Abstract
Metastatic tumours involving the brain overshadow primary brain neoplasms in frequency and are an important complication in the overall management of many cancers. Importantly, advances are being made in understanding the molecular biology underlying the initial development and eventual proliferation of brain metastases. Surgery and radiation remain the cornerstones of the therapy for symptomatic lesions; however, image-based guidance is improving surgical technique to maximize the preservation of normal tissue, while more sophisticated approaches to radiation therapy are being used to minimize the long-standing concerns over the toxicity of whole-brain radiation protocols used in the past. Furthermore, the burgeoning knowledge of tumour biology has facilitated the entry of systemically administered therapies into the clinic. Responses to these targeted interventions have ranged from substantial toxicity with no control of disease to periods of useful tumour control with no decrement in performance status of the treated individual. This experience enables recognition of the limits of targeted therapy, but has also informed methods to optimize this approach. This Review focuses on the clinically relevant molecular biology of brain metastases, and summarizes the current applications of these data to imaging, surgery, radiation therapy, cytotoxic chemotherapy and targeted therapy.
Introduction
Among the many undesirable effects of systemic cancer is metastatic spread to the brain, with subsequent deleterious effects on many critical functions controlled by this organ. Indeed, brain metastasis is an indicator of poor prognosis and nearly always determines a fatal outcome in patients with solid cancers. Currently, no effective measures are available to reliably prevent this event; therefore, intense vigilance for relevant symptoms is necessary to detect early involvement of the brain due to cancer metastases. Early confirmation of brain metastasis is critical to enable intervention to minimize irreversible damage of the nervous system.
Selective use of radiation therapy and surgery are the mainstay treatment for the management of many meta-static lesions in the brain, particularly if they become symptomatic; however, these modalities have many limitations depending on the location and characteristics of the tumour (Box 1), and owing to acute and delayed adverse effects. Efficacious therapies that can be administered systemically to avoid such pathological effects on the brain are scarce, due in part to limitations on brain uptake imposed by the blood–brain barrier (BBB). Nonetheless, gadolinium enhancement of MRI scans reveals that this barrier is incompetent in most brain metastases. The incorporation of targeted therapy in the systemic management of cancer has produced remarkable success, mainly at extracranial sites. Furthermore, innovative approaches such as pulse dosing and direct intratumoural delivery hold great promise in the therapeutic management of brain metastases.
Box 1. Surgery versus SRS for brain metastasis.
Surgery with or without WBRT* can be considered in tumours with the following characteristics:
▪ Mass effect (particularly relevant for metastases in the posterior fossa)‡
▪ Superficial and/or accessible location
▪ Maximal diameter >30–40 mm
▪ Radioresistant histology
▪ When a diagnosis is uncertain
SRS with or without WBRT* might be appropriate for tumours with the following features:
▪ Poor candidates for surgical resection
▪ Deep and/or inaccessible location
▪ Maximal diameter <20–30 mm
▪ Radiosensitive histology
▪ Situated close to the eloquent brain
*Assess systemic disease status along with the need for urgent decompression. ‡Consider multimodality treatment for multiple lesions. Abbreviations: SRS, stereotactic radiosurgery; WBRT, whole-brain radiation therapy.
This Review provides an overview of current management modalities for brain metastases, with particular emphasis on therapies that specifically target the key biological mechanisms involved in cancer development and treatment resistance. The epidemiology, biology and diagnosis of brain metastases, factors which are relevant to the management of this condition, are also discussed.
Epidemiology of brain metastases
The estimated prevalence of new brain metastases in the USA is between 7–14 persons per 100,000 based on population studies. On the basis of an official census of nearly 310 million people in the USA,1 the expected incidence of newly diagnosed patients with brain metastases is estimated to be between 21,651 to 43,301 per year.2 Furthermore, as the US population increased from 285 million people in 2000 to 310 million in 2010,1 the prevalence of metastases to the brain is expected to continue to increase in the future. Indeed, in a survey of Swedish individuals hospitalized from 1987 through 2006 and published in 2009,3 the annual age-adjusted incidence of hospitalization for brain metastases doubled from seven to 14 cases per 100,000 admissions. Interestingly, the primary cancer sites associated with brain metastases have varied over the past decades, reflecting underlying cancer incidence and mortality patterns. For example, in a comparison of patients with brain metastases treated in the years 1983–1989 versus 2005–2009 (n = 103 per cohort), Nieder et al.4 found a reduced incidence of primary lung cancers (52% versus 40%), an increased frequency of melanomas (5% versus 9%) and substantial increases in the number of patients with primary colorectal and kidney cancers (8% versus 24%), whereas breast cancer cases remained stable (17%). The global prevalence of brain metastases in patients with cancer is probably around 8.5–9.6%.5,6 On the basis of data from patients recorded in the Metropolitan Detroit Cancer Surveillance System between 1973 and 2001, the most common primary tumours responsible for brain metastases are lung cancer (19.9%), melanoma (6.9%), renal cancer (6.5%), breast cancer (5.1%) and colorectal cancer (1.8%).6
The true prevalence of brain metastases, however, might be far higher than reported in these surveys; asymptomatic brain metastases can go undetected, and symptomatic brain metastases might not be reported in patients with widespread metastatic disease.7 A high prevalence of undetected lesions was suggested in historical autopsy series,8,9 which identified brain metastases in 15–41% of patients with known primary cancers at the time of death, although lower estimates have been reported in similar studies.10 Moreover, brain metastases were identified in 40% of patients with melanoma,8 and in 16–30% of patients with breast cancer in whom autopsies were performed.11 These numbers far exceed those published in the aforementioned population-based studies.5,6 From an epidemiological standpoint, the low rate of postmortem autopsies performed in patients who died with malignant diseases, which currently stands at <5%,7 poses a challenge to future studies that aim to track the prevalence of brain metastases in patients with cancer.
Several hypotheses have been proposed to explain the reported increase in the incidence of brain metastases over the past decades. Certainly, improvements in imaging technologies during this period have led to increased detection of metastatic lesions; powerful neuro imaging modalities have become widely available and used in the detection of brain metastases, particularly MRI, which is presently used to assess approximately 64% of patients with cancer versus 2% 20 years ago.4 Another probable contributory factor is the global increase in cancer prevalence that has occurred during this time, and in particular those that have a predilection to metastasize to the brain, such as lung cancer.12 Improvements in the survival of patients with cancer due to earlier detection and improved treatment are also suggested to play a part in the increased incidence of brain metastases, and might have an increasingly prominent role as the incidence rates of breast and lung cancer now seem to be declining.13,14 The net effect of these phenomena is a rising number of individuals in the population at-risk of the development of brain metastases.3 The wipespread introduction of targeted therapies that have limited bioavailability in the brain might also have resulted in an iatrogenic increase in brain metastasis. For example, the monoclonal antibody trastuzumab is effective in the treatment of HER2-positive breast cancer; however, this targeted biological therapy has a limited capacity to enter the central nervous system (CNS) and therefore low efficacy therein,15,16 and an observed increase in brain metastases in trastuzumab-treated patients has led to the suggestion that this agent might make the CNS a potential ‘sanctuary’ site for meta-static disease.7 All of these factors likely contribute to the observed increase in incidence of brain metastases.17,18
The biology of brain metastases
Knowledge of the biology of brain metastasis is essential for the development and optimization of therapies for this disease. Various aspects of brain tumours have been identified as key factors in this regard, as detailed in the following sections.
The relevance of astrocytes
In 2011, Eichler et al.19 published an elegant Review on the biology of brain metastases in this journal. In this Review,19 the inefficient escape of cancer cells from a primary tumour site and colonization of the CNS was described. Nevertheless, the brain microenvironment, including brain vascular endothelial cells and stromal cells (microglia and astrocytes), provides growth and invasion advantages to the disseminated tumour cells.20,21 In elegant preclinical models, the normal neuro protective role of activated astrocytes was shown to extend to metastatic brain tumour cells in vitro after exposure to chemotherapeutic agents.20,22 Astrocytes are intimately involved in maintaining normal homeostasis of the brain microenvironment, accomplished through transport of nutrients to the neurons and facilitation of neural signal transduction. These mechanisms, usually deployed by activated astrocytes to protect injured neurons from apoptosis, could be co-opted to protect tumour cells in brain metastases from the cytotoxic effect of chemotherapeutic agents.20,21 Moreover, activated astrocytes also induced upregulation of survival genes, such as GSTA5, BCL2L1 and TWIST1, in co-cultured tumour cells, which was associated with increased resistance to chemotherapy in vitro.22 Thus, strategies targeting astrocytes or astrocyte-mediated tumour cell processes might have therapeutic potential in preventing or treating brain metastasis.
The importance of angiogenesis
The growth of metastatic brain tumours is critically dependent on angiogenesis, and thus therapies targeting this process might be important in the management of brain metastasis. Disordered angiogenesis results in structural and functional abnormality of tumour-associated blood vessels, characterized by defective endothelial cells, pericyte covering and basement membranes.23,24 These abnormalities can directly restrict the delivery of oxygen, leading to intratumoural hypoxia. Impaired efficacy of systemically administered anti-cancer therapeutics and agents used in radiation therapy due to limited perfusion of the cancer tumour bed and thus exposure to the drug, might lead to the establishment of functional sanctuary sites that enable the growth of cancer cells.25 Accordingly, the use of antiangiogenic therapies might be expected to decrease the efficacy of cytotoxic therapies; however, the opposite effect has in fact been reported, probably through ‘normalization’ of the disordered blood flow and reduced interstitial pressure within the tumour bed. Although the precise physiological mechanism by which antiangiogenic therapy enhances efficacy of other antitumour agents is not entirely clear, such normalization of tumour perfusion (and thus alleviation of hypoxia) are postulated to underlie this effect.26 Indeed, VEGFR2 blockade has been shown to result in a critical period of blood flow normalization, during which this approach optimally potentiates the effect of radiation therapy.27 This window of opportunity is characterized by an increase in tumour oxygenation, increased pericyte coverage of intratumoural vessels—mediated via upregulation of angiopoietin-1 (Ang-1)—and basement membrane degradation via activated matrix metalloproteinase enzymes.27 In a phase II clinical trial in primary glioblastoma,28 the pan-VEGFR small-molecule inhibitor, cediranib, combined with chemoradiation, improved tumour perfusion in a subset of patients. The increased perfusion of cancer tissue was associated with increased tumour oxygenation, which in turn correlated with changes in relevant angiogenic biomarkers, such as plasma levels of placenta growth factor (PGF) and soluble VEGFR2.28 Importantly, overall survival improved in this patient subset compared with the population with no improvement in tumour perfusion.28 Although these data are from patients with primary brain tumours, the biological mechanism elucidated might be pertinent to cerebral metastatic disease.
In a preclinical brain metastases model of HER2-amplified breast cancer based on orthotopic xenografting of human BT-474 cells in mice, extracranial disease was successfully controlled using the HER2 inhibitors trastuzumab (an anti-HER2 monoclonal antibody) or lapatinib (a tyrosine kinase inhibitor [TKI] that targets the EGFR and HER2), but these agents did not halt tumour growth in the brain.29 The addition of anti-VEGFR2 antibodies to the therapeutic protocol led to better control of tumour growth in the brain and improved survival, especially with combined lapatinib, trastuzumab and anti-VEGFR2 antibody treatment.29 In this instance, the therapeutic benefit of antiangiogenic therapy seemed to be manifested primarily as a reduced total and functional micro-vascular density and a marked increase in necrosis.29 The clinical promise of this strategy was demonstrated in a phase I study30 in 26 patients with breast cancer who were treated with the combination of trastuzumab, lapatinib and the VEGF-A inhibitor bevacizumab, with six of the 10 patients who had brain metastasis achieving prolonged stable disease lasting 6 months or more.
Cancer cell phenotypes in brain metastasis
Although our understanding of the biological changes in the tumour environment continues to expand, much remains to be learned about the specific mechanisms and characteristics of cancer cells that drive and/or facilitate colonization of the CNS. Further efforts focusing on identification and characterization of the molecular genetic underpinnings of CNS metastases are still needed, and are likely to lead to new opportunities for drug development in both the adjuvant and prophylactic settings. In this regard, Zhang et al.31 characterized circulating tumour cells (CTCs) from patients with breast cancer and identified a predictive signature for cancer cells with increased capacity for brain involvement, including a lack of expression of epithelial cell adhesion molecule (EpCAM) and positivity for HER2, EGFR, heparanase (HPSE) and Notch1 expression. Using a different approach, Bos et al.32 characterized the gene-expression profiles of brain infiltrating cancer cells from patients with metastatic breast cancer and identified prostaglandin G/H synthase 2 (also known as c yclooxygenase-2 [COX2]), the EGFR ligand heparin-binding EGF-like growth factor (HBEGF), and ST6GalNAc5, as mediators of cancer cell breach of the BBB. Future studies are now required to investigate the potential relevance of these findings to the therapeutic management of brain metastases.
MRI in diagnosis of brain metastases
Efficient detection of brain tumours early in the course of the disease is important to enable effective treatment to be provided, and advances in MRI have improved the identification of these brain metastases. Using MRI, multiple brain metastases are often visualized on contrast-enhanced T1-weighted images. Nonetheless, a solitary metastasis is not uncommon and can have some similarities in appearance to high-grade gliomas, such as evidence of central necrosis. Two major advances in MRI technique have been shown to produce data that enables differentiation between metastatic and primary tumours: magnetic resonance spectroscopy (MRS) and perfusion-weighted imaging (PWI). MRS characterizes regions of brain based on the abundance of specific metabolites. Spectra from MRS analyses of tumours differ from those characteristic of normal brain tissues; cancer tissues have increased levels of choline—a marker of cell p roliferation—and decreased levels of the neuronal biomarker N-acetylaspartate (NAA).33 In addition, PWI-derived relative cerebral blood volume (rCBV) measurements can be used to identify and quantify areas of neovascularization within the brain. Although choline to NAA ratios and rCBV values are similar in high-grade gliomas and brain metastases themselves, these characteristics are markedly different in the peritumoural region that lies outside the contrast-enhancing margins of these tumours. Whereas high-grade gliomas have a highly infiltrative nature, and thus have peritumoural regions containing infiltrating tumour cells, the tissues surrounding brain metastases usually contain no infiltrating cancer cells.34 As a result, the choline to NAA ratio in the peritumoural region of high-grade gliomas is typically substantially higher than normal brain, facilitating differential diagnosis of such tumours from brain metastases, the peritumoural regions of which have almost the same choline and NAA levels as normal brain.35 Similarly, the rCBV of peritumoural regions in high-grade glioma is higher than that of normal brain tissue, whereas rCBVs of peritumoural tissues associated with brain metastases and normal brain tissue are comparable.35,36
Importantly with regard to therapeutic intervention, techniques that promise to improve the early detection of metastatic brain tumours are currently being developed in vivo. For instance, in a mouse model of small meta-static breast tumours, infusions of recombinant human tumour necrosis factor (TNF) has been reported to induce selective permeabilization of the BBB to imaging tracers at sites of brain metastases.37 This method enabled the detection of smaller tumours than are currently visualized using standard imaging techniques.37 Of note, this approach increased the delivery of radiolabelled trastuzumab to metastatic lesions, whereby this biological agent is excluded without prior administration of TNF,37 suggesting applicability to therapy as well as diagnosis. Furthermore, human brain metastases shared similar, predominantly vascular, TNF receptor expression with tumours from mice, implying that clinical translation of this technique might be feasible.37 MRI using antibodies targeting vascular cell adhesion molecule-1 (VCAM-1) conjugated to microparticles of iron oxide has also been shown to enable early detection of metastatic brain tumours in two mouse models;38 this conjugate could be detected in tumours as early as the 1,000-cell stage. VCAM-1 was chosen as the targeted delivery vehicle due to its presence on endothelial cell membranes in developing tumour-associated blood vessels. Importantly, this approach could be clinically relevant, as pathology specimens from human metastatic brain tumours were found to have increased expression of VCAM-1 in vessels adjacent to even a monolayer of tumour cells, with the authors concluding that this methodology might enable the detection of metastases two to three orders of magnitudes smaller than is currently possible (0.3–3 × 105 cells rather than 107–108 cells).38 Nevertheless, further investigation and development of these technologies is needed to prove their safety and feasibility in humans.
Brain metastases—surgical management
Historical and current concepts
Advancements in neuroanaesthesia, instrumentation and imaging technologies, as well as improvements in standard tools, such as the operating microscope, now enable neurosurgeons to perform surgery more safely than ever before. Indeed, in 2010, the first evidence-based compendium for the treatment of patients with brain metastases published a level 1 recommendation for surgical resection combined with radiation therapy to prolong life in relatively young patients with good functional status and a newly diagnosed solitary brain metastasis.39 Prior to this formal guidance on the utility of surgery in patients with brain metastases, the benefits of this therapeutic option had been established in many studies. In 1990, Patchell et al.40 published a definitive study reporting that surgery followed by radiation therapy yielded a median survival of 40 weeks compared with 15 weeks in patients who received radiation alone. Shortly thereafter, Vecht et al.41 demonstrated a 4-month survival advantage for patients undergoing surgical resection in combination with radiation therapy compared with those receiving radiation therapy alone. Although no evidence from randomized controlled trials exists in support of surgery for multiple or recurrent metastatic lesions in the brain, a number of thorough retrospective studies and well-designed prospective studies have investigated the effect of surgery on survival in such cases.39,42,43 The currently available data indicate that resection of all lesions confers a similar survival advantage to resection of a single solitary metastasis.43,44 In cases of recurrent disease a considerable survival advantage, as well as improved quality of life, has been observed with repeat surgical resection.44,45
The use of stereotactic radiosurgery (SRS), involving noninvasive ablation of cells using high-dose radiation, is an option when conventional surgery is not considered for metastatic brain tumours. The Radiation Therapy Oncology Group (RTOG) 9508 study,48 published in 2004, showed a survival benefit in patients undergoing SRS and whole-brain radiation therapy (WBRT) compared with individuals treated with WBRT alone (6.5 months versus 4.9 months); 6-month functional scores were also improved in the SRS cohort.39,48 In addition, Kondziolka et al.49 reported substantial improvements in radio graphically assessed control of disease in patients with two to four brain metastases, according to the local failure rate at 1-year after treatment with SRS and WBRT (8%) compared with WBRT alone (100%). Notably, no class I evidence from adequately powered, randomized controlled studies comparing SRS to standard surgical resection exists, nor is class I data available on the role of resection followed by SRS, although a North American phase III trial relevant to the latter issue is currently underway.50 The current treatment paradigm describing the use of conventional surgery versus SRS in patients with brain metastasis is summarized in Box 1.
Surgical decision making
Perhaps the most critical aspect of the surgical management of brain metastasis is the decision to proceed with an operation. Careful patient selection based on the current body of evidence is of paramount importance. Currently, class I evidence is available in support of surgical resection followed by WBRT in patients with a newly diagnosed solitary brain metastasis, without advanced systemic disease, who spend more than 50% of their time out of bed.39 Additional surgical considerations include the accessibility and size of the lesion, as well as its relative proximity to eloquent brain and the degree of mass effects (pathology secondary to displacement of normal brain architecture by the tumour combined with the severity of biochemical response of the brain to tumour growth and invasion) or hydrocephalus (Box 1); the need for a definitive diagnosis is also an important factor. Thus, the ideal surgical patient would be a relatively young (aged <65 years), medically fit individual in need of a diagnosis, with excellent performance status and limited extracranial disease whose lesion is located in the right frontal pole. The data available on surgical resection of brain metastases, and consequently operative decision making, are less clear in considering an older patient with borderline performance status, multiple medical comorbidities, uncontrolled and/or diffuse extracranial disease, and multiple metastatic lesions located near the dominant frontal operculum and opposite insular region with mass effect upon the ventricular system resulting in hydrocephalus. Therefore, the surgeon must consider how best to maximize the benefits of surgery in each individual patient without causing undue harm.
In the past 5 years, a better understanding of the effect of postoperative complications has also emerged, further underscoring the need to perform surgery as safely as possible. For example, evidence from studies in high-grade glioma indicates that a new postoperative neurological deficit decreases survival up to 3–4 months, and any substantial postoperative complication negatively affects functional status and the patient’s ability to undergo subsequent radiation treatments, both of which are highly important factors in determining survival.51,52 At present, a multitude of surgical techniques and adjuncts are available to aid the provision of maximally effective, safe surgery and even more neurosurgical procedures are in the development pipeline, as discussed in the following sections.
Incorporation of new surgical techniques
Various advanced techniques and surgical adjuncts aimed at driving down the risk of morbidity and enhancing the benefit of surgery have been introduced into the neuro-surgical theatre. Although stereotactic intra operative guidance has been used for three decades, the value of this methodology has progressively grown as it has been integrated with multimodality imaging and advances in computing power, enabling improved 3D volumetric rendering of anatomy and function.46,53–56 At centres for the management of brain tumours, patients now commonly undergo extensive preoperative imaging to provide detailed information that facilitates intra operative navigation, including functional MRI, MRS and diffusion tractography, in addition to intra operative MRI (iMRI).57–62 iMRI provides valuable information regarding the extent of resection, as well as real-time intra operative feedback that enables compensation for brain shift during longer surgeries for complicated or extensive cases of brain metastasis.46,54,62,63 Initial studies evaluating the usefulness of iMRI for surgical removal of brain tumours have been confined largely to intrinsic lesions, and have indicated that surgeons continued resection 38% of the time after iMRI assessment of the completeness of resection following their initial attempt, which underscores the importance of intraoperative imaging in this patient population.64 The literature suggests at least level 2 evidence supports the utility of iMRI in improving the extent of resection, survival and quality of life in patients with glioma.62,65 Whether this therapeutic value will extend to metastatic brain tumours remains to be determined; however, as iMRI technology becomes more common, this issue will probably be resolved. Taken together, these technologies enable more insightful operative decision-making and risk assessment, even as surgeons are in the process of performing surgery.46,58,59,63
Additional intraoperative techniques within the neuro-surgical armamentarium include awake craniotomy and neurophysiological monitoring for functional assessment during resection, as well as other optical and molecular visualization technologies, including 5-aminolevulinic acid (5-ALA) fluorescence or fluorescein staining of malignant tissues within the operative bed. Multiple groups have obtained excellent clinical results using these optical and molecular visualization technologies for intraoperative mapping for resection of centrally located metastatic brain tumours.60,61 Feasibility studies have demonstrated that intraoperative confocal microscopy for histological markers and detection of tumour cells using fluorescein is a practical and useful tool.66,67
Future directions of surgical methods
In the coming years, routine use of surgical cryoablation or laser-heat ablation techniques, which incorporate real-time thermal feedback within the iMRI suite, will probably improve the stereotactic and minimally invasive treatment of spherical, well-circumscribed metastatic tumours in the brain, potentially obviating the need for radiation therapy for some lesions. Other molecular imaging technologies hold a great deal of promise, including Raman spectroscopy, a technology that can enable identification of brain tumour cells in vivo according to their molecular polarization potential.68 When used as an intraoperative probe device, this technology is sensitive enough to distinguish between areas of normal brain, brain tissues undergoing invasion by tumour cells and brain tumour tissue.68,69 Thus, one can envision this technology augmenting tumour surgery, subsequently leading to maximally safe, efficacious resection, or even automated targeted cancer cell destruction via focused ultrasound or other modalities. In addition, two clinical trials70,71 have been completed (with final results pending), and up to eight more studies are actively recruiting, in an effort to determine the utility of intraoperative 5-ALA-fluoresence-based guidance in safely maximizing the extent of resection in brain tumour surgery.72–79
Radiation therapy
Whole-brain radiation therapy
WBRT has historically been used as the primary non-surgical therapeutic modality for the treatment of brain metastasis (previously reviewed elsewhere80). This trend was due, in part, to the limited chemotherapeutic options demonstrated to be efficacious. On the basis of a recursive partitioning analysis of data from patients treated between 1979 and 1993 on previous RTOG protocols, even patients with brain metastasis who had the best prognosis had a median survival of only 7 months after WBRT alone.81 However, with improvements in systemic therapies for a variety of cancers, patient survival has now increased, even among those with metastatic disease.82 In this context, WBRT alone is increasingly found to be inadequate in the long-term control of brain metastasis. In addition, with these improved outcomes, many patients in whom control of brain disease is achieved with WBRT are surviving to experience the considerable neurocognitive sequelae and declines in quality of life that are associated with this treatment.83 The classic neuro cognitive toxicity associated with WBRT in adults is a moderate-to-severe dementia that occurs several months to years after treatment. DeAngelis et al.84 observed a 2–5% incidence of severe dementia in populations of patients who had undergone WBRT (with or without surgical resection) for brain metastases, although these authors estimated that a markedly higher incidence of dementia would have been found if less-severe cases of neurological decline were also included. An early neurocognitive decline, predominantly in verbal memory, occurring 1–4 months after WBRT has also been described.85 The degree of neuro-cognitive decline in patients with brain metastasis can be further confounded by the effects of metastasis at presentation or recurrence and therapeutic interventions (that is, chemotherapy) on cognitive function.
Combination with systemic therapies
WBRT and chemotherapy
The latest innovations and ongoing research on the use of radiation therapy for brain metastases are mainly aimed at exploring either adjuncts that can improve the control of brain disease with radiation, or strategies to limit the neurocognitive sequelae of WBRT. With regard to the latter approach, multiple RTOG studies evaluating dose escalation with altered WBRT fractionation schemes have not proven such approaches to be of benefit in patients with brain metastasis.86,87 However, other means of increasing local control of brain metastatic lesions and improving survival in selected patients using WBRT have proven more successful. In patients with a solitary brain metastasis, randomized studies have demonstrated improved overall survival when either surgery40,88 or SRS46 is combined with WBRT. Although these local therapies combined with WBRT could be beneficial for properly selected patients with a limited number of brain metastases, a substantial proportion of such patients would not qualify for these aggressive approaches due to location, size and/or number of metastases in the brain, or other oncological or medical issues.
Although different chemotherapies can penetrate the BBB to varying extents and have been evaluated in combination with WBRT for treatment of brain metastases, the therapeutic benefits of these agents in this context have been largely disappointing; the lipid soluble alkylating agent temozolomide, which can cross the BBB freely, has been combined with WBRT in phase II trials,89–92 but provided limited or no benefit compared with WBRT alone. Combining biologically targeted agents with brain irradiation might represent a more promising approach because of reduced normal tissue toxicity compared with approaches using nontargeted agents. However, the efficacy of this approach will probably vary depending on whether therapeutic concentrations of the particular agent used can be achieved within the brain.
WBRT and targeted treatments
Advantages of combining WBRT with targeted drugs, rather than traditional chemotherapies, could include potentially decreased toxicities and the opportunity for a biomarker-driven approach to disease management. An example of this approach was reported by Welsh et al.93 using the EGFR inhibitor erlotinib in combination with WBRT in the treatment of patients with brain metastases associated with non-small-cell lung cancer (NSCLC). In this phase II study,93 in which 40 patients completed the WBRT and erlotinib therapeutic regimen, the toxicity profile was acceptable—with no reported increase in neurotoxicity—and the median survival time was promising (11.8 months), with a median follow-up of 28.5 months in patients remaining alive at completion of the study. Interestingly, although a known EGFR status was not required for entry into this study, in the 17 patients with a known EGFR status, median survival times were 9.3 months in those with wild-type EGFR and 19.1 months in those with mutant EGFR.93 Conversely, data from the RTOG 0320 trial94 demonstrate no benefit of the addition of temozolomide or erlotinib to WBRT and SRS in patients with NSCLC and one to three meta-static lesions in the brain (Table 1), and in fact suggested better outcomes in the control arm (WBRT and SRS). However, allocation of treatment in this study was not biomarker-driven and the trial was severely under-powered, with much better outcomes in the control arm than would be expected based on the results of previous studies.48,94,95 Further studies with various chemo-therapies and targeted therapies should begin to define in what circumstances and according to which protocols such approaches can be combined with brain irradiation to optimally treat patients with brain metastasis.
Table 1.
Clinical studies of targeted therapies for brain metastases in lung cancer
| Study | Intervention | Design | Number of patients |
RR/SD/ DCR (%) |
PFS/OS (months) | Remarks |
|---|---|---|---|---|---|---|
| Ceresoli et al. (2004)231 |
Gefitinib | Prospective single arm phase II |
41 | 10/17/27 | 3/5 | Some patients had prior chemotherapy and WBRT |
| Wu et al. (2007)232 |
Gefitinib | Prospective single arm phase II |
40 | 38/45/83 | 9/15 | All patients were Asian individuals previously treated with chemotherapy; 26 patients treated with SRS or WBRT A single patient had a complete response in the brain |
| Welsh et al. (2013)93 |
Erlotinib and WBRT |
Prospective | 40 | 83/3/86 | 8/11.8 | RR of 89% and OS of 19.1 months in patients with EGFR-mutant tumours versus RR of 63% and OS of 9.3 months in patients with wild-type EGFR tumours |
| Kim et al. (2009)233 |
Gefitinib or erlotinib |
Prospective | 23 | 70/4/74 | 7.1/18.8 | Treatment-naive nonsmokers only |
| Sperduto et al. (2013)94 |
WBRT and SRS versus WBRT and SRS with either temozolomide or erlotinib |
Prospective randomized phase III |
126 out of 381 planned |
NR | WBRT and SRS: 8.1/13.4 WBRT, SRS and temozolomide: 4.6/6.3 WBRT, SRS and erlotinib: 4.8/6.1 |
Study was terminated prematurely due to poor accrual of patients |
| Hotta et al. (2004)234 |
Gefitinib | Retrospective | 14 | 43/57/100 | 8.8/NR | Median interval between XRT and gefitinib was 2 months |
| Porta et al. (2011)235 |
Erlotinib with or without XRT |
Retrospective | 17 with EGFR-mutant tumours; 39 with wild-type EGFR tumours |
EGFR-mutant tumours: 82/18/100 Wild-type EGFR tumours: 0/78/78 |
EGFR-mutant tumours: 11.7/12.9 Wild-type EGFR tumours: 5.8/3.1 |
8 patients with EGFR-mutant tumours received only erlotinib; 6 (75%) of these patients showed objective intracranial responses |
| Katayama et al. (2009)145 |
Erlotinib | Retrospective | 7 | 43/43/86 | NR/3 | All patients in whom previous gefitinib therapy was ineffective |
| Grommes et al. (2011)144 |
Erlotinib | Retrospective | 9 | 67/11/78 | 2.7/12 | Treatment with high-dose erlotinib (1,500 mg weekly) in patients with EGFR-mutant lung cancer in whom previous treatment with standard doses of an EGFR-targeted agent was not effective |
Abbreviations: DCR, disease-control rate; NR, not reported; OS, overall survival; PFS, progression-free survival; RR, response rate; SD, stable disease; SRS, stereotactic radiosurgery; WBRT whole-brain radiation therapy; XRT, external-beam radiation therapy.
WBRT and/or SRS?
Improvements in systemic therapies are prolonging the survival of patients with brain tumours,82 who therefore have a greater temporal risk of experiencing major neuro cognitive decline if they are treated with WBRT. To avoid such toxicity issues, SRS alone has been advocated in patients with better prognosis and a limited number of metastases.96,97 Two randomized studies95,98 have demonstrated that such patients (populations with either one to three or four lesions) receiving SRS alone had a similar survival to patients who received WBRT and SRS. A series of meta-analyses of randomized c ontrolled studies that investigated WBRT and SRS confirmed that WBRT did not enhance overall survival in patient with a limited number of brain metastases (up to four);99 however, reduced local and distant control of brain metastasis was observed after treatment with SRS alone compared with WBRT and SRS.99 Indeed, as might be expected, patients treated with SRS alone do experience increased recurrences of metastasis elsewhere in the brain, but salvage with either repeat SRS or WBRT results in survival comparable to initial treatment with WBRT and SRS.99
With respect to neurocognitive and performance outcomes, studies have demonstrated a considerable improvement in the preservation of neurocognitive function and performance status in patients treated with SRS alone compared with WBRT and SRS.98–100 A randomized study by the European Organisation for Research and Treatment of Cancer (EORTC) further supports the validity of using local therapy only (SRS or surgery) versus local therapy combined with WBRT in patients with one to three brain metastases, demonstrating no improvement in overall survival with the addition of WBRT.101 Of note, patients in the WBRT arm of the EORTC trial scored markedly worse on health-related quality-of-life measures, both at early and late time points after treatment.95 Pooled results from three randomized trials98,100,102 (comprising a total of 364 patients) have now been reported in abstract form103 and, in fact, revealed an apparent survival advantage in younger patients (<50 years) treated with SRS alone compared with WBRT and SRS; the reason for this surprising result is not entirely clear at this time. At present, the use of SRS alone in patients with more than three metastatic lesions in the brain can be considered, although routine use of this approach in such instances will require validation in additional randomized controlled trials.104
Although these findings argue against WBRT for patients with a limited number of brain metastases (fewer than 4), this treatment is still warranted in certain situations. For example, Slotman et al.105 reported improved overall survival, but no change in global health status, in patients with small-cell lung cancer (SCLC) randomly assigned to receive prophylactic cranial irradiation (PCI) versus no PCI. However, the use of PCI is not supported in the treatment of locally advanced stage III NSCLC based on the results of the RTOG 0214 trial,106 which found no difference in overall survival in patients randomized between the PCI with standard therapy (surgery and/or radiation therapy with or without chemotherapy) cohort and the observation cohort who received standard therapy only, despite a marked decrease in the rate of brain metastasis at 1-year follow up in the patients who underwent PCI. In addition, even the lower doses of WBRT (30 Gy in 15 fractions) used in this study resulted in considerable declines in memory,106 as measured using the Hopkins Verbal Learning Test (HVLT), a validated neurocognitive instrument with good sensitivity.107
For cases in which WBRT is warranted, reduction of the neurocognitive sequelae of this therapy might be possible, and indeed pharmacologic intervention has been explored as a means of preserving neuro cognitive function after WBRT. RTOG 0614, a randomized phase III study, evaluated the neurocognitive outcomes in patients with brain metastasis treated with WBRT with or without memantine, a drug that blocks N-methyl-d-aspartate (NDMA)-type glutamate receptors and is used to treat moderate-to-severe Alzheimer disease. In this study, >500 patients were randomized into two well-balanced cohorts, with substantially reduced decline in a number of neuro-cognitive parameters observed in the WBRT plus memantine arm in comparison with the WBRT plus placebo control arm.108 Taking a different approach to minimizing the risk of WBRT while maintaining therapeutic benefit, investigators at the University of Wisconsin109,110 have pioneered a technique using tomotherapy—which has since been adapted to enable treatment using conventional linear accelerator apparatus—to treat the whole brain while selectively ‘under-dosing’ the bilateral hippocampi, a small volume of brain that harbours active neural stem cells and is believed to be critical for the retention of short-term memories. These advanced radiotherapy approaches can potentially be used not only to selectively spare portions of the brain (that is, hippocampi), but might also be adapted to selectively expose known metastatic lesions to higher doses of radiation, possibly improving disease control. Multiple clinical trials using this simultaneous in-field boost strategy are currently open to accrual in the USA and Canada. Moreover, the use of hippocampal-avoidance WBRT (HA-WBRT) has already been tested in a multi-institutional setting. Preliminary results of the phase II RTOG 0933 trial,111 based on analysis of data from 100 eligible patients, were recently presented at the 2013 ASTRO annual meeting.111 The study findings suggested that patients who underwent HA-WBRT had improved neurocognitive functioning compared with the results expected in comparable patients receiving conventional WBRT, as measured using validated instruments including the HVLT in delayed and total recall.111 These results highlight the promise of the hippocampal sparing approach to WBRT, but require validation in phase III trials.
Systemic therapy for brain metastases
Patient survival following the development of brain metastasis is typically measured in weeks to months, although considerable variability is observed based on the size, number and location of the metastases, as well as the histological type of cancer involved. Whereas the overall 2-year survival rate in patients with brain meta stasis is 8.1%, 2-year survival after diagnosis of brain metastasis is less than 2% in patients with SCLC, but as high as 24% in patients with ovarian cancer.112 Contemporary data from patients with oligometastasis to the brain treated primarily with local surgical or radiation therapy reveal a more encouraging median overall survival of 16 months from the time of brain meta stasis diagnosis.113 As mentioned earlier, the proclivity of certain cancer types to spread to the brain is an intriguing phenomenon whose biological mechanisms remain to be clarified. The so-called ‘seed and soil’ hypothesis implicates key biological mechanisms that permit the development of metastatic tumour deposits in the brain. Signalling through HER2, EGFR, HPSE and Notch1-related pathways might mediate specific biological processes important to tumour growth and metastatic spread, including angiogenesis, epithelial–mesenchymal transition, anchorage independent growth and resistance to anoikis, as well as resistance to standard therapeutic interventions. Other than the use of antiangiogenic agents, the exploitation of these biological processes for therapeutic intervention in the context of brain m etastasis remains mostly limited to preclinical studies.114,115
The solid malignancies most frequently associated with brain metastasis are those of the lung, breast and kidney, and melanoma. As outlined above, the established treatment approaches for brain metastasis include surgical resection and radiation therapy, including SRS; at present, no standard cytotoxic chemotherapy exists for the treatment of secondary brain tumours. Instead, the patients in whom the disease is not amenable to local control with surgery or radiation are typically treated using the same cytotoxic chemotherapy employed for the treatment of extracranial disease. Alternatively, cytotoxic agents with good CNS penetration, such as topotecan, irinotecan, procarbazine, and carboplatin, are also employed for empiric therapy, even in cases in which these agents are not the standard therapy for the primary tumour site.
Improved knowledge of tumour biology has led to the identification of specific molecular drivers of cancer development and progression. Of note, insertions and/or deletions within the EGFR gene and EML4–ALK chromosomal translocation in lung cancer, BRAF mutation in melanoma, as well as amplification of the ERBB2 gene (encoding HER2) and HER2 protein over expression in breast cancer distinguish well-characterized, distinct subsets of cancer amenable to unique treatment approaches. Consequently, biologically targeted agents have become established therapies for these subsets of tumours with astounding effectiveness recorded in the clinic.116–119 Molecular characterization and targeted therapy for these solid organ malignancies have important implications for brain metastasis.
Firstly, that specific molecular drivers lead to increased predisposition of cancer cells to invade the CNS is plausible. This possibility is supported by the observation that ERBB2-amplified breast cancer has a higher propensity to metastasize to the brain.120 Furthermore, in pre clinical models, deregulated EGFR and HER2 signalling in co operation with activated HGFR (also known as c-Met) pathway induce an epithelial-to-mesenchymal phenotype transition, which can promote increased metastatic potential and higher likelihood of brain involvement.121
Secondly, the use of targeted agents is generally associated with superior efficacy and survival improvement, which has led to the approval of these agents for specific tumour subtypes. Consequently, increased longevity of patients with cancer treated with targeted biological agents might also increase the likelihood of brain meta-stasis over the course of the disease. This eventuality might result from failure of the targeted agents to eradicate micrometastatic deposits in the brain due to limited penetration through the BBB, or because of selective pressure leading to the emergence of treatment-resistant clones with increased capacity for invasion and meta stasis to distant sites. Paradoxically, the limited penetration of some targeted therapies into the brain could result in intracranial metastatic deposits that remain sensitive to these agents, even in the context of the development of drug resistance within the extracranial tumour compartments. Conversely, exposure of intracranial tumour deposits to subtherapeutic drug concentrations might promote the early development of drug resistance and isolated disease progression in the brain, while the extra-cranial disease remains sensitive to treatment. The likelihood of witnessing any of the aforementioned scenarios in a patient is dependent on several factors, such as the propensity of the specific tumour type to invade the brain, the ability of the therapeutic agent to cross the BBB and the burden of intracranial tumour deposits.
Finally, and most relevant to this discourse, is the possibility of incorporating biologically targeted therapy into the management paradigm for brain metastasis in patients whose tumours harbour genetic alterations that render them sensitive to such agents. The emerging role of targeted agents in the treatment of brain meta-stasis of solid tumour malignancies are highlighted in the follow ing sections, with particular emphasis on common cancer types, including lung cancer, breast cancer and melanoma, in which brain involvement is frequently observed; coincidentally, these cancers also represent those for which the greatest advance in targeted agents has been made during the past decade.
Brain metastasis in lung cancer
Background clinical biology
Metastatic CNS involvement is most frequently associated with advanced-stage lung cancer. Indeed, studies have suggested around 50% of patients with lung cancer will develop brain metastases at some time during the course of the disease.122,123 However, the pathogenetic pathways that underlie metastasis to the brain in lung cancer remain unclear.
The receptor tyrosine kinases have received much attention as mediators of both the initiation and progression of lung cancer. Activation of EGFR (via gene amplification, overexpression and/or mutation) can be observed in a large proportion of primary lung cancer cases, and thus this protein is a frontline therapeutic target (Figure 1). With regard to metastasis to the CNS, Sun et al.124 demonstrated that brain metastases expressed increased levels of EGF and phosphorylated EGFR compared with primary lung tumours derived from the same patients. In addition, Benedettini et al.125 discovered an association of HGFR expression and phosphorylation with brain metastasis. Similarly to EGF pathway components, HGFR was also found to be enriched in metastatic brain lesions compared with patient-matched primary tumour tissues, implicating this pathway in CNS metastasis.125 Interestingly, activation of the HGFR was shown to promote the EGFR-driven invasive capacity of NSCLCs in vitro, and inhibition of HGFR signalling decreased brain meta stasis in an EGFR-mutant cell population.126 Furthermore, PGF levels and associated triggering of VEGFR1 activation were shown to be increased in CNS metastases in patients with SCLC, the most aggressive lung cancer subtype.127 These proteins and signalling pathways thus represent promising therapeutic targets in brain meta stasis, and deserve further exploration in this capacity (Figure 1).
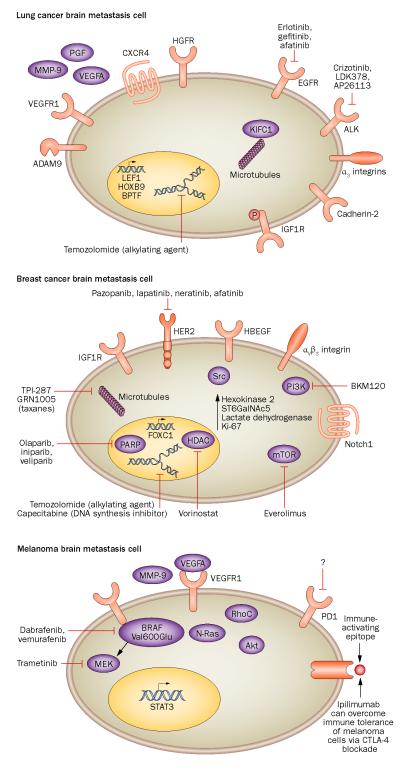
Figure 1.
The key molecular alterations in the primary cancers that most commonly metastasize to the brain. Important therapies that are currently available or are in clinical development and can cross the BBB are also shown. In lung cancer, expression of cadherin-2, KIFC1, and CXCR4 proteins and ADAM9 mRNA predicts metastasis, and phosphorylated IGF1R is associated with a poor prognosis. Furthermore expression of transcription factors such as LEF1, HOXB9 and BPTF is associated with a higher likelihood of brain metastases. Co-operative signalling involving EGFR and HGFR correlates with transition from an epithelial phenotype to a mesenchymal and invasive phenotype, and therapeutic agents targeting EGFR, as well as ALK, are important in the treatment of lung cancer brain metastases. In breast cancer, expression of IGF1R, αvβ3 integrin and Notch1 pathways increases the likelihood of metastasis. The presence of HBEGF, Ki-67 and ST6GalNAc5 increases tumour cell penetration of the BBB and hexokinase 2 and FOXC1 predict brain metastases and poorer survival. In melanomas, hyperactivation of the Akt cascade, expression of MMP-9 and RhoC and mutations in BRAF and N-Ras predict invasion and brain metastasis. The presence of VEGFA and STAT3 is associated with angiogenesis and proliferation, respectively. Immune-targeted therapies, particular blockade of CTLA-4-dependent inhibitory co-stimulatory signalling in T cells, can overcome immune tolerance of melanoma cells. Abbreviations: ADAM9, disintegrin and metalloproteinase domain-containing protein 9; BBB, blood–brain barrier; CTLA-4, cytotoxic T-lymphocyte antigen 4; CXCR4, CXC-motif chemokine receptor 4; FOXC1, forkhead box protein C1; HDAC, histone deacetylase; LEF1, lymphoid enhancer-binding factor 1; MMP-9, matrix metalloproteinase 9; PARP, poly-[ADP-ribose] polymerase; PGF, placenta growth factor; ST6GalNAc5, α-N-acetylgalactosaminide α-2,6-sialyltransferase 5; STAT3, signal transducer and activator of transcription 3.
Clinical specimens of CNS metastases and mouse models of CNS metastasis have revealed other genes associated with an invasive lung cancer cell phenotype. For example, the transcription factors LEF1 and HOXB9 are associated with metastasis of lung cancer cells to the brain (Figure 1).128 In addition, gene-expression analysis in tissue from 142 NSCLC tumours demonstrated that expression of three genes, encoding cadherin-2 (also known as N-cadherin; CDH2), kinesin-like protein KIFC1 (KIFC1) and nucleosome-remodeling factor subunit BPTF (BPTF), was predictive of a high risk of brain meta stasis.129 Phosphorylated insulin-like growth factor-1 receptor (IGF1R) was also associated with brain metastasis and poor prognosis.130
Studies have also provided insights into the potential mechanism of cell homing to, and infiltration of, the brain, which could represent another therapeutic avenue to explore (Figure 1). In particular, CXC-motif chemokine receptor 4 (CXCR4) was found to be expressed in 90% and 100% of samples isolated from primary tumours and brain metastases, respectively, in 32 patients with NSCLC and a solitary brain metastasis;131 this frequency of CXCR4 expression was markedly increased compared with tissue samples from NSCLC without metastatic brain tumours or other distant metastases.131 A separate study identified a subclone of EBC-1 cells with substantially higher levels of integrin α3 expression and an increased capacity for brain metastasis compared with parental EBC-1 cell line or EBC-1 cells that meta stasized to bone.132 ADAM9 mRNA, encoding disintegrin and metalloproteinase domain-containing protein 9, was also found to be overexpressed in EBC-1 cell lines that d emonstrate enhanced brain metastasis.133
Cytotoxic chemotherapy
Prior studies of traditional systemic chemotherapy agents showed encouraging therapeutic activity in lung-cancer-associated brain metastasis, especially in asymptomatic patients, often enabling avoidance of local intervention with surgery and radiation.134,135 Similarly, salvage therapy with cytotoxic chemotherapeutic agents owing to progression of disease after radiation therapy or surgical resection was also associated with clinical benefit, albeit modest.135 Temozolomide monotherapy is one of the most actively studied approaches for the treatment of brain metastasis in lung cancer, primarily owing to the high BBB penetrability of this drug. Whether as a monotherapy or in combination with radiation, the use of temozolomide achieved objective responses in approximately 5% and >60% of patients with brain metastasis, respectively, with median survival ranging between 4 and 9 months.135 Furthermore, temozolomide monotherapy was reported to induce objectively measured tumour shrinkage in patients with relapsed metastatic SCLC after prior treatment with one or two chemotherapy regimens.136 Interestingly, the therapeutic benefit of temozolomide in these patients was associated with MGMT hypermethylation in the tumour.136 This observation indicates the potential value of this molecular change to predict responsiveness of SCLC to temozolomide, owing to reduced MGMT gene expression and thus a decrease in the ability of the tumour cells to repair alkylation-induced DNA damage. This preliminary observation requires further validation. Aside from temozolomide monotherapy, combination chemotherapy regimens, such as carboplatin with paclitaxel, cisplatin with vinorelbine, or carboplatin with etoposide, have all been shown to induce objectively measured responses of metastatic brain lesions in approximately 20–45% of patients with lung cancer.137–139
EGFR inhibitor therapy
Erlotinib, gefitinib and afatinib are EGFR inhibitors currently approved in different parts of the world for the treatment of NSCLCs harbouring sensitizing mutations in the EGFR gene. In addition, orally administered gefitinib successfully controlled established intra cerebral tumours derived from EGFR-expressing epidermoid cancers.140 However, clinical evidence for efficacy of these agents in tumours affecting the CNS comes mainly from retrospective observational studies and case-series.140–144 In general, EGFR-targeting agents have a low capacity to penetrate into the cerebrospinal fluid (CSF), although erlotinib achieved a relatively higher level of CNS penetration, which might in part explain the improved control of brain metastasis that has been observed after erlotinib treatment, even in patients previously treated with gefitinib (Table 1).145 Nonetheless, the multitude of reports of patients who derived a substantial benefit of treatment with EGFR-targeted therapy, with control of brain metastases,141–144 eventually justified evaluation of this approach in larger case-series and prospective studies. Indeed, high-dose erlotinib (1,500 mg weekly) was associated with a partial control of CNS metastases and stable disease in 67% and 11% of patients, respectively, in a retrospective series of nine individuals with EGFR-mutant lung cancer (Table 1);144 the median time to progression of CNS metastases was 2.7 months (range, 0.8–14.5 months), and the median overall survival was 12 months (range, 2.5 months to outcome not reached).144 Furthermore, the use of EGFR kinase inhibitors rather than chemotherapy as frontline therapy of EGFR-mutant lung cancer also reduced the cumulative incidence of progressive CNS metastasis (HR = 0.56; 95% CI = 0.34–0.94): 6-month, 12-month and 24-month cumulative risk of CNS metastasis was 1%, 6%, and 21%, respectively, in patients treated with EGFR-targeted agents compared with 7%, 19% and 32%, respectively, in those who received chemotherapy (P = 0.026).146
Prospective studies testing the efficacy of EGFR-targeted agents in the treatment of brain metastasis have been mostly nonrandomized single-arm phase II studies. One such study evaluated the role of erlotinib as a radiosensitizing agent in 40 patients with brain meta-stasis arising from lung cancer irrespective of the EGFR status of the tumour, and demonstrated the safety of this approach and a disease control rate of >80% (Table 1).93 Likewise, an EGFR mutation was detected in 53% of tumours with tissue available for testing,93 which is a high proportion when compared with the expected rate of EGFR mutation in Western populations of patients with lung cancer, suggesting that EGFR mutations can confer increased propensity for brain involvement. Interestingly, objective response was achieved in 67% of patients treated with a combination of radiation therapy and erlotinib, and disease stabilization was observed in 11% of all enrolled patients. Although only an indirect comparison of these data is possible, this efficacy outcome was superior to historical experience with radiation therapy alone. Other studies that have published important findings regarding EGFR-targeted therapies are summarized in Table 1. Together these findings clearly warrant prospective validation in a randomized study to establish this approach as a standard treatment paradigm for brain metastasis in patients with EGFR-mutant lung cancer.
ALK-targeted agents
The EML4–ALK translocation is a genetic aberration that affects approximately 3–5% of all NSCLC. Of note, up to 30% of patients with ALK-positive lung cancer have been shown to develop brain metastasis.147 The ALK-targeting agent crizotinib is the first approved agent for the treatment of ALK-positive NSCLC,148,149 although newer agents with promising efficacy profiles, including LDK378 and AP26113 (Figure 1),150,151 are currently in clinical testing. Despite reports indicating poor penetration of crizotinib into the CSF,152 patients treated with ALK-targeted agents have frequently shown clinical response in the brain,148,150 suggesting a potential role for these agents in the treatment of brain metastasis. Crizotinib is a substrate for the ATP-binding cassette (ABC) drug efflux transporters, multidrug resistance protein 1 (also known as P-glycoprotein or ABC sub-family B member 1 [ABCG2]) and ABC subfamily G member 2 (ABCG2; also known as breast cancer resistance protein), which provides a potential reason for wide variability and overall poor accumulation of the drug in the brain.152 In support of this theory, ABCB1–/– and ABCG2–/– mice had a 25–70-fold higher brain concentration following oral administration of crizotinib compared with wild-type mice.152 Similar results were obtained when crizotinib was administered along with elacridar, an inhibitor of these efflux pumps.153 These preclinical data, together with clinical evidence of intracranial efficacy of crizotinib and other structurally similar ALK-targeted agents,154–156 indicates the potential utility of this class of agents for the treatment of brain metastasis of ALK-positive lung cancer. Potential strategies for incorporating ALK-inhibitor therapy might include combination of these agents with established surgical and radiation treatment approaches to improve outcome of symptomatic patients, or ALK-inhibitor monotherapy in lieu of surgical and radiation modalities in asymptomatic patients with small-volume disease.
Brain metastases in breast cancer
Background clinical biology
The pattern of metastatic spread to distant sites has been shown to vary according to the breast cancer subtype (luminal A, luminal B, basal-like and HER2-enriched subtypes).157,158 These molecular subtypes were initially identified by gene-expression profiling; however, an immunohistochemical (IHC) surrogate using the oestro gen receptor (ER), progesterone receptor (PR) and HER2, and other microarray assays have been utilized in determining the molecular subtype of breast tumours.159 Studies have revealed that basal-like and HER2-positive tumours are more likely to recur in the CNS.160 The timing of brain metastases also varies, with basal-like and triple-negative (that is, tumours negative for ER, PR and HER2 expression) tumours associated with higher rates of first recurrence in brain metastases.161 Median survival following the development of brain metastases is approximately 5–6 months across all breast cancer subtypes;162,163 however, retrospective studies have shown that median survival is shortest among patients with triple-negative tumours, ranging from 3–4 months.163,164 Although patients with HER2-positive disease have higher rates of brain metastases, median survival is much longer, typically >1 year.163,165 This finding might be due in part to the availability of effective HER2-directed systemic therapy for these patients. Discordance in ER, PR and HER2 status has been observed between the primary tumour and distant metastatic sites in 10–16% of patients with metastatic disease.166,167 Therefore, although biopsy of brain metastases might not be feasible, biopsy of other distant metastatic sites for confirmation of receptor status should be strongly considered to provide important prognostic information that could have a potential influence on treatment recommendations.
As well as mutations affecting the receptors described above, several other genetic changes have been implicated in the development of brain metastasis in patients with primary breast tumours. The sialyltransferase ST6GalNAc5, which is normally expressed in the brain, can be observed in breast cancers and associated brain metastases (Figure 1), highlighting the potential of brain-specific genes to facilitate CNS homing and colonization.32 In addition, p53 overexpression,168 elevated serum lactate dehydrogenase levels169 and a high Ki-67 labelling index170,171 are reported CNS metastasis risk factors in breast cancer. Positivity for hexokinase 2115 or Forkhead box protein C1 (FOXC1)172 expression is also associated with a higher incidence of brain meta-stases and poor patient survival in patients with breast cancer. Xenograft models of breast-cancer-associated brain metastasis have also implicated α β 173 v 3 integrins and activation of Notch pathway signalling in this process.174 Furthermore, Zhang et al.175 demonstrated Src hyper activation in a model of HER2-positive brain metastasis. IGF1R expression in cancer cells has also been associated with CNS meta stasis, and ablation of this receptor delayed metastasis in experimental models.176
Emerging experimental approaches highlight potential opportunity to target some of these metastasis mediators using systemic approaches to prevent the development of brain metastasis in at-risk patients. For example, in a mouse model of metastatic breast cancer, treatment with pazopanib, a multikinase inhibitor, 3 days after injection of human ERBB2-transfected breast cancer cell lines markedly reduced the development of both macrometastases and micrometastases by 73% and 39%, respectively.114 As expected based on observed inhibition of MEK and ERK activation by pazopanib in vitro, despite BRAF and KRAS mutations, reduced activation of the BRAF targets, ERK1/2 and MEK1/2 was seen in metastatic tumours after pazopanib treatment, whereas no change in microvessel density was demonstrated compared with vehicle-treated controls.114 Similarly, treatment of mice injected with a brain trophic 231-BR subline of the triple-negative MDA-MB-231 breast cancer cell line using vorinostat, a histone deacetylase inhibitor, prevented the development of brain meta stasis, in part, through induction of lethal DNA double-strand breaks.177 As these therapeutic agents are already in clinical use, testing of this therapeutic concept is likely to ensue once the molecular and genetic features of brain trophic cancer cells are better characterized.
Cytotoxic chemotherapy
Among patients with locally advanced and metastatic breast cancer, approximately 15–30% will develop brain metastases during the course of the disease.6,178 Several cytotoxic chemotherapies and targeted agents have been evaluated in clinical trials for the management of breast cancer brain metastasis. Temozolomide, the orally administered alkylating agent approved for treatment of primary brain tumours, has been investigated as a mono-therapy and in combination with other cytotoxic agents. Objective CNS response rates after temozolomide mono-therapy have been shown to be low (<5%), and median progression-free survival (PFS) was approximately 2 months.179,180 An evaluation of combination therapy comprising vinorelbine and temozolomide in patients with recurrent or progressive CNS disease showed similar results, with a transient, minor response in 1 of the 11 patients with breast cancer who were treated with this regimen.181 In a phase II study,182 the combination of cisplatin and temozolomide for the treatment of all solid tumours was associated with a 40% (6 of 15) partial CNS response rate among the patients with breast cancer who were enrolled.
The use of capecitabine, an inhibitor of DNA synthesis, as a monotherapy has been reported in the treatment of brain metastases associated with breast cancer.183,184 One report of a single institution experience183 in seven patients with breast cancer showed that three achieved complete response and another three experienced prolonged stable disease with capecitabine monotherapy; the median overall survival and PFS intervals were 13 and 8 months, respectively.183 Furthermore, the combination of capecitabine and temozolomide demonstrated an 18% response rate with a median time to progression of 12 weeks.185 Capecitabine in combination with lapatinib produced CNS response rates ranging from 20% to 38% among patients with HER2-positive breast cancer and brain metastases who had been heavily pretreated; lapatinib alone showed minimal activity in such patients.186–190 The combination of lapatinib and capecitabine has also been studied in patients with previously untreated HER2-positive breast cancer and brain metastases, and was associated with CNS objective response in approximately two-thirds of these individuals.191 Although these results are promising, randomized trials comparing this combination to radiation therapy will be needed before recommending this as first-line therapy for patients with HER2-positive breast cancer with brain metastases.
Novel systemic agents
The ideal chemotherapeutic agent for the treatment of brain metastasis in breast cancer would have activity against breast cancer and BBB permeability. GRN1005 is a peptide–taxane conjugate that gains entry into the CNS by targeting the low-density lipoprotein receptor-related protein 1, which is an endocytic receptor expressed on the surface of the BBB. On the basis of promising phase I data for this approach, a phase II trial in patients with breast-cancer-associated brain meta stases was initiated; however, interim analysis of the first 30 patients did not show any CNS responses,192 and the development of GRN1005 was discontinued. TPI-287 is a third-generation taxane microtubule-disrupting chemo-therapeutic agent (Figure 1), which has been shown to reduce the growth of breast cancer brain metastases in preclinical models;193 a phase II clinical trial with this agent is ongoing in patients with breast cancer and brain metastases.194
The HER2-directed TKIs neratinib and afatinib (Figure 1) are currently being evaluated in clinical trials to establish the activity of these drugs in CNS disease.195,196 Furthermore, a subset analysis of 58 Korean patients with brain metastasis treated as part of an expanded access programme with the combination of lapatinib and capecitabine demonstrated a PFS of 18.7 weeks and overall survival of 48.9 weeks (Table 2).197 Similar efficacy signal was reported from a retro spective analysis of Asian patients with brain metastasis who receive lapatinib or trastuzumab;198 a marked benefit was observed in patients treated with either of these anti-HER2 agents, with p rolonged overall survival (26 month versus 6 months).198
Table 2.
Clinical studies of targeted therapies for brain metastases in breast cancer
| Study | Intervention | Design | Number of patients |
RR/SD/ DCR (%) |
PFS/OS (months) |
Remarks |
|---|---|---|---|---|---|---|
| Yap et al. (2012)198 |
Lapatinib and/ or trastuzumab |
Retrospective | 114 | NR | NR/18.5 | Data represents a subpopulation of patients treated with anti-HER2 therapy after development of brain metastasis |
| Ro et al. (2012)197 |
Lapatinib | Expanded access programme |
58 | NR | 18.7*/48.9* | None |
Survival reported in weeks, not months. Abbreviations: DCR, disease-control rate; NR, not reported; OS, overall survival; PFS, progression-free survival; RR, response rate; SD, stable disease.
In addition, therapies targeting other kinases are being examined in this context. For example, several agents targeting angiogenesis are under investigation, including bevacizumab and sorafenib, a TKI targeting multiple receptors that mediate various aspects of cancer progression, such as angiogenesis and cell proliferation.199,200 Everolimus, an inhibitor of mTOR (Figure 1), in combination with exemestane was approved by the FDA for treatment of breast cancers positive for hormone receptors but negative for HER2 based on improved PFS associated with this approach.201 CNS responses to everolimus have been observed among patients with subependymal giant cell astrocytoma,202 and a study evaluating the combination of vinorelbine, trastuzumab and everolimus in patients with HER2-positive brain metastases is ongoing. In addition, shrinkage of CNS metastases in one patient treated with the PI3K inhibitor BKM120 in a phase I clinical trial203 has stimulated interest in evaluating agents targeting this kinase in patients with metastatic breast cancer involving the brain.
With regard to approaches targeting proteins other than kinases, synthetic lethality resulting from abrogation of the poly[ADP-ribose] polymerase (PARP) enzyme in vulnerable tumours that harbour deficient DNA repair machinery, such as BRCA1/2-deficient tumours, is another therapeutic paradigm under intense clinical investigation (Figure 1). Indeed, objective responses were observed in patients with BRCA-deficient ovarian and breast cancer treated with PARP inhibitor monotherapy (using olaparib).204 Similarly, combination of a PARP inhibitor (iniparib) with DNA-damaging cytotoxic agents (gemcitabine and carboplatin) initially showed intriguing efficacy, but a follow-up phase III study was negative.205 Further studies are required to assess the relevance of these approaches to the treatment of brain metastases. Given that ionizing radiation induces tumour cell death in part through DNA damage, interest has increased regarding the possible potentiation of radiation therapy when combined with PARP inhibition. A single-arm phase II study is currently testing whether the addition of the PARP inhibitor veliparib to WBRT is a safe and efficacious treatment approach in patients with brain metastasis; preliminary data from this study showed that the combination is safe, but the efficacy outcome is still awaited.206
Melanoma-associated brain metastasis
Background clinical biology
Melanomas account for 5–20% of primary tumours associated with CNS metastatic lesions.19 Indeed, brain metastases are common in melanoma and are the leading cause of death in metastatic melanoma.207 Although mela noma is known to have the capacity to metastasize to virtually every organ site in the body, brain metastases pose a particular therapeutic challenge because of the multiplicity of metastases, the lack of resectability of isolated or diffuse metastasis, radiation resistance and poor perfusion of therapeutic agents into brain metastases.
Whereas melanoma metastasis in the brain can be multifocal, these metastases appear morphologically different compared with multifocal primary glioblastomas in that they are often less invasive, and mediate their deleterious effect through mass effect and peri tumoral oedema. In terms of signalling, the most consistent event noted in brain metastases of melanoma is hyper-activation of the Akt cascade (Figure 1).208 Activation of Akt alone is sufficient to convert poorly tumourigenic melanoma cells to highly tumourigenic cells;209 thus, that Akt is activated in these metastases is unsurprising. Interactions between glial cells, which are part of the tumour stroma in brain metastases, are likely to activate Akt in the metastatic lesion. Akt activation can be dependent or independent of reactive oxygen species (ROS)-mediated signalling, although the combination of Akt and ROS activity is associated with local invasive growth, as in primary glioblastoma.210 One mechanism by which Akt signalling can be activated by ROS is through oxidative inactivation of PTEN, which is a redox-sensitive lipid phosphatase,211 and low levels of PTEN have been noted in both melanoma and brain metastases.208 Alternatively, PTEN is often deleted or mutated as a second mechanism of inactivation in such tumours.208 Interestingly, blockade of ROS signalling has also been demonstrated to block local invasion of glioblastoma in vivo.212
Studies have also demonstrated a high propensity for activating mutations in BRAF and/or NRAS to cause brain metastasis in melanomas.208 Indeed, these abnormalities represent the two most common subsets of oncogenic mutations in melanoma, although survival of patients with brain metastases does not seem to substantially differ according to mutation status of these genes.208 The most reliable prognostic factor in terms of overall survival in brain metastasis resulting from melanoma is an immune signature characterized by infiltration of T lymphocytes, especially CD8+ T cells, which is associated with improved long-term survival.213 Other genes associated with CNS metastases derived from primary melanomas are RHOC214,215 and MMP9,216 both of which are associated with tumour cell invasion. VEGFA217–219 and STAT3,220 which have roles in angiogenesis and cell growth, respectively, are also associated with advanced melanoma and metastasis (Figure 1).
Immunomodulatory therapy
The optimal treatment of brain metastasis related to melanoma is surgery, a point worth emphasizing; however, this approach might not be feasible in some cases given the location or multiplicity of lesions. In patients with small numbers of metastasis, SRS has been used, commonly with localized delivery of high doses of radiation (up to 24 Gy for large lesions), to overcome the ability of melanomas to resist double-stranded DNA breaks.221 The CTLA-4 immune checkpoint modulator, ipilimumab (Figure 1), has also been evaluated in a clinical trial that enrolled both steroid-naive and steroid-treated patients with melanoma and brain meta stasis (Table 3).222 Although no complete responses were observed in this trial, partial responses were achieved by patients in both the steroid-naive and steriod-exposed cohorts.222 In addition, follow-up therapy with this immunomodulatory agent is associated with occasional dramatic responses, not only at the site of radiation therapy but at distant sites as well. This phenomenon is known as the abscopal effect, and is thought to occur as a result of radiation-induced unmasking of tumour antigens that invokes an immunostimulatory effect.223,224 The clinical data thus suggests that immune control has an important influence on the prognosis of brain metastases and thera peutic measures that affect immune presentation might be beneficial in improving long-term survival.
Table 3.
Clinical studies of targeted therapies for brain metastases in melanoma
| Study | Intervention | Design | Number of patients |
RR/SD/DCR (%) |
PFS/OS (months) | Remarks |
|---|---|---|---|---|---|---|
| Long et al. (2012)226 |
Dabrafenib | Prospective | Cohort A: 89 Cohort B: 83 |
Cohort A: 39.2* Cohort B: 30.8* |
Cohort A: 16.1/33.1 Cohort B: 16.6/31.4 |
Cohort A: no prior treatment Cohort B: progressive brain metastases following prior therapy |
| Atkins et al. (2008)236 |
Temozolomide, thalidomide and WBRT |
Prospective | 39 | 8/18/26 | 7‡/4 | None |
| Margolin et al. (2012)222 |
Ipilimumab | Retrospective | 72 | 21/13/34 | NR | Response to therapy assessed using modified WHO criteria |
| Konstantinou et al. (2014)237 |
Ipilimumab | Retrospective | 38 | 5/11/16 | NR/3.3 | Analysis of expanded access programme data |
Intracranial response was reported in this study.
Survival reported in weeks, not months. Abbreviations: DCR, disease-control rate; NR, not reported; OS, overall survival; PFS, progression-free survival; RR, response rate; SD, stable disease; WBRT whole-brain radiation therapy.
Targeted molecular therapy
For several years, the mainstay of therapy for brain metastasis from a chemotherapeutic standpoint has been temozolomide. Subsequent to the discovery of BRAF mutations in a majority of melanomas, BRAF inhibitors have been increasingly used to treat this disease, as well as associated brain metastases (Figure 1). Initial evidence of the potential promise of BRAF-targeted agents in the clinical management of melanoma-related brain metastasis came from early phase clinical trials. In seven patients with brain metastases, a BRAF inhibitor induced CNS tumour shrinkage, and complete responses were observed in three patients.19 In a phase I trial, in which the majority of patients had BRAF-mutant melanomas, in addition to other BRAF mutant tumours, dabrafenib treatment was associated with a reduction in the size of brain lesions, and four patients achieved complete resolution of all brain lesions;225 normal serum lactate dehydro genase levels seemed to be a predictor of response to dabrafenib.225 The intriguing observation of intra cranial responses to dabrafenib in this phase I trial led to a prospective open-label phase II trial of dabrafenib in patients with previously untreated brain metastasis or with progressive brain lesions following initial local treatment (Table 3).226 Overall disease control in patients with metastases expressing Val600Glu (V600E) BRAF was 79%, but complete responses were rare, and the efficacy of this compound was generally decreased in the tumours with the Val600Lys (V600K) BRAF mutation subtype.226
BRAF inhibitors, such as dabrafenib and vemura fenib, are often combined in sequence with radiation therapy and/or immunotherapy. The optimal sequencing of these agents with immunothterapy and radiation therapy remains a subject of intense study; however, an emerging consensus seems to favour the initial use of the BRAF inhibitors (either alone or combined with a MEK inhibitor; Figure 1) for rapid tumour debulking, especially in patients with extensive or rapidly progressive disease, to achieve rapid symptom resolution and control of disease.227 Importantly, BRAF inhibition is presumed to increase the antigenicity of melanoma cells, and the above mentioned abscopal effect has also been observed in patients who have received sequential therapies, including those involving BRAF inhibition, stereotactic radiation and/or immunotherapy.227,228 In patients with limited disease burdens, or those who have asymptomatic brain metastases, immunotherapy (IL-2, CTLA-4-blockade with ipilimumab, or investigational antibodies targeting programmed cell death protein 1 or its ligand) is the preferred frontline therapeutic option.227 The immunomodulatory approach induces a slow but durable response that can be followed by BRAF inhibition.227 Nevertheless, determination of the optimal strategy for incorporating targeted therapies into cytoreductive strategies based on surgery and/or radiation therapy in patients with bulky, symptomatic brain metastasis should be the specific objective of future prospective studies.
Future perspectives
The main issue preventing the development of new and novel approaches to the management of brain meta stases is that our understanding of the biology of meta static disease of the brain is generally advancing at a slow pace. This slow progress limits the ability to take advantage of the currently available therapies, and the development of alternate therapies takes more time. This problem is currently apparent in the daily oncology practice, where the use of targeted and biological therapies for brain metastasis is relatively uncommon for the practical reason that few meaningful agents are available for the treatment of cases that are commonly observed therein; furthermore, access to such agents is limited by the rigorous requirements necessary for safe conduct of clinical trials, most of which exclude patients with active brain metastases. However, a large proportion of research effort and funding is being directed at resolving this problem, which is not surprising given the relatively high frequency of metastatic tumours affecting the brain, compared with primary cerebral neoplasms. At present, >300 clinical studies relating to therapy for meta static brain tumours are listed on the ClinicalTrials.gov database. Thus, private and govern mental funding organizations recognize the importance of the limitations facing the treatment of brain metastases. Now, clinicians involved in the manage ment of such individuals have the responsibility to ensure their patients have access to these studies to generate the data necessary to establish the efficacy of these novel therapies.
From a broader perspective, work being conducted by The Cancer Genome Atlas to elucidate gene expression profiles in various cancers will provide data on tumour biology, enabling the understanding of which cancers are more likely to metastasize to the brain, and why.214,215 These efforts are laying the ground work for potential primary prevention of brain metastasis and, more precisely, future targeted therapeutics for the treatment of metastatic disease.229,230 In addition, microRNA and even exosomal analysis might enable blood-based screening protocols to determine response to therapy and genetic profiling of metastatic disease, potentially obviating the need for invasive biopsies for tissue analyses.
Conclusions
Surgery and radiation therapy have important roles in the management of metastatic brain tumours and, depending on one’s definition, could be considered targeted therapies. As such, they remain the ‘go to’ modalities for the treatment of most metastatic brain tumours, particularly at the time of initial diagnosis. However, these interventions are responsible for well-recognized and substantial adverse events in some cases. Effective strategies to minimize these problems would be of great advantage to the patients with metastatic CNS lesions. The achievable technical improvements possible in surgical and radiation techniques are limited and ultimately medicinal means of management, probably relying on novel systemic targeted therapies, will be necessary to attain this goal and to improve outcomes in patients with brain metastases.
Promising forays into the use of systemically administered, biologically targeted agents, based on improved understanding of tumour molecular biology, include relatively low frequency but impactful responses of EGFR-mutant lung tumours to agents such as erlotinib. Encouragement also comes from the response to crizotinib in NSCLC with the EML4–ALK translocations, and especially to second generation ALK inhibitors such as LDK378. In addition, efficacy has been observed with the combination of trastuzumab and lapatinib in HER2-positive breast cancer, resulting in some control of CNS metastasis in patients with this disease. Finally, considerable hope can be garnered from the durable control of intracranial melanoma metastasis after treatment with dabrafenib and vemura fenib, potent inhibitors of BRAF-mutant melanoma cell function, and the immunomodulatory agent ipilimumab, in particular, in small-volume tumour deposits.
Future therapies for brain metastases will involve a comprehensive approach, utilizing a wealth of resources and adjuncts that are available or in development for the manage ment of patients with metastatic brain disease. Going forward, we can expect that these treatments will rely upon more powerful imaging technologies, the development of conceptually targeted treatments and more multi modal, minimally invasive procedures, with the intention of maximizing survival and quality of life in our patients.
Key points.
▪ An increased understanding of the molecular biology of metastatic processes, including cell migration, blood–brain barrier penetration, angiogenesis and tumour proliferation, is providing new opportunities for the development of targeted therapies
▪ Advances in MRI, incorporating spectroscopy and perfusion techniques, and tracers unique to metastases, provide additional information on responses to treatment and enable the earlier detection of new tumours
▪ Improvements in intraoperative tumour identification using MRI and fluorescent agents maximize the likelihood of complete tumour resection and minimize injury to normal tissue
▪ Reduction of radiation-induced cerebral injury and cognitive decline through repeated use of stereotactic radiosurgery or hippocampal-avoidance whole-brain radiotherapy provide useful options for individuals with advanced cerebral metastatic disease
▪ Targeted therapy is beneficial in molecularly-selected tumours, including erlotinib in EGFR-mutant lung tumours, crizotinib in lung carcinomas with EML4–ALK translocations, trastuzumab in HER2+ breast cancer and dabrafenib in BRAF-mutant melanoma
Review criteria.
The authors searched PubMed for English-language full-text manuscripts and abstracts published between 1980 and 2013. The search terms used, alone and in various combinations, were “brain metastasis”, “MRI”, “neurosurgery”, “radiation therapy”, “chemotherapy” and “targeted therapy”. These general searches were then refined considerably by each author depending on specific questions to be answered and needs for data to be confirmed or followed-up. The reference lists of the articles identified were also searched for additional relevant publications.
Footnotes
Author contributions T.K.O., J.A., A.Z., A.R. and M.K.M. researched the data for this article. T.K.O., J.A., A.Z., H.-K.G.S., A.M.R., S.N.K. and J.J.O. made substantial contributions to all other stages of the preparation of the manuscript for submission. In addition, T.R. and K.M.E. contributed substantially to discussion of content and the writing of the article. T.G.W., B.S., N.L.T. and M.K.M. also made considerable contributions to the writing of the article.
Competing interests The authors declare no competing interests.
References
- 1.US Census Bureau Census.gov [online] 2010 http://www.census.gov/prod/cen2010/briefs/c2010br-01.pdf.
- 2.Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of metastatic brain tumors. Neurosurg. Clin. N. Am. 2011;22:1–6. doi: 10.1016/j.nec.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Smedby KE, Brandt L, Backlund ML, Blomqvist P. Brain metastases admissions in Sweden between 1987 and 2006. Br. J. Cancer. 2009;101:1919–1924. doi: 10.1038/sj.bjc.6605373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieder C, Spanne O, Mehta MP, Grosu AL, Geinitz H. Presentation, patterns of care, and survival in patients with brain metastases: what has changed in the last 20 years? Cancer. 2011;117:2505–2512. doi: 10.1002/cncr.25707. [DOI] [PubMed] [Google Scholar]
- 5.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 6.Barnholtz-Sloan JS, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 7.Tabouret E, et al. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32:4655–4662. [PubMed] [Google Scholar]
- 8.Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv. Neurol. 1978;19:579–592. [PubMed] [Google Scholar]
- 9.Percy AK. Neoplasms of the central nervous system: epidemiologic considerations. Neurology. 1970;20:398–399. [PubMed] [Google Scholar]
- 10.Pickren JW, Lopez G, Tsukada Y, Lane WW. Brain metastases: an autopsy study. Cancer Treat. Symp. 1983;2:295–313. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52:2349–2354. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer institute Seer.cancer.gov [online] 2013 http://seer.cancer.gov/statfacts/html/lungb.html.
- 13.CDC Decline in Breast Cancer Incidence— United States, 1999–2003. MMWR Morb. Mortal. Wkly Rep. 2007;56:549–553. [PubMed] [Google Scholar]
- 14.CDC Press release Rates of new lung cancer cases drop in U. S. men and women [online] 2014 http://www.cdc.gov/media/releases/2014/p0109-lung-cancer.html.
- 15.Stemmler HJ, et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007;18:23–38. doi: 10.1097/01.cad.0000236313.50833.ee. [DOI] [PubMed] [Google Scholar]
- 16.Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J. Clin. Oncol. 2000;18:2349–2351. doi: 10.1200/JCO.2000.18.11.2349. [DOI] [PubMed] [Google Scholar]
- 17.Wen PY, Loeffler JS. Management of brain metastases. Oncol. (Williston Park) 1999;13:941–954. 957–961. discussion 961–962, 9. [PubMed] [Google Scholar]
- 18.American Cancer Society Cancer Facts & Figures 2013 [online] 2013 http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2013.
- 19.Eichler AF, et al. The biology of brain metastases—translation to new therapies. Nat. Rev. Clin. Oncol. 2011;8:344–356. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fidler IJ, Balasubramanian K, Lin Q, Kim SW, Kim SJ. The brain microenvironment and cancer metastasis. Mol. Cells. 2010;30:93–98. doi: 10.1007/s10059-010-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park ES, et al. Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. Proc. Natl Acad. Sci. USA. 2011;108:17456–17461. doi: 10.1073/pnas.1114210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SJ, et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia. 2011;13:286–298. doi: 10.1593/neo.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 24.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 25.Rampling R. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 1994;29:427–431. doi: 10.1016/0360-3016(94)90432-4. [DOI] [PubMed] [Google Scholar]
- 26.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 27.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Batchelor TT, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J. Clin. Oncol. 2010;28:2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kodack DP, et al. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc. Natl Acad. Sci. USA. 2012;109:E3119–E3127. doi: 10.1073/pnas.1216078109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pivot X, Bedairia N, Thiery-Vuillemin A, Espie M, Marty M. Combining molecular targeted therapies: clinical experience. Anticancer Drugs. 2011;22:701–710. doi: 10.1097/CAD.0b013e328345ffa4. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci. Transl. Med. 2013;5:180ra48. doi: 10.1126/scitranslmed.3005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bos PD, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sijens PE, et al. 1H MR spectroscopy in patients with metastatic brain tumors: a multicenter study. Magn. Reson. Med. 2005;33:818–826. doi: 10.1002/mrm.1910330612. [DOI] [PubMed] [Google Scholar]
- 34.Kelly PJ, et al. Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin. Proc. 1987;62:450–459. doi: 10.1016/s0025-6196(12)65470-6. [DOI] [PubMed] [Google Scholar]
- 35.Law M, et al. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology. 2002;222:715–721. doi: 10.1148/radiol.2223010558. [DOI] [PubMed] [Google Scholar]
- 36.Cha S. Neuroimaging in neuro-oncology. Neurotherapeutics. 2009;6:465–477. doi: 10.1016/j.nurt.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connell JJ, et al. Selective permeablization of the blood–brain barrier at sites of metastasis. J. Natl Cancer Inst. 2013;105:1634–1643. doi: 10.1093/jnci/djt276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serres S, et al. Molecular MRI enables early and sensitive detection of brain metastases. Proc. Natl Acad. Sci. USA. 2012;109:6674–6679. doi: 10.1073/pnas.1117412109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalkanis SN, et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J. Neurooncol. 2010;96:33–43. doi: 10.1007/s11060-009-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patchell RA, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 41.Vecht CJ, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann. Neurol. 1993;33:583–590. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 42.Katz HR. The relative effectiveness of radiation therapy, corticosteroids and surgery in the management of melanoma metastatic to the central nervous system. Int. J. Radiat. Oncol. Biol. Phys. 1981;7:897–906. doi: 10.1016/0360-3016(81)90006-7. [DOI] [PubMed] [Google Scholar]
- 43.Bindal RK, Sawaya R, Leavens ME, Lee JJ. Surgical treatment of multiple brain metastases. J. Neurosurg. 1993;79:210–216. doi: 10.3171/jns.1993.79.2.0210. [DOI] [PubMed] [Google Scholar]
- 44.Bindal RK, Sawaya R, Leavens ME, Hess KR, Taylor SH. Reoperation for recurrent metastatic brain tumors. J. Neurosurg. 1995;83:600–604. doi: 10.3171/jns.1995.83.4.0600. [DOI] [PubMed] [Google Scholar]
- 45.Al-Zabin M, Ullrich WO, Brawanski A, Proescholdt MA. Recurrent brain metastases from lung cancer: the impact of reoperation. Acta Neurochir. (Wien) 2010;152:1887–1892. doi: 10.1007/s00701-010-0721-7. [DOI] [PubMed] [Google Scholar]
- 46.Hatiboglu MA, Wildrick DM, Sawaya R. The role of surgical resection in patients with brain metastases. Ecancermedicalscience. 2013;7:308. doi: 10.3332/ecancer.2013.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwadate Y, Namba H, Yamaura A. Significance of surgical resection for the treatment of multiple brain metastases. Anticancer Res. 2000;20:573–577. [PubMed] [Google Scholar]
- 48.Andrews DW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 49.Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 1999;45:427–434. doi: 10.1016/s0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 50.US National Library of Medicine ClinicalTrials.gov [online] 2013 http://clinicaltrials.gov/ct2/show/NCT01372774?term=NCCTG-N107C&rank=1.
- 51.McGirt MJ, et al. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65:463–469. doi: 10.1227/01.NEU.0000349763.42238.E9. [DOI] [PubMed] [Google Scholar]
- 52.Gulati S, et al. The risk of getting worse: surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg. 2011;76:572–579. doi: 10.1016/j.wneu.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Youmans JR, Winn RH, editors. Youmans Neurological Surgery. Saunders/Elsevier; 2011. [Google Scholar]
- 54.Garber ST, Jensen RL. Image guidance for brain metastases resection. Surg. Neurol. Int. 2012;3:S111–S117. doi: 10.4103/2152-7806.95422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sills AK. Current treatment approaches to surgery for brain metastases. Neurosurgery. 2005;57:S24–S32. doi: 10.1227/01.neu.0000182763.16246.60. [DOI] [PubMed] [Google Scholar]
- 56.Levitt MR, Levitt R, Silbergeld DL. Controversies in the management of brain metastases. Surg. Neurol. Int. 2013;4:S231–S235. doi: 10.4103/2152-7806.111300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Narang J, et al. Differentiating treatment-induced necrosis from recurrent/progressive brain tumor using nonmodel-based semiquantitative indices derived from dynamic contrast-enhanced T1-weighted MR perfusion. Neuro. Oncol. 2011;13:1037–1046. doi: 10.1093/neuonc/nor075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eyüpoglu IY, Buchfelder M, Savaskan NE. Surgical resection of malignant gliomas—role in optimizing patient outcome. Nat. Rev. Neurol. 2013;9:141–151. doi: 10.1038/nrneurol.2012.279. [DOI] [PubMed] [Google Scholar]
- 59.Kellogg RG, Munoz LF. Selective excision of cerebral metastases from the precentral gyrus. Surg. Neurol. Int. 2013;4:66. doi: 10.4103/2152-7806.112189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weil RJ, Lonser RR. Selective excision of metastatic brain tumors originating in the motor cortex with preservation of function. J. Clin. Oncol. 2005;23:1209–1217. doi: 10.1200/JCO.2005.04.124. [DOI] [PubMed] [Google Scholar]
- 61.Walter J, Kuhn SA, Waschke A, Kalff R, Ewald C. Operative treatment of subcortical metastatic tumours in the central region. J. Neurooncol. 2011;103:567–573. doi: 10.1007/s11060-010-0420-5. [DOI] [PubMed] [Google Scholar]
- 62.Orringer DA, Golby A, Jolesz F. Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev. Med. Devices. 2012;9:491–500. doi: 10.1586/erd.12.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schackert G, Steinmetz A, Meier U, Sobottka SB. Surgical management of single and multiple brain metastases: results of a retrospective study. Onkologie. 2001;24:246–255. doi: 10.1159/000055087. [DOI] [PubMed] [Google Scholar]
- 64.Nimsky C, Ganslandt O, Tomandl B, Buchfelder M, Fahlbusch R. Low-field magnetic resonance imaging for intraoperative use in neurosurgery: a 5-year experience. Eur. Radiol. 2002;12:2690–2703. doi: 10.1007/s00330-002-1363-9. [DOI] [PubMed] [Google Scholar]
- 65.Kubben PL, et al. Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol. 2011;12:1062–1070. doi: 10.1016/S1470-2045(11)70130-9. [DOI] [PubMed] [Google Scholar]
- 66.Martirosyan NL, et al. Use of in vivo near-infrared laser confocal endomicroscopy with indocyanine green to detect the boundary of infiltrative tumor. J. Neurosurg. 2011;115:1131–1138. doi: 10.3171/2011.8.JNS11559. [DOI] [PubMed] [Google Scholar]
- 67.Sanai N, et al. Intraoperative confocal microscopy for brain tumors: a feasibility analysis in humans. Neurosurgery. 2011;68:282–290. doi: 10.1227/NEU.0b013e318212464e. [DOI] [PubMed] [Google Scholar]
- 68.Kircher MF, et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012;18:829–834. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalkanis SN, et al. Raman spectroscopy to distinguish grey matter, necrosis, and glioblastoma multiforme in frozen tissue sections. J. Neurooncol. doi: 10.1007/s11060-013-1326-9. http://dx.doi.org/10.1007/s11060-013-1326–9. [DOI] [PubMed]
- 70.US National Library of Medicine ClinicalTrials.gov [online] 2011 http://clinicaltrials.gov/ct2/show/NCT00671710?term=NCT00671710&rank=1.
- 71.US National Library of Medicine ClinicalTrials.gov [online] 2012 http://clinicaltrials.gov/ct2/show/NCT00241670?term=NCT00241670&rank=1.
- 72.US National Library of Medicine ClinicalTrials.gov [online] 2013 http://clinicaltrials.gov/ct2/show/NCT01445691?term=NCT01445691&rank=1.
- 73.US National Library of Medicine ClinicalTrials.gov [online] 2012 http://clinicaltrials.gov/ct2/show/NCT01128218?term=NCT01128218&rank=1.
- 74.US National Library of Medicine ClinicalTrials.gov [online] 2011 http://clinicaltrials.gov/ct2/show/NCT01351519?term=NCT01351519&rank=1.
- 75.US National Library of Medicine ClinicalTrials.gov [online] 2013 http://clinicaltrials.gov/ct2/show/NCT01502605?term=NCT01502605&rank=1.
- 76.US National Library of Medicine ClinicalTrials.gov [online] 2013 http://clinicaltrials.gov/ct2/show/NCT01116661?term=NCT01116661&rank=1.
- 77.US National Library of Medicine ClinicalTrials.gov [online] 2013 http://clinicaltrials.gov/ct2/show/NCT01575275?term=NCT01575275&rank=1.
- 78.US National Library of Medicine ClinicalTrials.gov [online] 2014 http://clinicaltrials.gov/ct2/show/NCT01502280?term=NCT01502280&rank=1.
- 79.US National Library of Medicine ClinicalTrials.gov [online] 2013 http://clinicaltrials.gov/ct2/show/NCT01811121?term=NCT01811121&rank=1.
- 80.Sahgal A, Soliman H, Larson DA. Whole-brain radiation therapy of brain metastasis. Prog. Neurol. Surg. 2012;25:82–95. doi: 10.1159/000331179. [DOI] [PubMed] [Google Scholar]
- 81.Gaspar L, et al. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 82.Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J. Natl Cancer Inst. 2011;103:714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li J, et al. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int. J. Radiat. Oncol. Biol. Phys. 2008;71:64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 84.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789–796. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 85.Welzel G. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2009;73:919–926. doi: 10.1016/j.ijrobp.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 86.Borgelt B, et al. The palliation of brain metastases: final results of the first two studies by the radiation therapy oncology group. Int. J. Radiat. Oncol. Biol. Phys. 1980;6:1–9. doi: 10.1016/0360-3016(80)90195-9. [DOI] [PubMed] [Google Scholar]
- 87.Murray KJ, et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: a report of the Radiation Therapy Oncology Group (RTOG) 9104. Int. J. Radiat. Oncol. Biol. Phys. 1997;39:571–574. doi: 10.1016/s0360-3016(97)00341-6. [DOI] [PubMed] [Google Scholar]
- 88.Noordijk EM, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int. J. Radiat. Oncol. Biol. Phys. 1994;29:711–717. doi: 10.1016/0360-3016(94)90558-4. [DOI] [PubMed] [Google Scholar]
- 89.Addeo R, et al. Phase 2 trial of temozolomide using protracted low-dose and whole-brain radiotherapy for nonsmall cell lung cancer and breast cancer patients with brain metastases. Cancer. 2008;113:2524–2531. doi: 10.1002/cncr.23859. [DOI] [PubMed] [Google Scholar]
- 90.Chua D, et al. Whole-brain radiation therapy plus concomitant temozolomide for the treatment of brain metastases from non-small-cell lung cancer: a randomized, open-label phase II study. Clin. Lung Cancer. 2010;11:176–181. doi: 10.3816/CLC.2010.n.022. [DOI] [PubMed] [Google Scholar]
- 91.Kouvaris JR, et al. Phase II study of temozolomide and concomitant whole-brain radiotherapy in patients with brain metastases from solid tumors. Onkologie. 2007;30:361–366. doi: 10.1159/000102557. [DOI] [PubMed] [Google Scholar]
- 92.Verger E, et al. Temozolomide and concomitant whole-brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2005;61:185–191. doi: 10.1016/j.ijrobp.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 93.Welsh JW, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J. Clin. Oncol. 2013;31:895–902. doi: 10.1200/JCO.2011.40.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sperduto PW, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int. J. Radiat. Oncol. Biol. Phys. 2013;85:1312–1318. doi: 10.1016/j.ijrobp.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aoyama H, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 96.Sneed PK, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int. J. Radiat. Oncol. Biol. Phys. 1999;43:549–558. doi: 10.1016/s0360-3016(98)00447-7. [DOI] [PubMed] [Google Scholar]
- 97.Sneed PK, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2002;53:519–526. doi: 10.1016/s0360-3016(02)02770-0. [DOI] [PubMed] [Google Scholar]
- 98.Chang EL, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 99.Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118:2486–2493. doi: 10.1002/cncr.26515. [DOI] [PubMed] [Google Scholar]
- 100.Aoyama H, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:1388–1395. doi: 10.1016/j.ijrobp.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 101.Kocher M, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J. Clin. Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soffietti R, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J. Clin. Oncol. 2013;31:65–72. doi: 10.1200/JCO.2011.41.0639. [DOI] [PubMed] [Google Scholar]
- 103.Sahgal A, et al. Individual patient data meta-analysis of randomized controlled trials comparing stereotactic radiosurgery alone to SRS plus whole brain radiation therapy in patients with brain metastasis. Int. J. Radiat. Oncol. Biol. Phys. 2013;87:1187. [Google Scholar]
- 104.Barani IJ, Larson DA, Berger MS. Future directions in treatment of brain metastases. Surg. Neurol. Int. 2013;4(Suppl. 4):S220–S230. doi: 10.4103/2152-7806.111299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Slotman B, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N. Engl. J. Med. 2007;357:664–672. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 106.Gore EM, et al. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of Radiation Therapy Oncology Group Study RTOG 0214. J. Clin. Oncol. 2011;29:272–278. doi: 10.1200/JCO.2010.29.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun A, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J. Clin. Oncol. 2011;29:279–286. doi: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brown PD, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gondi V, et al. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010;78:1244–1252. doi: 10.1016/j.ijrobp.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gutierrez AN, et al. Whole-brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: a planning study. Int. J. Radiat. Oncol. Biol. Phys. 2007;69:589–597. doi: 10.1016/j.ijrobp.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gondi V, et al. Memory preservation with conformal avoidance of the hippocampus during whole-brain radiotherapy (WBRT) for patients with brain metastases: primary endpoint results of RTOG 0933. Int. J. Radiat. Oncol. Biol. Phys. 2013;87:1186. [Google Scholar]
- 112.Hall WA, Djalilian HR, Nussbaum ES, Cho KH. Long-term survival with metastatic cancer to the brain. Med. Oncol. 2000;17:279–286. doi: 10.1007/BF02782192. [DOI] [PubMed] [Google Scholar]
- 113.Maclean J, et al. Multi-disciplinary management for patients with oligometastases to the brain: results of a 5 year cohort study. Radiat. Oncol. 2013;8:156. doi: 10.1186/1748-717X-8-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gril B, et al. Pazopanib reveals a role for tumor cell B-Raf in the prevention of HER2+ breast cancer brain metastasis. Clin. Cancer Res. 2011;17:142–153. doi: 10.1158/1078-0432.CCR-10-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Palmieri D, et al. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol. Cancer Res. 2009;7:1438–1445. doi: 10.1158/1541-7786.MCR-09-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shaw AT, et al. Clinical activity of the ALK inhibitor LDK378 in advanced, ALK-positive NSCLC [abstract] J. Clin. Oncol. 2013;31(Suppl.) abstract 8010. [Google Scholar]
- 117.Fukuoka M, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J. Clin. Oncol. 2011;29:2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 118.Sosman JA, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Slamon DJ, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 120.Kallioniemi OP, et al. Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Int. J. Cancer. 1991;49:650–655. doi: 10.1002/ijc.2910490504. [DOI] [PubMed] [Google Scholar]
- 121.Nie F, et al. Involvement of epidermal growth factor receptor overexpression in the promotion of breast cancer brain metastasis. Cancer. 2012;118:5198–5209. doi: 10.1002/cncr.27553. [DOI] [PubMed] [Google Scholar]
- 122.Gril B, et al. Translational research in brain metastasis is identifying molecular pathways that may lead to the development of new therapeutic strategies. Eur. J. Cancer. 2010;46:1204–1210. doi: 10.1016/j.ejca.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rahmathulla G, Toms SA, Weil RJ. The molecular biology of brain metastasis. J. Oncol. 2012;2012:723541. doi: 10.1155/2012/723541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sun M, et al. HER family receptor abnormalities in lung cancer brain metastases and corresponding primary tumors. Clin. Cancer Res. 2009;15:4829–4837. doi: 10.1158/1078-0432.CCR-08-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Benedettini E, et al. Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. Am. J. Pathol. 2010;177:415–423. doi: 10.2353/ajpath.2010.090863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Breindel JL, et al. EGF receptor activates MET through MAPK to enhance non-small cell lung carcinoma invasion and brain metastasis. Cancer Res. 2013;73:5053–5065. doi: 10.1158/0008-5472.CAN-12-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li B, et al. Elevated PLGF contributes to small-cell lung cancer brain metastasis. Oncogene. 2013;32:2952–2962. doi: 10.1038/onc.2012.313. [DOI] [PubMed] [Google Scholar]
- 128.Nguyen DX, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grinberg-Rashi H, et al. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clin. Cancer Res. 2009;15:1755–1761. doi: 10.1158/1078-0432.CCR-08-2124. [DOI] [PubMed] [Google Scholar]
- 130.Wu PF, et al. Phosphorylated insulin-like growth factor-1 receptor (pIGF1R) is a poor prognostic factor in brain metastases from lung adenocarcinomas. J. Neurooncol. 2013;115:61–70. doi: 10.1007/s11060-013-1194-3. [DOI] [PubMed] [Google Scholar]
- 131.Chen G, Wang Z, Liu XY, Liu FY. High-level CXCR4 expression correlates with brain-specific metastasis of non-small cell lung cancer. World J. Surg. 2011;35:56–61. doi: 10.1007/s00268-010-0784-x. [DOI] [PubMed] [Google Scholar]
- 132.Yoshimasu T, et al. Increased expression of integrin alpha3beta1 in highly brain metastatic subclone of a human non-small cell lung cancer cell line. Cancer Sci. 2004;95:142–148. doi: 10.1111/j.1349-7006.2004.tb03195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shintani Y, et al. Overexpression of ADAM9 in non-small cell lung cancer correlates with brain metastasis. Cancer Res. 2004;64:4190–4196. doi: 10.1158/0008-5472.CAN-03-3235. [DOI] [PubMed] [Google Scholar]
- 134.Minotti V, et al. Chemotherapy with cisplatin and teniposide for cerebral metastases in non-small cell lung cancer. Lung Cancer. 1998;20:93–98. doi: 10.1016/s0169-5002(98)00021-x. [DOI] [PubMed] [Google Scholar]
- 135.Kelly K, Bunn PA., Jr Is it time to reevaluate our approach to the treatment of brain metastases in patients with non-small cell lung cancer? Lung Cancer. 1998;20:85–91. doi: 10.1016/s0169-5002(98)00020-8. [DOI] [PubMed] [Google Scholar]
- 136.Pietanza MC, et al. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin. Cancer Res. 2012;18:1138–1145. doi: 10.1158/1078-0432.CCR-11-2059. [DOI] [PubMed] [Google Scholar]
- 137.Cortes J, et al. Front-line paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology. 2003;64:28–35. doi: 10.1159/000066520. [DOI] [PubMed] [Google Scholar]
- 138.Bernardo G, et al. First-line chemotherapy with vinorelbine, gemcitabine, and carboplatin in the treatment of brain metastases from non-small-cell lung cancer: a phase II study. Cancer Invest. 2002;20:293–302. doi: 10.1081/cnv-120001173. [DOI] [PubMed] [Google Scholar]
- 139.Robinet G, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Francais de Pneumo-Cancerologie (GFPC) Protocol 95–1. Ann. Oncol. 2001;12:59–67. doi: 10.1023/a:1008338312647. [DOI] [PubMed] [Google Scholar]
- 140.Heimberger AB, et al. Brain tumors in mice are susceptible to blockade of epidermal growth factor receptor (EGFR) with the oral, specific, EGFR-tyrosine kinase inhibitor ZD1839 (Iressa) Clin. Cancer Res. 2002;8:3496–3502. [PubMed] [Google Scholar]
- 141.Lai CS, Boshoff C, Falzon M, Lee SM. Complete response to erlotinib treatment in brain metastases from recurrent NSCLC. Thorax. 2006;61:91. doi: 10.1136/thx.2005.052233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yap TA, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J. Clin. Oncol. 2010;28:3965–3972. doi: 10.1200/JCO.2009.26.7278. [DOI] [PubMed] [Google Scholar]
- 143.Kuiper JL, Smit EF. High-dose, pulsatile erlotinib in two NSCLC patients with leptomeningeal metastases—one with a remarkable thoracic response as well. Lung Cancer. 2013;80:102–105. doi: 10.1016/j.lungcan.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 144.Grommes C, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13:1364–1369. doi: 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Katayama T, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J. Thorac. Oncol. 2009;4:1415–1419. doi: 10.1097/JTO.0b013e3181b62572. [DOI] [PubMed] [Google Scholar]
- 146.Heon S, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin. Cancer Res. 2012;18:4406–4414. doi: 10.1158/1078-0432.CCR-12-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Doebele RC, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer. 2012;118:4502–4511. doi: 10.1002/cncr.27409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shaw AT, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 149.Camidge DR, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–1019. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Camidge DR, et al. Updated results of a first-in-human dose-finding study of the ALK/EGFR inhibitor AP26113 in patients with advanced malignancies [abstract 3401]. Presented at the European Cancer Congress, 2013.2013. [Google Scholar]
- 151.Solomon B, Wilner KD, Shaw AT. Current status of targeted therapy for anaplastic lymphoma kinase-rearranged non-small cell lung cancer. Clin. Pharmacol. Ther. 2013;95:15–23. doi: 10.1038/clpt.2013.200. [DOI] [PubMed] [Google Scholar]
- 152.Costa DB, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J. Clin. Oncol. 2011;20:e443–e445. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 153.Tang SC, et al. Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int. J. Cancer. 2014;134:1484–1494. doi: 10.1002/ijc.28475. [DOI] [PubMed] [Google Scholar]
- 154.Kinoshita Y, Koga Y, Sakamoto A, Hidaka K. Long-lasting response to crizotinib in brain metastases due to EML4-ALK-rearranged non-small-cell lung cancer. BMJ Case Rep. doi: 10.1136/bcr-2013-200867. http://dx.doi.org/10.1136/bcr-2013-200867. [DOI] [PMC free article] [PubMed]
- 155.Kim YH, et al. High-dose crizotinib for brain metastases refractory to standard-dose crizotinib. J. Thor. Oncol. 2013;8:e85–e86. doi: 10.1097/JTO.0b013e31829cebbb. [DOI] [PubMed] [Google Scholar]
- 156.Gandhi L, Drappatz J, Ramaiya NH, Otterson GA. High-dose pemetrexed in combination with high-dose crizotinib for the treatment of refractory CNS metastases in ALK-rearranged non-small-cell lung cancer. J. Thorac. Oncol. 2013;8:e3–e5. doi: 10.1097/JTO.0b013e3182762d20. [DOI] [PubMed] [Google Scholar]
- 157.Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 158.Sorlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl Acad. Sci. USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guiu S, et al. Molecular subclasses of breast cancer: how do we define them? The IMPAKT 2012 Working Group Statement. Ann. Oncol. 2012;23:2997–3006. doi: 10.1093/annonc/mds586. [DOI] [PubMed] [Google Scholar]
- 160.Heitz F, et al. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur. J. Cancer. 2009;45:2792–2798. doi: 10.1016/j.ejca.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 161.Dawood S, et al. Incidence of brain metastases as a first site of recurrence among women with triple receptor-negative breast cancer. Cancer. 2012;118:4652–4659. doi: 10.1002/cncr.27434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fokstuen T, et al. Radiation therapy in the management of brain metastases from breast cancer. Breast Cancer Res. Treat. 2000;62:211–216. doi: 10.1023/a:1006486423827. [DOI] [PubMed] [Google Scholar]
- 163.Anders CK, et al. The prognostic contribution of clinical breast cancer subtype, age, and race among patients with breast cancer brain metastases. Cancer. 2011;117:1602–1611. doi: 10.1002/cncr.25746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Niwinska A, Murawska M, Pogoda K. Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT) Ann. Oncol. 2010;21:942–948. doi: 10.1093/annonc/mdp407. [DOI] [PubMed] [Google Scholar]
- 165.Melisko ME, Moore DH, Sneed PK, De Franco J, Rugo HS. Brain metastases in breast cancer: clinical and pathologic characteristics associated with improvements in survival. J. Neurooncol. 2008;88:359–365. doi: 10.1007/s11060-008-9578-5. [DOI] [PubMed] [Google Scholar]
- 166.Amir E, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J. Clin. Oncol. 2012;30:587–592. doi: 10.1200/JCO.2010.33.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Niikura N, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J. Clin. Oncol. 2012;30:593–599. doi: 10.1200/JCO.2010.33.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sezgin C, Gokmen E, Esassolak M, Ozdemir N, Goker E. Risk factors for central nervous system metastasis in patients with metastatic breast cancer. Med. Oncol. 2007;24:155–161. doi: 10.1007/BF02698034. [DOI] [PubMed] [Google Scholar]
- 169.Ryberg M, et al. Predictors of central nervous system metastasis in patients with metastatic breast cancer. A competing risk analysis of 579 patients treated with epirubicin-based chemotherapy. Breast Cancer Res. Treat. 2005;91:217–225. doi: 10.1007/s10549-005-0323-x. [DOI] [PubMed] [Google Scholar]
- 170.Musolino A, et al. Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer. 2011;117:1837–1846. doi: 10.1002/cncr.25771. [DOI] [PubMed] [Google Scholar]
- 171.Pestalozzi BC, et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann. Oncol. 2006;17:935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 172.Ray PS, et al. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 2010;70:3870–3876. doi: 10.1158/0008-5472.CAN-09-4120. [DOI] [PubMed] [Google Scholar]
- 173.Lorger M, Krueger JS, O’Neal M, Staflin K, Felding-Habermann B. Activation of tumor cell integrin αvβ3 controls angiogenesis and metastatic growth in the brain. Proc. Natl Acad. Sci. USA. 2009;106:10666–10671. doi: 10.1073/pnas.0903035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nam DHI. Activation of notch signaling in a xenograft model of brain metastasis. Clin. Cancer Res. 2008;14:4059–4066. doi: 10.1158/1078-0432.CCR-07-4039. [DOI] [PubMed] [Google Scholar]
- 175.Zhang S, et al. SRC family kinases as novel therapeutic targets to treat breast cancer brain metastases. Cancer Res. 2013;73:5764–5774. doi: 10.1158/0008-5472.CAN-12-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Saldana SM, et al. Inhibition of type I insulin-like growth factor receptor signaling attenuates the development of breast cancer brain metastasis. PLoS ONE. 2013;8:e73406. doi: 10.1371/journal.pone.0073406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Palmieri D, et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin. Cancer Res. 2009;15:6148–6157. doi: 10.1158/1078-0432.CCR-09-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J. Surg. Oncol. 1983;23:175–180. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 179.Trudeau ME, et al. Temozolomide in metastatic breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada—Clinical Trials Group (NCIC-CTG) Ann. Oncol. 2006;17:952–956. doi: 10.1093/annonc/mdl056. [DOI] [PubMed] [Google Scholar]
- 180.Siena S, et al. Dose-dense temozolomide regimen for the treatment of brain metastases from melanoma, breast cancer, or lung cancer not amenable to surgery or radiosurgery: a multicenter phase II study. Ann. Oncol. 2010;21:655–661. doi: 10.1093/annonc/mdp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Iwamoto FM, et al. A phase II trial of vinorelbine and intensive temozolomide for patients with recurrent or progressive brain metastases. J. Neurooncol. 2008;87:85–90. doi: 10.1007/s11060-007-9491-3. [DOI] [PubMed] [Google Scholar]
- 182.Christodoulou C, et al. Temozolomide (TMZ) combined with cisplatin (CDDP) in patients with brain metastases from solid tumors: a Hellenic Cooperative Oncology Group (HeCOG) phase II study. J. Neurooncol. 2005;71:61–65. doi: 10.1007/s11060-004-9176-0. [DOI] [PubMed] [Google Scholar]
- 183.Ekenel M, Hormigo AM, Peak S, Deangelis LM, Abrey LE. Capecitabine therapy of central nervous system metastases from breast cancer. J. Neurooncol. 2007;85:223–227. doi: 10.1007/s11060-007-9409-0. [DOI] [PubMed] [Google Scholar]
- 184.Wang ML, Yung WK, Royce ME, Schomer DF, Theriault RL. Capecitabine for 5-fluorouracil-resistant brain metastases from breast cancer. Am. J. Clin. Oncol. 2001;24:421–424. doi: 10.1097/00000421-200108000-00026. [DOI] [PubMed] [Google Scholar]
- 185.Rivera E, et al. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer. 2006;107:1348–1354. doi: 10.1002/cncr.22127. [DOI] [PubMed] [Google Scholar]
- 186.Lin NU, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 2008;26:1993–1999. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lin NU, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin. Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 188.Metro G, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann. Oncol. 2011;22:625–630. doi: 10.1093/annonc/mdq434. [DOI] [PubMed] [Google Scholar]
- 189.Sutherland S, et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases—the UK experience. Br. J. Cancer. 2010;102:995–1002. doi: 10.1038/sj.bjc.6605586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lin NU, et al. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J. Neurooncol. 2011;105:613–620. doi: 10.1007/s11060-011-0629-y. [DOI] [PubMed] [Google Scholar]
- 191.Batchelor T, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 192.Bourdeanu L, Reilly AA, Luu T. CNS metastases in breast cancer: a comparison report [abstract P6-11-12]. Presented at the San Antonio Breast Cancer Symposium.2013. [Google Scholar]
- 193.Fitzgerald DP, et al. TPI-287, a new taxane family member, reduces the brain metastatic colonization of breast cancer cells. Mol. Cancer Ther. 2012;11:1959–1967. doi: 10.1158/1535-7163.MCT-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.US National Library of Medicine ClinicalTrials.gov [online] 2013 http://clinicaltrials.gov/ct2/show/NCT01332630?term=NCT01332630&rank=1.
- 195.US National Library of Medicine ClinicalTrials.gov [online] 2014 http://clinicaltrials.gov/ct2/show/NCT01441596?term=NCT01441596&rank=1.
- 196.US National Library of Medicine ClinicalTrials.gov [online] 2013 http://clinicaltrials.gov/ct2/show/NCT01494662?term=NCT01494662&rank=1.
- 197.Ro J, et al. Clinical outcomes of HER2-positive metastatic breast cancer patients with brain metastasis treated with lapatinib and capecitabine: an open-label expanded access study in Korea. BMC Cancer. 2012;12:322. doi: 10.1186/1471-2407-12-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yap YS, et al. Brain metastases in Asian HER2-positive breast cancer patients: anti-HER2 treatments and their impact on survival. Br. J. Cancer. 2012;107:1075–1082. doi: 10.1038/bjc.2012.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.US National Library of Medicine ClinicalTrials.gov [online] 2014 http://clinicaltrials.gov/ct2/show/NCT01004172?term=NCT01004172&rank=1.
- 200.US National Library of Medicine ClinicalTrials.gov [online] 2013 http://clinicaltrials.gov/ct2/show/NCT01281696?term=NCT01281696&rank=1.
- 201.Baselga J, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Curran MP. Everolimus: in patients with subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Paediatr. Drugs. 2012;14:51–60. doi: 10.2165/11207730-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 203.Bendell JC, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 204.Fong PC, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 205.O’Shaughnessy J, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N. Engl. J. Med. 2011;364:205–214. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 206.Mehta MP, et al. Phase I safety and pharmacokinetic (PK) study of veliparib in combination with whole brain radiation therapy (WBRT) in patients (pts) with brain metastases [abstract 2013] Int. J. Radiat. Oncol. Biol. Phys. 2012;84(Suppl.):S269–S270. [Google Scholar]
- 207.Byrne TN, Cascino TL, Posner JB. Brain metastasis from melanoma. J. Neurooncol. 1983;1:313–317. doi: 10.1007/BF00165714. [DOI] [PubMed] [Google Scholar]
- 208.Davies MA, et al. Integrated molecular and clinical analysis of Akt activation in metastatic melanoma. Clin. Cancer Res. 2009;15:7538–7546. doi: 10.1158/1078-0432.CCR-09-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Govindarajan B, et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J. Clin. Invest. 2007;117:719–729. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bonner MY, Arbiser JL. Targeting NADPH oxidases for the treatment of cancer and inflammation. Cell. Mol. Life Sci. 2012;69:2435–2442. doi: 10.1007/s00018-012-1017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Boivin B, Zhang S, Arbiser JL, Zhang ZY, Tonks NK. A modified cysteinyl-labeling assay reveals reversible oxidation of protein tyrosine phosphatases in angiomyolipoma cells. Proc. Natl Acad. Sci. USA. 2008;105:9959–9964. doi: 10.1073/pnas.0804336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Munson JM, et al. Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Sci. Transl. Med. 2012;4:127–136. doi: 10.1126/scitranslmed.3003016. [DOI] [PubMed] [Google Scholar]
- 213.Hamilton R, et al. Pathologic and gene expression features of metastatic melanomas to the brain. Cancer. 2013;119:2737–2746. doi: 10.1002/cncr.28029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Arpaia E, et al. The interaction between caveolin-1 and Rho-GTPases promotes metastasis by controlling the expression of alpha5-integrin and the activation of Src, Ras and ERK. Oncogene. 2012;31:884–896. doi: 10.1038/onc.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 216.Arnold SM, et al. Expression of p53, bcl-2, E-cadherin, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinases-1 in paired primary tumors and brain metastasis. Clin. Cancer Res. 1999;5:4028–4033. [PubMed] [Google Scholar]
- 217.Kusters B, et al. Differential effects of vascular endothelial growth factor A isoforms in a mouse brain metastasis model of human melanoma. Cancer Res. 2003;63:5408–5413. [PubMed] [Google Scholar]
- 218.Kusters B, et al. Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res. 2002;62:341–345. [PubMed] [Google Scholar]
- 219.Yano S, et al. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res. 2000;60:4959–4967. [PubMed] [Google Scholar]
- 220.Xie TX, et al. Activation of STAT3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 221.Shaw E, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. Int. J. Radiat. Oncol. Biol. Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 222.Margolin K, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 223.Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br. J. Radiol. 1975;48:863–866. doi: 10.1259/0007-1285-48-574-863. [DOI] [PubMed] [Google Scholar]
- 224.Stamell EF, et al. The abscopal effect associated with a systemic anti-melanoma immune response. Int. J. Radiat. Oncol. Biol. Phys. 2013;85:293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Falchook GS, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893–1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Long GV, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 227.Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 2013;14:e60–e69. doi: 10.1016/S1470-2045(12)70539-9. [DOI] [PubMed] [Google Scholar]
- 228.Okwan-Duodu D, Pollack BP, Lawson D, Khan MK. Role of radiation therapy as immune activator in the era of modern immunotherapy for metastatic malignant melanoma. Am. J. Clin. Oncol. doi: 10.1097/COC.0b013e3182940dc3. http://dx.doi.org/10.1097/COC.0b013e3182940dc3. [DOI] [PubMed]
- 229.Martinez N, Boire A, Deangelis LM. Molecular interactions in the development of brain metastases. Int. J. Mol. Sci. 2013;14:17157–17167. doi: 10.3390/ijms140817157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ceresoli GL, et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann. Oncol. 2004;15:1042–1047. doi: 10.1093/annonc/mdh276. [DOI] [PubMed] [Google Scholar]
- 232.Wu C, et al. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer. 2007;57:359–364. doi: 10.1016/j.lungcan.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 233.Kim JE, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer. 2009;65:351–354. doi: 10.1016/j.lungcan.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 234.Hotta K, et al. Effect of gefitinib (‘Iressa’, ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer. 2004;46:255–261. doi: 10.1016/j.lungcan.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 235.Porta R, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur. Respir. J. 2011;37:624–631. doi: 10.1183/09031936.00195609. [DOI] [PubMed] [Google Scholar]
- 236.Atkins MB, et al. Temozolomide, thalidomide, and whole brain radiation therapy for patients with brain metastasis from metastatic melanoma: a phase II Cytokine Working Group study. Cancer. 2008;113:2139–2145. doi: 10.1002/cncr.23805. [DOI] [PubMed] [Google Scholar]
- 237.Konstantinou MP, et al. Ipilimumab in melanoma patients with brain metastasis: a retrospective multicentre evaluation of thirty-eight patients. Acta Derm. Venereol. 2014;94:45–49. doi: 10.2340/00015555-1654. [DOI] [PubMed] [Google Scholar]