Abstract
IMPORTANCE
Freedom from symptoms is an important determinant of a good death, but little is known about symptom occurrence during the last year of life.
OBJECTIVE
To evaluate the monthly occurrence of physical and psychological symptoms leading to restrictions in daily activities (ie, restricting symptoms) among older persons during the last year of life and to determine the associations of demographic and clinical factors with symptom occurrence.
DESIGN, SETTING, AND PARTICIPANTS
Prospective cohort study. Comprehensive assessments were completed every 18 months, and monthly interviews were conducted to assess the presence of restricting symptoms. Of 1002 nondisabled community-dwelling individuals 70 years or older in greater New Haven, Connecticut, eligible to participate, 754 agreed and were enrolled between 1998 and 1999.
MAIN OUTCOMES AND MEASURES
The primary outcome was the monthly occurrence of restricting symptoms as a dichotomous outcome. The monthly mean count of restricting symptoms was a secondary outcome.
RESULTS
Among the 491 participants who died after their first interview and before June 30, 2011, mean age at death was 85.8 years, 61.9% were women, and 9.0% were nonwhite. The mean number of comorbid conditions was 2.4, and 73.1% had multimorbidity. The monthly occurrence of restricting symptoms was fairly constant from 12 months before death (20.4%) until 5 months before death (27.4%), when it began to increase rapidly, reaching 57.2% in the month before death. In multivariable analysis, age younger than 85 years (odds ratio [OR], 1.30 [95% CI, 1.07–1.57]), multimorbidity (OR, 1.38 [95% CI, 1.09–1.75]), and proximity to time of death (OR, 1.14 per month [95% CI, 1.11–1.16]) were significantly associated with the monthly occurrence of restricting symptoms. Participants who died of cancer had higher monthly symptom occurrence (OR, 1.80 [95% CI, 1.03–3.14]) than participants who died of sudden death, although this difference was only marginally significant (P = .04). Symptom burden did not otherwise differ substantially according to condition leading to death.
CONCLUSIONS AND RELEVANCE
Restricting symptoms are common during the last year of life, increasing substantially approximately 5 months before death. Our results highlight the importance of assessing and managing symptoms in older patients, particularly those with multimorbidity.
Freedom from symptoms is consistently identified by seriously ill persons, their family members, and clinicians as an important determinant of a good death.1 Despite the clinical relevance of end-of-life symptoms, scant data are available about their occurrence or determinants. Symptom occurrence is assumed to increase during the final stages of life on the basis of studies demonstrating a high burden of symptoms in moribund patients.2 Studies that have observed patients longitudinally have focused on select clinical populations, such as seriously ill, hospitalized patients,3 patients with cancer,4 or those in hospice.5 Relatively little is known, however, about the evolution of symptom occurrence in the final months of life among a diverse group of older persons. This information could be used to help guide efforts to improve end-of-life care.
To address this gap in knowledge, we examined symptoms that led to restrictions in daily activities among older persons during the last year of life, hereafter referred to as restricting symptoms. Recognizing that symptoms can range in severity and duration from mild and fleeting to severe and longer lasting, we chose to focus on symptoms leading to restricted activity because they are more likely to be of importance to patients and caregivers. Participants were enrolled in a community-based, longitudinal study that included monthly assessments of a diverse array of physical and psychological symptoms. We examined the occurrence of restricting symptoms during the last year of life and evaluated associations between demographic and clinical characteristics and the monthly occurrence of these symptoms. We were particularly interested in the relationship between conditions leading to death and symptom burden, given differential use of hospice and palliative care services by terminal condition.
Methods
Study Population
Participants were drawn from an ongoing longitudinal study, previously described in detail,6 involving 754 older persons. Eligible participants were community-dwelling, nondisabled, and at least 70 years old at the time of enrollment. Exclusion criteria were substantial cognitive impairment with no available proxy, life expectancy less than 12 months (at the time of eligibility assessment), plans to move out of the area, or inability to speak English. Of the 1002 persons eligible for the study, 754 agreed to participate and were enrolled between 1998 and 1999. Of these, 20 dropped out of the study, 2 died before their first monthly interview, and 491 others, comprising the current analytic sample, subsequently died through June 30, 2011. The research protocol was approved by the Yale Human Investigation Committee.
Data Collection
Comprehensive home-based assessments were completed at baseline and subsequently at 18-month intervals, and telephone interviews were completed monthly. The completion rate of monthly interviews in the last year of life was 98.6%. Because 23 deaths occurred in the first year, the mean (SD) duration of follow-up was 11.8 (1.3) months. During the comprehensive assessments, data were collected on demographic characteristics, cognitive impairment (defined as a Folstein Mini–Mental State7 examination score <20), and the presence of 9 self-reported, physician-diagnosed comorbid conditions, including hypertension, myocardial infarction, heart failure, stroke, cancer, diabetes mellitus, hip fracture, arthritis, and chronic lung disease. For descriptive purposes and to fulfill federal regulations regarding the inclusion of minority participants in studies funded by the US National Institutes of Health, participants were asked to identify their race and ethnicity. Consistent with prior work,8,9 multimorbidity was defined as the presence of 2 or more comorbid conditions. During monthly telephone interviews, data were collected on restricting symptoms. Deaths were ascertained from the next of kin or another knowledgeable person, as well as review of obituaries.
When participants were too ill to complete the monthly interviews, proxy data were obtained using a standard protocol.10 We required that proxies were cognitively intact and lived with the participant or saw the participant regularly. Of the 5776 monthly interviews during the last year of life, 42.2% were completed by proxies. The use of proxies increased as death approached, with 34.8% and 68.3% of the interviews completed by proxies 12 months and 1 month prior to death, respectively. To determine the accuracy of proxy reports, we conducted interviews with 20 participants and their designated proxies (separately) for 6 months and compared their responses to questions assessing the occurrence of restricted activity. Concordance was substantial, with κ = 0.66 (95% CI, 0.50–0.83).
Assessment of Restricting Symptoms
During the monthly interviews, the occurrence of restricting symptoms was ascertained using a standard protocol described previously.6 First, participants were asked 2 questions related to restricted activity: “Since we last talked, have you stayed in bed for at least half a day due to an illness, injury, or other problem?” and “Since we last talked, have you cut down on your usual activities due to an illness, injury, or other problem?” Second, if participants answered “yes” to either of these questions, they were asked whether they had developed any of 24 prespecified symptoms and problems since the last interview.11–14 Third, immediately after each “yes” response to a specific symptom or problem, participants were asked, “Did this cause you to stay in bed for at least half a day or to cut down on your usual activities?” During pilot testing among 20 persons, we found that the test-retest reliability (mean time between assessments, 4.1 days) of this protocol was high, with of κ 0.90 for the presence or absence of restricted activity and of κ 0.75 or greater for the presence or absence of all restricting symptoms. The present analysis focused on the occurrence of 15 restricting symptoms, including fatigue; dizziness or unsteadiness; memory or thinking problem; swelling in feet or ankles; cold or influenza symptoms; musculoskeletal pain; shortness of breath; depression; anxiety; poor eyesight; arm or leg weakness; nausea, vomiting, or diarrhea (ascertained through a single question); urinary problems; difficulty sleeping; and chest pain or tightness. The primary outcome was the monthly occurrence of restricting symptoms as a dichotomous outcome. We also considered the monthly mean count of restricting symptoms as a secondary outcome.
Classification of Conditions Leading to Death
To classify conditions leading to death, we used a previously described protocol15,16 that included 6 categories: cancer, advanced dementia, organ failure, frailty, sudden death, and other conditions. Information about these terminal conditions was obtained from death certificates coded by a certified nosologist and from the comprehensive assessment immediately preceding death. To enhance the specificity of information obtained from death certificates, we required that terminal conditions appear as the immediate or underlying cause of death. Each decedent was assigned a single condition leading to death using a previously described hierarchy, ie, from cancer to advanced dementia to organ failure to frailty to sudden death.15,16
Statistical Analysis
Using information from the comprehensive assessment immediately preceding the study period (ie, last year of life), demographic and clinical characteristics were compared between decedents who experienced restricting symptoms during the last year of life and those who did not. Monthly occurrence of each specific symptom was calculated by dividing the number of persons with that symptom in each month by the total number of persons reporting in that same month. We calculated monthly occurrence of 1 or more restricting symptoms by dividing the number of participants with at least 1 restricting symptom in a given month by the number of participants interviewed in that same month. This monthly occurrence of restricting symptoms was plotted across the last 12 months of life using spline-based interpolation.
To evaluate the primary outcome, ie, monthly occurrence of restricting symptoms as a dichotomous variable, we used logistic regression with generalized estimating equations to adjust for repeated measurements. The logistic model evaluated the multivariable associations between the independent variables and monthly occurrence of 1 or more restricting symptoms using odds ratios (ORs). Odds ratios indicate the adjusted odds of restricting symptoms in participants with each risk factor compared with those without the risk factor. The independent variables considered were age, sex, race, educational attainment, multimorbidity, time (in months) prior to death, cognitive impairment, and condition leading to death. Although the primary objective was to evaluate the occurrence of 1 or more restricting symptoms during the last year of life, we also examined the monthly mean count of restricting symptoms. A Poisson model based on generalized estimating equations was used to evaluate this secondary outcome with the same covariates used in the logistic regression model.
The monthly occurrence of participants who had at least 1 restricting symptom was also plotted for each of the conditions leading to death. Because visual inspection of the graphs depicting the occurrence of 1 or more restricting symptoms indicated a notable change in slope closer to the time of death, we fit a Bayesian linear change-point regression model that estimated time prior to death when the slopes changed statistically, and the corresponding 95% credible intervals (the Bayesian counterpart of confidence intervals). The Bayesian approach assumes that the change-point time is an unknown parameter to be estimated, in contrast to the traditional approach, which chooses the change-point time that yields the best model fit.17 P < .05 (2-tailed) was considered statistically significant. All analyses were performed using SAS, version 9.3 (SAS Institute),18 and graphs were produced with R, version 2.14.2 (R Foundation for Statistical Computing).19
Results
The characteristics of the study population are shown in Table 1. The mean age was 85.8 years, nearly two-thirds of the sample were female, and 9.0% were nonwhite. Approximately two-thirds had completed at least high school, the mean number of comorbid conditions was 2.4, and nearly three-quarters of the sample had multimorbidity. The most common condition leading to death was frailty (28.1%), followed by organ failure (20.8%) and cancer (18.5%). Sudden death was relatively rare (2.9%). The characteristics of decedents with and without restricting symptoms were generally quite similar, with the exception of nonwhite race, cancer, and heart failure, which were more common in the group with symptoms.
Table 1.
Characteristics of Decedents
| Characteristica | Overall (n = 491) | Restricting Symptoms (n = 281) | No Restricting Symptoms (n = 210) | P Valueb |
|---|---|---|---|---|
| Age, mean (SD), y | 85.8 (5.9) | 85.6 (5.8) | 86.0 (6.1) | |
| Age <85 y, No. (%) | 199 (40.5) | 119 (42.3) | 80 (38.1) | .34 |
| Female sex, No. (%) | 304 (61.9) | 175 (62.3) | 129 (61.4) | .85 |
| Nonwhite race,c No. (%) | 44 (9.0) | 33 (11.7) | 11 (5.2) | .01 |
| Education <12 y, No. (%) | 168 (34.2) | 96 (34.2) | 72 (34.3) | .98 |
| Cognitive impairment,d No. (%) | 100 (20.4) | 54 (19.2) | 46 (21.9) | .46 |
| Individual comorbid conditions, No. (%) | ||||
| Hypertension | 310 (63.1) | 173 (61.6) | 137 (65.2) | .40 |
| Arthritis | 220 (44.8) | 135 (48.0) | 85 (40.5) | .10 |
| Myocardial infarction | 119 (24.2) | 65 (23.1) | 54 (25.7) | .51 |
| Diabetes mellitus | 117 (23.8) | 65 (23.1) | 52 (24.8) | .68 |
| Lung disease | 117 (23.8) | 74 (26.3) | 43 (20.5) | .13 |
| Cancer | 111 (22.6) | 74 (26.3) | 37 (17.6) | .02 |
| Heart failure | 76 (15.5) | 53 (18.9) | 23 (11.0) | .02 |
| Stroke | 76 (15.5) | 39 (13.9) | 37 (17.6) | .26 |
| Hip fracture | 51 (10.4) | 29 (10.3) | 22 (10.5) | .95 |
| Comorbid conditions, No., mean (SD) | 2.4 (1.3) | 2.5 (1.3) | 2.3 (1.4) | .14 |
| Multimorbidity,e No. (%) | 359 (73.1) | 214 (76.2) | 145 (69.1) | .08 |
| Conditions leading to death, No. (%) | ||||
| Frailty | 138 (28.1) | 75 (26.7) | 63 (30.0) | .42 |
| Organ failure | 102 (20.8) | 61 (21.7) | 41 (19.5) | .56 |
| Cancer | 91 (18.5) | 55 (19.6) | 36 (17.1) | .49 |
| Advanced dementia | 79 (16.1) | 42 (14.9) | 37 (17.6) | .43 |
| Other | 67 (13.7) | 39 (13.9) | 28 (13.3) | .86 |
| Sudden death | 14 (2.9) | 9 (3.2) | 5 (2.4) | .59 |
Characteristics were determined during the most proximate comprehensive assessment prior to death.
P values were calculated from the t test for continuous age and χ2 statistic for all others.
Race was self-reported; nonwhite category included Hispanics.
Cognitive impairment was defined as a Folstein Mini–Mental State examination score of less than 20.
Multimorbidity was defined as the presence of at least 2 comorbid conditions.
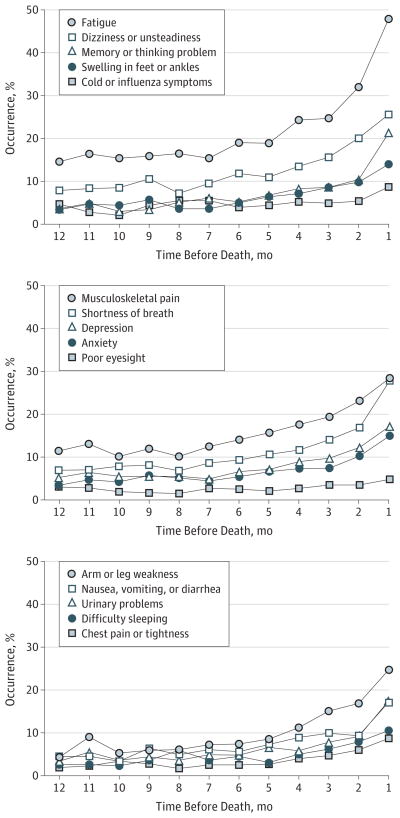
The monthly occurrence of each symptom during the last year of life is shown in Figure 1. The occurrence of all symptoms increased during the study period, most notably beginning 5 months before death. Relative to the other symptoms considered, poor eyesight increased modestly (from 3.0% 12 months before death to 4.8% in the month before death). Fatigue was the most common symptom throughout the study period, followed by musculoskeletal pain, dizziness or unsteadiness, and shortness of breath.
Figure 1. Occurrence of Each Restricting Symptom in the Last Year of Life.
Monthly occurrence of each specific symptom was calculated by dividing the number of participants in each month with that symptom by the total number of participants reporting in that same month. The 15 symptoms were divided among 3 panels to enhance visual clarity.
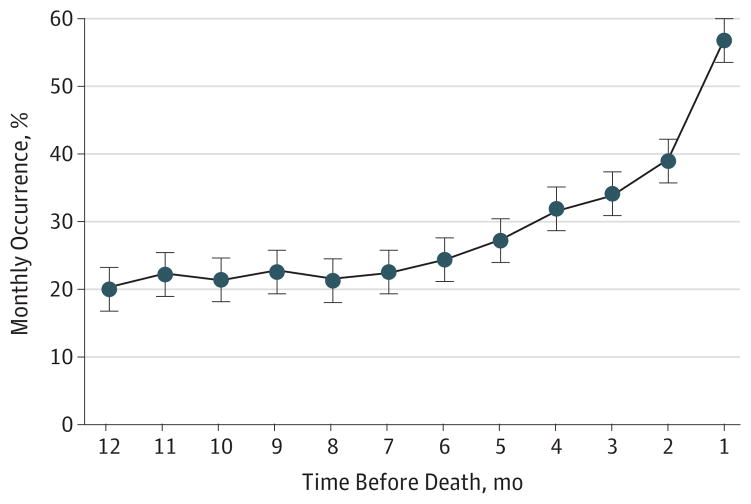
As depicted in Figure 2, the monthly occurrence of 1 or more restricting symptoms was fairly constant from 12 months prior to death (20.4%) until approximately 5 months prior to death (27.4%), when it began to increase rapidly, reaching 57.2% in the month prior to death. According to the Bayesian change-point model, the time at which the slope rose was 5.2 months before death, with a 95% credible interval of 3.1 to 8.7 months.
Figure 2. Monthly Occurrence of 1 or More Restricting Symptoms in the Last Year of Life.
Monthly occurrence was calculated by dividing the number of participants with 1 or more restricting symptoms in that month by the number interviewed in the same month. Bars denote 1 standard error around mean values.
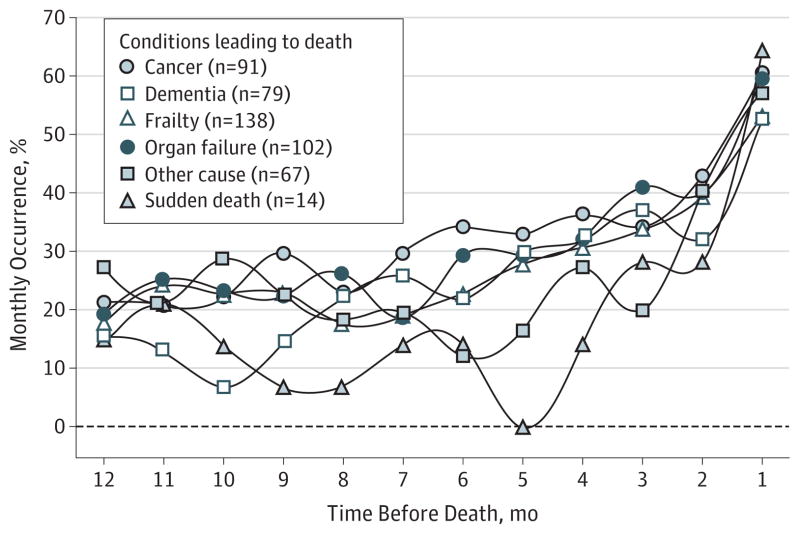
Figure 3 plots the monthly occurrence of 1 or more restricting symptoms stratified by the condition leading to death. Participants who died of cancer generally had the highest occurrence of restricting symptoms, but the patterns were otherwise similar across the decedent groups. According to the Bayesian change-point models, the time at which the slopes rose ranged from 4.7 months before death (for “other conditions”) to 5.8 months before death (for dementia), with credible intervals overlapping by at least 90% in all cases (data not reported), indicating that they were not significantly different from one another. In the month prior to death, the occurrence of restricting symptoms exceeded 50% for all decedent groups.
Figure 3. Monthly Occurrence of 1 or More Restricting Symptoms in the Last Year of Life by Condition Leading to Death.
Monthly occurrence was calculated for each condition leading to death by dividing the number of participants with 1 or more restricting symptoms in that month by the number interviewed in that month.
Table 2 provides the multivariable associations between the demographic and clinical characteristics and the monthly occurrence of at least 1 restricting symptom during the last year of life. Younger age (odds ratio [OR], 1.30 [95% CI, 1.07–1.57]), multimorbidity (OR, 1.38 [95% CI, 1.09–1.75]), and greater proximity to the time of death (OR, 1.14 per month [95% CI, 1.11–1.16]) were significantly associated with the monthly occurrence of 1 or more restricting symptoms. When the analysis was performed with sudden death used as the reference category, only participants who died of cancer had a significantly higher rate of monthly occurrence of at least 1 restricting symptom (OR, 1.80 [95% CI, 1.03–3.14]), although this difference was only marginally significant (P = .04).
Table 2.
Multivariable Associations With Occurrence of Restricting Symptomsa
| Characteristicb | Odds Ratio (95% CI) | P Valuec |
|---|---|---|
| Age <85 y | 1.30 (1.07–1.57) | .009 |
| Female sex | 1.16 (0.96–1.39) | .12 |
| Nonwhite raced | 1.28 (0.97–1.70) | .08 |
| Education <12 y | 1.05 (0.86–1.28) | .64 |
| Multimorbidity | 1.38 (1.09–1.75) | .008 |
| Month in last year of life | 1.14 (1.11–1.16) | <.001 |
| Cognitive impairmente | 1.06 (0.76–1.47) | .74 |
| Conditions leading to deathf | ||
| Cancer | 1.80 (1.03–3.14) | .04 |
| Dementia | 1.34 (0.74–2.41) | .34 |
| Frailty | 1.51 (0.87–2.61) | .14 |
| Organ failure | 1.70 (0.98–2.95) | .06 |
| Other | 1.37 (0.78–2.41) | .27 |
All variables shown in this table have been dichotomized, except for month in last year of life, which was considered as a continuous variable, with increasing proximity to time of death associated with increased risk of occurrence of restricting symptoms, which were defined as 1 or more of the following 15 symptoms: fatigue; dizziness or unsteadiness; memory or thinking problem; swelling in feet or ankles; cold or influenza symptoms; musculoskeletal pain; shortness of breath; depression; anxiety; poor eyesight; arm or leg weakness; nausea, vomiting, or diarrhea; urinary problems; difficulty sleeping; and chest pain or tightness.
Characteristics were determined during the most proximate comprehensive assessment prior to death.
All P values are from logistic regression with generalized estimating equations in SAS Proc Genmod.
Race was self-reported; nonwhite category included Hispanics.
Cognitive impairment was defined as a Folstein Mini–Mental State examination score of less than 20.
Participants dying of sudden death were considered the referent category.
The mean (SD) count of restricting symptoms also rose as the time of death approached, from 4.0 (2.2) restricting symptoms 12 months prior to death to 5.0 (2.6) in the month prior to death. In the multivariable Poisson analysis, multimorbidity (RR, 1.39 [95% CI, 1.12–1.74]) and greater proximity to the time of death (RR, 1.12 per month [95% CI, 1.10–1.14]) were significantly associated with mean number of restricting symptoms. There were no significant differences in the mean count of symptoms according to the condition leading to death.
Discussion
In this prospective study, we found that restricting symptoms were common among older persons during the last year of life, being experienced by nearly half of all decedents in the month before death. The monthly occurrence of restricting symptoms was relatively constant until 5 months prior to death, at which time it increased steadily until the last month of life. Several factors were associated with symptom occurrence, including age less than 85 years, multimorbidity, proximity to time of death, and death of cancer. Our results highlight the need to integrate symptom assessment and management into the routine care of older persons with comorbid illness, particularly those with multimorbidity.
Despite the importance of minimizing symptoms at the end of life, to our knowledge, ours is the first large-scale study with frequent, serial assessments of symptoms among the same group of decedents. Reassessing the presence of symptoms at monthly intervals enhances the likelihood that our findings accurately depict temporal trends over the last year of life. Our study has several additional strengths, including low rates of missing data and losses to follow-up. The validity of our results is further strengthened by the high reliability of our symptom assessments. Our focus on symptoms leading to restricted activity enhances the clinical relevance of our findings because proper management of these symptoms may substantially improve physical function and quality of life while reducing caregiver burden.
Whereas the assessment of pain has been the focus of much attention in previous research20–22 and in clinical practice standards,23 we evaluated a broad range of physical and psychological symptoms that are common, and potentially treatable, among seriously ill older persons. The most common symptom experienced was fatigue, followed by musculoskeletal pain, dizziness, and shortness of breath. Although fatigue and dizziness may be viewed as inevitable consequences of aging and illness, there are a number of treatable causes, including hypothyroidism, anemia, and polypharmacy. Therapeutic decisions in older persons must be premised on a careful consideration of risks vs benefits. In the case of potent analgesics (such as narcotics) to alleviate restricting pain, the potential risks are particularly serious, including confusion and falls, but judicious use may improve overall physical function in many cases. Adding to the complexity, physical symptoms such as pain and dyspnea reflect not only pathophysiologic derangements but also the individual patient’s experience of the resulting physical sensation.24 Prior research has shown that dyspnea in patients with heart failure is significantly affected by depression and by patients’ perceived control over their condition.25 The high rates of restricting depression and anxiety in the last year of life (up to 27.4% and 23.0%, respectively) in our sample further suggest that increased screening and treatment of these conditions is warranted.
Consistent with previous studies,20,26 we found few differences in symptom burden according to condition leading to death. Participants who died of cancer had higher monthly symptom occurrence, but the mean number of symptoms experienced was the same across all conditions leading to death. These findings suggest that a focus on symptom management should be standard in the care of all seriously ill older persons. The challenges of precise prognostication regarding life expectancy represent a major barrier to more widespread use of hospice care, which is predicated on patients having a relatively short life expectancy.
In contrast to hospice, palliative care focuses on providing relief from the symptoms and stress of any serious illness irrespective of life expectancy or diagnosis (eg, cancer vs noncancer). The primary goal is to improve quality of life for both the patient and the family, and palliative care can be provided concurrently with curative treatment.27 Notably, among the demographic and clinical characteristics considered in our analyses, multimorbidity had one of the strongest associations with symptom occurrence. Our results highlight the need for palliative services for older persons, particularly in the context of multimorbidity. This need is underscored by previous work that demonstrates a high burden of symptoms among a nondecedent cohort of older adults.28
Restricting symptoms were less common among persons aged 85 years or older, consistent with previous end-of-life studies that have reported decreasing occurrence of serious symptoms with advancing age.20–22 This finding may reflect greater focus on symptom management in the oldest old, in accordance with goals of care. Although it is possible that reporting of symptoms was compromised in this group, the presence of cognitive impairment was not associated with symptom occurrence.
There are several issues that should be considered when interpreting our results. First, given the high prevalence of multimorbidity, the identification of a single condition leading to death may be an oversimplification. Second, because participants were members of a single health plan in a small urban area, our results may not be generalizable to older persons in other settings. However, the demographic characteristics of our cohort are similar to those of the US population as a whole, with the exception of race or ethnicity.10 Third, persons with disability (N = 244) or limited life expectancy at the time of eligibility assessment (N = 3) were not included in the parent study. Although the mortality rate (68%) during the 13-year study period was high, it is possible that the inclusion of these persons with poor prognosis would have altered our findings. Fourth, an increasing proportion of interviews were completed by proxy respondents as death approached. Although we found substantial agreement between participants’ and proxies’ reports of activity restriction, it is possible that proxies may underreport or overreport symptoms leading to restricted activity at the end of life. However, the inclusion of proxy reports mirrors clinical reality because clinicians must often rely on caregivers to provide information about symptoms in older persons at the end of life when they are unable to verbally communicate themselves. Fifth, we did not have information about treatment of symptoms, so it is not possible to discern whether our findings represent underdiagnosis or undertreatment of restricting symptoms. Sixth, although we refer to inclusion of “15 restricting symptoms,” some are actually groups of symptoms. Seventh, because only symptoms leading to restricted activity were ascertained, the overall burden of symptoms in the last year of life was likely underestimated. Finally, whereas prior work has highlighted limitations in the use of the retrospective study design in studying end-of-life care,29 our objective was not to critique care delivered in the last year of life. Furthermore, given the uncertainties of prognostication for life expectancy, retrospective assessments of decedents remain an important methodology for examining end-of-life experiences.30
In conclusion, symptoms that lead to restrictions in daily activities are common at the end of life, with notable increases beginning in the last 5 months prior to death. Given the challenges to precise prognostication regarding life expectancy in clinical practice, our results highlight the importance of assessing and managing symptoms in all older patients with comorbid illness, particularly those with multimorbidity, even when they are not clearly at the end of life. Expanded use of palliative care services in such patients may improve their function and quality of life and reduce caregiver burden.
Acknowledgments
Funding/Support: The work for this article was supported by a grant from the National Institute on Aging (NIA) (R37AG17560). The study was conducted at the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342). Dr Gill is the recipient of an NIA Midcareer Investigator Award in Patient-Oriented Research (K24AG021507). Dr Chaudhry is the recipient of an NIA Beeson Career Development Award (K23AG030986).
Role of the Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Disclosures: None reported.
Additional Contributions: Linda Leo-Summers, MPH, provided technical assistance in the preparation of this manuscript.
Author Contributions: Drs Chaudhry and Gill had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the analyses.
Study concept and design: Chaudhry, Gill.
Acquisition of data: Gahbauer, Gill.
Analysis and interpretation of data: Chaudhry, Murphy, Sussman, Allore, Gill.
Drafting of the manuscript: Chaudhry, Murphy, Gahbauer.
Critical revision of the manuscript for important intellectual content: Chaudhry, Murphy, Sussman, Allore, Gill.
Statistical analysis: Chaudhry, Murphy, Gahbauer, Allore.
Obtained funding: Gill.
Administrative, technical, and material support: Chaudhry, Gill.
Study supervision: Gill.
References
- 1.Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284(19):2476–2482. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 2.Ventafridda V, Ripamonti C, De Conno F, Tamburini M, Cassileth BR. Symptom prevalence and control during cancer patients’ last days of life. J Palliat Care. 1990;6(3):7–11. [PubMed] [Google Scholar]
- 3.Desbiens NA, Mueller-Rizner N, Connors AF, Jr, Wenger NS, Lynn J SUPPORT Investigators. The symptom burden of seriously ill hospitalized patients. J Pain Symptom Manage. 1999;17(4):248–255. doi: 10.1016/s0885-3924(98)00149-3. [DOI] [PubMed] [Google Scholar]
- 4.Mercadante S, Casuccio A, Fulfaro F. The course of symptom frequency and intensity in advanced cancer patients followed at home. J Pain Symptom Manage. 2000;20(2):104–112. doi: 10.1016/s0885-3924(00)00160-3. [DOI] [PubMed] [Google Scholar]
- 5.Owens MR, Simmons B, Gibson PS, Weeks D. A longitudinal study of pain in hospice and pre-hospice patients. Am J Hosp Palliat Care. 2001;18(2):124–128. doi: 10.1177/104990910101800211. [DOI] [PubMed] [Google Scholar]
- 6.Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135(5):313–321. doi: 10.7326/0003-4819-135-5-200109040-00007. [DOI] [PubMed] [Google Scholar]
- 7.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 8.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 9.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition—multimorbidity. JAMA. 2012;307(23):2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291(13):1596–1602. doi: 10.1001/jama.291.13.1596. [DOI] [PubMed] [Google Scholar]
- 11.Rakowski W, Julius M, Hickey T, Verbrugge LM, Halter JB. Daily symptoms and behavioral responses: results of a health diary with older adults. Med Care. 1988;26(3):278–297. doi: 10.1097/00005650-198803000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Verbrugge LM, Ascione FJ. Exploring the iceberg: common symptoms and how people care for them. Med Care. 1987;25(6):539–569. [PubMed] [Google Scholar]
- 13.Brody EM, Kleban MH. Day-to-day mental and physical health symptoms of older people: a report on health logs. Gerontologist. 1983;23(1):75–85. doi: 10.1093/geront/23.1.75. [DOI] [PubMed] [Google Scholar]
- 14.Tinetti ME, Mendes de Leon CF, Doucette JT, Baker DI. Fear of falling and fall-related efficacy in relationship to functioning among community-living elders. J Gerontol. 1994;49(3):M140–M147. doi: 10.1093/geronj/49.3.m140. [DOI] [PubMed] [Google Scholar]
- 15.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA. 2003;289(18):2387–2392. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 16.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362(13):1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry D, Hartigan JA. A Bayesian analysis for change point problems. J Am Stat Assoc. 1993;88(421):309–319. [Google Scholar]
- 18.SAS User’s Manual. Cary, NC: SAS Institute; 2009. Version 9.3. [Google Scholar]
- 19.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 20.Smith AK, Cenzer IS, Knight SJ, et al. The epidemiology of pain during the last 2 years of life. Ann Intern Med. 2010;153(9):563–569. doi: 10.1059/0003-4819-153-9-201011020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17(3):417–431. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- 22.Desbiens NA, Mueller-Rizner N, Connors AF, Jr, Hamel MB, Wenger NS. Pain in the oldest-old during hospitalization and up to one year later. J Am Geriatr Soc. 1997;45(10):1167–1172. doi: 10.1111/j.1532-5415.1997.tb03765.x. [DOI] [PubMed] [Google Scholar]
- 23.Phillips DM Joint Commission on Accreditation of Healthcare Organizations. JCAHO pain management standards are unveiled. JAMA. 2000;284(4):428–429. doi: 10.1001/jama.284.4.423b. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong TS. Symptoms experience: a concept analysis. Oncol Nurs Forum. 2003;30(4):601–606. doi: 10.1188/03.ONF.601-606. [DOI] [PubMed] [Google Scholar]
- 25.Heo S, Doering LV, Widener J, Moser DK. Predictors and effect of physical symptom status on health-related quality of life in patients with heart failure. Am J Crit Care. 2008;17(2):124–132. [PubMed] [Google Scholar]
- 26.Bekelman DB, Rumsfeld JS, Havranek EP, et al. Symptom burden, depression, and spiritual well-being: a comparison of heart failure and advanced cancer patients. J Gen Intern Med. 2009;24(5):592–598. doi: 10.1007/s11606-009-0931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palliative Care and Hospice Care Across the Continuum. [Accessed September 25, 2012];Center to Advance Palliative Care website. www.capc.org/palliative-care-across-the-continuum.
- 28.Sha MC, Callahan CM, Counsell SR, Westmoreland GR, Stump TE, Kroenke K. Physical symptoms as a predictor of health care use and mortality among older adults. Am J Med. 2005;118(3):301–306. doi: 10.1016/j.amjmed.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Bach PB, Schrag D, Begg CB. Resurrecting treatment histories of dead patients: a study design that should be laid to rest. JAMA. 2004;292(22):2765–2770. doi: 10.1001/jama.292.22.2765. [DOI] [PubMed] [Google Scholar]
- 30.Barnato AE, Lynn J. Resurrecting treatment histories of dead patients. JAMA. 2005;293(13):1591–1592. doi: 10.1001/jama.293.13.1591-b. [DOI] [PubMed] [Google Scholar]