]
- Kang KN, Lee YS. RNA aptamers: a review of recent trends and applications. Adv Biochem Eng Biotechnol. 2013;131:153–169. doi: 10.1007/10_2012_136. [DOI] [PubMed] [Google Scholar]
- Liu J, You M, Pu Y, Liu H, Ye M, Tan W. Recent developments in protein and cell-targeted aptamer selection and applications. Curr Med Chem. 2011;18:4117–4125. doi: 10.2174/092986711797189619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann AP, Somasunderam A, Nieves-Alicea R, Li X, Hu A, Sood AK, Ferrari M, Gorenstein DG, Tanaka T. Identification of thioaptamer ligand against E-selectin: potential application for inflamed vasculature targeting. PLoS ONE. 2010;5(9):e13050, 1–11. doi: 10.1371/journal.pone.0013050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss JA. HIV/AIDS Review. Radiol Technol. 2013;84:247–267. quiz p 268–270. [PubMed] [Google Scholar]
- Meng L, Yang L, Zhao X, Zhang L, Zhu H, Liu C, Tan W. Targted delivery of chemotherapy agents using a liver cancer-specific aptamer. Plos one. 2012;7 (4):e33434. doi: 10.1371/journal.pone.0033434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X, Castanares M, Mukherjee A, Lupold SE. Nucleic acid aptamers: clinical applications and promising new horizons. Curr Med Chem. 2011;18:4206–4214. doi: 10.2174/092986711797189600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasut G, Veronese FM. PEG conjugates in clinical development or use as anticancer agents: an overview. Adv Drug Deliv Rev. 2009a;61:1177–1188. doi: 10.1016/j.addr.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Pasut G, Veronese FM. PEGylation for improving the effectiveness of therapeutic biomolecules. Drugs Today (Barc) 2009b;45:687–695. doi: 10.1358/dot.2009.45.9.1416421. [DOI] [PubMed] [Google Scholar]
- Peeters M, Aghokeng AF, Delaporte E. Genetic diversity among human immunodeficiency virus-1 non-B subtypes in viral load and drug resistance assays. Clin Microbiol Infect. 2010;16:1525–1531. doi: 10.1111/j.1469-0691.2010.03300.x. [DOI] [PubMed] [Google Scholar]
- Scanlon ML, Vreeman RC. Current strategies for improving access and adherence to antiretroviral therapies in resource-limited settings. HIV AIDS (Auckl) 2013;5:1–17. doi: 10.2147/HIV.S28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KS, Borkowski S, Kurreck J, Stephens AW, Bald R, Hecht M, Friebe M, Dinkelborg L, Erdmann VA. Application of locked nucleic acids to improve aptamer in vivo stability and targeting function. Nucleic Acids Res. 2004;32:5757–5765. doi: 10.1093/nar/gkh862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefah K, Shangguan D, Xiong X, O’Donoghue MB, Tan W. Development of DNA aptamers using Cell-SELEX. Nat Protoc. 2010;5:1169–1185. doi: 10.1038/nprot.2010.66. [DOI] [PubMed] [Google Scholar]
- Song KM, Lee S, Ban C. Aptamers and their biological applications. Sensors (Basel) 2012;12:612–631. doi: 10.3390/s120100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stray KM, Callebaut C, Glass B, Tsai L, Xu L, Muller B, Krausslich HG, Cihlar T. Mutations in multiple domains of Gag drive the emergence of in vitro resistance to the phosphonate-containing HIV-1 protease inhibitor GS-8374. J Virol. 2013;87:454–463. doi: 10.1128/JVI.01211-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehlitz B, Reinemann C, Linkorn S, Stoltenburg R. Aptamers for pharmaceuticals and their application in environmental analytics. Bioanal Rev. 2012;4:1–30. doi: 10.1007/s12566-011-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram P, Wower J, Byrne ME. A nanoscale drug delivery carrier using nucleic acid aptamers for extended release of therapeutic. Nanomedicine. 2012;8:1143–1151. doi: 10.1016/j.nano.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Teissier E, Penin F, Pecheur EI. Targeting cell entry of enveloped viruses as an antiviral strategy. Molecules. 2010;16:221–250. doi: 10.3390/molecules16010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel WH, Bair T, Peek AS, Liu X, Dassie J, Stockdale KR, Behlke MA, Miller FJ, Jr, Giangrande PH. Rapid identification of cell-specific, internalizing RNA aptamers with bioinformatics analyses of a cell-based aptamer selection. PLoS One. 2012;7:e43836. doi: 10.1371/journal.pone.0043836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiviyanathan V, Gorenstein DG. Aptamers and the next generation of diagnostic reagents. Proteomics Clin Appl. 2012;6:563–573. doi: 10.1002/prca.201200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombelli S, Mascini M. Aptamers biosensors for pharmaceutical compounds. Comb Chem High Throughput Screen. 2010;13:641–649. doi: 10.2174/1386207311004070641. [DOI] [PubMed] [Google Scholar]
- Ulrich M, Linda KC, Sheena A, Stephen CH, Ellis LR. The human immunodeficiency virus gp120 binding site on CD4: delineation by quantitative equilibrium and kinetic binding studies of mutants in conjunction with a high-resolution CD4 atomic structure. J Exp Med. 1992;176:507–517. doi: 10.1084/jem.176.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainberg MA, Mesplede T, Quashie PK. The development of novel HIV integrase inhibitors and the problem of drug resistance. Curr Opin Virol. 2012;2:656–662. doi: 10.1016/j.coviro.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Wang J, Yan Y, Garrett TPJ, Liu J, Rodgers DW, Garlick RL, Tarr GE, Husain Y, Reinherz EL, Harrison SC. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature. 1990;348:411–413. doi: 10.1038/348411a0. [DOI] [PubMed] [Google Scholar]
- Wang T, Ray J. Aptamer-based molecular imaging. Protein Cell. 2012;3:739–754. doi: 10.1007/s13238-012-2072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Zhao N, Zeng Z, Chang CC, Zu Y. Combination of an aptamer probe to CD4 and antibodies for multicolored cell phenotyping. Am J Clin Pathol. 2010;134:586–593. doi: 10.1309/AJCP55KQYWSGZRKC. [DOI] [PubMed] [Google Scholar]
- Zhao N, Fogg JM, Zechiedrich L, Zu Y. Transfection of shRNA-encoding Minivector DNA of a few hundred base pairs to regulate gene expression in lymphoma cells. Gene Ther. 2011a Mar;18(3):220–4. doi: 10.1038/gt.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Bagaria HG, Wong MS, Zu Y. A nanocomplex that is both tumor cell-selective and cancer gene-specific for anaplastic large cell lymphoma. J Nanobiotechnology. 2011b;9:2. doi: 10.1186/1477-3155-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, You J, Zeng Z, Li C, Zu Y. An Ultra pH-Sensitive and Aptamer-Equipped Nanoscale Drug-Delivery System for Selective Killing of Tumor Cells. Small. 2013 Oct 25;9(20):3477–84. doi: 10.1002/smll.201202694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.