Abstract
In adult humans, the prefrontal cortex possesses wider minicolumns and more neuropil space than other cortical regions. These aspects of prefrontal cortex architecture, furthermore, are increased in comparison to chimpanzees and other great apes. In order to determine the developmental appearance of this human cortical specialization, we examined the spatial organization of neurons in four cortical regions (frontal pole [Brodmann’s area 10], primary motor [area 4], primary somatosensory [area 3b], and prestriate visual cortex [area 18]) in chimpanzees and humans from birth to approximately the time of adolescence (11 years of age). Horizontal spacing distance (HSD) and gray level ratio (GLR) of layer III neurons were measured in Nissl-stained sections. In both human and chimpanzee area 10, HSD was significantly higher in the post-weaning specimens compared to the pre-weaning ones. No significant age-related differences were seen in the other regions in either species. In concert with other recent studies, the current findings suggest that there is a relatively slower maturation of area 10 in both humans and chimpanzees as compared to other cortical regions, and that further refinement of the spatial organization of neurons within this prefrontal area in humans takes place after the post-weaning periods included here.
Keywords: minicolumn, evolution, comparative neuroanatomy, biological anthropology
The development of the spatial organization of neurons in the neocortex is an arena of research with important implications for a wide array of fields, including genetics (Chen et al., 2012) and pathology (Kana et al., 2011). Horizontal spatial organization incorporates both modular and vertical characteristics of the cerebral cortex, including the arrangement of neurons into minicolumns (DeFelipe, 2005; Galuske et al., 2000; Mountcastle, 1997). Minicolumns are composed of vertically arrayed pyramidal cells and their related axons and dendrites (Buxhoeveden and Casanova, 2002; Peters and Sethares, 1996), and are considered to reflect the migration destination of radial units of postmitotic cells generated in fetal life in the ventricular zone (Rakic, 1995). Variation in spacing between minicolumns hints at changes in other aspects of neuroanatomy, including the morphology and distribution of dendrites and axons associated with pyramidal neurons (Allman et al., 2002). Features of dendritic arborization may influence neuronal functioning, including the size, branching pattern, and the number and distribution of synapses (Elston et al., 2007). Furthermore, the addition of minicolumns in fetal brain development is proposed to be one evolutionary mechanism by which the cerebral cortex may increase in size (Rakic and Kornack, 2007). The present study examines the postnatal development of the horizontal spatial organization of neurons in the neocortex of humans and chimpanzees during the pre-and post-weaning periods.
Direct comparisons of humans with our close phylogenetic relatives, the great apes (common chimpanzees, bonobos, gorillas, orangutans), are necessary in order to understand what is evolutionarily distinctive in human brain organization. However, such comparative analyses of the spatial organization of neurons in the cerebral cortex are relatively scarce, despite the close phylogenetic relationship that great apes share with humans. A recent study (Semendeferi et al., 2011) identified significant differences in area 10, with HSD in humans being 30% larger than in the frontal pole of the other species. HSD in humans was second largest in area 4, followed by area 3b and then area 17. With the exception of area 10, humans shared overlapping HSD and GLR values with the apes for all other areas examined. Although area 4 was the region with the largest HSD values in the great apes, HSD in area 4 did not differ between humans and apes, suggesting that the absolute increase in the size of area 10 human values was the cause of the observed divergence between humans and apes. Together with other studies examining similar parameters in Broca’s area (Schenker et al., 2008) and dorsolateral prefrontal cortex (area 9) as well as areas 3b, 4, and 17 (Casanova et al., 2006), these results suggest that humans have more space for connections between neuronal cell bodies in the prefrontal cortex compared to apes. These findings of a rostral to caudal gradient have recently been independently replicated (Spocter et al., 2012) using different specimens in an analysis of the neuropil fraction (which is similar to GLR) in area 10, Broca’s area (area 45), frontoinsular cortex, area 4, primary auditory cortex (area 41/42), and the planum temporale (area 22).
The present study focuses on the postnatal development of the spatial organization of cells in layer III in human and chimpanzee (Pan troglodytes) specimens ranging from birth to the juvenile period, in areas 10, 4, 3b, and 18. We sought to examine the developmental progression of changes in minicolumn dimensions and neuropil proportions that lead to the unique phenotype observed in adult humans, with increased space for interconnectivity in the prefrontal cortex (area 10) (Semendeferi et al., 2011). In both humans and chimpanzees, it has been shown that synaptogenesis occurs over the juvenile period and pruning of excess synapses takes place through adolescence, with a delay in the development of dendritic branching and spines in the prefrontal cortex relative to sensorimotor regions (Bianchi et al., in press; Huttenlocher and Dabholkar, 1997; Travis et al., 2005). Myelination likewise occurs later in prefrontal cortex than in other regions of both humans and chimpanzees, though overall myelin development is extended uniquely in humans beyond puberty (Miller et al., 2012). The major goal of the current study was to determine at what point the distinctive patterns of cortical neuron spatial organization observed in adult humans and chimpanzees emerges in development.
Material and Methods
Specimen and Tissue Preparation
The sample for the current study consisted of sixteen human specimens, ranging in age from birth to 11 years, and thirteen chimpanzees, ranging in age from birth to 11 years (Table 1). Chimpanzee specimens were collected from various research institutions, where they were housed according to each institution’s Animal Care and Use Committee guidelines and either died of natural causes or were euthanized humanely.
Table 1.
Specimens by species, name, sex, and age.
| Species | Specimen | Sex | Age |
|---|---|---|---|
| Human | RPSL-B-10-60* | F | Stillborn |
| RPSL-B-197-61* | F | Stillborn | |
| RPSL-B-210-61* | M | Stillborn | |
| RPSL-B-84-60* | F | Stillborn | |
| RPSL-B-138-61* | F | 14 days | |
| RPSL-W-160-64* | F | 48 days | |
| RPSL-B-123-61* | M | 7 months | |
| MU-93-65* | M | 8 months | |
| MU-89-65+ | M | 3.5 years | |
| MU-92-65+ | F | 4 years | |
| MU-108-66+, S | F | 4 years | |
| MU-94-65+, S | M | 5 years | |
| MU-91-65+, S | M | 6 years | |
| MU-90-65+ | M | 6.5 years | |
| MU-118-66+, S | F | 9.5 years | |
| MU-101-65+ | F | 11 years | |
| Chimpanzee | Brain 1* | M | Stillborn |
| Brain 2* | F | Stillborn | |
| Brain 3* | M | 1 day | |
| Brain 4* | M | 6 days | |
| Brain 5* | M | 10 days | |
| Brain 6* | M | 1 years | |
| Brain 7* | F | 1.5 years | |
| Brain 8* | F | 2 years | |
| Brain 9+ | M | 5 years | |
| Brain 10+ | M | 5 years, 4 months | |
| Brain 11+ | M | 6 years | |
| Brain 12+ | M | 9 years | |
| Brain 13+ | M | 11 years |
Indicates a specimen that was processed in the sagittal plane.
Indicates specimens included in the pre-weaning group.
Indicated specimens included in the post-weaning group.
All human and chimpanzee specimens were fixed postmortem in 10% formalin and sectioned at 35 or 40 µm thickness, respectively. All human specimens were held at the Yakovlev-Haleem Collection at the National Museum of Health and Medicine in Washington, DC. The human sections were from complete series of histologically processed brains and were stained with cresyl violet to identify Nissl substance. Chimpanzee samples were from dissected blocks collected from brain specimens. Blocks from the left hemisphere of approximately 3 cm for each region of interest were sectioned. As noted in Table 1 the majority of specimens were sliced in the coronal plane and four were sliced in the sagittal plane. We have previously shown that plane of cut has no effect on our minicolumn measurements (Semendeferi et al., 2011). In the human specimens, images were collected from the right hemisphere as consistently as possible; in cases of damage, the left hemisphere was sampled instead. We have no reason to suspect that the inclusion of data from both hemispheres would affect the findings; hemispheric asymmetry in minicolumn width and neuropil spacing was not found in our previous studies (Semendeferi et al., 2011; Spocter et al., 2012) and has, to date, only been found in the planum temporale (Chance et al., 2006), an area not included in the present study. Adult human and chimpanzee specimens used in a previous study (Semendeferi et al., 2011) and mentioned in the Discussion were sectioned at 20 µm, following a different processing protocol, with the exception of one chimpanzee that was sectioned at 15 µm. Absolute values in µm are directly comparable between species and cortical areas within each study, but not between studies.
Quantification of Spatial Organization
To identify the regions of interest (ROI) in all specimens, we employed a combination of topographical and cytoarchitectural criteria. The definitions supplied by Geyer and colleagues (Geyer et al., 1996; Geyer et al., 2000; Geyer et al., 1999) were used for both area 3 and area 4. Area 18 was defined using the work of Amunts and colleagues (Amunts et al., 2000; Amunts and Zilles, 2001). Delineation of area 10 was determined using previously published work that described its cytoarchitecture in humans and apes (Semendeferi et al., 1994; Semendeferi et al., 2001) as well as other sources (Kononova, 1938; Kononova, 1949; Kononova, 1955; von Economo and Koskinas, 1925; Sanides, 1962; Sanides, 1964)). While these studies identified some variability within the frontopolar cortex, they did not claim differences large enough to form separate cortical areas. Images of the frontal pole in the human brains were captured from throughout the extent of area 10, including its dorsal, medial and orbital surface. There were locations even in the most convoluted parts of the cortex where the minicolumnar formations could be seen, since the size of each captured ROI is 700–900×1100 µm. In the chimpanzee brains, area 10 was sampled from its dorsal surface. All parameters were measured in cortical layer III, an approach that matches that taken in our previous work (Buxhoeveden et al., 2001a; Schenker et al., 2008; Semendeferi et al., 2011). Layer III is a common focus of minicolumn analyses for several reasons: it typically displays the clearest and most visible linear organization; columns in layer III are generally representative, although not identical, of the size of a minicolumn throughout the depth of the cortex in adults (Buxhoeveden et al., 1996); and the supragranular layers play a critical role in transcolumnar and corticocortical processing.
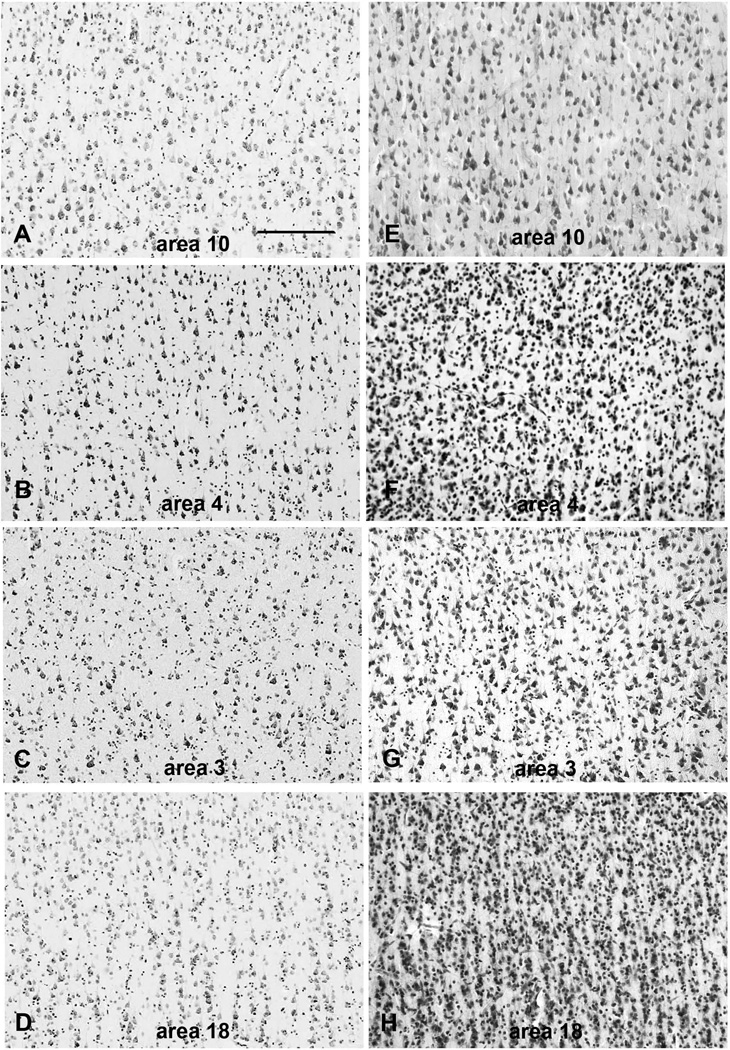
Three to six images from each ROI were taken (Fig. 1) and digitized. Images were captured in a consistent fashion from the walls of the gyrus where cell bodies tend to exhibit a more perpendicular orientation to the cortical surface, avoiding the sulcal depths and gyral crowns. We obtained scale calibration from the use of a micrometer photographed at the same resolution and magnification as the images. Photomicrographs for the chimpanzee specimens were captured using a Nikon H600L microscope with a 10x CFI Plan Aprochromat lens (N.A. 0.20), attached to a Dell workstation via an Optronics Microfire video camera. For the chimpanzee specimens, all images were coded before analysis and the rater was blinded to the specimen investigated. Photomicrographs for the human specimens were captured at the National Museum of Health and Medicine using an Olympus Provis AX80 Research Photomicrographic System Microscope attached to a Dell workstation via an Olympus DP70 camera. Due to the design of the Yakovlev collection, coding the human specimens was not possible and the rater was not blind. The collection of images from large segments of layer III depended on the quality of the tissue in the large human and chimpanzee brains; nevertheless, data collection was performed in a consistent manner.
Figure 1.
Photomicrographs of layer III in all four regions of interest in both human and chimpanzee specimens. (A–D). Human specimens. (E–H). Chimpanzee specimens. Images represent different developmental ages and individuals. Scale bar = 200 µm.
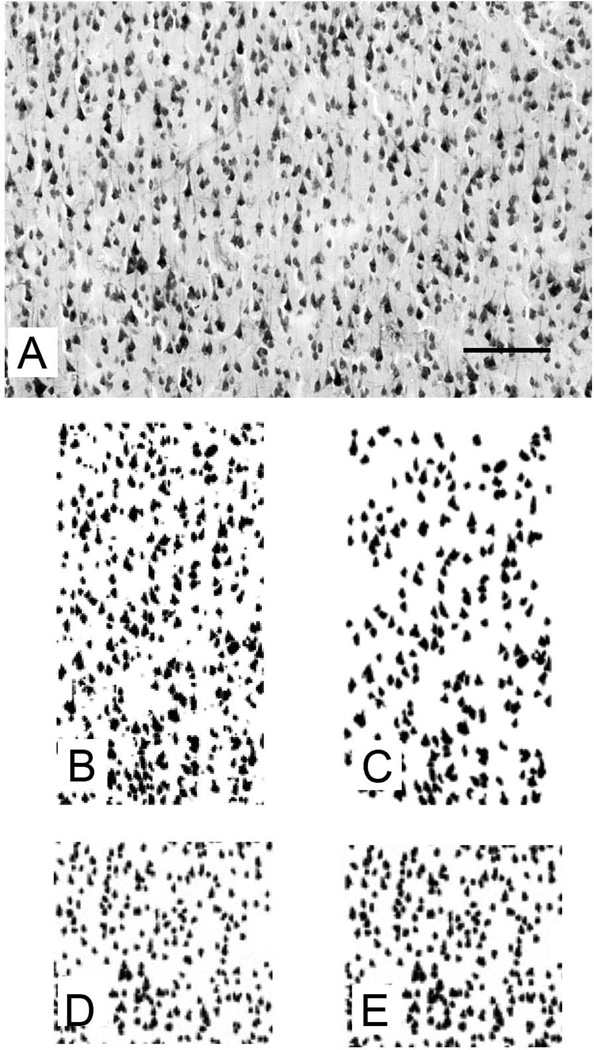
After being captured, each image was examined for artifacts such as blood vessels and uneven lighting that would affect the accuracy of the binary image. Images that had poor lighting or other artifacts were not analyzed. Each image was subjected to two processes (Fig. 2): thresholding (to exclude cells smaller than 20 pixels), and watershedding (for edge detection). The use of a threshold eliminated small cells, such as glia and smaller interneurons, and allowed us to focus on the pyramidal cells that comprise most of layer III. Afterwards, each image was converted into a binary image using Image J software that was modified by a method described elsewhere (Buxhoeveden et al., 2006). The program detects the location of cell bodies across the region of interest as it descends the ROI in the vertical plane. Human input was limited to the determination of the threshold level and the boundary of the ROI within each image. Photoshop CS5 was used to manipulate brightness and contrast levels in the published micrographs.
Figure 2.
Analytical processes used in the current study (A) Photomicrograph of layer III in chimpanzee area 10.(B) The digitized binary image is shown prior to the threshholding process. (C) The digitized binary image is shown after the thresholding process, which excludes cells smaller than 20 pixels. (D) Horizontal spacing distance (HSD) is calculated based on the edge-to-edge measures of cells in the horizontal axis, and provides a measure of the average spacing distance between cells. Gray level ratio (GLR) is the fraction of the converted binary image that is gray, which reveals how much of the image is occupied by stained cell bodies. Locations largely occupied by cells have high GLR values. HSD and GLR are generally correlated and when cells are located further away from each other, HSD tends to be higher and GLR lower. Nevertheless GLR is more sensitive than HSD to possible differences in cell size in addition to spacing of cells. (E) The binary image shown here comes from the exact same ROI shown in D, but the cells were artificially enlarged (fattened) to demonstrate the value of both measures. Despite the striking difference in appearance, the spacing of the cells as reflected in HSD remains identical between D and E, but GLR changes dramatically. A higher GLR value is indicative of greater density of neurons due to their number or their size, or combination thereof. Scale bar (on A) = 100 µm.
The two parameters used for this study are the horizontal spacing distance, HSD, and the gray level ratio or GLR. HSD provides the average spacing distance between neurons for the entire ROI, and was calculated based on the edge-to-edge measures of cells in the horizontal axis. GLR calculates the fraction of the converted binary image that is gray, which displays how much of the image is occupied by stained cell bodies (Buxhoeveden et al., 2001b). A higher GLR value is due to more neurons, larger neurons, or a combination of the two. GLR and HSD were calculated from the same binary image, but they were derived as independent variables. In general HSD and GLR correlate inversely; when cells are located further away from each other, HSD tends to be higher and GLR lower. Additionally, the GLR may be considered the inverse of the measure of neuropil fraction, which has been reported in other comparative studies of human and great ape neocortex (Spocter et al., 2012). However, on rare occasions HSD may not vary statistically significantly in the same region where GLR does or vice versa.
Together, these two parameters describe aspects of minicolumnar morphology and, therefore we used the term “minicolumn” throughout this paper. The term “wider minicolumns” refers to an increase in the horizontal spacing between neuronal cell bodies that, together with a decrease in GLR, is indicative of increased intracolumnar and intercolumnar neuropil space in layer III.
Analysis
To compare the developmental changes that occur in humans and chimpanzees, two species with different life history trajectories, the specimens were divided into two age groups: those younger than weaning age (pre-weaning), and those older than weaning age but not yet sexually mature (post-weaning). Average weaning age is 4.5 years for chimpanzee infants, and 2.8 years for human infants (Alvarez, 2000; Robson and Wood, 2008). All data analysis was performed using IBM SPSS Statistics 21 for Macintosh. Repeated measures ANOVA tests were performed on both HSD and GLR, with region as the within-subjects factor and both species (chimpanzee or human) and age group (pre-weaning or post-weaning) as between-subjects factors. For GLR, Greenhouse-Geisser corrections were used on all comparisons to correct for a lack of sphericity (Mauchly’s test, p = 0.033). Post-hoc analyses for both HSD and GLR were performed using a Bonferroni correction for multiple comparisons (p = 0.003125). P-values for F tests with degrees of freedom corrected by the Greenhouse-Geisser are given in the Results section.
Results
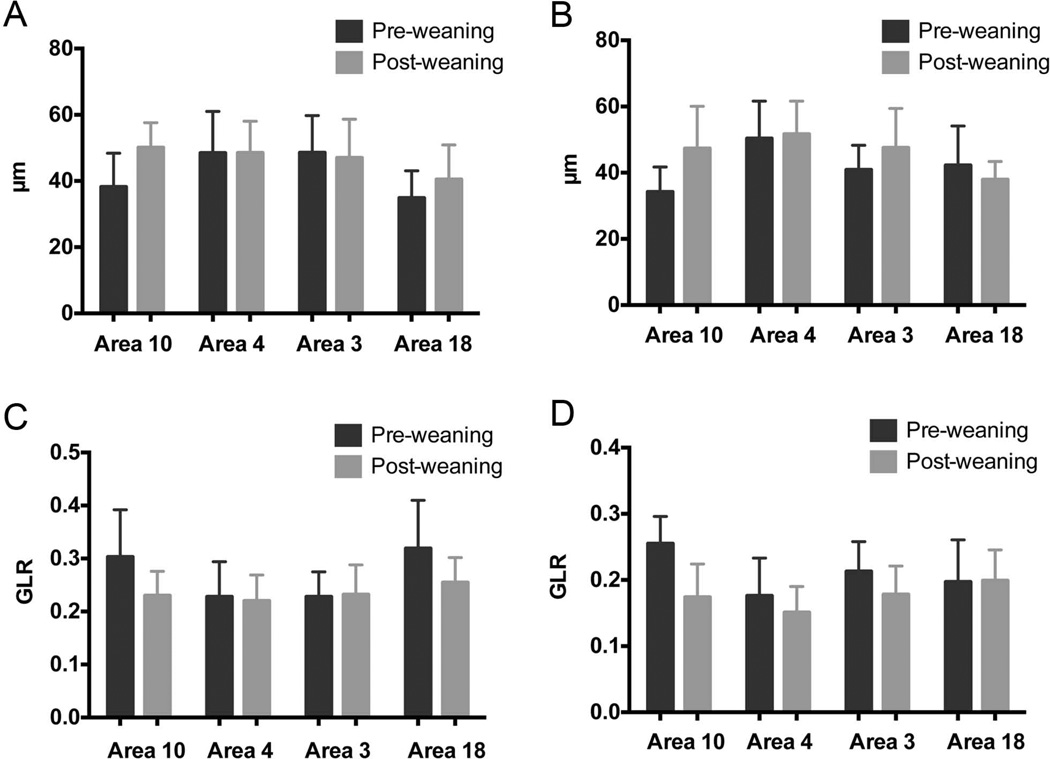
When data from both species and age groups were combined, there was a significant main effect for cortical region differences in HSD (F = 12.103, p = 0.000001, df = 3; Fig. 3A). Post hoc tests using a Bonferroni correction for multiple comparisons revealed that area 4 possessed significantly larger HSD than area 10, and area 18 possessed significantly smaller HSD than areas 10, 4, and 3b. There was also a significant main effect for cortical region differences in GLR (F = 9.001, p = 0.000017, df = 2.575; Fig. 3B). The post-hoc comparisons indicated that area 4 had smaller values for GLR than areas 10, 3b, and 18.
Figure 3.
(A) Average horizontal spacing distance (HSD) in human specimens, in both age groups, and for all four regions. (B) Average HSD in chimpanzee specimens, in both age groups, and for all four regions. (C) Average gray level ratio (GLR) in human specimens, in both age groups, and for all four regions. (D) Average GLR in chimpanzee specimens, in both age groups, and for all four regions. Note that HSD increases and GLR decreases in both species with age in area 10.
For HSD, the interaction effect between region and age group was significant (F = 3.960, p = 0.01, df = 3), as was the interaction among region, age group, and species (F = 2.755, p = 0.05, df = 3). Follow-up post hoc tests indicated that in humans, HSD in area 10 increases markedly after weaning (p = 0.001). In the pre-weaning specimens, mean HSD in area 10 was 38.23 µm, while mean HSD in the older post-weaning human specimens was 50.08 µm (Fig. 3 A). For GLR, the interactions both of region and species (F = 2.686, p = 0.049, df = 3) and of region, species, and age group (F = 2.665, p = 0.05, df = 3) were likewise significant. In the chimpanzees, spacing distance (as measured by both HSD and GLR), increased significantly in the frontal pole (area 10) after weaning (HSD p = 0.00022; GLR p = 0.000002) (Fig. 3 B C).
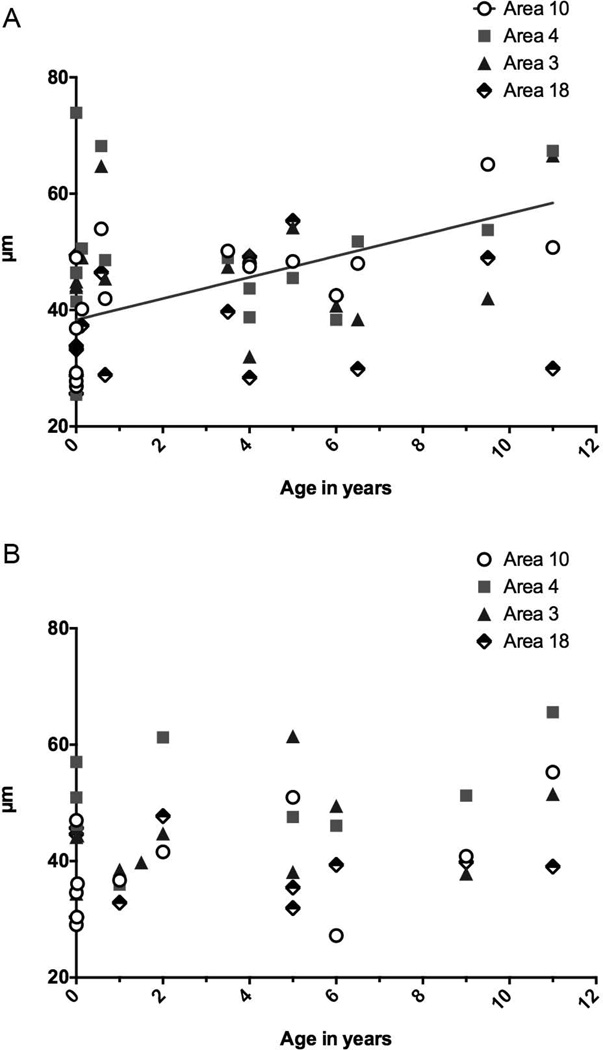
No other developmental differences were statistically significant in humans, and minicolumn size in area 4 and area 3b did not differ significantly between the pre-and post-weaning groups. Spacing distance in area 18 increased in humans by approximately 5 µm over development, though this was not statistically significant. As can be seen in Figure 4A, which plots the mean HSD values for every individual human specimen by chronological age, there is a trend for HSD in area 10 to increase with age, during the protracted development of the human frontal lobe. HSD in area 4 exhibits the most variance across specimens, with the most pronounced variability among the youngest specimens. HSD in areas 3b and 18 do not change much over the developmental period studied. We plotted linear, quadratic, and cubic curves for the data displayed in Figure 4A to determine best fit. Humans had a significant linear increase in HSD values with age in area 10 (r2 = 0.424, p = 0.006), but no other best-fit curves were significant.
Figure 4.
(A) Average horizontal spacing distance (HSD) for each individual human specimen, plotted against age in years or fractions thereof. Stillborns are listed as 0 years. (B) Average HSD for each individual chimpanzee specimen, plotted against age in years of fractions thereof. The linear best-fit curve for area 10 in humans is displayed on the graph, as it was the only significant best-fit curve in either species.
Similarly, no other developmental differences were significant in the chimpanzees, though spacing distance also increased slightly in the somatosensory cortex (area 3b). In the youngest chimpanzee specimens, those under weaning age, HSD was largest in area 4 (mean = 50.37 µm), followed by area 18 (mean = 42.24 µm), area 3b (40.88 µm), and then area 10 (mean = 34.23 µm); see Figure 3B. GLR was largest in area 10 (0.255), followed by area 3b (0.213), area 18 (0.197), and then area 4 (0.176; see Fig. 3D). In older chimpanzees, HSD was still largest in area 4 (51.64 µm in post-weaning juveniles). In Figure 4B, which plots the mean HSD values for every individual chimpanzee specimen by their chronological age, there was a trend for HSD in areas 10, 3b, and 4 to increase with age, though no best fit curves were significant for the data plotted in Figure 4B. In area 10, this trend of increasing HSD with age appears to be driven by the particularly low HSD values of the youngest, newborn specimens.
Discussion
Recent studies suggest that the human prefrontal cortex has increased space available for interneuronal connectivity compared to apes (Casanova and Tillquist, 2008; Schenker et al., 2008; Semendeferi et al., 2011; Spocter et al., 2012). In order to identify at what point in development this human specialization emerges, we examined the spatial organization of neurons in the frontal pole (area 10), primary motor (area 4), primary somatosensory (area 3), and prestriate visual cortex (area 18) in humans and chimpanzees, in specimens ranging from newborn to 11 years old.
Humans and chimpanzees, having diverged 6–7 million years ago (Cheng, 2007), are far more similar to each other in terms of brain development than they are to other living primates, according to comparative studies that examined a range of species (DeSilva and Lesnik, 2006; Leigh, 2004). At birth, the human brain is 26.9–29.5% of its adult size (meta-analysis from (DeSilva and Lesnik, 2006) while chimpanzees achieve 39.5–40.1% of their adult brain size by birth (DeSilva and Lesnik 2006; but see also (Vinicius, 2005). This is a marked departure from macaques, which are born with brains ~70% of the adult size (Passingham, 1982), and from whom humans diverged 30 million years ago (Cheng, 2007). In humans, adult brain size is reached between the ages of 5 to 7 years (Coqueugniot and Hublin, 2012; Leigh, 2004) and by age 4–5 in chimpanzees (Robson and Wood, 2008). Humans similarly share more in common cognitively and behaviorally with chimpanzees than they do with macaque monkeys (Matsuzawa, 2013).
Recent longitudinal magnetic resonance imaging (MRI) research suggests that in both species, most of early postnatal brain volume growth is attributable to increases in white matter (Sakai et al., 2011). Additionally, chimpanzees and humans share a long period of synaptogenesis (Bianchi et al., in press), which occurs in both species throughout the juvenile period; this pattern contrasts with macaques, in which synaptogenesis is completed in infancy (Rakic et al., 1986). This extended development is likely related to the importance of early social learning for developing proficiency at adult skills, which is evident in chimpanzees (Biro et al., 2003) as well as humans (Boyd et al., 2011; Tomasello, 1999).
The current study found some key developmental similarities between humans and chimpanzees. In both species, HSD in area 10 is considerably greater in post-weaning specimens than in the pre-weaning specimens. The later maturation of both association cortex and more rostral regions of the human cortex, particularly the frontal lobe, has been established by examining neuronal density (Shankle et al., 1999), dendritic and axonal growth (Schade and Van Groenou, 1961; Shankle et al., 1999; Travis et al., 2005), overall gray matter development (Gogtay et al., 2004), and cerebral energy metabolism (Chugani and Phelps, 1986). The present findings support this pattern, and further suggest that prolonged prefrontal development also characterizes chimpanzees, which is consistent with observations that dendrites of prefrontal pyramidal neurons of chimpanzees develop later than they do in sensorimotor cortices (Bianchi et al., in press). Importantly, regions with later development in humans are the very same regions that experienced the greatest degree of expansion in human evolution (Hill et al., 2010).
There were no significant differences between pre-and post-weaning specimens in either species in the other three regions studied; the visual, somatosensory, and motor cortices are known to develop earlier in the maturing human brain (Becker et al., 1984; Gogtay et al., 2004) and chimpanzee brain (Bianchi et al., in press). Thus, the developmental trajectory up through the juvenile period is prolonged for both species selectively in the frontal pole, and likely other association cortical regions. By the time of adulthood, dendrites in area 10 display more complex branching patterns in both chimpanzees and humans than they do in sensorimotor and visual cortices (Jacobs et al., 2001; Bianchi et al., 2012), which correlates with the finding that synaptogenesis occurs later in area 10 in both species (Bianchi et al., 2012; Travis et al., 2005), as regions with later development tend to have more complex dendritic branching (Jacobs et al., 1997; Jacobs et al., 2001).
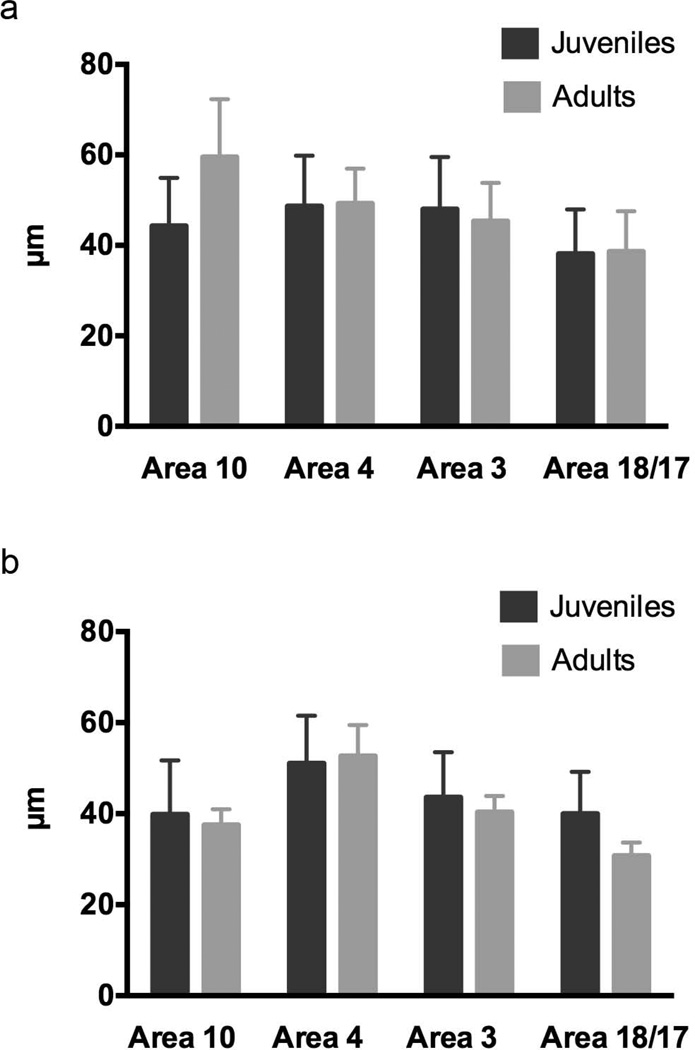
There are, however, important developmental differences between the species when the current study is interpreted in concert with other data on the timing of cortical maturation. In chimpanzees, myelination of area 10 is completed by sexual maturity, while in humans it continues past the time of adolescence (Miller et al., 2012). Additionally, because post-adolescent adult humans have significantly greater minicolumns spacing and neuropil fraction in area 10 than great apes (Semendeferi et al., 2011; Spocter et al., 2012), this implies that HSD continues to increase in humans after puberty, but does not in chimpanzees (Fig. 5). Only the GLR measurement in area 10 was significantly different between post-weaning humans and post-weaning chimpanzees. Furthermore, the expression of genes related to synaptogenesis in the prefrontal cortex peaks later in humans than in chimpanzees or macaque monkeys (Liu et al., 2012), suggesting that synaptic refinement may occur over a longer developmental period. Consistent with this idea, it has been reported that dendritic spines in the prefrontal cortex of humans continue the process of pruning into early adulthood, during the third decade of life (Petanjek et al., 2011). Such exceptionally slow and delayed neocortical maturation might be associated with the generally protracted growth of humans, who possess an evolutionarily distinct phase of slow childhood growth and a later adolescence growth spurt (Bogin, 2009; Crews and Bogin, 2010).
Figure 5.
(A) Average horizontal spacing distance (HSD) in all juvenile human specimens from the present study and for all adult human specimens in our previous study (Semendeferi et al., 2011). (B) Average horizontal spacing distance (HSD) in all juvenile chimpanzee specimens from the present study and for both adult chimpanzee specimens in our previous study. Note that the absolute values from our previous study (µm in y axis) are not directly comparable with the current study due to tissue processing differences (See Methods). Note also that area 17 rather than area 18 was examined in the previous study.
Neurons in areas that complete development later in post-adolescent adulthood, such as the frontal lobe, have more complex dendritic trees than those that mature earlier, such as area 4s and 18 (Jacobs et al., 1997; Jacobs et al., 2001; Travis et al., 2005). Similarly, it has been found that projections from layer III neurons in human prefrontal cortex have more branched and spiny dendritic arbors than in temporal, occipital, or parietal cortex (Elston et al., 2005; Elston and Rosa, 1997; Jacobs et al., 2001; Petanjek et al., 2008). The long-range, corticocortical projections of layer III neurons (Casanova, 2007; Hof et al., 1995; Lewis et al., 2002) in particular are thought to be critically involved in working memory and other higher-order cognitive processes in primates (Elston et al., 2006; Fuster, 2000a; Fuster, 2000b), suggesting that the reported differences in dendritic tree structure are related to the evolution of cognition (but see Zeba et al., 2008). The developmental pattern identified in this study is thus consistent with the later development and more complex dendrites of the human prefrontal cortex.
In recent years many studies have sought to determine the most functionally significant differences, as well as similarities, between human and chimpanzee brains. The human brain is roughly three times the size that of chimpanzees and other great apes, yet there are many intriguing similarities. Although the human and chimpanzee genomes only differ by a few percent (Mikkelsen et al., 2005; Preuss, 2012), this divergence still translates to hundreds of genetic differences, some of which might have functional consequences for neurodevelopment. Of note, one of these differences has been proposed to play a role in the prolonged development of the human prefrontal cortex; around 2.5 million years ago, when humans diverged from chimpanzees, SRGAP2, a gene related to slower dendritic and synaptic spine development, was duplicated (Dennis et al., 2012). While human and chimpanzee brains exhibit many similarities in their development throughout infancy and childhood, there are also differences, such as those identified in the current study, that might be involved in the divergence in cognitive capacities that are present in adults of each species Such examination, regarding species-specific developmental trajectories, can help to place the human brain in biological and evolutionary context. Altriciality in humans, characterized by relatively slow maturation, appears to be particularly evident in the prolonged development of the prefrontal cortex (Petanjek et al., 2011) and is likely related to the remarkable capacity for learning and communication of our own species.
Acknowledgments
Other acknowledgments: The authors wish to thank the Busch Gardens Zoo (Tampa Bay, FL), the Henry Doorly Zoo (Omaha, NE), the Milwaukee County Zoo (Milwaukee, WI) and the Yerkes National Primate Research Center (Atlanta, GA) for contributing ape brain specimens and the National Museum of Health and Medicine for access to the Yakovlev collection of human specimens. We also thank Natalie Schenker for advice regarding statistical analysis, Peter Frase for assistance with data processing, and Anthony Shirley for assistance with photographing the Yakovlev collection.
Grant sponsor: NSF; Grant numbers: BCS-0515484, BCS-0549117, and BCS-0824531;
Grant sponsor: NIH; Grant number: NS042867; Grant sponsor: the James S. McDonnell Foundation; Grant numbers: 22002078 and 220020293; Grant Sponsors: the Chancellor’s Interdisciplinary Collaboratory Scholarship, and the Kavli Institute for Brain and Mind at the University of California, San Diego.
Footnotes
Conflict of interest statement: None declared.
Statement of Authors contributions to the work:
Study concept and design: K.T., K.S., C.C.S., P.R.H., W.H.D., A.J.F., S.J.S., W.B.B., M.J.M. Acquisition of data: K.T., C.D.S. Statistical analysis: K.T., C.C.S. Analysis and interpretation of data: K.T., D.B., P.R.H., K.S., C.C.S. Drafting of the manuscript: K.T., K.S. Critical revision of the manuscript for important intellectual content: K.S., C.C.S., P.R.H., C.D.S., D.B., W.H.D., A.J.F., S.J.S., W.B.B., M.J.M. Administrative, technical, and material support: K.S., C.C.S., P.R.H., A.J.F., C.D.S., S.J.S, W.B.B., M.J.M., W.D.H.
Literature cited
- Allman JM, Hakeem A, Watson K. Two phylogenetic specializations in the human brain. Neuroscientist. 2002;8:335–346. doi: 10.1177/107385840200800409. [DOI] [PubMed] [Google Scholar]
- Alvarez HP. Grandmother hypothesis and primate life histories. Am J of Phys Anthropol. 2000;113:435–450. doi: 10.1002/1096-8644(200011)113:3<435::AID-AJPA11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann's areas 17 and 18 brought into stereotaxic space - Where and how variable? Neuroimage. 2000;1:66–84. doi: 10.1006/nimg.1999.0516. [DOI] [PubMed] [Google Scholar]
- Amunts K, Zilles K. Advances in cytoarchitectonic mapping of the human cerebral cortex. Neuroimaging Clin N Am. 2001;11:151–169. [PubMed] [Google Scholar]
- Barbas H. Pattern in the laminar origin of corticocortical connections. J Comp Neurol. 1986;252:415–422. doi: 10.1002/cne.902520310. [DOI] [PubMed] [Google Scholar]
- Becker LE, Armstrong DL, Chan F, Wood MM. Dendritic development in human occipital cortical neurons Dev Brain Res. 1984;1:117–124. doi: 10.1016/0165-3806(84)90083-x. [DOI] [PubMed] [Google Scholar]
- Bianchi S, Stimpson CD, Bauernfeind AL, Schapiro SJ, Baze WB, McArthur MJ, Bronson E, Hopkins WD, Semendeferi K, Jacobs B, Hof P, Sherwood CC. Dendritic morphology of pyramidal neurons in the chimpanzee neocortex: regional specializations and comparison to humans. Cereb Cortex in press. 2012 doi: 10.1093/cercor/bhs239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi S, Stimpson CD, Duka T, Larsen MD, Janssen WG, Collins Z, Bauernfeind AL, Schapiro SJ, Baze WB, McArthur MJ, Hopkins WD, Wildman DE, Lipovich L, Kuzawa CW, Jacobs B, Hof PR, Sherwood CC. Synaptogenesis and development of pyramidal neuron dendritic morphology in the chimpanzee neocortex resembles humans. Proc Natl Acad Sci USA in press. 2013 doi: 10.1073/pnas.1301224110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matsuzawa T. Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Anim Cognit. 2003;6:213–223. doi: 10.1007/s10071-003-0183-x. [DOI] [PubMed] [Google Scholar]
- Bogin B. Childhood, adolescence, and longevity: a multilevel model of the evolution of reserve capacity in human life history. Am J Hu Biol. 2009;21:567–577. doi: 10.1002/ajhb.20895. [DOI] [PubMed] [Google Scholar]
- Boyd R, Richerson PJ, Henrich J. The cultural niche: Why social learning is essential for human adaptation. Proc Natl Acad Sci USA. 2011;108:10918–10925. doi: 10.1073/pnas.1100290108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxhoeveden D, Lefkowitz W, Loats P, Armstrong E. The linear organization of cell columns in human and nonhuman anthropoid Tpt cortex. Anat Embryol. 1996;194:23–36. doi: 10.1007/BF00196312. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden DP, Casanova M. The minicolumn hypothesis in neuroscience. Brain. 2002;125:935–951. doi: 10.1093/brain/awf110. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden DP, Switala AE, Roy E, Litaker M, Casanova MF. Morphological differences between minicolumns in human and nonhuman primate cortex. Am J Phys Anthropol. 2001a;115:361–371. doi: 10.1002/ajpa.1092. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden DR, Hasselrot U, Buxhoeveden NE, Booze RM, Mactutus CF. Microanatomy in 21 day rat brains exposed prenatally to cocaine. Int J Dev Neurosci. 2006;24:335–341. doi: 10.1016/j.ijdevneu.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden DR, Switala AE, Litaker M, Roy E, Casanova MF. Lateralization of minicolumns in human planum temporale is absent in nonhuman primate cortex. Brain Behav Evol. 2001b;57:349–358. doi: 10.1159/000047253. [DOI] [PubMed] [Google Scholar]
- Casanova MF. The neuropathology of autism. Brain Pathol. 2007;17:422–433. doi: 10.1111/j.1750-3639.2007.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Tillquist CR. Encephalization, emergent properties, and psychiatry: A minicolumnar perspective. Neuroscientist. 2008;14:101–118. doi: 10.1177/1073858407309091. [DOI] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IAJ, Switala AE, van Engeland H, Heinsen H, Steinbusch HWM, Hof PR, Trippe J, Stone J, Schmitz C. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Chance SA, Casanova MF, Switala AE, Crow TJ, Sn Minicolumnar structure in Heschl's gyrus and planum temporale: Asymmetries in relation to sex and callosal fiber number. Neuroscience. 2006;143:1041–1050. doi: 10.1016/j.neuroscience.2006.08.057. [DOI] [PubMed] [Google Scholar]
- Chen C-H, Gutierrez ED, Thompson W, Panizzon MS, Jernigan TL, Eyler LT, Fennema-Notestine C, Jak AJ, Neale MC, Franz CE, Lyons MJ, Grant MD, Fischl B, Seidman LJ, Tsuang MT, Kremen WS, Dale AM. Hierarchical genetic organization of human cortial surface area. Science. 2012;335:1634–1636. doi: 10.1126/science.1215330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. Visualizing columnar architectures using high-field fMRI. Prog Nat Sci. 2007;17:19–23. [Google Scholar]
- Chugani HT, Phelps ME. Maturational changes in cerebral function in infants determined by F-18-DG positron emission tomography. Science. 1986;231:840–843. doi: 10.1126/science.3945811. [DOI] [PubMed] [Google Scholar]
- Coqueugniot H, Hublin JJ. Age-related changes of digital endocranial volume during human ontogeny: Results from an osteological reference collection. Amer J Phys Anthropol. 2012;147:312–318. doi: 10.1002/ajpa.21655. [DOI] [PubMed] [Google Scholar]
- Crews DE, Bogin B. Growth, development, senescence, and aging: a life history perspective. In: Larsen CS, editor. A Companion to Biological Anthropology. Hoboken, NJ: Blackwell Publishing Limited; 2010. pp. 124–152. [Google Scholar]
- DeFelipe J. Reflections of the structure of the cortical minicolumn. In: Casanova M, editor. Neocortical Modularity and the Cell Minicolumn. New York: Nova Biomedical; 2005. pp. 57–92. [Google Scholar]
- DeFelipe J, Alonso-Nanclares L, Arellano J, Ballesteros-Yanez I, Benavides-Piccione R, Munoz A. Specializations of the cortical microstructure of humans. In: Preuss T, editor. Evolution of Neural Systems. Amsterdam: Elsevier; 2007. pp. 167–190. [Google Scholar]
- Dennis MY, Nuttle X, Sudmant PH, Antonacci F, Graves TA, Nefedov M, Rosenfeld JA, Sajjadian S, Malig M, Kotkiewicz H, Curry CJ, Shafer S, Shaffer LG, de Jong PJ, Wilson RK, Eichler EE. Evolution of human-specific neural SRGAP2 genes by incomplete segmental duplication. Cell. 2012;149:912–922. doi: 10.1016/j.cell.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilva J, Lesnik J. Chimpanzee neonatal brain size: Implications for brain growth in Homo erectus. J Hum Evol. 2006;51:207–212. doi: 10.1016/j.jhevol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, DeFelipe J. A study of pyramidal cell structure in the cingulate cortex of the macaque monkey with comparative notes on inferotemporal and primary visual cortex. Cereb Cortex. 2005;15:64–73. doi: 10.1093/cercor/bhh109. [DOI] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, Elston A, Zietsch B, Defelipe J, Manger P, Casagrande V, Kaas JH, Xu Specializations of the granular prefrontal cortex of primates: Implications for cognitive processing. Anat Rec. 2006;288A:26–35. doi: 10.1002/ar.a.20278. [DOI] [PubMed] [Google Scholar]
- Elston GN. Specialization of the neocortical pyramidal cell during primate evolution. In: Preuss TM, editor. Evolution of nervous systems: a comprehensive reference. Volume 4. Primates: Amsterdam:Elsevierp; 2007. pp. 191–242. [Google Scholar]
- Elston GN, Rosa MGP. The occipitoparietal pathway of the macaque monkey: Comparison of pyramidal cell morphology in layer III of functionally related cortical visual areas. Cereb Cortex. 1997;7:432–452. doi: 10.1093/cercor/7.5.432. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Cortical dynamics of memory. Int J Psychophysiol. 2000a;35:155–164. doi: 10.1016/s0167-8760(99)00050-1. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Executive frontal functions. Exp Brain Res. 2000b;133:66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- Galuske RAW, Schlote W, Bratzke H, Singer W. Interhemispheric asymmetries of the modular structure in human temporal cortex. Science. 2000;289:1946–1949. doi: 10.1126/science.289.5486.1946. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Zilles K. Functional neuroanatomy of the primate isocortical motor system. Anat Embryol. 2000;202:443–474. doi: 10.1007/s004290000127. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex 1. Microstructural organization and interindividual variability. Neuroimage. 1999;10:63–83. doi: 10.1006/nimg.1999.0440. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci USA. 2010;107:13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Nimchinsky EA, Morrison JH. Neurochemical phenotype of corticocortical connections in the macaque monkey: quantitative analysis of a subset of neurofilament protein-immunoreactive projection neurons in frontal, parietal, temporal, and cingulate cortices. J Comp Neurol. 1995;362:109–133. doi: 10.1002/cne.903620107. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: A quantitative Golgi study. J Comp Neurol. 1997:661–680. [PubMed] [Google Scholar]
- Jacobs B, Schall M, Prather M, Kapler E, Driscoll L, Baca S, Jacobs J, Ford K, Wainwright M, Treml M. Regional dendritic and spine variation in human cerebral cortex: a quantitative Golgi study. Cereb Cortex. 2001;11:558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- Kana RK, Libero LE, Moore MS. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys Life Reviews. 2011;8:410–437. doi: 10.1016/j.plrev.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Kononova EP. Variability of the structure of the cerebral cortex. The frontal region of adult human 2. Communication. Trudy Instituta Mozga. 1938:213–274. [Google Scholar]
- Kononova EP. Frontal region. In: Sarkissov SA, Fillimonoff IN, Preobrazenskaja NS, editors. Cytoarchitectonics of the cerebral cortex of man. Moscow: Medgiz; 1949. pp. 309–343. [Google Scholar]
- Kononova EP. Frontal region. In: Sarkissov SA, Fillimonoff IN, Kononova EP, editors. Atlas of the cytoarchitectonics of the cerebral cortex. Moscow: Medgiz; 1955. pp. 108–167. [Google Scholar]
- Leigh SR. Brain growth, life history, and cognition in primate and human evolution. Am J Primatol. 2004;62:139–164. doi: 10.1002/ajp.20012. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Burgos GG. Specificity in the functional architecture of primate prefrontal cortex. J Neurocytol. 2002;31:265–276. doi: 10.1023/a:1024174026286. [DOI] [PubMed] [Google Scholar]
- Liu X, Somel M, Tang L, Yan Z, Jiang X, Guo S, Yuan Y, He L, Oleksiak A, Zhang Y, Li N, Hu Y, Chen W, Qiu Z, Paabo S, Khaitovich P. Extension of cortical synaptic development distinguishes humans from chimpanzees and macaques. Genome Res. 2012;22:611–622. doi: 10.1101/gr.127324.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa T. Evolution of the brain and social behavior in chimpanzees. Curr Opin in Neurobiol. 2013;23:1–7. doi: 10.1016/j.conb.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Hillier LW, Eichler EE, Zody MC, Jaffe DB, Yang SP, Enard W, Hellmann I, Lindblad-Toh K, Altheide TK. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2005;437:69–87. doi: 10.1038/nature04072. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AMM, Sestan N, Wildman DE, Lipovich L, Kuzawa CW, Hof PR, Sherwood CC. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci USA. 2012;109:16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Passingham RE. The human primate. San Francisco: Freeman; 1982. [Google Scholar]
- Petanjek Z, Judas M, Kostovic I, Uylings HBM. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: A layer-specific pattern. Cereb Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HBM, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sethares C. Myelinated axons and the pyramidal cell modules in monkey primary visual cortex. J Comp Neurol. 1996;365:232–255. doi: 10.1002/(SICI)1096-9861(19960205)365:2<232::AID-CNE3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Human brain evolution: From gene discovery to phenotype discovery. Proc Natl Acad Sci USA. 2012;109:10709–10716. doi: 10.1073/pnas.1201894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind - a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldmanrakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Rakic P, Kornack D. The development and evolutionary expansion of the cerebral cortex in primates. In: Preuss TM, editor. Evolution of Nervous Systems. Volume 4. Primates: Amsterdam:Elsevierp; 2007. pp. 243–259. [Google Scholar]
- Robson SL, Wood B. Hominin life history: reconstruction and evolution. J Anat. 2008;212:394–425. doi: 10.1111/j.1469-7580.2008.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Mikami A, Tomonaga M, Matsui M, Suzuki J, Hamada Y, Tanaka M, Miyabe-Nishiwaki T, Makishima H, Nakatsukasa M, Matsuzawa T. Differential prefrontal white matter development in chimpanzees and humans. Curr Biol. 2011;21:1397–1402. doi: 10.1016/j.cub.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Sanides F. Monograf für Neurologie und Psychologie. Vol. 98. Berlin: Springer; 1962. Die Architektonik des Menschlichen Stirnhirns; pp. 1–203. [Google Scholar]
- Sanides F. The cyto-myeloarchitecture of the human frontal lobe and its relation to phylogenetic differentiation of the cerebral cortex. J Hirnforsch. 1964;6:269–292. [PubMed] [Google Scholar]
- Schade JP, Van Groenou WB. Structural organization of human cerebral cortex. Acta Anatomica. 1961;47:74–111. doi: 10.1159/000141802. [DOI] [PubMed] [Google Scholar]
- Schenker NM, Buxhoeveden DP, Blackmon WL, Amunts K, Zilles K, Semendeferi K. A comparative quantitative analysis of cytoarchitecture and minicolumnar organization in Broca's area in humans and great apes. J Comp Neurol. 2008;510:117–128. doi: 10.1002/cne.21792. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Armstrong E, Ciochon R, Damasio H, Van Hoesen G. Evolution of the hominoid prefrontal cortex: Imaging analysis of areas 10 and 13. Am J Phys Anthropol Suppl. 1994;18:179. [Google Scholar]
- Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: A comparative study of area 10. Am J Phys Anthropol. 2001;114:224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Teffer K, Buxhoeveden D, Park MS, Bludau S, Amunts K, Travis K, Buckwalter J. Spatial organization of neurons in the frontal pole sets humans apart from great apes. Cereb Cortex. 2011;21:1485–1497. doi: 10.1093/cercor/bhq191. [DOI] [PubMed] [Google Scholar]
- Shankle WR, Rafii MS, Landing BH, Fallon JH. Approximate doubling of numbers of neurons in postnatal human cerebral cortex and in 35 specific cytoarchitectural areas from birth to 72 months. Ped Dev Pathol. 1999;2:244–259. doi: 10.1007/s100249900120. [DOI] [PubMed] [Google Scholar]
- Spocter MA, Hopkins WD, Barks SK, Bianchi S, Hehmeyer AE, Anderson SM, Stimpson CD, Fobbs AJ, Hof PR, Sherwood CC. Neuropil distribution in the cerebral cortex differs between humans and chimpanzees. J Comp Neurol. 2012;520:2917–2929. doi: 10.1002/cne.23074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M. The human adaptation for culture. Ann Rev Anthropol. 1999;28:509–529. [Google Scholar]
- Travis K, Ford K, Jacobs B. Regional dendritic variation in neonatal human cortex: A quantitative Golgi study. Dev Neurosci. 2005;27:277–287. doi: 10.1159/000086707. [DOI] [PubMed] [Google Scholar]
- Vinicius L. Human encephalization and developmental timing. J Hum Evol. 2005;49:762–776. doi: 10.1016/j.jhevol.2005.08.001. [DOI] [PubMed] [Google Scholar]
- von Economo CV, Koskinas GN. Die Cytoarchitectonik der Hirnrinde des erwachsenen Menschen. Berlin: Julius Springer; 1925. [Google Scholar]
- Yabuta NH, Callaway EM. Cytochrome-oxidase blobs and intrinsic horizontal connections of layer 2/3 pyramidal neurons in primate V1. Vis Neurosci. 1998;15:1007–1027. doi: 10.1017/s0952523898156018. [DOI] [PubMed] [Google Scholar]
- Zeba M, Jovanov-Milosevic N, Petanjek Z. Quantitative analysis of basal dendritic tree of layer IIIc pyramidal neurons in different areas of adult human frontal cortex. Coll Antropol. 2008;32:161–169. [PubMed] [Google Scholar]