Abstract
In the past 20–25 years, there have been a number of studies published on handedness in nonhuman primates. The goal of these studies has been to evaluate whether monkeys and apes show patterns of hand preference that resemble the right-handedness found in the human species. The extant findings on handedness in nonhuman primates have revealed inconsistent evidence for population-level handedness within and between species. In this article, I discuss some of the methodological and statistical challenges to comparative studies of handedness in human and nonhuman primates. I further offer a framework for developing some consensus on evaluating the validity of different handedness measures and the characterization of individual hand preferences.
Keywords: handedness, primates, laterality, measurement validity, comparative analysis
INTRODUCTION
Though there is some variation between cultures, all human populations studied to date have been observed to exhibit or self-report preferring to use the right hand for a majority of manual actions (Annett, 1985; Perelle & Ehrman, 1994; Porac & Coren, 1981). Evidence of right handedness has also been reported in so-called nontraditional societies, though it is expressed less strongly and is largely task specific compared to data from Westernized cultures (Marchant, McGrew, & Eibl-Eibesfeldt, 1995). Not with-standing these caveats, these results minimize the possibility that reports of right-handedness in Westernized cultures were attributable to social or cultural norms but rather reflect an inherent biological trait in our species. There is also evidence that handedness is linked to language lateralization, though the association is relatively weak. It has been shown that 96% of right-handed individuals are left hemisphere dominant for language in comparison to 70% of left-handed people (Knecht et al., 2000).
The evidence of near uniform right-handedness in humans coupled with the link with language lateralization has resulted in a number of saltational hypotheses regarding the evolution of hemispheric specialization. By saltational, I am suggesting that there is a qualitative difference in the expression of behavioral and brain asymmetries in humans compared to nonhumans. Specifically, the alleged uniquely human trait of right-handedness has been hypothesized to be a consequence of other human specific adaptations such as language, bipedalism, tool use, bimanual coordination and tool-making (Bradshaw & Rogers, 1993; Crow, 2009). This basic view of the evolution of handedness was the accepted lore until about 20–25 years ago, even though up to that point, there had been only limited research on the topic of handedness in nonhuman primates (Ettlinger, 1988; Lehman, 1993; Warren, 1980).
MacNeilage, Studdert-Kennedy, and Lindblom (1987) reopened the question of handedness in nonhuman primate handedness by providing a provocative theory on the origins of handedness that was linked to adaptations associated with postural motor control. Unlike previously saltational views, the so-called postural origins theory advocated continuity in primate handedness by emphasizing species variation that was linked to changes in postural organization within and between different primate taxonomic groups. If nothing else, one consequence of this theory was the emergence of a large number of studies on handedness in nonhuman primates that focused on the question of population-level handedness. Indeed, since 1987, there have been more than 50 published studies on handedness in nonhuman primates, a value that reflects the increased research interest in this topic. Nearly all of the studies are descriptive in nature and have focused on testing whether a given species shows population-level handedness for a specific task or set of tasks (Hopkins, 2006; Hopkins et al., 2012; Papademetriou, Sheu, & Michel, 2005). Population-level handedness is defined as a significant proportion of individuals having the same hand preference within a sample of subjects.
There seems to be uniform agreement that the comparative results on handedness in nonhuman primates are, in many instances, inconsistent between and sometimes within species (Hopkins, 2006; Papademetriou et al., 2005). The goal of this article is not to review the plethora of studies on handedness in nonhuman primates but rather to focus on evaluating and discussing why there are inconsistent results within and between species. A number of potential explanations have been offered including sampling bias, task variation, testing artifact or differences between settings (captive vs. wild populations of subjects) (Cashmore, Uomini, & Chapelain, 2008; Hopkins, 1999; Hopkins & Cantalupo, 2005; Marchant & McGrew, 1991; McGrew & Marchant, 1997). In this article, I address in particular (a) the role that different tasks might play in the assessment of hand preference and (b) the statistical approaches used to characterize individual hand preference and how this influences the interpretation of hand preference. At the heart of this discussion is how best to approach the measurement and characterization of handedness in nonhuman primates within the framework of comparative psychology and ethology. Further to this point, the goal in many studies of handedness in nonhuman primates is to compare the findings to the distribution of handedness in humans. This is certainly laudable but there are important differences in both the approach and measurement of handedness in humans that differ quite dramatically from those employed with nonhuman primates. Therefore, I further offer some ways going forward that might facilitate the comparative analysis of handedness between human and nonhuman primate handedness.
HANDEDNESS DISTRIBUTIONS
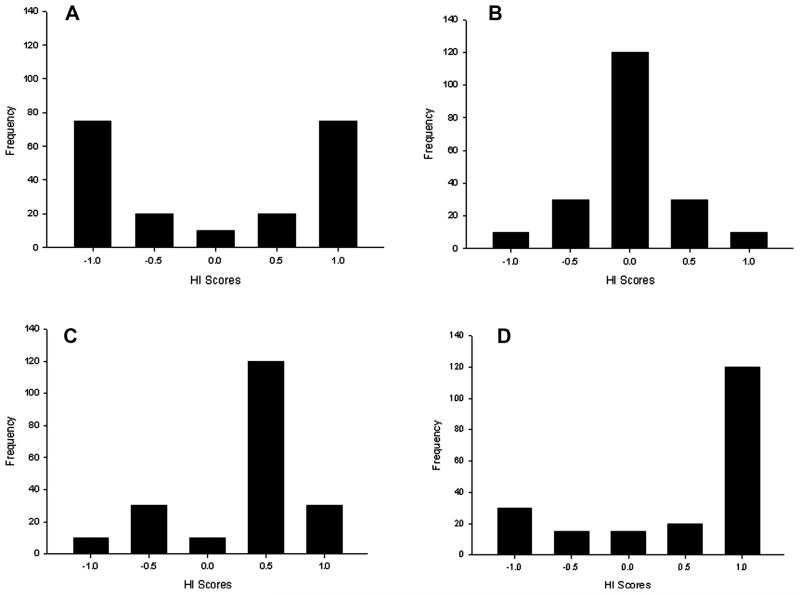
As a segue into the discussion about variation in hand preference, shown in Figure 1 are four theoretical distributions of handedness for any given measure. These include bimodal, normal, skewed, and J-shaped. Individually binned handedness index (HI) scores are plotted on the x-axis and the frequency distribution of handedness based on a hypothetical sample of 200 individuals on the y-axis. HI scores are derived following the formula [HI = (R − L)/(R + L)] where R and L represent the frequency in left and right hand use. HI scores range from −1.0 (exclusive left hand use) to +1.0 (exclusive right-hand use). In terms of handedness, the bimodal and normal distributions would not reveal significant population-level biases but for very different reasons. In the bimodal distribution, there are roughly equal numbers of right- and left-handed individuals and very few subjects with no preference (HI scores on or around zero). In contrast, for the normal distribution, there are very few right- and left-handed subjects and the majority of the subjects do not show an individual hand preference (i.e., most have HI scores on or around zero). For the skewed and J-shaped distributions, each task reflected in these data elicits a population-level bias but again for slightly different reasons. Skewed distributions are those in which the data might be normally distributed but the sample is skewed above or below the theoretical value of 0, which is what would be assumed in a normal distribution. The modal handedness pattern in the skewed distribution is shifted positively but is not on the extreme end of the distribution of scores. In contrast, for the J-shaped distribution, this task elicits strong hand preferences at one end of the distribution and is the one most often used to describe human handedness.
FIGURE 1.
Four theoretical distributions in hand preference. (a) binomial (b) normal (c) skewed, and (d) J-shaped.
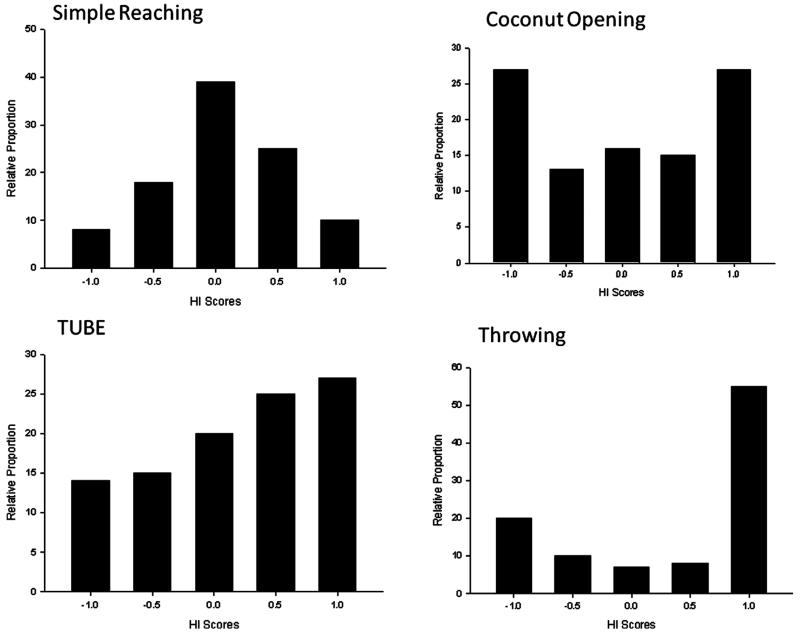
There is very good evidence that hand preference data in nonhuman primates can similarly assume the binomial, normal, skewed, and J-shape distributions. For example, shown in Figure 2 are the distributions of hand preference based on HI scores for four measures of hand use in chimpanzees based on previous studies in my laboratory including simple reaching, coconut opening, the TUBE task, and throwing (Hopkins, Russell, Cantalupo, Freeman, & Schapiro, 2005; Hopkins, Russell, Hook, Braccini, & Schapiro, 2005; Hopkins, Russell, & Cantalupo, 2007; Hopkins et al., 2005). As can be seen, simple reaching conforms largely to a normal distribution while coconut opening is bimodally distributed. One sample t tests on the HI scores for each of these measures are not significant but, like the hypothetical distributions, coconut opening appears to be a better measure because it reliably elicits robust hand preferences in each individual. One sample t tests on the HI scores for manual gestures and throwing are both significant and show that the distributions are rightward for these two tasks. Throwing takes on the J-shaped while manual gestures have a skewed distribution.
FIGURE 2.
Relative frequency distributions in hand preference for 4 different measures of handedness in chimpanzees.
CHALLENGE #1: ASSESSING HAND PREFERENCES
With the discussion of handedness distributions above, the first challenge I address pertains to what constitutes a valid or good measure of handedness. The prescribed goal of any study on handedness is to assess whether individual subjects show a hand preference. In my view, an argument can be made that tasks which result in a normal distribution in handedness are psychometrically poor measures of hand preference because, in point of fact, these behaviors or tasks do not elicit significant hand preferences in the majority of individuals within a sample, This stands in contrast, to the bimodal, skewed and J-shaped distributions where a majority of individuals show a preference. Of course, it may be the case that handedness in some species is normally distributed, no matter what the task or measure, and this simple fact should not be discounted or ignored. Notwithstanding, if the goal is to measure hand preference, then it seems logical that researchers should identify and quantify measures or behaviors that elicit consistent biases in hand use within the same individual. Yet, there are studies that claim a lack of population-level handedness in nonhuman primates that fail this very simple criterion. For instance, hand use for everyday behaviors or so-called spontaneous actions have been reported in wild chimpanzees from Gombe and Mahale (Marchant & McGrew, 1996; McGrew & Marchant, 2001), in Hanuman langurs (Mittra, Fuentes, & McGrew, 1997) and capuchin monkeys (Panger, 1998). In all four of these reports, the majority of subjects show no hand preference (i.e., they were classified as nonpreferent). However, we know from other studies in these same species (save langurs for which there are no other data), that individual subjects can show consistent hand preferences for some behaviors like tool use, bimanual feeding and coordinated bimanual actions such as the TUBE task (Bogart et al., 2012; Corp & Byrne, 2004; Lilak & Phillips, 2007; Llorente et al., 2010; Marchant & McGrew, 2007; Meunier & Vauclair, 2007; Spinozzi, Castornina, & Truppa, 1998). Thus, in my view, to claim that a species is not lateralized on the basis of data in which none of the measures or behaviors elicits consistent hand use is at a minimum premature and unjustified. It is just as likely and arguably more parsimonious to conclude that the behaviors of interest (or convenience) are poor measures of the construct called “handedness” and in all probability these types of tasks are highly susceptible to situational factors or other extraneous variables.
Even further, if the goal is to compare the distribution of hand preference between humans and other primates, then these types of studies in nonhumans are really problematic because of the nature of test development for handedness assessment in humans. Specifically, handedness in adult humans is typically measured using a questionnaire that has been explicitly developed to measure consistency in hand use for actions or items on which most individuals report using a preferred hand. In developing a handedness measurement instrument, tasks or items on a questionnaire that fail to elicit consistent hand preferences are omitted or removed because they are viewed as having little construct validity. If the same criteria were applied to the measure of nonhuman primate handedness, a number of studies or tasks used to assess individual hand preferences would be deemed unacceptable. For instance, in the study on spontaneous hand use in wild chimpanzees by McGrew and Marchant (2001), none of the 10 most common behaviors measured elicited significant hand preferences in the majority of individuals. On the basis of these findings, McGrew and Marchant (2001) concluded that wild chimpanzees are not lateralized but I would offer an alternative interpretation: wild chimpanzees are not lateralized for those specific actions and therefore they are poor measures of hand use and do not actually measure the construct of interest. Some have called for expansion of handedness studies to include measures of everyday activity (Cashmore, 2009) but I believe this is ill-advised and would not lead to any type of conceptual or methodological advancement in the study of handedness, unless methods are adopted that distinguish between different types of actions or the context in which they are produced (see Aruguete, Ely, & King, 1992; Forrester, Leavens, Quaresmini, & Vallortigara, 2011; Forrester, Quaresmini, Leavens, Mareschal, & Thomas, 2013; Forrester, Quaresmini, Leavens, Spiezio, & Vallortigara, 2012). In my view, as in the case in human handedness, some measures of nonhuman primate handedness are better than others, at least when “better” is defined by the sensitivity of the task to elicit consistent hand preferences in a majority of individuals. If the majority of individuals fail to show hand preferences for a given task within a study, then I believe the validity of the measure can be reasonably questioned.
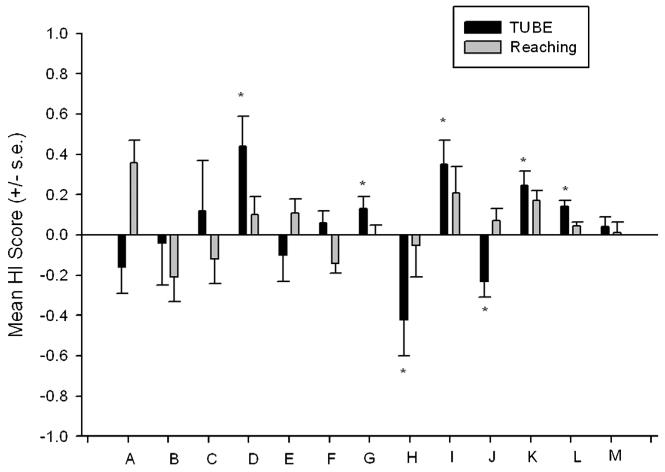
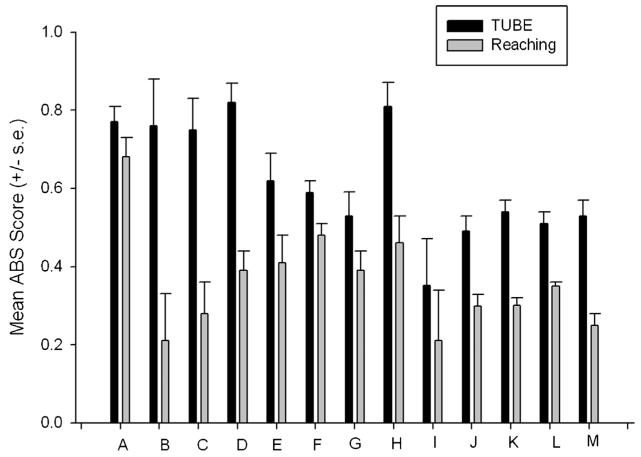
An example of this point comes from comparative studies of hand preference for the TUBE task compared to simple reaching. Shown in Figures 3 and 4 are the mean HI and ABS-HI scores for the TUBE and simple reaching tasks in 13 studies in which these two tasks have been measured and results reported (Bennett, Suomi, & Hopkins, 2008; Chapelain, Hogervorst, Mbonzo, & Hopkins, 2011; Hopkins et al., 2011; Lilak & Phillips, 2007; Meguerditchian, Donnot, Molesti, Francioly, & Vauclair, 2012; Meunier & Vauclair, 2007; Schmitt, Melchisedech, Hammerschmidt, & Fischer, 2008; Schweitzer, Bec, & Blois-Heulin, 2007; Spinozzi et al., 1998; Vauclair, Meguerditchian, & Hopkins, 2005). As can be seen in Figure 3, population-level handedness was revealed in seven studies for the TUBE task but in none of the studies for simple reaching. From Figure 4, it can be clearly seen that the strength in hand preference for the TUBE task is higher in every single study compared to simple reaching. Thus, the TUBE task elicits stronger hand preferences at the individual level in all studies and population-level hand preference in more then 50% of the studies. In short, all things being equal, the TUBE task is a better measure of hand preference than simple reaching. The fact that individual variation in hand preferences for the TUBE task but not simple reaching are linked to asymmetries in the motor-hand area of the precentral gyrus reinforces the argument that this task better reflects underlying specializations in the brain (Dadda, Cantalupo, & Hopkins, 2006; Hopkins & Cantalupo, 2004; Phillips & Sherwood, 2005).
FIGURE 3.
Mean HI scores (±SE) for hand use on the TUBE and simple reaching tasks in 13 studies. A, Meguerditchian et al. (2012); B, Meunier & Vauclair (2007); C, Lilak & Phillips (2007); D, Spinozzi et al. (1998); E, Schmitt et al. (2008); F, Bennett et al. (2008); G, Vauclair et al. (2005); H, Schweitzer et al. (2007); I, Llorente et al. (2009); J–M, Hopkins (1993), Hopkins and de Waal (1995), Hopkins et al. (2011), and Hopkins et al. (2005b).
FIGURE 4.
Mean ABS-HI scores (±SE) or hand use on the TUBE and simple reaching tasks in 13 studies. See Figure 3 captions for references. ABS-HI scores are the absolute values of the HI scores. ABS = scores vary from 0 (no hand preference) to1 (exclusive hand use).
To be clear, I would not want to suggest that simple reaching is a poor measure of handedness in all circumstances nor in all species. There is certainly abundant evidence that factors like posture, the role of visual feedback and even grip morphology can have a significant impact of handedness for simple reaching in a variety of species (Fagot, Drea, & Wallen, 1991; Hook-Costigan & Rogers, 1997; Hopkins, 1995; Hopkins, Russell, Hook, et al., 2005; Jones-Engel & Bard, 1996; LaCreuse, Parr, Smith, & Hopkins, 1999; Laurence, Wallez, & Blois-Heulin, 2011; Schweitzer et al., 2007; Spinozzi & Cacchiarelli, 2000; Spinozzi, Truppa, & Lagana, 2004; Tonooka & Matsuzawa, 1995). The simple reaching measure described above pertains only to the conditions in which a piece of food is thrown into the subject’s enclosure and they are free to locomote to that location and reach for the food without any postural or visual constraints on their responding. In other words, the subjects have the maximum degree of freedom to reach for the food. One reason the TUBE task, or for that matter any task requiring bimanual coordination, may elicit stronger hand preferences is because it limits the degree of freedom for hand use to be influenced by situational or other factors. The same logic applies to the variation in hand use for simple reaching when factors like posture or visual feedback are constrained by the test.
The challenge of task selection is not restricted to the analysis of single measures of hand use but is also highly germane to discussions of what is described as task specialization versus true handedness. McGrew and Marchant (1997) have proposed a five level analysis of handedness that distinguishes between no preferences to exclusive hand use within tasks and between subjects. What is defined a true handedness is consistent hand use across multiple tasks in all subjects within a population. In contrast, evidence of population-level hand use for a single task in a significant majority of subjects is labeled as task specialization. Presumably, human handedness is defined as true handedness because the working assumption is that most individuals prefer the same hand across multiple tasks (or test items on a questionnaire).
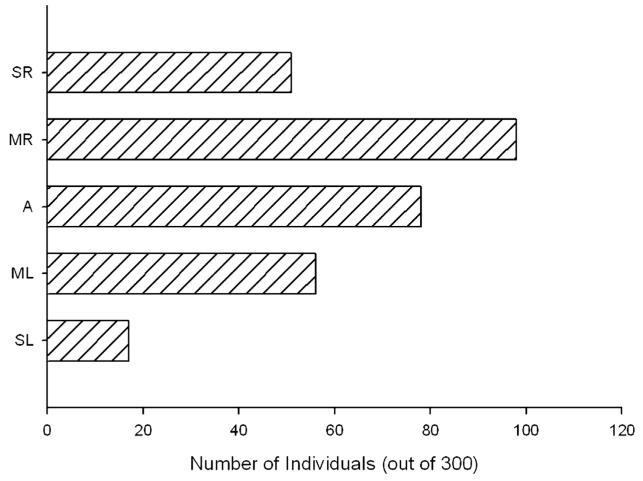
The problem here is that which tasks are selected as a basis for assessing consistency in hand use across multiple measures will influence the outcome. For instance, Hopkins, Gardner, Mingle, Reamer, and Schapiro (in press) examined consistency in hand preference for four measures of hand use in a sample of 300 chimpanzees. Subjects that had the same hand preference for all four tasks were classified as strongly left- or right-handed. Subjects that had the same preference for three of the four tasks were classified as either moderately right- or left-handed. Chimpanzees that preferred their left hand for two tasks and their right for the remaining two were classified as ambidextrous. The distribution hand preferences based on this classification scheme are shown in Figure 5. As can be seen, there are significantly more right- than left-handed subjects but the modal pattern was moderately right-handed. That is, the distribution is skewed right-ward but not J-shaped, as is typically found for human hand preferences, which would be manifest as the modal pattern being strongly right-handed. One interpretation is that captive chimpanzees show a rightward shift in handedness but it is not as extreme as that reported in humans. However, an alternative explanation can be offered that is related to task selection. The four measures in the Hopkins et al. (in press) study were simple reaching, tool use, the TUBE task and manual gestures. Only two of the four measures show a skewed distribution in hand preference and none were J-shaped. These measures were chosen largely because we had data on the most subjects for these tasks. In other words, they were chosen for their convenience more than any other criteria. This is markedly different then the rationale for choosing different measures of handedness in humans. Questionnaire items of specific behavioral tasks are selected because they (a) elicit consistent hand preferences in most individuals and (b) they typically elicit a right or left hand preference in self-reported right- or left-handed people, and (c) hand preference distributions for each questionnaire item are skewed or J-shaped. Thus, tasks such as drawing or throwing are not chosen for convenience but rather because they typically elicit strong individual hand preferences in most people. If there is really a desire to define and measure true handedness in human and nonhuman primates then, at a minimum, measures or behaviors that fail to elicit consistent hand preferences should be not be included in the battery of tasks.
FIGURE 5.
Handedness distribution based on consistency in hand use across 4 tasks. SL, strongly left-handed; ML, moderately left-handed; A, ambidextrous; MR, moderately right-handed and SR, strongly right-handed. Data regraphed from Hopkins et al. (in press).
CHALLENGE #2: THE INDEPENDENCE OF DATA POINTS OR THE “POOLING FALLACY” PROBLEM
It has been suggested that the recording of hand use in some situations is biased due to a lack of independence of data points or what has sometimes been described as the pooling fallacy (Machlis, Dodd, & Fentress, 1985). At the heart of the matter is how to treat hand use responses when they occur as repeated or sequences of actions without intervening events. For example, a number of investigators have tested for handedness in primates using a measure called the TUBE task including New World monkeys (Lilak & Phillips, 2007; Meguerditchian et al., 2012; Meunier & Vauclair, 2007; Spinozzi et al., 1998), Old World monkeys (Bennett et al., 2008; Schmitt et al., 2008; Schweitzer et al., 2007; Vauclair et al., 2005; Zhao, Hopkins, & Li, 2012) and great apes (Chapelain & Hogervorst, 2009; Chapelain et al., 2011; Hopkins et al., 2011; Llorente et al., 2010). With the TUBE task, a food with adhesive properties is smeared on the inside edges of poly-vinyl-chloride (PVC) pipes (or a similar type object) and handed to the subject. The subjects typically pick up the TUBE, hold it in one hand and extract the food with a digit from the opposite hand. Often times, the subject repeats the extract and feed action without any intervening events. It has been suggested by some that when recording repeated actions, such as in the TUBE task, each data point is not independent of the other because the first response predicts the each subsequent hand use response and this might lead to a bias representation in hand use (McGrew & Marchant, 1997). Thus, to have independent data points, it is critical that the actions be broken up in some way and that hand use responses only be recorded when they have been separated by some intervening event that presumably puts the hands back to some equal probability of use. Intervening events would be things like dropping the tube and picking up a new one or switching hand use.
There are a number of limitations with the argument that bouts of hand use must be the level of analysis and these have been discussed extensively elsewhere and I will not rehash them here (Hopkins, 1999; Hopkins & Cantalupo, 2005). Briefly though, first, there is no evidence that hand preferences differ when recording hand use at the level of bouts or individual events, at least when quantified on a continuous scale of measurement (Hopkins, in press-b). Second, if one is only recording bouts and not individual hand use events that occur within a bout, then potential asymmetries in hand use sequences are omitted and important information regarding hand use is lost. Indeed, Martin and Bateson (1986) suggest that the best way to record event behaviors, such as hand use, is using focal sampling with continuous recording. This has been the dominant approach in studies of hand use in nonhuman primates. If other methods are more effective or lead to different outcomes in hand preference then these findings have yet to be reported in the literature.
Lastly, the concepts “independence of data points” or the “pooling fallacy” are not being correctly applied in these circumstances. Independence of data points or the pooling fallacy refer to the concern that each data point used in a statistical analysis represent a single, independent subject’s score. That is to say, if a researcher recorded hand use from 50 subjects and tested for asymmetries by just summing the total number of left and right hand responses, they would be statistically violating the independence of data points because left or right hand use by some subjects may contribute more or less to the total number of observed responses. Based on the existing literature on handedness in nonhuman primates, the pooling fallacy or independence of data points problems never occur. Most scientists classify each subject as right, left or nonpreferent and the number of subjects in each handedness category is compared using chi-square or some other related test. Alternatively, HI scores are derived for each subject and the mean HI score is tested against a hypothetical value of zero using a one-sample t-test. Whether recording hand use at the level of bouts or events, in all studies, the data points are independent of each other because each score is associated with one subject. The problem is that the concepts of independence of data points or the pooling fallacy are being misapplied to the measurement of individual hand preferences as it relates to recording events or bouts of hand use. Whether recording bouts or events in hand use, the data points can never be statistically independent of each other when they are recorded in the same individual.
I believe what those advocating the bouts levels of analysis for handedness are really concerned about is what is defined as auto-correlated responses. Auto-correlated responses are those that are correlated with each other as a function of the time of separation between them. The problem of auto-correlated responses in the behavioral sciences is more problematic when considering instantaneous compared to continuous recording but, nonetheless, the concern is that if sampling intervals are not far enough apart then there will be a tendency to oversample certain behaviors. Thus, for hand use events that are repeated as sequences, the argument is that by recording each response, the events are auto-correlated because they occur in close temporal proximity and have not been separated by some intervening event. This is a valid methodological point (not statistical) but there are two problems with this argument as it pertains to the measurement of hand preference. First, as noted above, in a number of studies where HI scores based on bouts and events of hand have been recorded, the correlations are positive and significant, suggesting that the operationally defined intervening events are ineffective in limiting auto-correlated responses. In other words, bouts of hand use appear to be auto-correlated too; thus, if the goal of recording bouts is to limit auto-correlated responses then none of the methods currently being employed are effective. Second, and more importantly, why would scientists strive to prevent auto-correlation in the measurement of hand preference? To demonstrate individual hand preferences, hand use must be consistent across time, whether the intervals separating the events are 1 s, 1 min, 1 day, or 1 month. Efforts to remove auto-correlation in other behavioral studies strive to create sampling intervals that increase the probability that one will sample a behavior at time point N that differs from time point N − 1. If this is a necessary condition for the measurement of hand preference, then it leads to me to ask, how does one ever demonstrate a significant hand preference?
CHALLENGE #3: QUANTIFYING HAND PREFERENCES AS A DISCRETE OR CONTINUOUS TRAIT
The primary method for measuring adult handedness in humans is to provide subjects with a questionnaire and ask them to self-report their preferred hand use for a variety of actions. For example, the Edinburgh is a commonly used questionnaire that consists of 10 handedness items such as writing, throwing, using a spoon, striking a match, etc. (Oldfield, 1971). For each item, the subjects are asked to indicate whether they show strong (++), less strong (+) or indifferent (+/+) left or right hand for each item. The subject responses are then assigned scores from 1 to 5 corresponding to strongly left to strongly right for each item. The scores are then summed across the items to create the EHI score and subjects are classified as left-handed, right-handed or ambidextrous based on their EHI value. I note here that the cut-points used to characterize individual hand preferences vary quite significantly across studies depending on the aims of the study, which by itself, seems problematic (Salmaso & Longoni, 1985). Notably, what constitutes a mixed-handed or ambidextrous subject is directly related to the cut-points applied to the continuously scaled EHI measure.
The use of questionnaires is common in studies of handedness in adult humans but for those scientists interested in the development of handedness or handedness in some clinical populations, questionnaires are not always desirable and in some cases implausible when the subjects lack the ability to understand the verbal instructions of the task. In these cases, scientists must adopt measures that require direct measurement of hand use during structured or unstructured testing circumstances. For example, Coren, Porac, and Duncan (1981) tested hand preference in a sample of preschool children and young adults by recording their hand use when ask to execute four different actions including throwing, pointing, pick up a crayon and draw, and touch your nose with your finger. The children were then classified as right-, left-, or mixed-handed based on the consistency in their hand use across the four behaviors.
The first challenge in the characterization of hand preference in human and nonhuman primates is the approach. In many studies of handedness in humans, particularly adults, hand preference classification is based on consistency in hand use across multiple items or tasks. In contrast, in nonhuman primates, hand preference classification is derived from z-scores calculated on the basis of frequencies in left and right hand use for a single measure. Indeed, in nonhuman primates, there are very few studies that have characterized individual hand preferences on the basis of consistency in hand use across multiple tasks (Diamond & McGrew, 1994; Hopkins et al., in press; Humle & Matsuzawa, 2009; Lilak & Phillips, 2007; Llorente, Mosquera, & Fabre, 2009). Thus, at the more general level of analysis, classification of human handedness emphasizes consistency in hand use across multiple items whereas nonhuman primates handedness is centered around frequency in hand use within a single task.
A second challenge is the use of z-scores as the basis for characterizing individual hand preferences in human and nonhuman primates. The most common approach in the nonhuman primate literature is to adopt z-score cut-points of ±1.96. Subjects with z-scores ≥1.96 or ≤−1.96 are classified as right- and left-handed. Subject with z-scores >−1.96 or <+1.96 are classified as nonpreferent or ambiguously handed. I emphasize here that this is the common approach used with nonhuman primates but this is virtually never done in assessing hand preferences in adult humans, even though there is absolutely nothing preventing researchers from adopting this approach, even with questionnaire data. z-scores as well as HI scores are often used to characterize handedness in young children because, like nonhuman primates, hand preference is assessed by measuring frequency in left and right hand use for specific actions; thus, this approach is more similar to that used with nonhuman primates. Notwith-standing, in studies with human children, there is considerable variation in the cut-point values used to characterize individual hand preferences on the basis of z-scores or HI scores. For example, Fagard and Marks (2000) used z-score cut-points of −1.0 and +1.0 to classify toddlers as left- or right-handed where as Michel, Sheu, and Brumley (2002) used two criteria including −1.0 and +1.0 0r −1.65 and +1.65 to characterize individual left- and right-hand preferences in 7–11 month infants. In a study by Coren et al. (1981), subjects were classified as right- or left-handed for each task on the basis of the sign of their HI score (positive = right-handed, zero or negative = left-handed). In short, studies with nonhuman primates use very conservative criteria for classifying subjects as right- or left-handed compared to studies in developing human children and, particularly, adult humans.
The differences in statistical approaches to the characterization of hand preferences in human and nonhuman primates are problematic for several reasons. First, differences in cut-points used to classify right- and left-handed subjects have a direct influence on the number of ambiguously handed subjects. More conservative cut-point lead to a higher number of subjects classified as ambiguously-handed, which as noted above, is the primary criteria for determining whether a hand preference task elicits a “significant” hand preference. Second, with conservative cut-points such as ±1.96, there is the potential to commit Type II error or to falsely accept the null hypothesis (i.e., no hand preference). That is to say, there may be subjects that are moderately right handed as manifest by a positive z-score that would be classified as nonpreferent based on current practices. These subjects are arguably “right-handed” but do not meet the magical p < .05 criteria. This is particularly problematic when there is variation in the number of hand use responses used to derive the z-score. For instance, a subject with three left and seven right hand responses would have a z-score of +1.26 and an HI score of .40 while a subject with 30 left and 70 right hand responses would have a z-score of +4.00 and an HI score of .40. From this example, one can see that the HI scores are identical between these two subjects but the z-scores differ dramatically due to differences in the number of observations in hand use. These two hypothetical subjects differ only in the ability of the experimenter to be confident in their hand preference classification but my concern is that in an effort to define subjects as “significantly” handed we are potentially overlooking subtle, quantitative differences in hand preferences between species.
I would further argue that investigators are sacrificing external validity at the considerable expense of internal validity in the measurement of handedness in nonhuman primates. As an example, imagine I tested 100 chimpanzees for handedness and all 100 individuals preferred the left hand for three responses and the right for seven. Based on current statistical and inferential practices, the conclusion would be that the chimpanzees are nonpreferent because in none the individual cases would the subjects’ z-scores exceed 1.95. In contrast, I would argue that the chimpanzees show a small but consistent population-level right hand bias. I will let the readers decide which characterization best describes these hypothetical data.
CHALLENGE #4: COMPARING HANDEDNESS IN WILD VERSUS CAPTIVE PRIMATES
Another important issue is the lack of handedness data from wild populations of nonhuman primates compared to those generated from captive individuals. Nearly all studies of handedness in wild populations have come from great apes (Biro, Sousa, & Matsuzawa, 2006; Boesch, 1991; Bogart et al., 2012; Byrne & Byrne, 1991; Corp & Byrne, 2004; Humle & Matsuzawa, 2009; Lonsdorf & Hopkins, 2005; Marchant & McGrew, 2007; McGrew & Marchant, 1992, 1996; Parnell, 2001; Peters & Rogers, 2007; Rogers & Kaplan, 1995; Sugiyama, Fushimi, Sakura, & Matsuzawa, 1993) with very few from lesser apes (Morino, 2011) New World (Garber, Gomes, & Bicca-Marques, 2008; Panger, 1998; Smith & Thompson, 2011) Old World (Zhao, Gao, Li, & Wantanabe, 2008; Zhao et al., 2012) or prosimian primate species (Rigamonti, Spiezio, Poli, & Fazio, 2005). This is an important issue for two reasons. First, some have suggested that captive subjects have been raised in a human right-hand constructed environment and this may potentially bias their hand preferences in a small but nonetheless significant manner. On related manner, some captive primates have been raised by humans whereas others have not and it might be argued that direct human contact might result in some subjects adopting a more human-like right handedness (Llorente et al., 2009). For instance, McGrew and Marchant (1997) and more recently Cashmore et al. (2008) have suggested that rearing or setting differences may explain the discrepancy between findings on handedness in wild compared to captive chimpanzees. Second, it has been suggested that some of the tasks used in captive populations have little ecological validity in relation to behaviors and task encountered by wild populations. For instance, recording hand use when reaching from different postures that are rarely exhibited in the natural environment may lead to some significant results but how this test might generalize to wild settings is not clear (Braccini, Lambeth, Schapiro, & Fitch, 2010).
The role of setting differences on hand preference has been a particular focal point of debate when considering handedness findings in chimpanzees. In captive chimpanzees, evidence of population-level right handedness have been found for several measures including coordinated bimanual actions, manual gesture and throwing (Hopkins, in press-a; Hopkins, Taglialatela, Leavens, Russell, & Schapiro, 2010). In contrast, evidence of population-level handedness in wild chimpanzees have been less apparent within a given study; however, I have previously argued that sample sizes are usually relatively small in wild compared to captive populations and this limits the ability to detect the existence of population-level handedness, specifically within single communities of wild individuals (Hopkins, 2006). When hand preference data for similar behaviors are combined across different wild chimpanzee communities, the evidence of population-level hand use becomes more evident. To illustrate this point, shown in Table 1 are published data on hand use for different behaviors (mostly tool use) in wild chimpanzees. As can be seen, based on my assessment of the extant data, wild chimpanzees show population-level right handedness for wadge-dipping/leaf sponging and ant-dipping and left handedness for termite fishing. No population-level bias is found for throwing and nut-cracking. Also, as noted above, there are no population-level biases in hand use for spontaneous actions in wild chimpanzees either but, unlike measures of tool-use, these behaviors do not elicit significant hand preferences at the individual level in a majority of the subjects to their validity is questionable.
Table 1. Summary of Handedness Data for Tool Use From Wild Chimpanzees.
| #L | #R | #A | Mean HI | t-Value | |
|---|---|---|---|---|---|
| Termite fishing(1) | 46 | 24 | 7 | −.237 | −2.77* |
| Ant dipping(2) | 7 | 22 | 10 | .292 | 2.21* |
| Wadge dip/leaf sponge(3) | 9 | 27 | 4 | .336 | 3.10* |
| Nut cracking(4) | 32 | 44 | 13 | .117 | 1.51 |
| Pestle pounding(5) | |||||
| Pound action | 7 | 10 | 2 | .012 | .78 |
| Reach action | 4 | 15 | 1 | .341 | 2.34* |
| Algae dip(6) | 5 | 9 | 0 | .037 | .21 |
| Throwing(7) | 1 | 3 | 12 | .089 | 1.65+ |
Reference to each of the behaviors are provided here:
Significant population-level handedness at p < .05.
Significant population-level bias at p < .10.
Several other observations in hand use should be noted in wild chimpanzees and other great apes. In the chimpanzees from Mahale, sex differences in handedness for bimanual feeding have been reported with males showing a significant left hand bias and females showing a right hand bias (Corp & Byrne, 2004). Similarly, for termite fishing, when the data are combined across sites, there is a borderline significant sex difference with greater left handedness among male compared to female chimpanzees (Bogart et al., 2012). Lastly, population-level right handedness has been reported for bimanual feeding in wild gorillas (Byrne & Byrne, 1991) and leading limb in brachiated locomotion in wild orangutans (Peters & Rogers, 2007) while left hand preferences have been found for water drinking in wild siamangs (Morino, 2011).
One challenge in comparing the data on hand preference in wild and captive populations is the lack of consistency the types of measures used to assess hand preference. In short, setting differences are confounded with task variation. For instance, most studies in wild chimpanzees have focused on tool use whereas very few have in captive populations. Further-more, the extent to which tasks or paradigms used with captive populations can perfectly model the sensory and motor demands of certain tasks in the wild present some challenges. For example, Hopkins, Russell, Schaeffer, Gardner, and Schapiro (2009) tested handedness for a simulated termite fishing tasks in a sample of captive chimpanzees and the result were somewhat similar to reports in wild chimpanzees. Nonetheless, the sticks used for dipping and the size of the hole into which the subjects probed in the Hopkins et al. (2009) article were both larger than what is often reported in wild chimpanzees. Whether this makes a significant difference in the expression of asymmetry in hand use is unclear but comparison between wild and captive chimpanzees is difficult because the tasks differ so dramatically, rendering any meaningful comparisons very difficult if not impossible.
A second challenge is problems in the operational definitions used to describe specific actions. For instance, nut-cracking has been described as a “complex bimanual task” in wild chimpanzees and, in this sense, one might equate the motor demands for this behavior to those used to describe bimanual feeding or for tasks involving coordinated bimanual actions, such as the TUBE task. For nut-cracking in wild chimpanzees, bimanual implies that the chimpanzee used both hands but the hands are not being used in a coordinated manner; however, one hand holds the hammer and strikes the nut while the other hand independently places the nut on the anvil. It is not the case that the chimpanzee holds the nut with one hand while simultaneously hammering with the opposite hand. In contrast, for something like the TUBE task, the subjects is holding the tube with one hand and simultaneously extracting the food with the opposite hand. Though both of these tasks are described as complex bimanual actions, in the case of the TUBE task, the hands are working in a complementary manner with one hand assuming a subordinate and one assuming a dominant role when their actions are coordinated and directed toward a single object. In contrast, for nut-cracking, the two hands are being used but not in a complementary fashion but rather as distinct, independent motor actions. These may seem like trivial differences but they may prove to be important distinctions in the measurement of handedness (see Lambert, in press; Meguerditchian, Calcutt, Lonsdorf, & Ross, 2010).
SUMMARY AND A PROPOSAL FOR CONSENSUS
There are several simple solutions that may help to resolve much of the confusion regarding handedness in human and nonhuman primates. First, with respect to handedness measurement, only behaviors or tasks that elicit consistent biases at the individual level of analysis should be considered in the assessment of population-level handedness. Behaviors or tasks that fail to elicit consistent hand preferences should either not be considered valid measures of hand use or, at a minimum, be recognized for their limitations. Though I do not believe that researchers should ignore hand use for different kinds of unimanual actions, it seems increasingly clear that tasks that require coordinated bimanual actions appear to elicit more robust hand preferences at both the individual and population-level in different primate species (see Figs. 3 and 4). Though findings on measures, like the TUBE task, have largely come from experimental studies, I would emphasize that there are likely parallels in wild populations for some kinds of actions such as a grooming, tool manufacture and feeding where role differentiation of the hands might similarly have an influence on hand preference (Byrne & Byrne, 1991; Hopkins, Russell, Remkus, Freeman, & Schapiro, 2007; Zhao, Gao, & Li, 2010). In my view, those studying handedness in nonhuman primates need to focus on quantifying measures of hand use that are (1) less prone to influence by situational factors (Meunier, Blois-Heulin, & Vauclair, 2011; Meunier, Vauclair, & Fagard, 2012), (2) reliably elicit significant hand preferences at the individual level, and (3) focus on measures that are conceptually important and related to theory rather than selected on the basis of convenience.
In terms of the methods of collecting handedness data, I have no reservations and, indeed, strongly believe that researchers should record both bouts (properly done) and events in hand use for tasks or behaviors that are produced in sequences (Hopkins, in press-b). Reporting frequencies in left and right hand use for both levels of analysis would be useful for characterizing individual hand preference; however, I also strongly believe that the continued claim and insistence by some that recording bouts of hand use is the only useful and scientifically acceptable means of recording hand use for repeated actions is completely unfounded. There is no empirical evidence that recording bouts instead of events of hand use leads to different findings. Furthermore, the methods currently being employed to record bouts of hand use are ineffective in preventing the perceived problem of auto-correlated responses.
There are several ways in which the characterization of handedness can be standardized that would facilitate comparative studies of handedness (see Hopkins, in press-b, for discussion). First, if handedness is characterized on a continuous scale of measurement, mean HI scores and associated measures of variability could be used to characterize hand reference for different species. As an alternative, rather than use HI scores, one could use descriptive statistics based on raw z-scores to characterize a sample of handedness scores. One advantage to this approach is that the z-scores are all scaled to the normal distribution; thus task and particularly species variation can be compared in standard units of measurement. Lastly, efforts to characterize individual hand preferences within and between tasks that emphasize consistency instead of frequency in hand use would greatly facilitate comparison with findings in human subjects (Hopkins et al., in press).
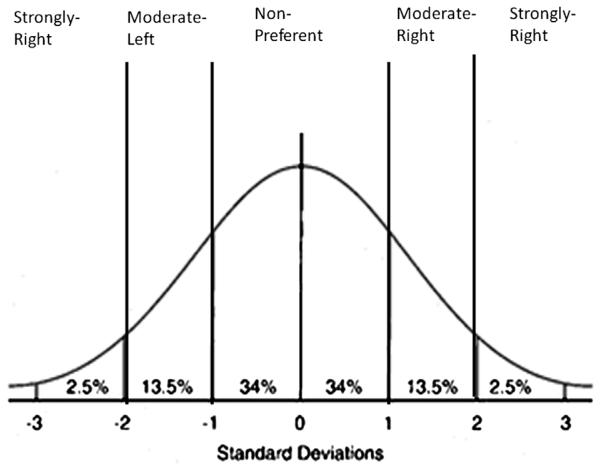
In terms of handedness classification, I would propose that the range of hand preference categories be expanded to include moderate left- and right-handed groups (see Fig. 6). Specifically, because individual binomial z-scores are tested against the probabilities of the normal distribution, it seems only logical to adapt cut-points that conform to the standard units of variation within the normal distribution. In the normal distribution, μ = 0 and the SD 1. Therefore, individuals with z-scores ≥1.0 or z ≤ −1.0 have values that are at least one standard deviation unit from 0 (or no hand preference). In this approach, subjects with z-scores ≥1.96 or ≤ −1.96 would be classified as strongly right- and strongly left-handed. Subjects with z-scores ≥1.0 but <1.96 would be classified as moderately right-handed and subjects with z-scores ≤ −1.0 but < −1.96 would be classified as moderately left-handed. Subjects with z-scores > −1.0 and <1.0 would be classified as nonpreferent.
FIGURE 6.
Proposed classification scheme for individual handedness based on z-scores. Strongly right (z ≥ +1.96), Moderate-right (z ≥ +1.0 and z < +1.96), Non-preferent (z > −.99 and z < +.99), Moderate-left (z ≤ −1.0 and z > −1.96) and Strongly left (z ≤ −1.96).
Finally, there is need for additional studies in captive but particularly wild monkeys and apes. The extent to which research in captive and wild settings can draw on similar methods or tasks is somewhat limited for obvious reasons but, nonetheless, effort should be made to try and adopt similar measures (see Zhao et al., 2012). At a minimum, for observational studies of naturally occurring behavior, the emphasis should be on behaviors that require coordination of the hands that are not subject to situational or other extraneous factors. More importantly, the behaviors of interest should be selected on their ability to elicit consistent hand preferences at the individual level of analysis. Increased attention to the selection of behaviors or tasks for handedness assessment will provide a better framework for evaluating potential setting and species differences in handedness in primates, including humans.
Acknowledgments
Contract grant sponsor: NIH
Contract grant numbers: MH-92923, NS-42867, NS-73134, HD-56232, HD-60563
Footnotes
American Psychological Association and Institute of Medicine guidelines for the treatment of animals were followed during all aspects of this study.
REFERENCES
- Annett M. Left, right, hand, and brain: The right-shift theory. Lawrence Erlbaum Associates; London: 1985. [Google Scholar]
- Aruguete MA, Ely EA, King JE. Laterality in spontaneous motor activity of chimpanzees and squirrel monkeys. American Journal of Primatology. 1992;27:13–21. doi: 10.1002/ajp.1350270303. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Suomi SJ, Hopkins WD. Effects of early adverse experiences on behavioral lateralisation in rhesus monkeys (Macaca mulatta) Laterality. 2008;13(3):282–292. doi: 10.1080/13576500802022216. [DOI] [PubMed] [Google Scholar]
- Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matsuzawa T. Cultural innovation and transmission of tool use in wild chimpanzees: Evidence from field experiments. Animal Cognition. 2003;6(4):213–223. doi: 10.1007/s10071-003-0183-x. [DOI] [PubMed] [Google Scholar]
- Biro D, Sousa C, Matsuzawa T. Ontogeny and cultural propagation of tool use by wild chimpanzees at Bossou, Guinea: Case studies in nut cracking and leaf folding. In: Matsuzawa T, Tomonaga T, Tanaka M, editors. Cognitive development of chimpanzees. Springer; New York: 2006. pp. 476–507. [Google Scholar]
- Boesch C. Handedness in wild chimpanzees. International Journal of Primatology. 1991;6:541–558. [Google Scholar]
- Bogart SL, Pruetz JD, Ormiston LK, Russell JL, Meguerditchian A, Hopkins WD. Termite fishing laterality in the Fongoli savanna chimpanzees (Pan troglodytes verus): Further evidence of a left hand preference. American Journal of Physical Anthropology. 2012;149:591–598. doi: 10.1002/ajpa.22175. [DOI] [PubMed] [Google Scholar]
- Braccini S, Lambeth SP, Schapiro SJ, Fitch WT. Bipedal tool use strengthens chimpanzee hand preferences. Journal of Human Evolution. 2010;58:234–241. doi: 10.1016/j.jhevol.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw B, Rogers L. The evolution of lateral asymmetries, language, tool-use and intellect. Academic Press; San Diego: 1993. [Google Scholar]
- Byrne RW, Byrne JM. Hand preferences in the skilled gathering tasks of mountain gorillas (Gorilla gorilla berengei) Cortex. 1991;27:521–536. doi: 10.1016/s0010-9452(13)80003-2. [DOI] [PubMed] [Google Scholar]
- Cashmore L. Can hominin “handedness” be accuartely assessed? Annals of Human Biology. 2009;36(5):624–641. doi: 10.1080/03014460902956733. [DOI] [PubMed] [Google Scholar]
- Cashmore L, Uomini N, Chapelain A. The evolution of handedness in humans and great apes: A review and current issues. Journal of Anthropological Sciences. 2008;86:7–35. [PubMed] [Google Scholar]
- Chapelain A, Hogervorst E. Hand preferences for bimanual coordination in 29 bonobos (Pan paniscus) Behavioural Brain Research. 2009;196:15–29. doi: 10.1016/j.bbr.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Chapelain A, Hogervorst E, Mbonzo P, Hopkins WD. Hand preferences for bimanual coordination in 77 bonobos (Pan paniscus): Replication and extension. International Journal of Primatology. 2011;32:491–510. [Google Scholar]
- Coren S, Porac C, Duncan P. Lateral preference behaviors in preschool children and young adults. Child Development. 1981;52(2):443–450. [Google Scholar]
- Corp N, Byrne RW. Sex difference in chimpanzee handedness. American Journal of Physical Anthropology. 2004;123:62–68. doi: 10.1002/ajpa.10218. [DOI] [PubMed] [Google Scholar]
- Crow TJ. A theory of the origin of cerebral asymmetry: Epigenetic variation superimposed on a fixed right-shift. Laterality. 2009;15(3):289–303. doi: 10.1080/13576500902734900. [DOI] [PubMed] [Google Scholar]
- Dadda M, Cantalupo C, Hopkins WD. Further evidence of an association between handedness and neuroanatomical asymmetries in the primary cortex of chimpanzees (Pan troglodytes) Neuropsychologia. 2006;44:2482–2486. doi: 10.1016/j.neuropsychologia.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond AC, McGrew WC. True handedness in the Cotton-top tamarin (Saguinus oedipus)? Primates. 1994;35(1):69–77. [Google Scholar]
- Ettlinger GF. Hand preference, ability and hemispheric specialization. How far are these factors related in the monkey? Cortex. 1988;24:389–398. doi: 10.1016/s0010-9452(88)80002-9. [DOI] [PubMed] [Google Scholar]
- Fagard J, Marks A. Unimanual and bimanual tasks and the assessment of handedness in toddlers. Developmental Science. 2000;3(2):137–147. [Google Scholar]
- Fagot J, Drea C, Wallen K. Asymmetrical hand use in rhesus monkeys (Macaca mulatta) in tactually and visually regulated tasks. Journal of Comparative Psychology. 1991;105:260–268. doi: 10.1037/0735-7036.105.3.260. [DOI] [PubMed] [Google Scholar]
- Forrester GS, Leavens DA, Quaresmini C, Vallortigara G. Target animacy influences gorilla handedness. Animal Cognition. 2011;14:903–907. doi: 10.1007/s10071-011-0413-6. [DOI] [PubMed] [Google Scholar]
- Forrester GS, Quaresmini C, Leavens DA, Mareschal D, Thomas MSC. Human handedness: An inherited evolutionary trait. Behavioral Brain Research. 2013;237:200–206. doi: 10.1016/j.bbr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Forrester GS, Quaresmini C, Leavens DA, Spiezio C, Vallortigara G. Target animacy influences chimpanzee handedness. Animal Cognition. 2012;15(6):1121–1127. doi: 10.1007/s10071-012-0536-4. [DOI] [PubMed] [Google Scholar]
- Garber PA, Gomes DF, Bicca-Marques JC. Experimental field study of hand preference in wild black-horned (Cebus nigritus) and white-faced (Cebus capucinus): Evidence for individual and species differences. Animal Cognition. 2008;11(3):401–411. doi: 10.1007/s10071-007-0130-3. [DOI] [PubMed] [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Hand preferences in New World primates. International Journal of Comparative Psychology. 1997;9:173–207. [Google Scholar]
- Hopkins WD. Posture and reaching in chimpanzees (Pan) and orangutans (Pongo) Journal of Comparative Psychology. 1993;17:162–168. doi: 10.1037/0735-7036.107.2.162. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences in juvenile chimpanzees: Continuity in development. Developmental Psychology. 1995;31:619–625. [Google Scholar]
- Hopkins WD. On the other hand: Statistical issues in the assessment and interpretation of hand preference data in non-human primates. International Journal of Primatology. 1999;20:851–866. [Google Scholar]
- Hopkins WD. Comparative and familial analysis of handedness in great apes. Psychological Bulletin. 2006;132(4):538–559. doi: 10.1037/0033-2909.132.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD. Behavioral and brain asymmetries in chimpanzees: A case for continuity. Annals of the New York Academy of Sciences. 1288:17–35. doi: 10.1111/nyas.12109. in press-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD. Independence of data points in the measurement of handedness: Statistical problem or urban myth? American Journal of Physical Anthropology. 151(1):151–157. doi: 10.1002/ajpa.22248. in press-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Handedness in chimpanzees is associated with asymmetries in the primary motor but not with homologous language areas. Behavioral Neuroscience. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Individual and setting differences in the hand preferences of chimpanzees (Pan troglodytes): A critical analysis and some alternative explanations. Laterality. 2005;10:65–80. doi: 10.1080/13576500342000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, de Waal FBM. Behavioral laterality in captive bonobos (Pan paniscus): Replication and extension. International Journal of Primatology. 1995;16:261–276. [Google Scholar]
- Hopkins WD, Gardner M, Mingle M, Reamer L, Schapiro SJ. Within- and between-task consistency in hand use as a means of characterizing hand preferences in captive chimpanzees (Pan troglodytes) Journal of Comparative Psychology. doi: 10.1037/a0031071. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Phillips KA, Bania A, Calcutt SE, Gardner M, Russell JL, Schapiro SJ. Hand preferences for coordinated bimanual actions in 777 great apes: Implications for the evolution of handedness in Hominins. Journal of Human Evolution. 2011;60:605–611. doi: 10.1016/j.jhevol.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Pika S, Liebal K, Bania A, Meguerditchian A, Gardner M, Schapiro SJ. Handedness for manual gestures in great apes: A meta-analysis. In: Pika S, Liebal K, editors. Current developments in non-human primate gesture research. John Benjamins Publishing; Amsterdam: 2012. [Google Scholar]
- Hopkins WD, Russell J, Cantalupo C, Freeman H, Schapiro S. Factors influencing the prevalence and handedness for throwing in captive chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 2005;119(4):363–370. doi: 10.1037/0735-7036.119.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Hook M, Braccini S, Schapiro S. Simple reaching is not so simple: Association between hand use and grip preferences in captive chimpanzees. International Journal of Primatology. 2005;26(2):259–277. doi: 10.1007/s10764-005-2924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, Cantalupo C. Neuroanatomical correlates of handedness for tool use in chimpanzees (Pan troglodytes): Implication for theories on the evolution of language. Psychological Science. 2007;18(11):971–977. doi: 10.1111/j.1467-9280.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, Freeman H, Buehler N, Reynolds E, Schapiro SJ. The distribution and development of handedness for manual gestures in captive chimpanzees (Pan troglodytes) Psychological Science. 2005;16(6):487–493. doi: 10.1111/j.0956-7976.2005.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, Remkus M, Freeman H, Schapiro SJ. Handedness and grooming in Pan troglodytes: Comparative analysis between findings in captive and wild chimpanzees. International Journal of Primatology. 2007;28:1315–1326. [Google Scholar]
- Hopkins WD, Russell JL, Schaeffer JA, Gardner M, Schapiro SJ. Handedness for tool use in captive chimpanzees (Pan troglodytes): Sex differences, performance, heritability and comparion to the wild. Behaviour. 2009;146:1463–1483. doi: 10.1163/156853909X441005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Taglialatela J, Leavens DA, Russell JL, Schapiro SJ. Behavioral and brain asymmetries in chimpanzees. In: Lonsdorf EV, Ross SR, Matsuzawa T, editors. The mind of the chimpanzee. University of Chicago Press; Chicago: 2010. pp. 60–74. [Google Scholar]
- Humle T, Matsuzawa T. Laterality in hand use across four tool use behaviors among the wild chimpanzees of Bossou, Guinea, West Africa. American Journal of Primatology. 2009;71:40–48. doi: 10.1002/ajp.20616. [DOI] [PubMed] [Google Scholar]
- Jones-Engel LE, Bard KA. Precision grips in young chimpanzees. American Journal of Primatology. 1996;39:1–15. doi: 10.1002/(SICI)1098-2345(1996)39:1<1::AID-AJP1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- LaCreuse A, Parr LA, Smith HM, Hopkins WD. Hand preferences for a haptic task in chimpanzees(Pan troglodytes) International Journal of Primatology. 1999;20(6):867–881. [Google Scholar]
- Lambert M. Hand preference for bimanual and unimanual feeding in captive gorillas: Extension in a second colony of apes. American Journal of Physical Anthropology. :641–647. doi: 10.1002/ajpa.22095. in press. [DOI] [PubMed] [Google Scholar]
- Laurence A, Wallez C, Blois-Heulin C. Task complexity, posture, age, sex: which is the main factor influencing manual laterality in captive Cercocebus torquatus torquatus? Laterality. 2011;16(5):586–606. doi: 10.1080/1357650X.2010.501338. doi: 10.1080/1357650X.2010.501338. [DOI] [PubMed] [Google Scholar]
- Lehman RAW. Manual preference in prosimians, monkeys, and apes. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. Springer-Verlag; New York: 1993. pp. 107–124. [Google Scholar]
- Lilak AL, Phillips KA. Consistency in hand preference across low-level and high-level tasks in capuchin monkeys (Cebus apella) American Journal of Primatology. 2007;69:1–12. doi: 10.1002/ajp.20485. [DOI] [PubMed] [Google Scholar]
- Llorente M, Mosquera M, Fabre M. Manual laterality for simple reaching and bimanual coordinated task in naturalistic housed. Pan troglodytes International Journal of Primatology. 2009;30:183–197. [Google Scholar]
- Llorente M, Palou L, Carrasco L, Riba D, Mosquera M, Colell M, Feliu O. Population-level right handedness for a coordinated bimanual task in naturalistic housed chimpanzees: Replication and extension in 114 animals from Zambia and Spain. American Journal of Primatology. 2010;71:1–10. doi: 10.1002/ajp.20895. [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV, Hopkins WD. Wild chimpanzees show population level handedness for tool use. Proceedings of the National Academy of Sciences United States of America. 2005;102:12634–12638. doi: 10.1073/pnas.0505806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlis L, Dodd PWD, Fentress JC. The pooling fallacy: Problems arising when individuals contribute more than one observation to the data set. Zeitschrift fur Tierpsychologie. 1985;68(3):201–214. [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behavioral and Brain Sciences. 1987;10:247–303. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of function in apes: A meta-analysis of methods. Journal of Human Evolution. 1991;21:425–438. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of limb function in wild chimpanzees of Gombe National Park: Comprehensive study of spontaneous activities. Journal of Human Evolution. 1996;30:427–443. [Google Scholar]
- Marchant LF, McGrew WC. Ant fishing by wild chimpanzees is not lateralised. Primates. 2007;48:22–26. doi: 10.1007/s10329-006-0020-3. [DOI] [PubMed] [Google Scholar]
- Marchant LF, McGrew WC, Eibl-Eibesfeldt I. In human handedness universal? Ethological analyses from three traditional cultures. Ethology. 1995;101(3):239–258. [Google Scholar]
- Martin P, Bateson P. Measuring behaviour: An introductory guide. Cambridge University Press; Cambridge: 1986. [Google Scholar]
- McGrew WC, Marchant LF. Chimpanzees, tools, and termites: Hand preference or handedness? Current Anthropology. 1992;33:114–119. [Google Scholar]
- McGrew WC, Marchant LF. On which side of the apes? In: McGrew WC, Marchant LF, Nishida T, editors. Great ape societies. Cambridge University Press; Cambridge: 1996. pp. 255–272. [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in non-human primates. Yearbook of Physical Anthropology. 1997;40:201–232. [Google Scholar]
- McGrew WC, Marchant LF. Ethological study of manual laterality in the chimpanzees of the Mahale mountains, Tanzania. Behaviour. 2001;138:329–358. [Google Scholar]
- Meguerditchian A, Calcutt SE, Lonsdorf EV, Ross SR. Captive gorillas are right-handed for bimanual feeding. American Journal of Physical Anthropology. 2010;141:638–645. doi: 10.1002/ajpa.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguerditchian A, Donnot J, Molesti S, Francioly R, Vauclair J. Sex difference in squirrel monkeys’ handedness for unimanual and bimanual tasks. Animal Behaviour. 2012;83:635–643. [Google Scholar]
- Meunier H, Blois-Heulin C, Vauclair J. A new tool for measuring hand preference in non-human primates: Adaptation of Bishop’s Quantifying Hand Preference task for olive baboons. Behavioural Brain Research. 2011;218:1–7. doi: 10.1016/j.bbr.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Meunier H, Vauclair J. Hand preferences on unimanual and bimanual tasks in white-face capuchins. American Journal of Primatology. 2007;69:1064–1069. doi: 10.1002/ajp.20437. [DOI] [PubMed] [Google Scholar]
- Meunier H, Vauclair J, Fagard J. Human infants and baboons show the same pattern of handedness for a communicative gesture. PLoS ONE. 2012;7(3):e33959. doi: 10.1371/journal.pone.0033959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel GF, Sheu CF, Brumley MR. Hand use preferences from 7 to 11 months of age as revealed by latent class analysis. Developmental Psychobiology. 2002;40:1–13. [PubMed] [Google Scholar]
- Mittra ES, Fuentes A, McGrew WC. Lack of hand preference in wild hanuman langurs (Presbytis entellus) American Journal of Physical Anthropology. 1997;103:455–461. doi: 10.1002/(SICI)1096-8644(199708)103:4<455::AID-AJPA3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Morino L. Left-hand rpeference for a complex manual task in a populaiton of wild siamangs (Sympha-langus syndactylus) International Journal of Primatology. 2011;32:793–800. [Google Scholar]
- Nishida T, McGrew WC, Marchant LF. Wild chimpanzees at Mahale are not manually lateralised for throwing. Pan Africa News Letter. 19(2):1–3. in press. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Panger MA. Hand preference in free-ranging white-throated capuchins (Cebus capucinus) in Costa Rica. International Journal of Primatology. 1998;19:133–163. [Google Scholar]
- Papademetriou E, Sheu CF, Michel GF. A meta-analysis of primate hand preferences for reaching and other hand-use measures. Journal of Comparative Psychology. 2005;119:33–48. doi: 10.1037/0735-7036.119.1.33. [DOI] [PubMed] [Google Scholar]
- Parnell RJ. Hand preference for food processing in wild western lowland gorillas (Gorilla gorilla gorilla) Journal of Comparative Psychology. 2001;115:365–375. [PubMed] [Google Scholar]
- Perelle IB, Ehrman L. An international study of human handedness: The data. Behavior Genetics. 1994;24:217–227. doi: 10.1007/BF01067189. [DOI] [PubMed] [Google Scholar]
- Peters HH, Rogers LJ. Limb use and preferences in wild orang-utans during feeding and locomotor behavior. American Journal of Primatology. 2007;69:1–15. doi: 10.1002/ajp.20483. [DOI] [PubMed] [Google Scholar]
- Phillips K, Sherwood CC. Primary motor cortex asymmetry correlates with handedness in capuchin monkeys (Cebus apella) Behavioral Neuroscience. 2005;119:1701–1704. doi: 10.1037/0735-7044.119.6.1701. [DOI] [PubMed] [Google Scholar]
- Porac C, Coren S. Lateral preferences and human behavior. Springer; New York: 1981. [Google Scholar]
- Rigamonti MM, Spiezio C, Poli MD, Fazio F. Laterality of manual fucntion in foraging and positional behavior in wild indri (Indri indri) American Journal of Primatology. 2005;65:27–38. doi: 10.1002/ajp.20095. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Kaplan G. Hand preferences and other lateral biases in rehabilitated orangutans, Pongo pygmaeus pygmaeus. Animal Behaviour. 1995;51:13–25. [Google Scholar]
- Salmaso D, Longoni AM. Problems in the assessment of handedness. Cortex. 1985;21:533–549. doi: 10.1016/s0010-9452(58)80003-9. [DOI] [PubMed] [Google Scholar]
- Schmitt V, Melchisedech S, Hammerschmidt K, Fischer J. Hand preferences in barbary macaques (Macaca sylvanus) Laterality. 2008;13(2):143–157. doi: 10.1080/13576500701757532. [DOI] [PubMed] [Google Scholar]
- Schweitzer C, Bec P, Blois-Heulin C. Does the complexity of the task influence laterality in De Brazza’s monkeys (Cercopithecus neglectus)? Ethology. 2007;113:993–994. [Google Scholar]
- Smith HM, Thompson CL. Observations of hand preference in wild groups of white-faced sakis (Pithecia pithecia) in Suriname. American Journal of Primatology. 2011;73:655–664. doi: 10.1002/ajp.20942. [DOI] [PubMed] [Google Scholar]
- Spinozzi G, Cacchiarelli B. Manual laterality in haptic and visual reaching tasks by tufted capuchin monkeys (Cebus apella). An association between hand preference and hand accuracy for food discrimination. Neuropsychologia. 2000;38(13):1685–1692. doi: 10.1016/s0028-3932(00)00080-4. [DOI] [PubMed] [Google Scholar]
- Spinozzi G, Castornina MG, Truppa V. Hand preferences for unimanual and coordinated-bimanual tasks in tufted capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 1998;112:183–191. [Google Scholar]
- Spinozzi G, Truppa V, Lagana T. Grasping behavior in tufted capuchin monkeys (Cebus apella): Grip types and manual laterality for picking up a small food item. American Journal of Physical Anthropology. 2004;125:30–41. doi: 10.1002/ajpa.10362. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y. Tool-use for catching ants by chimpanzees at Bossou and Monts Nimba, West Africa. Primates. 1995;36:193–205. [Google Scholar]
- Sugiyama Y, Fushimi T, Sakura O, Matsuzawa T. Hand preference and tool use in wild chimpanzees. Primates. 1993;34:151–159. [Google Scholar]
- Tonooka R, Matsuzawa T. Hand preferences in captive chimpanzees (Pan troglodytes) in simple reaching for food. International Journal of Primatology. 1995;16:17–34. [Google Scholar]
- Vauclair J, Meguerditchian A, Hopkins WD. Hand preferences for unimanual and coordinated bimanual tasks in baboons (Papio anubis) Cognitive Brain Research. 2005;25:210–216. doi: 10.1016/j.cogbrainres.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiological Psychology. 1980;8:351–359. [Google Scholar]
- Zhao D, Gao X, Li B. Hand preference for spontaneously unimanual and bimanual coordinated tasks in wild Sichuan snub-nosed monkeys: Implications for hemispheric specialization. Behavioural Brain Research. 2010;208:85–89. doi: 10.1016/j.bbr.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Zhao D, Gao X, Li B, Wantanabe K. First wild evidence of neonate nipple preference and maternal cradling laterality in Old World monkeys: A preliminary study from Rhinopithecus roxellana. Behavioral Processes. 2008;77(3):364–368. doi: 10.1016/j.beproc.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Zhao D, Hopkins WD, Li B. Handedness in nature: First evidence of manual laterality on bimanual coordinated tube task in wild primates. American Journal of Physical Anthropology. 2012;148:36–44. doi: 10.1002/ajpa.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]