Abstract
Practicing certain visual tasks leads, as a result of a process termed “perceptual learning,” to a significant improvement in performance. Learning is specific for basic stimulus features such as local orientation, retinal location, and eye of presentation, suggesting modification of neuronal processes at the primary visual cortex in adults. It is not known, however, whether such low-level learning affects higher-level visual tasks such as recognition. By systematic low-level training of an adult visual system malfunctioning as a result of abnormal development (leading to amblyopia) of the primary visual cortex during the “critical period,” we show here that induction of low-level changes might yield significant perceptual benefits that transfer to higher visual tasks. The training procedure resulted in a 2-fold improvement in contrast sensitivity and in letter-recognition tasks. These findings demonstrate that perceptual learning can improve basic representations within an adult visual system that did not develop during the critical period.
Keywords: plasticity, development, visual cortex, spatial interactions
Amblyopia is characterized by several functional abnormalities in spatial vision (for reviews see refs. 1-4), including reductions in visual acuity (VA), contrast-sensitivity function (CSF), and vernier acuity as well as spatial distortion (5), abnormal spatial interactions (6, 7), and impaired contour detection (8, 9). In addition, amblyopic individuals suffer from binocular abnormalities such as impaired stereoacuity and abnormal binocular summation. The visual deficiencies are thought to be irreversible after the first decade of life (10-12), by which time the developmental maturation window has been terminated. The loss of vision is thought to result from abnormal operation of the neuronal network within the primary visual cortex, particularly of orientation-selective neurons and their interactions (13). The perceptual learning procedure described in this study was designed to train this network by efficiently stimulating these neuronal populations and effectively promoting their spatial interactions.
Spatial interactions in human vision can be probed by contrast detection of a localized target in the presence of flankers (Fig. 1). These experiments show that the contrast threshold of a foveal Gabor signal (GS) is reduced in the presence of cooriented and coaligned (collinear) high-contrast GS flankers (14-18). The excitatory interaction is range-dependent and is maximal for target-flanker separation of approximately three times the GS wavelength. Smaller separations can raise the target threshold, depending on flanker contrast and phase (19). Single-unit recordings suggest that the underlying mechanisms reside within the primary visual cortex (20, 21). Neuronal responses in the visual cortex are tuned for location, orientation, and spatial frequency. Recent evidence from studies in the cat and the monkey (20, 22-25) shows that neuronal responses in the primary visual cortex are modulated by remote image parts, with both excitatory and inhibitory effects observed, depending on stimulus contrast and configuration. Psychophysical and electrophysiological results show abnormal interactions in amblyopic patients (6, 7), with an extended range of inhibition. It is possible that the abnormal visual input to the amblyopic visual system during early development produces distorted patterns of activity in the visual cortex, leading to abnormal development of connectivity.
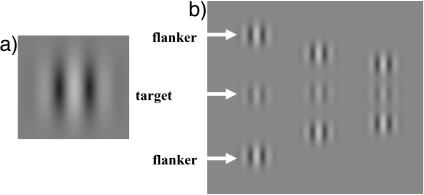
Fig. 1.
Visual stimuli used during training. (a) Single GS (see Methods). (b) Triplets consisting of a target GS and two flankers used in the lateral-masking experiments. Three different target-flanker separations were used. Target contrast is enhanced for demonstration. In all experiments reported here, the GS spatial SD was equal to the wavelength (σ = λ). The spatial frequency and orientation of flankers were always set to those of the target.
Methods
The results reported here are from a prospective, randomized, masked, controlled study. Included were patients, between 9 (when usually no treatment is offered) and 55 years old, with unilateral amblyopia secondary to strabismus and/or anisometropia. Best-corrected VA in the amblyopic eyes ranged from 6/9 to 6/30, measured on early treatment of diabetic retinopathy study (ETDRS) charts. Each patient (or parent/legal guardian) signed an informed consent form approved by the local institutional review board. A total of 77 amblyopic patients (divided into two groups) and 16 subjects with normal vision (control group) participated in the study. Their clinical details and treatment history are described in Table 1 and Fig. 2. Best-corrected VA was measured on one of three randomly selected ETDRS charts [LogMAR (log minimum angle of resolution) scale] by clinicians who were blinded to the subgroup (treatment or control) to which the examinee had been allocated. Each study participant had two to four weekly treatment sessions of ≈30 min each, totaling 45 ± 15 sessions (mean ± SD).
Table 1. Clinical details and treatment history.
| Range of 1st treated spatial frequency
|
Category
|
No. of patients
|
||||
|---|---|---|---|---|---|---|
| Initial VA | Aniso | Strab | Age, years | |||
| Treatment group (amblyopic) | ||||||
| 1st group | 3-12 | 0.41 ± 0.14 | 23 | 21 | 35 ± 13 | 44 |
| 5.9 ± 2.8 | ||||||
| 2nd group | 1.5-12 | 0.38 ± 0.12 | 8 | 11 | 39 ± 9.6 | 19 |
| 2.7 ± 1.6 | ||||||
| Subtotal | 31 | 32 | 63 | |||
| Control (amblyopic) | ||||||
| 1st group | 0.5 | 0.41 ± 0.12 | 7 | 3 | 38.2 ± 9.4 | 10 |
| 2nd group | 1.5-12 | 0.43 ± 0.12 | 3 | 1 | 41 ± 11.3 | 4 |
| Subtotal | 10 | 4 | 14 | |||
| Total amblyopia | 41 | 36 | 77 | |||
| Control (normal vision) | 3-12* | N/A | N/A | 32.8 ± 15 | 16 | |
| 7.7 ± 3.6 | ||||||
N/A, not applicable; Aniso, anisometropic; Strab, strabismic.
Fig. 2.
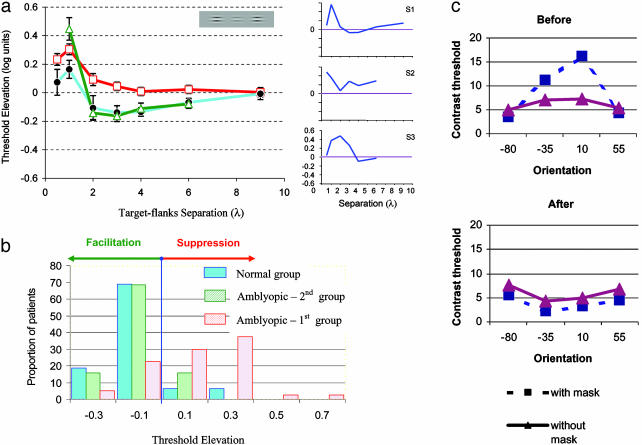
Lateral-masking curves. (a Left) Comparison exposing the absence of lateral facilitation in the data of the amblyopic patients. Error bars denote ±1 SE. (a Right) Masking curves for three amblyopic patients (S1-S3, selected to demonstrate variability), which show variable amounts of increased suppression, pointing to abnormal connectivity. Data from untrained subjects were obtained during the first lateral-masking session and were averaged across subjects: first group of amblyopic patients (open squares; n = 40; 40 of 44 completed these sessions); second group of amblyopic patients (open triangles; n = 19); nonamblyopic subjects (filled circles; n = 16). Spatial frequencies ranged from 3 to 12 cpd (mean ± SD, 5.9 ± 2.8; n = 40) for the first treatment group, from 1.5 to 6 cpd (2.7 ± 1.6; n = 19) for the second treatment group, and from 3 to 12 (7.7 ± 3.6 cpd; n = 16) for the control group. Past medical records and childhood photographs were obtained whenever possible. Of the 77 amblyopes, 58 had been treated by occlusion in the past (occlusion treatment had been initiated in 12 patients before the age of 3 years, in 21 patients between the ages of 3 and 5 years, in 21 patients between 5 and 9 years, in 3 patients aged ≥9 years, and 1 patient could not recall the age at which the occlusion treatment started). Fourteen subjects had received no treatment in the past, and in five patients information was not available. (b) The sum of the threshold elevations (2-6λ) was recorded for each patient from the first treatment session. The mean threshold elevation was 0.13 log units ± 0.03 (mean ± SE) for the first amblyopic group (n = 40 of 44), -0.12 log units ± 0.02 for the second amblyopic group (n = 19), and -0.12 ± 0.03 (n = 16) for the group with normal vision. (c) Contrast-detection thresholds for Gabor targets in the presence and absence of flankers for an amblyopic patient with astigmatism. Thresholds were tested with flankers at a distance of 3λ from the target. Data obtained before the first training session show strong lateral suppression around the orientation corresponding to the astigmatism axis (maximal blur), whereas after training the suppression disappeared and some facilitation was observed for all orientations. These experiments were carried out with the eyes optically corrected.
Each patient was subjected to a comprehensive evaluation including detailed ophthalmic history focused on amblyopia-related factors including age of diagnosis, past treatments, and family history. They also all underwent a detailed ophthalmologic examination, which included cycloplegic refraction. Ocular movements were checked, ocular alignment for distance and near was tested by cover tests, and binocular functions were examined with the Worth-4-dot and Titmus stereo tests. On the basis of the collected data, the type of amblyopia was evaluated carefully. CSF was measured at baseline, after the treatment, and at follow-up visits by using a wall-mounted chart (S.W.C.T., Stereo Optical Company, Chicago) (26) from a distance of 3 m with controlled room lighting (≈140 cd/m2, within the range of 68-240 cd/m2 specified by the manufacturer). These grating stimuli subtended a visual angle of 1.4° at all frequencies.
The stimuli used for treatment were local gray-level gratings (the GS) with spatial frequencies of 1.5-12 cycles per degree (cpd) modulated from a background luminance of 40 cd·m-2 (Fig. 1). In all experiments, the SD of the GS was equal to the wavelength (σ = λ). Stimuli were presented on a Philips (Eindhoven, the Netherlands) multiscan 107P color monitor using a personal computing system. The effective size of the monitor screen was 24 × 32 cm, which at a viewing distance of 150 cm subtends a visual angle of 9 × 12°. The study participants were treated in a dark cubicle in which the only ambient light came from the display screen.
Contrast threshold was measured by a procedure in which the subject was required to choose between two alternatives (two alternatives forced choice). The target was presented in one of two images, each lasting 80-320 msec, at an interval of 500 msec. The subject, seated 1.5 m from the screen and wearing the best optical correction with the nonamblyopic eye occluded, was required to detect the target, which was shown in only one of the two presentations. A visible fixation circle indicated the location of the target between presentations. Subjects activated the presentation of each pair of images at their own pace. They were informed of a wrong answer by an auditory feedback after each pair of presentations.
A standard training session included a GS contrast-detection task (Fig. 1), with and without flanking collinear high-contrast patches. Thresholds for the contrast-detection task were measured with a one-up/three-down staircase (with steps of 0.1 log units), which is used to estimate the stimulus strength at the 79% accuracy level.
Before training started, two sessions were devoted to measuring the basic spatial functions such as contrast sensitivity and spatial interactions, the latter representing degrees of cortical suppression and facilitation. From these sessions we chose the initial training parameters; the starting spatial frequency and the global orientation were set to the highest spatial frequency, where for the worst global orientation, the contrast threshold was lower than twice the normal value but not >15%. During training sessions, the spatial frequency and orientation of the stimuli were changed, starting with lower spatial frequencies (set according to the criteria described above) and moving progressively to higher ones, with four orientations at each spatial frequency. Subsequent sessions were designed individually by using an automated and computerized decision-maker algorithm, depending on the patient's performance during the previous session in relation to a standard performance of subjects without amblyopia. Sessions included training on a lateral interaction task including detection of isolated GSs with varied parameters (14-16). A typical session included 10-15 blocks with different target-flanker separations. The spatial frequency and orientation in any given treatment session were kept constant.
The training procedure described above was used for all amblyopic patients in the treatment groups. Patients in the second treatment group were trained on the same algorithm, but the starting spatial frequency was set to one octave below the frequency set for the first group (second group; see Table 1). Subjects in the control (placebo) group were given similar tasks, namely detection of GS targets. For the 10 subjects who served as controls for the first treatment group, the attributes of the stimuli, such as contrast (high) and spatial frequency (low), were fixed and remained unmodified between and within the training sessions. These subjects therefore each achieved a perfect performance. For the other four subjects who served as controls for the second treatment group, the attributes of the target were modified by using the algorithm used for this treatment group. Two of these subjects were given low-contrast targets without flankers, and the other two practiced with flankers but the spatial frequency and orientation were modified at high contrast. According to the approved protocol for the first group, which stipulates that treatment be terminated in cases in which no visual improvement is detected after 12 consecutive sessions, nine subjects in the first control group attended 12 sessions and one subject attended 16 sessions. In the second group, this rule was withdrawn from the protocol, and three of the four control subjects from this group therefore continued performing for 20, 24, and 28 sessions each. The VA of the patients in the treatment group improved, and their treatment therefore was continued for ≈45 ± 15 sessions (mean ± SD).
Results
The results of this study provide evidence for deficient connectivity in amblyopia. Lateral-masking curves (see Methods) are presented in Fig. 2a, in which amblyopic vision (with corrected optics) is compared with normal vision. Examples of interindividual differences between participants are shown for three representative patients (S1-S3). Results for the two groups of amblyopic patients are presented, with the initial spatial frequency set to match two different sensitivity criteria (see Methods). The first treatment group (n = 40 of 44; open squares) was tested initially with a range of spatial frequencies from 3 to 12 cpd (mean = 5.9, SD = 2.8 across participants), whereas the second treatment group (n = 19; open triangles) was tested with a lower range of 1.5 to 6 cpd (mean = 2.7, SD = 1.6). The range of spatial frequencies for the normal-sighted subjects in the second group (n = 16) was the same as for the first treatment group (3-12 cpd; mean = 7.7, SD = 3.6). The data clearly show that facilitation is absent in amblyopic patients in whom the range of inhibition in the higher spatial frequencies is larger than normal (first amblyopic group). This inhibitory effect is reminiscent of the well known crowding phenomenon typical of amblyopia (27). Results with lower spatial frequencies (second amblyopic group) show a close-to-normal facilitation, in agreement with the well known normal vision of amblyopic individuals with low spatial-frequency stimuli (1-4).
Next, the individual threshold elevation, averaged across the range of 2-6λ, was taken to quantify facilitation strength, and its distribution is presented in Fig. 2b. The average threshold elevation for the first group of amblyopic patients (mean ± SE) was 0.18 ± 0.1 log units (suppression, n = 40), -0.11 ± 0.02 for the second group of amblyopic patients (facilitation, n = 19), and -0.12 ± 0.03 for normal-sighted subjects. These results demonstrate that abnormal spatial interactions in amblyopia correlate with sensitivity reduction and can account for some of the individual differences observed here and elsewhere (6, 7). Of special interest here is a subgroup of amblyopic patients with astigmatic eyes. In this subgroup, the optical distortion in the eye is not isotropic but shows stronger blur in one axis (28). Lateral interactions in these patients were probed in different directions (orientations) with the optics of the eyes corrected. Thresholds for the target, with (solid red line) and without (dashed blue line) flankers, are presented in Fig. 2c, measured at target-flanker separation of 3λ for four different orientations for an amblyopic patient with high astigmatism at -10°. Thresholds show strong inhibitory effects along the astigmatic axis (having the maximal distortion, cylindrical power) while being close to normal at an orientation orthogonal to it. Because anisometropia develops late in the developmental period (1-4), possibly after stabilization of refraction (29), this result suggests a strong link between the abnormalities found in the lateral interactions and the developmental pressure put on the amblyopic brain.
Perceptual learning improves visual performance on many basic tasks (30) independently of the trainee age (31), with evidence pointing to modifications in the adult visual cortex during training (32). Several studies point to plasticity of spatial interactions in adults resulting from repetitive practice on the target-flanker task. Both an increased range of excitatory interactions (15) and a reduced short-range inhibition (19) were observed in normal-sighted subjects and have been reported in monkeys (33). The high stimulus specificity observed in the learning studies (15, 30) points to activity-dependent plasticity of the visual cortex, in which the specific connections activated during training are being modified to improve performance.
We show here that by probing a wide range of cortical interactions using a large set of suitable stimuli, it is possible to improve the underdeveloped spatial interactions in adults with amblyopia. Furthermore, amblyopic patients with improved connectivity perform better on standard visual tests (VA) and show improved vision.
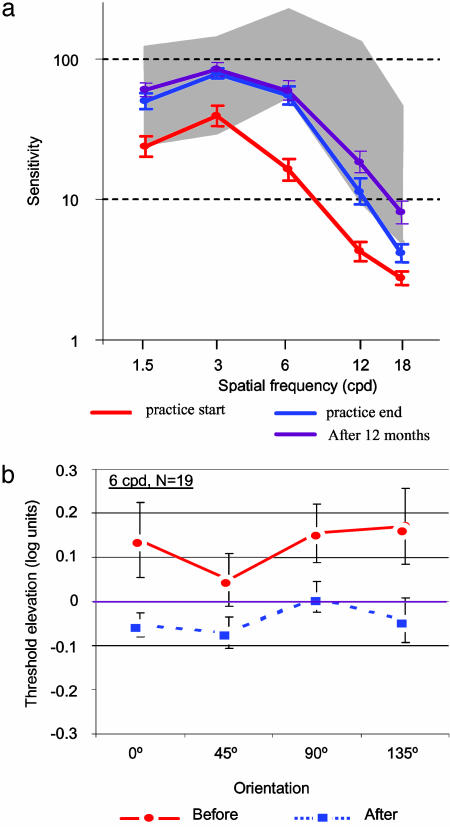
A standard training session comprised a task requiring contrast detection of a small grating patch (a GS; see Fig. 1) with and without flanking collinear high-contrast patches. In the course of the training sessions (up to 80), the size (spatial frequency) and orientation of the stimuli were changed, starting with lower spatial frequencies and moving progressively to the higher ones, with four orientations at each size (see Methods). In addition, the CSF of each amblyopic patient was measured by using a standard contrast-sensitivity chart (26) before and after training. The CSF of amblyopic patients showed higher thresholds (lower sensitivity) than those obtained by normal-sighted subjects, with the low spatial frequencies in the lower range of normal (not significantly different from normal) and the high spatial frequencies showing the strongest sensitivity loss. Training resulted in a significant improvement in sensitivity for all spatial frequencies, with the high spatial-frequency range improving to within the normal range (Fig. 3 a). The CSF improved by a factor of 2.21, 2.12, 2.93, 4.23, and 2.05 (purple line, 12 months after treatment) for spatial frequencies of 1.5, 3, 6, 12, and 18 cpd, respectively, reaching statistical significance (P < 0.05) at each of these frequencies. Furthermore, the lateral-inhibitory effects, demonstrated in Fig. 2, were reduced significantly after training, as shown in Fig. 3 b (22 amblyopic patients, second group, 6 cpd), and showed no inhibition after learning (improvement of 0.15 log units, 40%). A correlation coefficient (r) of 0.68 was found between improvement of facilitation (suppression reduction) and VA, meaning that the improvement in lateral facilitation can account for 46% of the improvement in VA.
Fig. 3.
CSFs. (a) CSF for the first treatment group (n = 39, tested after 1 year) before and after training. Sensitivity improved by a factor of ≈2 across the range tested, reaching normal performance (gray shaded area) for all spatial frequencies tested except for the highest one (18 cpd). Tests carried out 12 months after the training was terminated showed complete retention and additional improvement on spatial frequencies of 12 and 18 cpd. CSFs were estimated by using a sine-wave contrast test with constant grating size (1.4°; see Methods) (26). (b) Reduced lateral inhibition after training. Data show threshold elevation for targets separated from flankers by a distance of 3λ (for which facilitation is near-maximal in normal-sighted subjects, σ = λ); values are means for 19 patients tested at 6 cpd before and after treatment. Threshold elevation is compared at four orientations to allow for possible anisotropy (astigmatism). The initial suppression, observed with all four orientations tested during the second training session, was removed by training (up to 12 sessions). The improvement is ≈0.15 log units. Error bars denote SEM of ±1.
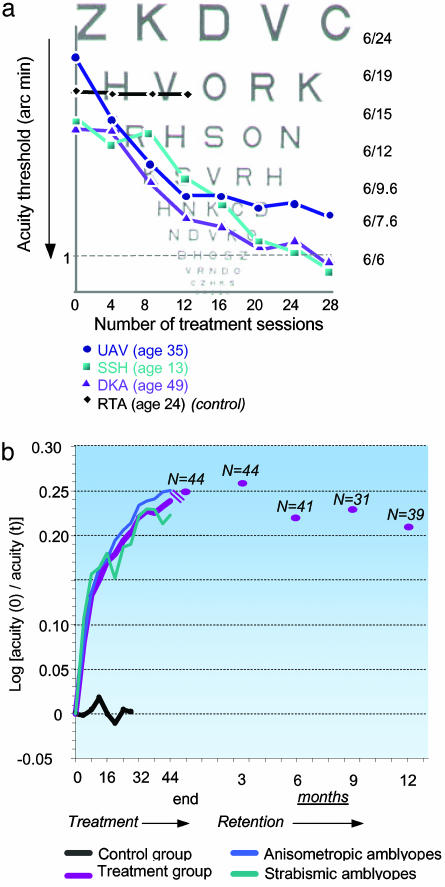
VA, similar to letter identification, requires pattern mapping by the subject into one of many learned categories. Because spatial filters in the visual cortex filter any incoming visual information, they are expected also to limit performance on letter identification (34). In particular, the increased lateral inhibition described above is expected to degrade letter identification (crowding effect) when the letter to be identified is surrounded by other letters, as in standard VA tests (4, 27). Thus, the practice-induced reduction of inhibitory interactions is expected to improve VA. This expectation was confirmed by the VA tests (ETDRS) that the study participants took regularly through the training period. Fig. 4a presents data from three patients, showing a marked improvement in spatial resolution over 28 sessions, and reaching a normal VA performance (i.e., ≥6/7.6, Snellen equivalent). The control (placebo) subject shown was trained with high-contrast targets without flankers and showed no improvement. In Fig. 4b we present group VA scores taken at intervals of four training sessions for both treatment groups (n = 63) and both control (n = 14) groups. Performance is documented in terms of the gain in spatial resolution relative to the initial threshold. The combined treatment group showed a rapid 35% improvement (0.13 log units) during the first eight sessions, followed by a slower learning rate, reaching 78% gain (0.25 log units) after 48 training sessions. Subjects in the control group, who practiced with the target only (high or low contrast, n = 10 and 2, respectively) without flankers, showed stable scores. The two control subjects who practiced with high-contrast targets and flankers also showed no improvement in VA. An independent-groups t test, performed after the 12th sessions (the last session of the first control group), showed that the probability of the results of a control subject belonging to the corresponding treatment group by chance was <0.2% (P < 0.002). When treated patients are considered, only 2 of the 44 in the first treatment group (4.5%) showed an improvement <0.05 LogMAR (0.5 ETDRS lines), whereas 7 of 10 subjects (70%) in the first control group were within this range. One subject from this control group improved by 0.08 LogMAR, and two improved by 0.06 LogMAR. The mean improvement after 12 sessions for patients from both treatment groups (n = 63) was 0.15 ± 0.01 (mean ± SE) LogMAR, compared with 0.01 ± 0.02 for all the control subjects (n = 14). This difference is highly significant (t test for independent groups, P < 0.0001). Three control subjects (second group) continued for >12 sessions. Whereas the patients in the treatment group continued to improve significantly (by 30%) between sessions 12 and 24 (from 0.15 ± 0.01 to 0.2 ± 0.01), these three control subjects did not improve at all (from 0.03 ± 0.04 to 0.02 ± 0.04).
Fig. 4.
Learning curves. (a) VA learning curves for three amblyopic patients and one control subject (RTA). Whereas the patients going through the training schedule showed a marked improvement in VA, the control subject, practicing with only highly discriminable (high-contrast) targets, showed no improvement. The actual chart, viewed at 3 m, has measured letters that are ≈7.3 times larger than the chart shown. (b) VA learning curves: group data (two treatment groups, n = 63; control group, n = 14). A relatively rapid improvement in VA during the first eight sessions is followed by a phase of slower learning. Learning seems to occur at the same rate for anisometropic amblyopic patients (n = 32) and strabismic amblyopic patients (n = 14). Testing of retention after 3 (n = 44), 6 (n = 41), 9 (n = 31), and 12 (n = 39) months disclosed only a slight decrement of performance.
The dramatic difference observed between the treatment and control groups clearly showed that repetition of VA tests did not contribute to improvement in VA. Also, the results pointed to the critical dependence of learning on features of the stimuli on which subjects received training (target contrast, presence of flankers), indicating that it is not only the practice itself that improves performance. Overall, the average VA of both treatment groups achieved an improvement of 78% (median, 66%), although the gain showed a relatively large scatter of 7-307%, with the average best-corrected VA after treatment reaching 0.16 ± 0.02 (better than 6/9). An improvement of two or more ETDRS lines was achieved by 43 patients (68%), and 40 of the 48 patients (83%) who started with a VA worse than 6/12 ended their treatment with a VA better than 6/12. The improvement in VA was not significantly dependent on age (P = 0.66), indicating that plasticity is not limited by age, or on amblyopia type (strabismic or anisometropic; P = 0.55), but it was positively correlated with initial deficit (P < 0.05). Subjects in the control group showed a relatively uniform gain: between -13% and 20% of the initial VA.
Discussion
The results of this study provide persuasive evidence for cortical plasticity in human adults. Previous studies have used “psychoanatomical” methods (35) to probe cortical modules undergoing changes during learning of basic visual tasks by measuring learning specificity to low-level visual features. The present approach is aimed at the (re)construction of functionalities that were not acquired during development. We showed here that the distorted pattern of spatial integration in amblyopia is development-dependent and correlated with the optical distortion (e.g., as in meridional amblyopia). Because the optical distortions were not corrected during the critical period in most amblyopic eyes (until diagnosed), the input from that eye to the cortex was abnormal, leading to abnormal development of the visual cortex. The reduced lateral inhibition shown by our patients after training thus can be attributed to undoing of the developmental damage incurred during the “plastic” period in early life. Alternatively, if amblyopia is associated with an early history of a sensory obstacle that hinders additional development of low-level visual functions (2), our results might suggest that the training was successful in restoring these functions, although not necessarily within the same neuronal networks that were affected during the critical period.
Perceptual learning is stimulus- and task-specific (30, 36-38). These features are used often to predict the anatomical site at which learning takes place. Vernier acuity was shown to improve with practice in both adults with normal vision (39) and adult amblyopic patients (37, 38). Although those studies showed that repetition of a vernier acuity task can improve performance on another task (similarly limited by VA) by the use of similar stimuli (lines and letters), we showed here that VA could be improved while practicing a very different and functionally more basic task (contrast detection) by using stimuli (GSs) different than those used for the acuity tests (letters). This improvement might be a consequence of the practiced stimulus set being multidimensional; its effect was to improve the early processing of the visual system and consequently all higher levels of processing that depend on the quality of the low-level visual representation. Persistence of the improved visual function showed that the learning is not just a temporary adaptation effect but a long-lasting change in the visual cortex, consistent with previous studies of perceptual learning (30, 36). In addition, transfer of the improvement to nonpracticed tasks (e.g., VA) precludes the possibility of improvement caused by a specific “practice” effect of the trained task. This study shows improvement of contrast-detection thresholds in adults with amblyopia. This improvement was achieved only for the patients in the treatment groups, all of whom practiced with flankers, whereas control subjects (n = 14), who practiced either on high- or low-contrast GSs without flankers, did not improve at all.
The possibility that “front-end” sources such as accommodation and eye movements might account for our learning effect is unlikely. VA tests for the amblyopic eye were carried out with full hyperopic correction, as revealed under cycloplegia. Also, the VA tests were performed at a distance of 3 m, at which accommodation is only 1/3 diopter and therefore plays very small role. Many of our patients were of an age at which accommodation amplitude is limited (almost absent above the age of 40 years in amblyopic individuals). Improvement of the fixation function is also unlikely to account for the VA improvement, because patients with eccentric fixation were excluded. There were no significant differences between strabismic and anisometropic patients, and the latter are known to suffer much less, if at all, from fixation problems. Interestingly, astigmatic patients, who often show good vision with stimuli oriented in one direction (thus no fixation problems) but not another, also showed improvement in performance. Moreover, the absolute contrast threshold of many strabismic patients is within the normal range, ruling out the possibility of a front-end source, yet they show deficiencies in lateral interactions, as expected from their known distorted form perception. Strabismic participants also were shown to improve their VA during training. Moreover, if accommodation, fixation, or both are responsible for the visual improvement, the amblyopic control patients should have improved similarly, but they showed no improvement at all.
A treatment that shares some common theoretical basis with the present treatment is the CAM stimulator (40-42). This instrument, used to be applied in conjunction with occlusion in children, is a rotating disk with black and white stripes aimed at exposing the visual cortex to various spatial frequencies. Controlled studies of the efficacy of treatment with the CAM stimulator found no significant differences between treatment and control groups (10, 41). Although both methods (the present one and the CAM) are neurophysiologically based, they differ in several important aspects. The contrast in the CAM treatment was high (as in our control group), whereas in our treatment groups we used low-contrast targets with high-contrast flankers. In the CAM treatment, attention was focused on an irrelevant task (drawing), whereas the patients in our study were instructed to attend to the foveal target. In the CAM treatment, many orientations and spatial frequencies were exposed within a short time period regardless of the level of the improvement or depth of amblyopia; in our study only one orientation and spatial frequency was presented in each session, with stimulus parameters individually set to match the subject's progress. Also, the moving peripheral stimulation in the CAM might have distracted the subjects' attention from the foveal stimulation, whereas our method provides an efficient and selective stimulation of the fovea.
No treatment is currently available for adults with amblyopia (4, 10-12). In young children, amblyopia is treated primarily with eye-patching, forcing the “lazy eye” to function by covering the eye that sees better. This treatment, by prolonged occlusion of the good eye, is considered impractical in adult amblyopia (12, 43). The efficiency of patching is negatively correlated with age at treatment (43); the probability of failure is 7.9 times higher for the 11- to 20-year age group than for children 0-3 years old. The treatment success rate is somewhat difficult to assess, because it lacks an accepted definition (10, 12), but it is mostly between 60% and 70% in young children (43), comparable with the success rate found here in adults.
The present results support the use of a structured method, targeted at the specific deficiencies in amblyopia, to improve vision of adults. It is possible that the perceptual learning method used here can be generalized to other sensory and nonsensory brain modules suffering from developmental problems.
Abbreviations: VA, visual acuity; CSF, contrast-sensitivity function; GS, Gabor signal; ETDRS, early treatment of diabetic retinopathy study; LogMAR, log minimum angle of resolution; cpd, cycles per degree.
This study was supported in part by NeuroVision (Wellesley, MA).
References
- 1.Hess, R. F., Field, W. & Watt, R. J. (1990) Vision: Coding and Efficiency (Cambridge Univ. Press, Cambridge, U.K.).
- 2.Levi, D. M. & Carkeet, A. (1993) in Early Visual Development, Normal and Abnormal, ed. Simons, K. (Oxford Univ. Press, New York), pp. 391-407.
- 3.Levi, D. M. (1991) Spatial Vision (Macmillan, London).
- 4.Ciuffreda, K. J., Levi, D. M. & Selenow, A. (1991) Amblyopia: Basic and Clinical Aspects (Butterworth-Heinemann, Stoneham, MA).
- 5.Sireteanu, R., Lagreze, W. D. & Constantinescu, D. H. (1993) Vision Res. 33, 677-690. [DOI] [PubMed] [Google Scholar]
- 6.Polat, U., Sagi, D. & Norcia, A. M. (1997) Vision Res. 37, 737-744. [DOI] [PubMed] [Google Scholar]
- 7.Levi, D. M., Hariharan, S. & Klein, S. A. (2002) Vision Res. 42, 1379-1394. [DOI] [PubMed] [Google Scholar]
- 8.Hess, R. F., McIlhagga, W. & Field, D. J. (1997) Vision Res. 37, 3145-3161. [DOI] [PubMed] [Google Scholar]
- 9.Kovacs, I., Polat, U., Pennefather, P. M., Chandna, A. & Norcia, A. M. (2000) Vision Res. 40, 1775-1783. [DOI] [PubMed] [Google Scholar]
- 10.Greenwald, M. J. & Parks, M. M. (1999) in Clinical Ophthalmology, ed. Duane, T. (Harper and Row, Hagerstown, MD), Vol. 1.
- 11.American Academy of Ophthalmology Preferred Practice Patterns Committee, Pediatric Ophthalmology Panel: Amblyopia (1997) Preferred Practice Pattern: Amblyopia (Am. Acad. Ophthalmol., San Francisco).
- 12.Prieto-Diaz, J. (2000) Strabismus (Butterworth-Heinemann, Boston).
- 13.Polat, U. (1999) Spat. Vis. 12, 143-162. [DOI] [PubMed] [Google Scholar]
- 14.Polat, U. & Sagi, D. (1993) Vision Res. 33, 993-999. [DOI] [PubMed] [Google Scholar]
- 15.Polat, U. & Sagi, D. (1994) Proc. Natl. Acad. Sci. USA 91, 1206-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polat, U. & Sagi, D. (1994) Vision Res. 34, 73-78. [DOI] [PubMed] [Google Scholar]
- 17.Bonneh, Y. & Sagi, D. (1998) Vision Res. 38, 3541-3553. [DOI] [PubMed] [Google Scholar]
- 18.Polat, U. & Norcia, A. M. (1996) Vision Res. 36, 2099-2109. [DOI] [PubMed] [Google Scholar]
- 19.Zenger, B. & Sagi, D. (1996) Vision Res. 36, 2497-2513. [DOI] [PubMed] [Google Scholar]
- 20.Polat, U., Mizobe, K., Pettet, M. W., Kasamatsu, T. & Norcia, A. M. (1998) Nature 391, 580-584. [DOI] [PubMed] [Google Scholar]
- 21.Crook, J. M., Engelmann, R. & Lowel, S. (2002) Exp. Brain Res. 143, 295-302. [DOI] [PubMed] [Google Scholar]
- 22.Kapadia, M. K., Ito, M., Gilbert, C. D. & Westheimer, G. (1995) Neuron 15, 843-856. [DOI] [PubMed] [Google Scholar]
- 23.Levitt, J. B. & Lund, J. S. (1997) Nature 387, 73-76. [DOI] [PubMed] [Google Scholar]
- 24.Sillito, A. M., Grieve, K. L., Jones, H. E., Cudeiro, J. & Davis, J. (1995) Nature 378, 492-496. [DOI] [PubMed] [Google Scholar]
- 25.Sengpiel, F., Baddeley, R. J., Freeman, T. C., Harrad, R. & Blakemore, C. (1998) Vision Res. 38, 2067-2080. [DOI] [PubMed] [Google Scholar]
- 26.Ginsburg, A. P. (1984) Am. J. Optom. Physiol. Opt. 61, 403-407. [DOI] [PubMed] [Google Scholar]
- 27.Stuart, J. A. & Burian, H. M. (1962) Am. J. Ophthalmol. 53, 471-477. [PubMed] [Google Scholar]
- 28.Mitchell, D. E., Freeman, R. D., Millodot, M. & Haegerstrom, G. (1973) Vision Res. 13, 535-558. [DOI] [PubMed] [Google Scholar]
- 29.Atkinson, J. (2000) The Developing Visual Brain (Oxford Univ. Press, New York).
- 30.Sagi, D. & Tanne, D. (1994) Curr. Opin. Neurobiol. 4, 195-199. [DOI] [PubMed] [Google Scholar]
- 31.Fahle, M. & Daum, I. (1997) Neuropsychologia 35, 1583-1589. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert, C. D., Sigman, M. & Crist, R. E. (2001) Neuron 31, 681-697. [DOI] [PubMed] [Google Scholar]
- 33.Crist, R. E., Li, W. & Gilbert, C. D. (2001) Nat. Neurosci. 4, 519-525. [DOI] [PubMed] [Google Scholar]
- 34.Solomon, J. A. & Pelli, D. G. (1994) Nature 369, 395-397. [DOI] [PubMed] [Google Scholar]
- 35.Julesz, B. (1995) Dialogues on Perception (MIT Press, Cambridge, MA).
- 36.Gilbert, C. D. (1998) Physiol. Rev. 78, 467-485. [DOI] [PubMed] [Google Scholar]
- 37.Levi, D. M. & Polat, U. (1996) Proc. Natl. Acad. Sci. USA 93, 6830-6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levi, D. M., Polat, U. & Hu, Y. S. (1997) Invest. Ophthalmol. Vis. Sci. 38, 1493-1510. [PubMed] [Google Scholar]
- 39.Fahle, M., Edelman, S. & Poggio, T. (1995) Vision Res. 35, 3003-3013. [DOI] [PubMed] [Google Scholar]
- 40.Campos, E. (1995) Surv. Ophthalmol. 40, 23-39. [DOI] [PubMed] [Google Scholar]
- 41.Schor, C., Gibson, J., Hsu, M. & Mah, M. (1981) Am. J. Optom. Physiol. Opt. 58, 930-938. [DOI] [PubMed] [Google Scholar]
- 42.Campbell, F. W., Hess, R. F., Watson, P. G. & Banks, R. (1978) Br. J. Ophthalmol. 62, 748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flynn, J. T., Schiffman, J., Feuer, W. & Corona, A. (1998) Trans. Am. Ophthalmol. Soc. 96, 431-450. [PMC free article] [PubMed] [Google Scholar]