Abstract
In multiple sclerosis, in which brain tissue becomes permeable to blood proteins, extravascular fibrin deposition correlates with sites of inflammatory demyelination and axonal damage. To examine the role of fibrin in neuroinflammatory demyelination, we depleted fibrin in two tumor necrosis factor transgenic mouse models of multiple sclerosis, transgenic lines TgK21 and Tg6074. In a genetic analysis, we crossed TgK21 mice into a fibrin-deficient background. TgK21fib-/- mice had decreased inflammation and expression of major histocompatibility complex class I antigens, reduced demyelination, and a lengthened lifespan compared with TgK21 mice. In a pharmacologic analysis, fibrin depletion, by using the snake venom ancrod, in Tg6074 mice also delayed the onset of inflammatory demyelination. Overall, these results indicate that fibrin regulates the inflammatory response in neuroinflammatory diseases. Design of therapeutic strategies based on fibrin depletion could potentially benefit the clinical course of demyelinating diseases such as multiple sclerosis.
Keywords: autoimmunity, anticoagulants, ancrod, extracellular matrix
Fibrin, as the final product of the coagulation cascade, plays a major role in blood clotting. However, the role of fibrin is not restricted to the blood, since components of the coagulation cascade reside within tissues and can stimulate extravascular fibrin formation (1). Studies of fibrin deposition in human diseases (2-5), in combination with experiments from gene-targeted mice deficient in fibrin (6), have shown that a wide range of pathological conditions, such as glomerulonephritis, lung ischemia, and rheumatoid arthritis, are exacerbated by fibrin deposition.
Compromised vasculature in the nervous tissue is a pathogenic manifestation apparent in traumatic injuries, such as spinal cord, optic nerve, and sciatic nerve injury, as well as in central nervous system (CNS) diseases with autoimmune characteristics, such as multiple sclerosis (MS) (7). Blood-brain barrier (BBB) disruption precedes clinical symptoms in MS patients (8), and fibrin is deposited in the lesions (9, 10), apparently before cerebral tissue injury and demyelination (11). Fibrin deposition also coincides with areas of demyelination (12), as well as with areas of axonal damage (13). In addition, in experimental autoimmune encephalomyelitis (EAE), an autoimmune animal model of MS, there is increased coagulation activation before symptom development (14). Pharmacologic depletion of fibrin in EAE ameliorates clinical symptoms, suggesting that fibrin plays a role in CNS inflammatory demyelination (15, 16). Furthermore, inhibition of fibrin formation by attenuation of thrombin activity is protective in the injured optic nerve (17).
Although extravascular fibrin(ogen) is present at sites of inflammatory demyelination in MS, and experiments in rodents have established a deleterious role for fibrin in nervous system pathogenesis (18), the cellular mechanisms of fibrin action in the CNS have not been investigated. In this study we examined the effects of fibrin depletion in two tumor necrosis factor (TNF)-transgenic animal models for MS (19). These animal models have previously revealed aspects of TNF action in the CNS (20, 21), provided a mechanism for TNF-induced demyelination (22, 23), and provided mouse models for MS (24-26). The work presented here, derived from experiments of genetic and pharmacologic depletion of fibrin in TNF transgenic mice, indicates that fibrin stimulates the immune response in the CNS and is a regulator of disease pathogenesis in neuroinflammatory demyelination.
Materials and Methods
Generation of TgK21fib-/- Transgenic Mice. TgK21 mice die by 5 weeks of age (27) and cannot be used for breeding. TgK21 mice heterozygous for the p55 TNF receptor (TgK21p55+/-) exhibit clinical symptoms of paralysis by 8 months of age (28). In this study we crossed 2-month-old TgK21p55+/- mice with mice deficient for the fibrinogen Aα chain (fib-/-) (29) and obtained TgK21p55+/-fib+/- mice. We further crossed TgK21p55+/-fib+/- mice with fib+/- mice and obtained TgK21fib-/-, as well as TgK21fib+/+ littermates. In all experiments TgK21fib+/+ littermates were used as controls. To determine the onset of disease and lifespan, mice were monitored daily and scored for clinical symptoms.
Systemic Defibrinogenation. For the pharmacological depletion of fibrinogen we used Tg6074 mice (30). Mice were depleted of fibrinogen by delivering 3 units of ancrod per day by using mini osmotic pumps (model 2002; Alza) filled with 250 units/ml ancrod (Sigma) as described (31, 32).
Histopathological Analysis. Dissection of brain and spinal cords, embedding in paraffin and cryostat sectioning, hematoxylin and luxol fast blue/periodic acid-Schiff staining, and immunohistochemical staining were performed as described (28). The primary antibodies were goat anti-human fibrin(ogen) (Chemicon; 1/500) and rat anti-mouse MHC class I (BMA Biomedicals; 1/200). Bound antibody was visualized by using the avidin-biotin-peroxidase complex (Vectastain Elite ABC kit; Vector Laboratories) and 3-amino-9-ethylcarbazole (AEC) (Sigma) as a chromogen. Staining specificity for the fibrin(ogen) antibody was confirmed by using tissue from fib-/- mice. Images were collected by using an Axiophot Zeiss microscope with an Axiocam camera.
In Situ Zymography. For in situ zymographies (33), 10-μm unfixed cryostat spinal cord sections were prepared. Sections were covered by a casein overlay containing 1% low-melting-point agarose and 25 μg/ml plasminogen, and then covered with a coverslip. Plasminogen was prepared from human plasma (34). Control experiments were performed with overlay mixtures lacking plasminogen. Conversion of plasminogen into plasmin, which in turn lysed the insoluble casein, resulted in the appearance of lytic zones within 6 h. Zones of plasmin-dependent caseinolysis appeared as black areas when photographed under dark-field illumination on a Nikon microscope.
Cell Culture. RAW264.7 murine macrophage cell line (American Type Culture Collection) was maintained in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen). For 5-bromodeoxyuridine (BrdUrd) proliferation assay, cells were cultured for 48 h either with addition of 1 μg/ml lipopolysaccharide (LPS) from Escherichia coli O55:B5 (Sigma catalog no. L 6529) or on plates coated with 50 μg/ml plasminogen-free fibrinogen (Calbiochem) or 50 μg/ml fibronectin (Sigma). Proliferation assay was performed by using the Cell Proliferation BrdU colorimetric ELISA (Roche Diagnostics). For immunocytochemistry, cells were cultured on glass coverslips, fixed with 1:1 acetone/methanol, blocked with 3% BSA (Sigma), and incubated with FITC-conjugated anti-mouse CD11b (1:50, eBioscience, San Diego). Results represent the means of four independent experiments performed in triplicate. Statistical analysis was performed by using Student's t test.
Results
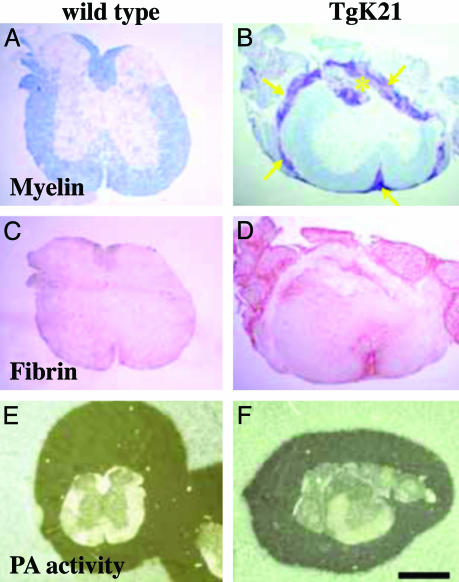
Fibrin Deposition Precedes Demyelination and Correlates with Demyelinating Plaques in TgK21 Mice. TgK21 mice show inflammatory demyelinating lesions in the spinal cord (27, 28). To determine whether fibrin was present in the CNS of TgK21 mice, we stained cross sections of spinal cords with an antibody against fibrin(ogen). Wild-type (WT) mice showed normal spinal cord histology (Fig. 1A) and no fibrin deposition (Fig. 1C). In contrast, TgK21 mice showed inflammation and demyelination in their spinal cord (Fig. 1B) and fibrin deposition (Fig. 1D). Fibrin deposition in the spinal cord of TgK21 mice was present in white matter areas (Fig. 2A), whereas inflammatory cells were still confined in the meninges and demyelination had not yet been initiated (Fig. 2B). In addition, active demyelinating plaques (Fig. 2D) were also immunoreactive for fibrin (Fig. 2C). This was not a general effect of BBB disruption, because fibrin deposition occurred within the white matter and was absent from the gray matter (Fig. 2), where extensive IgG staining could be observed (28). Overall, these results show that fibrin is present at white matter areas before the onset of demyelination and colocalizes with areas of active demyelination.
Fig. 1.
Inflammatory demyelinating lesions of TgK21 mice have fibrin deposition but normal amounts of plasminogen activator activity. Luxol fast blue staining counterstained with nuclear red shows normal histology in WT mice (A), whereas TgK21 spinal cords have meningeal inflammation (arrows) and myelin loss (asterisk) (B). Immunostaining for fibrin(ogen) shows that WT mice do not have fibrinogen in their spinal cord (C), whereas TgK21 spinal cords are immunoreactive for fibrin(ogen) (D). In situ zymography on spinal cord sections shows proteolytic activity in both normal (E) and TgK21 (F) mice. (Bar = 722 μm for A-D, 1.6 mm for E and F.)
Fig. 2.
Fibrin deposition precedes demyelination and correlates with demyelinating plaques in TgK21 mice. Immunohistochemistry for fibrin(ogen) shows deposition of fibrin (A) at areas of meningeal inflammation before the onset of demyelination (B). In addition, fibrin immunoreactivity (C) is detected at areas the spinal cord associated with inflammation and loss of myelin (D). A and C and B and D represent high-magnification images of Fig. 1 D and B, respectively. (Bar = 125 μm.)
Inflammatory Demyelinating Lesions of TgK21 Mice Do Not Show Up-Regulation of Plasminogen Activator Activity. Fibrin deposited in tissues can be cleared by the plasminogen activator/plasmin fibrinolytic system. We therefore examined the fibrinolytic capacity of the CNS tissue of the TgK21 mice. In situ zymography on spinal cord sections of WT (Fig. 1E) and TgK21 (Fig. 1F) mice and gel zymography experiments on spinal cord extracts (not shown) revealed no up-regulation in the plasminogen activator activity in TgK21 mice compared with WT.
Genetic Depletion of Fibrin(ogen) Increases the Lifespan and Delays the Clinical Symptoms of the TgK21 Mice. To determine whether fibrin depletion could affect inflammatory demyelination, we crossed TgK21 mice into a fibrin-deficient background and generated TgK21fib-/- mice. TgK21fib-/- demonstrated a 1.6-fold lifespan increase (6.4 ± 0.4 weeks; n = 11) compared with TgK21fib+/+ littermate mice (4.2 ± 0.3 weeks; n = 9, P < 0.01) (Fig. 1 A). TgK21 mice showed paralysis at 3 weeks, whereas TgK21fib-/- mice did not develop paralysis until 5 weeks. Both TgK21 and TgK21fib-/- mice show no clinical symptoms until the development of paralysis, and they both live for 1 week after the onset of paralysis. Overall, the phenotypic differences between TgK21 and TgK21fib-/- mice suggest that fibrin affects the onset and not the severity of the disease in TNF-induced inflammatory demyelination.
Genetic or Pharmacologic Depletion of Fibrin(ogen) Decreases Inflammation and Delays the Onset of Demyelination in TNF Transgenic Mouse Models of MS. We next performed histopathological analysis to investigate the effects of fibrin depletion on the onset and progress of inflammatory demyelination. At 4 weeks, when TgK21fib+/+ mice were paralyzed and TgK21fib-/- were phenotypically normal, mice were killed and histopathological analysis of spinal cord sections was performed. TgK21fib+/+ mice showed fibrin deposition (Fig. 3B), whereas as expected TgK21fib-/- mice showed no immunoreactivity for fibrin (Fig. 3C). Hematoxylin/eosin staining showed inflammation in TgK21fib+/+ mice (Fig. 3D), whereas TgK21fib-/- mice showed only very mild meningeal inflammation (Fig. 3E).
Fig. 3.
Fibrin(ogen) facilitates inflammatory demyelination. (A) TgK21fib-/- demonstrate a 1.6-fold lifespan increase (6.4 ± 0.4 weeks; n = 11) compared with TgK21fib+/+ littermate mice (4.2 ± 0.3 weeks; n = 9, P < 0.01). Immunohistochemistry for fibrin shows fibrin deposition in TgK21 spinal cord (B), but fibrin is absent from TgK21fib-/- spinal cord (C). Hematoxylin/eosin staining shows accumulation of inflammatory cells in TgK21 spinal cord (D), whereas there is minor inflammation in TgK21fib-/- spinal cord (E). Immunohistochemistry for MHC class I shows the presence of cytotoxic cells in TgK21 (F), which are absent from TgK21fib-/- (G). Myelin staining with luxol fast blue (blue) counterstained with periodic acid-Schiff stain (phagocytic macrophages, purple) shows active demyelination in TgK21 (H), whereas there are no lesions in TgK21fib-/- (I). (Bar = 170 μm.)
Fibrin(ogen) regulates the survival (35, 36) and migration capacity of neutrophils in vitro (37) and the production of cytokines (38) and chemokines (39). To investigate whether fibrin could affect the cytotoxic properties of the immune response, we examined the expression of MHC class I as a marker for lymphocyte and macrophage activation (40, 41). TgK21fib+/+ mice showed expression of MHC class I (Fig. 3F), whereas TgK21fib-/- mice were negative for MHC class I expression (Fig. 3G). TgK21fib+/+ mice showed active demyelination (Fig. 3H), whereas TgK21fib-/- showed no signs of myelin loss (Fig. 3I). Overall, these results suggest that fibrin deposition in the CNS, which occurs after disruption of the BBB, is a facilitator for the development of inflammation.
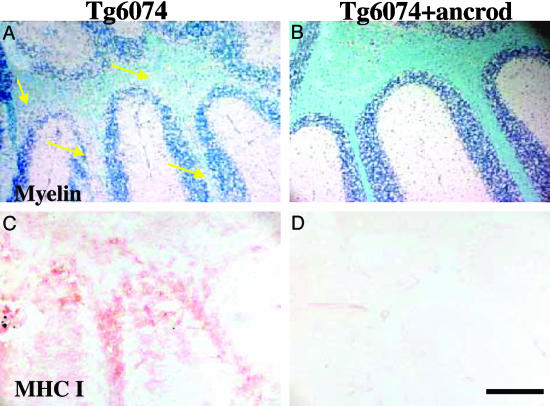
To complement our genetic experiments, we examined demyelination after pharmacologic depletion of fibrin. Administration of ancrod, a Malayan pit viper (Calloselasma rhodostoma) venom protein, drastically reduces plasma fibrinogen levels and fibrin deposition (42). Ancrod is administrated by mini osmotic pumps (31), which because of size limitations cannot be inserted in mice less than 4 weeks of age. Because TgK21 mice die at 4.2 weeks of age, we chose Tg6074 TNF transgenic mice (28, 30), a model of inflammatory demyelination with onset of disease at 6 weeks. Immunostaining for fibrin(ogen) showed that similarly to TgK21 demyelinating lesions, Tg6074 lesions also exhibited fibrin deposition (data not shown). Ancrod was administered to 4-week-old Tg6074 mice for 2 weeks. At 6 weeks, mice were killed together with littermate controls and histopathological analysis was performed. Six-week-old Tg6074 mice showed myelin vacuolation and demyelination in the cerebellum (Fig. 4A arrows). In contrast, fibrin-depleted Tg6074 mice showed no signs of demyelination (Fig. 4B). Examination of MHC class I immunoreactivity, as a marker of cytotoxic activity in the cerebellum, showed that while there was an up-regulation of MHC class I in Tg6074 mice (Fig. 4C), MHC class I gene expression was not detected in fibrin-depleted Tg6074 mice (Fig. 4D). Therefore, consistent with experiments with genetic depletion of fibrinogen, pharmacological depletion also delayed the onset of inflammatory demyelination and down-regulated the immune response.
Fig. 4.
Pharmacologic depletion of fibrin(ogen) delays the onset of inflammatory demyelination. Tg6074 transgenic mice show myelin loss at the cerebellum by 6 weeks of age (A, arrows), whereas Tg6074 mice pretreated with ancrod for 2 weeks show no myelin alteration by 6 weeks of age (B). Tg6074 mice show up-regulation of MHC class I genes in the cerebellum (C), whereas fibrin-depleted Tg6074 do not show any signs of inflammatory cells or expression of immunologic markers characteristic of cytotoxic immune response (D). (Bar = 200 μm.)
Fibrinogen Induces Macrophage Activation. Because genetic or pharmacologic depletion of fibrin delayed the onset of inflammation in the CNS, we sought to investigate the effects of fibrin on macrophages, the major cell type that contributes to both TNF-induced (43) and autoimmune-induced (44) inflammatory demyelination. To investigate the effects of fibrin in macrophages we used the murine macrophage cell line RAW 264.7, which acquires an activated dendritic morphology upon LPS induction (45). Immunofluorescence using an antibody against CD11b, a macrophage cell marker, shows that RAW cells acquire distinct dendritic-like morphology after 24 h in culture in the presence of LPS (Fig. 5B), compared with unstimulated RAW cells cultured on plastic (Fig. 5A). RAW cells cultured on fibrinogen-coated surface exhibit morphologic changes similar to those of the LPS-treated cells (Fig. 5C). Phase-contrast microscopy shows that the fibrinogen-induced morphologic alterations of macrophages (Fig. 5F) resemble the morphologic alterations induced by LPS (Fig. 5E), characterized by a dramatic increase in size, larger nuclei, prominent nucleoli, extended processes, and cytoplasm with increased granularity, compared with untreated RAW cells (Fig. 5D). Quantitation reveals 62.1 ± 9.8% of cells with activated morphology upon LPS induction, 24.5 ± 3.2% upon fibrinogen stimulation, and 4.2 ± 0.8% of untreated cells (Fig. 5G). Activation of macrophages and their differentiation to dendritic cells is associated with a decrease in proliferation (46). LPS, a potent activator of macrophages, is known to inhibit their proliferation (47). To examine whether fibrin affects macrophage proliferation, we compared BrdUrd incorporation into cells grown on plastic, LPS-coated, or fibrinogen-coated plates. We observed that fibrin decreased macrophage proliferation by 37% compared with proliferation of macrophages on uncoated surface (Fig. 5H). Fibronectin did not have any effect on altering macrophage morphology (Fig. 5G) or proliferation (Fig. 5H), suggesting that fibrinogen specifically affects macrophage functions. Our results, taken together with the stimulatory effects of fibrinogen on secretion of proinflammatory cytokines, such as IL-1β (38, 48) and TNF (49), and secretion of chemokines, such as macrophage inflammatory protein-1 α (MIP-1α), MIP-1β, and MIP-2 (39) by macrophage cells suggest that fibrinogen is a potent mediator of macrophage activation.
Fig. 5.
Fibrinogen induces differentiation in macrophages. Untreated CD11b-immunostained RAW 264.7 macrophage cells (A) show undifferentiated, macrophage-like morphology. LPS- (1 μg/ml; B) or fibrinogen- (50 μg/ml; C) stimulated RAW264.7 cells show a morphological transformation to dendritic-like cells. LPS-stimulated cells (E) show morphologic alterations similar to those of fibrinogen-stimulated cells (F). Untreated cells (D) are round and smaller. (G) Quantitation of cells with activated morphology shows a statistically significant increase in activation upon fibrinogen stimulation, compared with untreated or fibronectin-stimulated cells. (H) Proliferation assay shows a statistically significant decrease of macrophage proliferation upon fibrinogen induction, compared with untreated or fibronectin-stimulated cells. Results are presented as means ± SE. Statistical analysis was performed by using Student's t test. (Scale bar = 45μm for A-C, 25 μm for D-F.)
Discussion
In this study we investigated the effects of fibrin during inflammatory demyelination in the CNS. Our data show that fibrin deposition precedes and regulates the onset of inflammatory demyelination, and they demonstrate that genetic or pharmacologic fibrin depletion ameliorates both clinical symptoms and the severity of the inflammatory response in the nervous system. Vascular pathology, which allows the leakage of blood proteins in the brain parenchyma, has been considered to participate in the development of MS lesions (50). Use of magnetic resonance imaging (MRI) has established that disruption of the vasculature integrity precedes clinical symptoms in MS patients (8). Fibrin deposition is associated with a variety of vascular abnormalities in MS lesions, including vessel wall disruption that renders the nervous system permeable to cells and proteins derived from the blood (11). Our data identify fibrin as a major blood-derived component that regulates the induction of the inflammatory response and demyelination in the nervous system.
The recognition of fibrinogen by members of three major families of integrins, β1 (α5β1), β2 (CD11b/CD18 and CD11c/CD18) and β3 (αvβ3), expressed on leukocytes, macrophages, and monocytes, points out the importance of this molecule in adhesion, migration, and activation of the major cellular players of inflammation (51). Fibrin-depleted mice showed a dramatic reduction in MHC class I expression and inflammatory cell number at the spinal cord, suggesting a decrease in the number of activated immune cells. Our in vitro findings indicate that fibrin induces macrophage activation and inhibits their proliferation. Previous studies have demonstrated that fibrinogen and fibrin induce macrophage secretion of proinflammatory cytokines (38, 48, 49) and chemokines (37). Activated macrophages, proinflammatory cytokines, and chemokines are known participants in the pathogenesis of MS by orchestrating the cytotoxic immune response and contributing to myelin degradation (52). Because fibrin deposition in the CNS of the TNF transgenic mice is an early event that precedes the development of demyelinated plaques, its role in the induction of the inflammatory response in CNS could be envisaged. In cell-transferred EAE, where lymphoid cells are already activated before transfer, there is no difference in inflammation after prophylactic fibrin depletion (16). This result further supports a role for fibrin in CNS inflammation primarily in the induction and not the effector phase of inflammatory demyelination, because it indicates that fibrin does not affect inflammation when inflammatory cells are activated ex vivo. Comparative analysis of EAE induced by active immunization using myelin peptides or adoptive transfer using activated immune cells, would determine the effects of fibrin in the induction and effector phases of autoimmune-driven demyelination. Overall, these results could be of potential interest for MS, because fibrin deposition occurs before cerebral parenchymal inflammation and demyelination (11) and could therefore be a potential regulator of the cytotoxic response responsible for the initiation of the immunologic attack against myelin.
Demyelinating lesions in MS are initially perivascular and inflammatory in nature (40, 53). Both in our animal model and in MS, fibrin deposition correlates with areas of demyelination (12) as well as areas of axonal damage (13). In addition to the presence of fibrin in MS plaques of active demyelination, fibrin deposition persists even in inactive lesions (10), suggesting an inability of the CNS to degrade fibrin. Impaired fibrinolysis, attributed to impaired activity of tissue plasminogen activator (tPA), has recently been reported in MS (54). In EAE mice deficient for tPA show increased severity and delayed recovery from the neurological dysfunction (55). Interestingly, the gliotic white matter shows no fibrinolytic activity, suggesting that the MS plaque has a decreased innate capacity to degrade fibrin (56). Similarly, fibrin deposition persists in the spinal cord of TgK21 mice and there is no increase in fibrinolytic activity compared with the uninjured spinal cord. Our previous evidence from the peripheral nervous system (PNS), which spontaneously regenerates after injury, showed that the fibrinolytic activity is up-regulated after injury (32) and fibrin degradation correlates with nerve regeneration (57). The innate inability of the demyelinated CNS to degrade fibrin within the plaques could represent a mechanism contributing to decreased repair and regeneration in the injured CNS. Because fibrin depletion positively affects EAE even in the presence of inflammation, such additional mechanisms could considerably contribute to the progression and clinical severity of inflammatory demyelination. Taken together, these results suggest that the differential regulation of fibrinolytic activity between the PNS and CNS might be a factor that determines the regeneration capacity of the nervous tissue after injury or disease associated with BBB leakage.
Our data suggest that fibrin could be a potential therapeutic target in MS. Fibrinolytic therapies aiming directly at the dissolution of the fibrin clot might be a safer approach than the exogenous administration of proteases, such as tPA, because there is evidence that tPA, through laminin degradation (58), contributes to neuronal death in models of excitotoxic neuronal death (59) and cerebral ischemia (60). MS is a disease with a profound heterogeneity in its clinical course, neuroradiological appearance of the lesions, and involvement of susceptibility gene loci. For these reasons, response to therapy is varied (61). In general, therapeutic intervention in demyelinating diseases depends greatly on the etiology of demyelination. The positive effect of prophylactic fibrin depletion in models of inflammatory demyelination with diverse etiology and histopathologic characteristics (reviewed in ref. 62) indicates the potential for therapeutic benefits of anticoagulants that target fibrin in inflammatory demyelination.
Acknowledgments
We are indebted to Jay Degen and George Kollias for providing us with the fibrinogen-deficient and the TgK21 mice, respectively, for helpful discussions, and for their genuine interest in this work. TgK21 mice were obtained from the Hellenic Pasteur Institute, Athens, Greece. We thank Stella Tsirka for insightful discussions, Werner Lesslauer for providing us with the p55-/- deficient mice, and Alexander Tarakhovsky for insightful suggestions on the manuscript. We thank Zu-Lin Chen for the preparation of human plasminogen and Lynn Corrigan for technical assistance with mouse genotyping. We thank Tal Nuriel and Shoana Willis for assistance with the preparation of the manuscript. We are grateful to Palmer Taylor and his lab members for their generosity for letting us use an inverted phase-contrast microscope and an ELISA plate reader and to Moses V. Chao for providing us with space to house the TgK21 mouse colony at New York University Medical Center animal facility. Some of the work included here was conducted at the National Center for Microscopy and Imaging Research at San Diego, which is supported by National Institutes of Health Grant RR04050 awarded to Dr. Mark Ellisman. This work was supported by National Institutes of Health Grants NS 35704 and NS 38472 (to S.S.) and by The Wadsworth Foundation Award and the National Multiple Sclerosis Society Research Grant RG 3370 (to K.A.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BBB, blood-brain barrier; EAE, experimental autoimmune encephalomyelitis; LPS, lipopolysaccharide; MS, multiple sclerosis; TNF, tumor necrosis factor.
References
- 1.Furie, B. & Furie, B. C. (1988) Cell 53, 505-518. [DOI] [PubMed] [Google Scholar]
- 2.Colvin, R. B., Johnson, R. A., Mihm, M. C., Jr., & Dvorak, H. F. (1973) J. Exp. Med. 138, 686-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy, G. A., Leibowitz, J. L. & Edgington, T. S. (1981) J. Exp. Med. 154, 1150-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neale, T. J., Tipping, P. G., Carson, S. D. & Holdsworth, S. R. (1988) Lancet 2, 421-424. [DOI] [PubMed] [Google Scholar]
- 5.Labarrere, C. A., Nelson, D. R. & Faulk, W. P. (1998) Am. J. Med. 105, 207-213. [DOI] [PubMed] [Google Scholar]
- 6.Degen, J. L., Drew, A. F., Palumbo, J. S., Kombrinck, K. W., Bezerra, J. A., Danton, M. J., Holmback, K. & Suh, T. T. (2001) Ann. N.Y. Acad. Sci. 936, 276-290. [DOI] [PubMed] [Google Scholar]
- 7.Huber, J. D., Egleton, R. D. & Davis, T. P. (2001) Trends Neurosci. 24, 719-725. [DOI] [PubMed] [Google Scholar]
- 8.Kermode, A. G., Thompson, A. J., Tofts, P., MacManus, D. G., Kendall, B. E., Kingsley, D. P., Moseley, I. F., Rudge, P. & McDonald, W. I. (1990) Brain 113, 1477-1489. [DOI] [PubMed] [Google Scholar]
- 9.Sobel, R. A. & Mitchell, M. E. (1989) Am. J. Pathol. 135, 161-168. [PMC free article] [PubMed] [Google Scholar]
- 10.Claudio, L., Raine, C. S. & Brosnan, C. F. (1995) Acta Neuropathol. 90, 228-238. [DOI] [PubMed] [Google Scholar]
- 11.Wakefield, A. J., More, L. J., Difford, J. & McLaughlin, J. E. (1994) J. Clin. Pathol. 47, 129-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon, E. E. & Prineas, J. W. (1994) J. Neuropathol. Exp. Neurol. 53, 625-636. [DOI] [PubMed] [Google Scholar]
- 13.Gveric, D., Hanemaaijer, R., Newcombe, J., van Lent, N. A., Sier, C. F. & Cuzner, M. L. (2001) Brain 124, 1978-1988. [DOI] [PubMed] [Google Scholar]
- 14.Inaba, Y., Ichikawa, M., Inoue, A., Itoh, M., Kyogashima, M., Sekiguchi, Y., Nakamura, S., Komiyama, A. & Koh, C. (2001) J. Neurol. Sci. 185, 89-93. [DOI] [PubMed] [Google Scholar]
- 15.Paterson, P. Y. (1976) Fed. Proc. 35, 2428-2434. [PubMed] [Google Scholar]
- 16.Inoue, A., Koh, C. S., Shimada, K., Yanagisawa, N. & Yoshimura, K. (1996) J. Neuroimmunol. 71, 131-137. [DOI] [PubMed] [Google Scholar]
- 17.Friedmann, I., Yoles, E. & Schwartz, M. (2001) J. Neurochem. 76, 641-649. [DOI] [PubMed] [Google Scholar]
- 18.Akassoglou, K. & Strickland, S. (2002) Biol. Chem. 383, 37-45. [DOI] [PubMed] [Google Scholar]
- 19.Probert, L. & Akassoglou, K. (2001) Glia 36, 212-219. [DOI] [PubMed] [Google Scholar]
- 20.Lock, C., Oksenberg, J. & Steinman, L. (1999) Ann. Rheum. Dis. 58, Suppl. 1, I121-I128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selmaj, K. W. (2000) Ann. Rheum. Dis. 59, Suppl. 1, I94-I102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benveniste, E. N. (1997) Chem. Immunol. 69, 31-75. [DOI] [PubMed] [Google Scholar]
- 23.Merrill, J. E. & Scolding, N. J. (1999) Neuropathol. Appl. Neurobiol. 25, 435-458. [DOI] [PubMed] [Google Scholar]
- 24.Owens, T., Wekerle, H. & Antel, J. (2001) Nat. Med. 7, 161-166. [DOI] [PubMed] [Google Scholar]
- 25.Becher, B., Prat, A. & Antel, J. P. (2000) Glia 29, 293-304. [PubMed] [Google Scholar]
- 26.Pouly, S. & Antel, J. P. (1999) J. Autoimmun. 13, 297-306. [DOI] [PubMed] [Google Scholar]
- 27.Akassoglou, K., Probert, L., Kontogeorgos, G. & Kollias, G. (1997) J. Immunol. 158, 438-445. [PubMed] [Google Scholar]
- 28.Akassoglou, K., Bauer, J., Kassiotis, G., Pasparakis, M., Lassmann, H., Kollias, G. & Probert, L. (1998) Am. J. Pathol. 153, 801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suh, T. T., Holmback, K., Jensen, N. J., Daugherty, C. C., Small, K., Simon, D. I., Potter, S. & Degen, J. L. (1995) Genes Dev. 9, 2020-2033. [DOI] [PubMed] [Google Scholar]
- 30.Probert, L., Akassoglou, K., Pasparakis, M., Kontogeorgos, G. & Kollias, G. (1995) Proc. Natl. Acad. Sci. USA 92, 11294-11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busso, N., Peclat, V., Van Ness, K., Kolodziesczyk, E., Degen, J., Bugge, T. & So, A. (1998) J. Clin. Invest. 102, 41-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akassoglou, K., Kombrinck, K. W., Degen, J. L. & Strickland, S. (2000) J. Cell Biol. 149, 1157-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sappino, A. P., Madani, R., Huarte, J., Belin, D., Kiss, J. Z., Wohlwend, A. & Vassalli, J. D. (1993) J. Clin. Invest. 92, 679-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deutsch, D. G. & Mertz, E. T. (1970) Science 170, 1095-1096. [DOI] [PubMed] [Google Scholar]
- 35.Pluskota, E. & D'Souza, S. E. (2000) Eur. J. Biochem. 267, 4693-4704. [DOI] [PubMed] [Google Scholar]
- 36.Whitlock, B. B., Gardai, S., Fadok, V., Bratton, D. & Henson, P. M. (2000) J. Cell Biol. 151, 1305-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sans, E., Delachanal, E. & Duperray, A. (2001) J. Immunol. 166, 544-551. [DOI] [PubMed] [Google Scholar]
- 38.Perez, R. L., Ritzenthaler, J. D. & Roman, J. (1999) Am. J. Respir. Cell Mol. Biol. 20, 1059-1066. [DOI] [PubMed] [Google Scholar]
- 39.Smiley, S. T., King, J. A. & Hancock, W. W. (2001) J. Immunol. 167, 2887-2894. [DOI] [PubMed] [Google Scholar]
- 40.Traugott, U. (1987) J. Neuroimmunol. 16, 283-302. [DOI] [PubMed] [Google Scholar]
- 41.Bauer, J., Rauschka, H. & Lassmann, H. (2001) Glia 36, 235-243. [DOI] [PubMed] [Google Scholar]
- 42.Bell, W. R., Shapiro, S. S., Martinez, J. & Nossel, H. L. (1978) J. Lab. Clin. Med. 91, 592-604. [PubMed] [Google Scholar]
- 43.Kassiotis, G., Bauer, J., Akassoglou, K., Lassmann, H., Kollias, G. & Probert, L. (1999) Eur. J. Immunol. 29, 912-917. [DOI] [PubMed] [Google Scholar]
- 44.Huitinga, I., van Rooijen, N., de Groot, C. J., Uitdehaag, B. M. & Dijkstra, C. D. (1990) J. Exp. Med. 172, 1025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saxena, R. K., Vallyathan, V. & Lewis, D. M. (2003) J. Biosci. 28, 129-134. [DOI] [PubMed] [Google Scholar]
- 46.Valledor, A. F., Comalada, M., Xaus, J. & Celada, A. (2000) J. Biol. Chem. 275, 7403-7409. [DOI] [PubMed] [Google Scholar]
- 47.Vadiveloo, P. K., Vairo, G., Novak, U., Royston, A. K., Whitty, G., Filonzi, E. L., Cragoe, E. J., Jr., & Hamilton, J. A. (1996) Oncogene 13, 599-608. [PubMed] [Google Scholar]
- 48.Perez, R. L. & Roman, J. (1995) J. Immunol. 154, 1879-1887. [PubMed] [Google Scholar]
- 49.Fan, S. T. & Edgington, T. S. (1993) J. Immunol. 150, 2972-2980. [PubMed] [Google Scholar]
- 50.Lassmann, H. (2003) J. Neurol. Sci. 206, 187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ugarova, T. P. & Yakubenko, V. P. (2001) Ann. N.Y. Acad. Sci. 936, 368-385. [DOI] [PubMed] [Google Scholar]
- 52.Brosnan, C. F. & Raine, C. S. (1996) Brain Pathol. 6, 243-257. [DOI] [PubMed] [Google Scholar]
- 53.Traugott, U., Reinherz, E. L. & Raine, C. S. (1983) Science 219, 308-310. [DOI] [PubMed] [Google Scholar]
- 54.Gveric, D. H. B., Petzold, A., Lawrence, D. A. & Cuzner, M. L. (2003) Brain 126, 1-9. [DOI] [PubMed] [Google Scholar]
- 55.Lu, W., Bhasin, M. & Tsirka, S. E. (2002) J. Neurosci. 22, 10781-10789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirsch, H. E., Blanco, C. E. & Parks, M. E. (1981) J. Neuropathol. Exp. Neurol. 40, 271-280. [DOI] [PubMed] [Google Scholar]
- 57.Akassoglou, K., Yu, W.-M., Akpinar, P. & Strickland, S. (2002) Neuron 33, 861-875. [DOI] [PubMed] [Google Scholar]
- 58.Chen, Z. L. & Strickland, S. (1997) Cell 91, 917-925. [DOI] [PubMed] [Google Scholar]
- 59.Tsirka, S. E., Gualandris, A., Amaral, D. G. & Strickland, S. (1995) Nature 377, 340-344. [DOI] [PubMed] [Google Scholar]
- 60.Wang, Y. F., Tsirka, S. E., Strickland, S., Stieg, P. E., Soriano, S. G. & Lipton, S. A. (1998) Nat. Med. 4, 228-331. [DOI] [PubMed] [Google Scholar]
- 61.Lucchinetti, C., Bruck, W., Parisi, J., Scheithauer, B., Rodriguez, M. & Lassmann, H. (2000) Ann. Neurol. 47, 707-717. [DOI] [PubMed] [Google Scholar]
- 62.Probert, L., Eugster, H. P., Akassoglou, K., Bauer, J., Frei, K., Lassmann, H. & Fontana, A. (2000) Brain 123, 2005-2019. [DOI] [PubMed] [Google Scholar]