Abstract
SALL4 transcription factor is associated with embryonic cell pluripotency and has been shown as a useful immunohistochemical marker for germ cell tumors. However, information of SALL4 distribution in normal human tissues and non germ-cell tumors is limited. In this study we examined normal human tissues and 3215 tumors for SALL4 expression using a monoclonal antibody 6E3 and automated immunohistochemistry. In a 10th week embryo, SALL4 was expressed in ovocytes, intestine, kidney, and some hepatocytes. In adult tissues, it was only detected in germ cells. SALL4 was consistently expressed in all germ cell tumors except some trophoblastic tumors and mature components of teratomas, where it was selectively expressed in intestinal-like and some squamous epithelia. In non germ-cell carcinomas, SALL4 was detected in 20% of cases or more of serous carcinoma of ovary, urothelial high-grade carcinoma, and gastric adenocarcinoma (especially the intestinal type). SALL4 was only rarely (≤5%) expressed in mammary, colorectal, prostatic, and squamous cell carcinomas. Many SALL4 positive carcinomas showed poorly differentiated patterns and some showed positivity in most tumor cells mimicking the expression in germ cell tumors. SALL4 was commonly expressed in rhabdoid tumors of kidney and extrarenal sites, and in Wilms tumor. Expression of SALL4 was rare in other mesenchymal and neuroendocrine tumors but was occasionally detected in melanoma, desmoplastic small round cell tumor, epithelioid sarcoma, and rhabdomyosarcoma. All hematopoietic tumors were negative. SALL4 is an excellent marker of non-teratomatous germ cell tumors, but it is also expressed in other tumors, sometimes extensively. Such expression may reflect stem-cell like differentiation and must be considered when using SALL4 as a marker for germ cell tumors. Observed lack of other pluripotency factors, OCT4 and NANOG, in SALL4-positive non-germ cell tumors can also be diagnostically helpful.
Keywords: SALL4, germ cell tumor, serous carcinoma, urothelial carcinoma, gastric adenocarcinoma, cholangiocarcinoma, small cell carcinoma, immunohistochemistry, OCT4, NANOG
INTRODUCTION
Sal-like protein 4 (SALL4) is a zinc-finger transcription factor expressed in embryonic stem cells and important during embryonic development, as studied in murine embryos.1 SALL4 is a master regulator of embryonal pluripotency and forms a regulatory network with other pluripotency-related transcription factors OCT4 (POU5F1) and NANOG. 2-4 SALL4 is important for early development and homozygous loss-of-function mutants are embryonic lethal. 5 Haploinsufficiency true truncating heterozygous mutations causes Okihiro syndrome (closely related if not same as Duane-radial ray syndrome and acro-renal-ocular syndrome) associated with ray defects involving thumbs and other parts of limbs, deficient eye movements, renal malformations, and deafness. 6,7 On the other hand, induced overexpression causes acute myeloid leukemia in mice indicating oncogenic potential. 8
Whereas SALL4 is highly expressed in early embryo, SALL4 gene is switched off during mouse development, remaining expressed into adulthood only in germ cells. 9 Although known to be expressed in adult germ cells such as ovocytes 10 and spermatogonia 11, its distribution in human tissues is incompletely characterized. Immunohistochemical detection of SALL4 protein has been suggested useful in the detection of germ cell tumors of the testis, ovary, mediastinum and at metastatic sites. 11-14 However, only small numbers of non-germ cell tumors were evaluated in these studies.
SALL4 has also been detected in some non germ-cell carcinomas, such as subsets of gastric carcinomas 15, but many studies on carcinomas have been based on RNA expression and not on tissue immunohistochemistry. 16,17
In this study we systematically evaluated human normal tissues and 3215 epithelial, mesenchymal, neuroectodermal, and hematolymphoid neoplasms to determine the tissue distribution of SALL4 and evaluated its specificity for germ cell tumors. SALL4-positive tumors were further evaluated with two other pluripotency markers: OCT4, and NANOG.
MATERIALS AND METHODS
Normal tissues and the 3215 tumors were derived from surgical specimens. The tumors analyzed in this study were arranged in hand-made multitumor blocks containing 30-60 tumors each, as previously described. 18 Various tumors used for this study were extensively immunohistochemically characterized.
Immunohistochemical staining starting from deparaffinization and ending with hematoxylin counterstaining was performed in Leica Bond automated immunostainer. The mouse monoclonal antibody to SALL4 (clone 6E3) was obtained from Biocare Medical, Concord, CA and was diluted 1:200. Mouse monoclonal antibody OCT3/4 (clone N1NK) was obtained from Novocastra/Leica, Bannockburn, IL and diluted 1:100. Rabbit monoclonal antibody to NANOG (clone D73G4) was obtained from Cell Signaling Technology, Danvers, MA and diluted 1:750. Application of each of the primary antibodies was preceded by heat induced epitope retrieval in Leica Bond using Leica high-pH buffer (25 min). The primary antibody was incubated for 30 min. Testicular seminoma slide containing 20 cases was used as a positive control.
The results were scored for estimated percentage of positive cells. Immunostaining limited to sporadic positive nuclei was disregarded out of caution based on experience that such staining in sporadic cells may be obtained by the epitope retrieval only. SALL4 did not yield significant cytoplasmic staining and cytoplasmic staining only was not considered positive. Selected SALL4-positive tumors were also evaluated for two other pluripotency markers: OCT3/4, and NANOG. For these markers, seminoma was used as a positive control and only nuclear staining was scored.
RESULTS
Normal tissues
In a 10-week-old fetus, SALL4-positive elements included germ cells of a gonad (probably ovary) which were strongly positive, primitive renal tubules, early glomerular epithelia, intestinal epithelia, and approximately 25% of hepatocytes. The latter showed weaker yet still distinct positivity. Skin, mesothelia, and all mesenchymal and neural tissues (brain, spinal cord) were negative.
In normal adult tissues, positivity was detected only in germ cells (spermatogonia of testis), whereas skin, breast, salivary gland, respiratory and gastrointestinal epithelia, liver, pancreas, prostate, endometrium, thyroid, and squamous epithelia of the oral region were negative, as were mesenchymal, neural, and lymphoid tissues.
Germ cell tumors
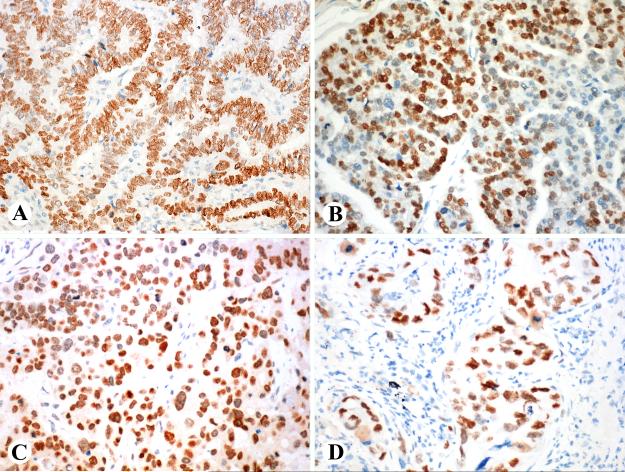
SALL4 expression in germ cells tumors is shown in Table 1. All seminomas (n= 85) were strongly and nearly uniformly positive (Fig. 2A), except one tumor, which was focally positive (10% of tumor cells). All embryonal carcinomas (n = 30) and yolk sac tumors (n = 9) or such components in combined tumors were strongly positive with practically uniform nuclear staining (Fig. 2B). Trophoblastic components of testicular germ cell tumors (n = 4) and uterine choriocarcinomas (n = 3) also contained positive cells, but the positivity varied so that larger cells including the syncytiotrophoblastic elements were typically negative (Fig. 2C). One testicular choriocarcinoma and one placental site trophoblastic tumor were entirely negative.
Fig. 2.
SALL4 in germ cell tumors. A, Seminoma cells are strongly positive but lymphocyte-rich septa are negative. B. Yolk sac tumor epithelium is strongly positive. C. In a trophoblastic component of a testicular germ cell tumor, small trophoblasts are positive but the larger cells including syncytiotrophoblasts are negative. D. In mature components of teratoma, intestinal epithelia is uniformly positive and squamous epithelia focally positive.
In differentiated components of testicular teratomas (all adult patients), the SALL4-positivity varied. In all, 6/10 cases contained positive cells, often in low numbers. Positive elements included mature-appearing intestinal goblet cell-containing epithelia and isolated squamous epithelial cells (Fig. 2D). Stromal connective tissues, smooth muscle, and cartilage were negative.
Non germ-cell carcinomas
Expression of SALL4 in epithelial neoplasms is summarized in Table 1. Ovarian serous carcinoma had the highest frequency of SALL4-positive cases among any specific non-germ cell carcinomas (23/78, 29%). Although most of the positive cases were high-grade carcinomas, also a serous tumor of low malignant potential was positive with extensive staining of the epithelia (Fig. 3A). The percentage of SALL4-positive cells varied 5-70% (median, 20%). Five cases had 50% or more of SALL4-positive tumor cells. All ovarian endometrioid and clear cell carcinomas were negative.
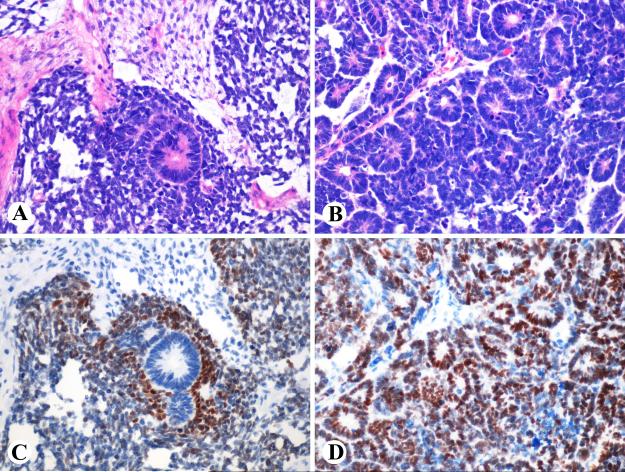
Fig. 3.
A. Ovarian serous tumor of low malignant potential, B. Urothelial high-grade in situ carcinoma, C. poorly differentiated endometrial carcinoma with a solid pattern, and D. Stroma of sarcomatoid endometrial carcinoma are SALL4-positive.
Urothelial carcinomas contained SALL4-positive cells in 21/96 cases (3-100% of positive cells, median 25%). All positive cases were high grade tumors (20 invasive, 1 in situ carcinoma) (Fig. 3B).
Endometrial carcinomas were SALL4-positive in 8/114 cases (7%). One of these cases was mismatch repair-deficient tumor (MLH1-, PMS2-). All positive examples were high-grade tumors often showing rudimentary if any glandular differentiation (Fig. 3C). In one case, sarcomatoid stroma was positive and glandular elements negative (Fig. 3D).
Gastric adenocarcinomas showed SALL4-positive cells in 24/102 cases (24%). The percentage of positive cells varied 3-100% (median, 25%). A great majority of positive tumors had intestinal-like differentiation (Fig. 4A) and at least focally expressed CDX2 (19/24) and keratin 20 (19/23). One hepatoid carcinoma (Fig. 4B) was also strongly SALL4-positive (100%), whereas diffuse signet ring cell carcinomas were only rarely positive (1/22).
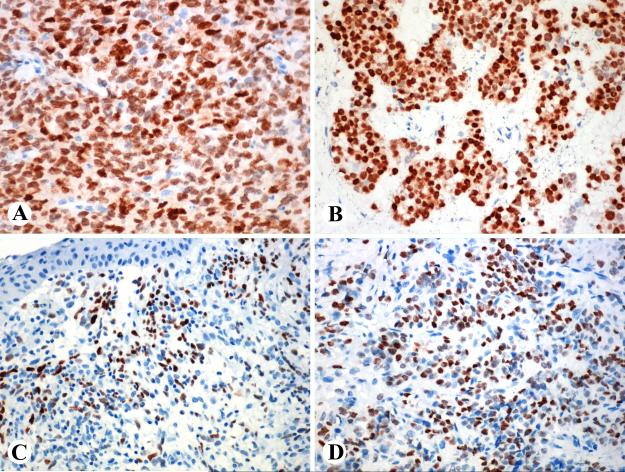
Fig. 4.
SALL4-positive carcinomas. A. Gastric carcinoma of intestinal type. B. Gastric hepatoid carcinoma. C. Poorly differentiated pancreatic adenocarcinoma with a solid pattern. D. Pulmonary adenocarcinoma with acinar differentiation.
Pancreatic adenocarcinomas contained SALL4-positive cells in 7/93 cases (8%). Four moderately differentiated gland-forming tumors had 5-20% of positive cells, and three poorly differentiated tumors forming solid sheets, including one sarcomatoid example, had 20, 50, and 100% of positive tumor cells (Fig. 4C).
Colorectal adenocarcinomas were only rarely positive (9/305, 3%). The number of positive cells varied 5-100% (median, 28%). Of the positive cases, 3 were liver metastases and they had 5%, 60% and 100% of tumor cells positive. Only one of the positive cases was mismatch repair-deficient (negative for MLH1 and PMS2).
Pulmonary adenocarcinomas contained SALL4-positive cells in 8/140 cases (6%), often in large numbers (Fig. 4D). Most examples were poorly differentiated tumors and the content of positive cells varied 10-100% (median, 75%). Pulmonary small cell carcinomas contained SALL4-positive cells in 5/26 cases (19%), with 5-30% of positive cells (median, 5%).
Mammary ductal carcinomas were only rarely SALL4-positive (5/208, 2%). Two cases contained 5% and two had 20% of positive nuclei, and in one case, all tumor cell nuclei were positive. Four of these cases were positive for estrogen receptor and also strongly positive (3+) for HER2 with uniform membrane labeling, whereas one case was triple negative (ER-, PR-, HER2-). All lobular carcinomas were negative.
Renal carcinomas of the common types were uniformly negative. Squamous cell carcinomas of different locations all showed a low frequency of SALL4-expression detected in <5% of cases in each category. All positive examples were poorly differentiated, nonkeratinizing squamous cell carcinomas.
Metastatic carcinomas of unknown origin showed the highest frequency of any non-germ-cell tumor (5/16, 31%). These keratin-positive tumors were composed of solid sheets of cohesive epithelial cells without gland formation. One case was also positive for OCT4 (a possible germ cell tumor). These tumors lacked “organ-specific” markers such as TTF1, CDX2, and GATA3.
A representative number of SALL4-positive non-germ cell epithelial neoplasms examined for OCT4 (n = 80) and NANOG (n = 76) were all negative. Whereas OCT4 immunostaining also lacked cytoplasmic staining, several cases showed variable, often strong cytoplasmic NANOG-positivity without nuclear immunoreactivity.
Non-epithelial and unclassified neoplasms
A renal rhabdoid tumor from a 27 year-old man had 30% tumor cells positive. This tumor was also INI-1 deficient (Fig. 5). Two extrarenal rhabdoid tumors in children were similarly SALL4-positive. Wilms tumors were variably SALL4-positive. In some cases, only tubular elements were positive (Fig. 6A,B), whereas in some “triphasic” examples, the positivity was restricted to blastema while epithelial tubules and stroma were negative (Fig. 6 C,D, Table 2).
Fig 5.
A, B. A renal rhabdoid tumor has inclusion-like eosinophilic cytoplasm. Tumor cells are partly SALL4-positive and they show loss of INI1.
Fig. 6.
Two examples of Wilms tumor. A. A triphasic tumor with epithelial tubules, purple staining blastema, and pale staining stroma (middle up). B. A tumor predominantly consisting of epithelial tubules. C. The triphasic tumor shows SALL4-expression in the blastema, whereas the epithelial tubules and stroma are negative. D. In the example with tubular differentiation, tubular epithelia are positive.
Other mesenchymal, neuroectodermal, and lymphoid tumors were almost uniformly negative for SALL4 (Table 2). However, two metastatic malignant melanomas (3% of all cases), both positive for S100 protein and at least focally for HMB45, and MelanA, contained 50% and nearly 100% of SALL4-positive tumor cells (Fig. 7A). One desmoplastic small round cell tumor positive for keratins and desmin was also extensively SALL4-positive (Fig. 7B), as was an embryonal rhabdomyosarcoma of uterine cervix (Fig. 7C) and an epithelioid sarcoma (Fig. 7D).
Fig. 7.
A. Metastatic melanoma and B. Desmoplastic stromal tumor are here strongly SALL4-positive, a rare event among these tumors. C. Uterine cervical embryonal rhabdomyosarcoma (botryoid rhabdomyosarcoma) has a high number of SALL4-positive cells in the subepithelial zone. D. An exceptionally SALL4-positive epithelioid sarcoma.
DISCUSSION
In this study we examined the expression of SALL4, a pluripotency-related transcription factor in normal and neoplastic human tissues in order to determine its specificity for germ cell tumors and discover possible non-germ cell targets for diagnostic immunohistochemistry. SALL4 is expressed in germ cells and is an excellent marker for malignant germ cell tumors in that most of these tumors are strongly and uniformly positive, a feature less commonly found in non germ-cell tumors.
In our study, seminomas, embryonal carcinomas, and yolk sac tumors were equally positive, as previously reported. 10-14 However, SALL4 does not seem to be expressed in all trophoblastic tumors, based on our relatively small sample. Although it is at least focally detected in all choriocarcinomas, it is absent in larger, perhaps more differentiated cells, and especially syncytiotrophoblasts. Therefore, negative results do not necessarily rule out trophoblastic differentiation.
Differentiated components (studied here in adult testicular germ cell tumors) showed limited SALL4-positivity indicating that SALL4 is no longer present during somatic differentiation. The teratoma components that were exceptionally positive include intestinal like epithelia, and this mirrors the fetal SALL4-expression in intestinal epithelia. Absence of SALL4 in many germ cell tumor elements with somatic differentiation also indicates that this marker may not be able to detect somatic derivatives of germ cell tumors, such as squamous cell carcinomas originating from teratomas.
Based on our observations, SALL4 is by no means specific for germ cell tumors. It is expressed in diverse types of carcinomas although more commonly only focally in this context. The non germ-cell tumors showing highest percentages of cases with SALL4 expression (close to 20% or more) were ovarian serous carcinoma, gastric adenocarcinoma, hepatic cholangio-carcinoma, pulmonary small cell carcinoma, and urothelial carcinoma. In many carcinoma categories, SALL4-positivity had predilection to poorly differentiated tumors, such as carcinomas with solid undifferentiated patterns, with SALL4-positivity often correlating with low level of differentiation apparently reflecting a stem-cell like phenotype. Whether SALL4-expression in non germ-cell tumors has any clinical significance in term of prognosis or chemotherapy response, requires further study.
Gastric intestinal type carcinomas (most of them shown here as CDX2 and CK20-positive) seem to be among the most commonly positive carcinomas often showing extensive SALL4-positivity. In their SALL4-positivity they resemble early embryonic intestinal epithelia. A previous study reported SALL4 expression in 15% of gastric carcinomas with predilection to tumors with intestinal differentiation and often associated with synchronous liver metastases. 15 However, of some reason, colorectal carcinomas, also of intestinal phenotype, only rarely were SALL4 positive indicating that interm of SALL4, they do not have fetal intestinal phenotype. The finding that many SALL4-positive colon carcinomas were metastases, suggests the possibility that SALL4-positive cells may be enriched in metastases, possibly induced or selected by chemotherapy.
For SALL4 expression in non germ cell tumors, our results differ from some previous studies that were based on relatively small numbers of cases. One study found that all ovarian serous carcinomas were SALL4-negative (0/23) 10 Another study reported all 12 lung cancers and 8 breast cancers negative for SALL4, while noting weak SALL4 expression in 6/18 gastric adenocarcinomas in up 20-25% of tumor cells. 14 Both of these studies used the same antibody clone as in our study but from a different source. However, use of a different detection system may be the source of the variance of results.
Because a small number of cases in many carcinoma categories had extensive SALL4-positivity in virtually all tumor cells, similar to that seen in germ cell tumors, even extensive SALL4-positivity cannot be considered specific for germ cell tumors. SALL4-positivity in pelvic tumors such as ovarian and urothelial carcinomas is especially significant for the differential diagnosis with germ cell tumors. There is no information of SALL4 in ovarian serous carcinomas or urothelial carcinomas. However, SALL4 expression in urothelial carcinomas may reflect oncofetal expression, as SALL4 is transiently expressed in the ureteric bud. 19
In ductal carcinomas of the breast, SALL4 was only rarely expressed (2%) but when present had a predilection to estrogen receptor positive and strongly HER2-positive cases, which constituted 80% of positive ductal carcinomas (one was a triple negative tumor). As HER2 may be selectively expressed in cancer stem cell populations in ductal carcinomas 20, SALL4 expression in HER2+ cancers may further define a subset of ductal carcinomas rich in stem cells. Possible clinical and oncologic correlation to SALL4 expression in ductal carcinomas of the breast requires further study. There are only scant observations on SALL4 in breast cancer, all based on MRNA expression studies. One study reported elevated SALL4 mRNA in 86% of breast cancers. 21 Compared with this, immunohistochemically detected expression is markedly more infrequent.
In this study, SALL4 was not detected in hepatocellular carcinomas beyond expression in isolated cells, which was not considered positive as nuclear staining in sporadic cells can be potentially obtained with the epitope retrieval only. While 2 previous studies also reported the absence of SALL4 expression in hepatocellular carcinoma contrasting gastric hepatoid carcinoma 15,22, a third study showed common SALL4 expression in hepatocellular carcinoma, however often in a clump-like nuclear pattern and usually limited to focal areas. 23
In general SALL4 was only rarely expressed in mesenchymal and neuroectodermal tumors. However, two notable exceptions were rhabdoid tumors (renal and extrarenal) and Wilms tumors, a majority of which contained SALL4-positive components, although unpredictably in terms of epithelial vs. stromal expression. Previous studies have detected SALL4 in both of these tumors. 24-26 The apparent rarity of SALL4 expression in epithelioid sarcoma suggests that this feature may distinguish epithelioid sarcoma from rhabdoid tumor, which has overlapping features in morphology as well as loss of INI1/SMARCB1 protein.
Among the exceptional other SALL4-positive mesenchymal or neuroectodermal tumors were 2 melanomas (3%), 1 desmoplastic small round cell tumor (8%), 1 embryonal rhabdomyosarcoma (2%) and 1 epithelioid sarcoma (5%) indicating rare and sporadic immunohistochemical expression of SALL4 in these tumors. Some of these tumors could be clinicopathologically mimic germ cell tumors, for example paratesticular rhabdomyosarcomas. Notably, SALL4-positive non germ-cell carcinomas or the rare SALL4-positive mesenchymal or neuroectodermal tumors were negative for OCT3/4 and NANOG, two other pluripotency markers indicating that secondary testing with those two antibodies may help to evaluate germ cell vs. non germ-cell origin of SALL4-positive tumors of unknown lineage.
However, in the context of germ cell tumors, one has to consider that many nonseminomatous germ cell tumors, such as yolk sac and trophoblastic tumors, are negative for OCT3/4 and NANOG so that SALL4 is the only one positive of these 3 germ cell/pluripotency markers. 12 Therefore, additional markers may be necessary to distinguish yolk sac tumor from Sall4-positive non germ-cell carcinomas, especially the histologic and clinicopathologic mimics, such as ovarian carcinomas. Glypican-3 has been shown useful in this respect based on one study that found nearly all ovarian serous and mucinous carcinomas negative but yolk sac tumors positive. However, 17% of clear cell carcinomas had at least some glypican-3 positivity in one study.27 Another study found even higher percentage (44%) of clear cell carcinomas to be glypican-3 positive. 28 These observations indicate some immunohistochemical overlap in the antigen patterns of yolk sac tumor and clear cell carcinoma, so that careful morphologic observation and additional criteria are needed for their separation.
SALL4 has also been reported expressed and pathogenetically important in acute myeloid leukemia so that it has been considered an oncogene in this context. 8 Expression has been also reported in various lymphomas, multiple myeloma, and acute lymphoblastic leukemia at the mRNA level. 29 Our immunohistochemical studies on myeloid sarcomas (tissue equivalent of acute myeloid leukemia) and various lymphomas failed to detect expression by immunohistochemistry. This suggests that expression of SALL4 protein in these tumors must be lower than present in germ cell tumors and SALL4-positive non germ-cell carcinomas. However, lack of immunohistochemically detected expression may not necessarily rule out biological role of SALL4 in hematopoietic/lymphoid tumors.
In conclusion, we examined >3000 human tumors for SALL4, a pluripotency and germ cell marker. In addition to its nearly consistent expression in germ cell tumors, SALL4 is frequently expressed in ovarian serous, urothelial, gastric intestinal carcinomas, cholangiocarcinomas, and small cell carcinomas, sometimes in a majority of tumor cells, similar to germ cell tumors. In addition, sporadic other carcinomas, melanomas and sarcomas can also be positive. This non-germ cell tumor expression of SALL4 may reflect stem cell-like phenotype of positive tumors and has to be considered when using SALL4 as a marker to evaluate possible germ cell differentiation. Clinical correlation of SALL4 expression in non-germ cell tumors should be further studied in terms of possible significance for prognosis and chemotherapy response.
Supplementary Material
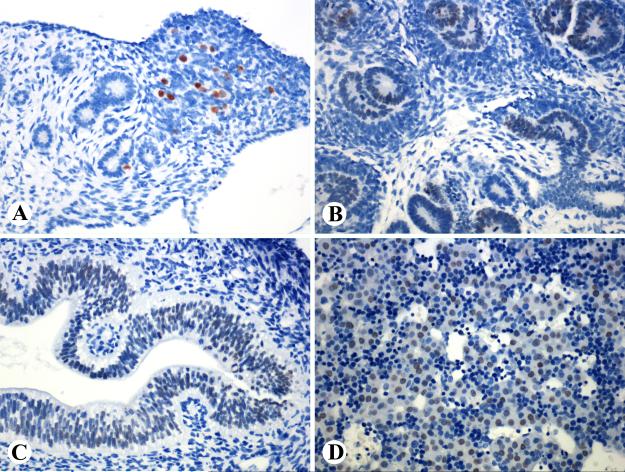
Fig. 1.
SALL4 expression in a 10 week-old fetus. A. Ovarian germ cells are strongly positive. B-D. Non-germ cell elements, such as developing tubular and glomerular epithelia, intestinal epithelia, and a portion of hepatocytes, show weaker yet distinct positivity.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article. This work was supported as a part of NCI's intramural research program.
REFERENCES
- 1.Yang J, Chai L, Fowles TC, et al. Genome-wide analysis reveals SALL4 to be a major regulator of pluripotency in murine-embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:19756–19761. doi: 10.1073/pnas.0809321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Tam WL, Tong GQ, et al. SALL4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat Cell Biol. 2006;8:1114–1123. doi: 10.1038/ncb1481. [DOI] [PubMed] [Google Scholar]
- 3.Wu Q, Chen X, Zhang J, et al. SALL4 interacts with nanog and co-occupies Nanog genomic sites in embryonic stem cells. J Biol Chem. 2006;281:24090–24094. doi: 10.1074/jbc.C600122200. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Q, Chipperfield H, Melton DA, Wong WH. A gene regulatory network in mouse embryonic stem cells. ProcNatl Acad Sci U S A. 2007;104:16438–16443. doi: 10.1073/pnas.0701014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warren M, Wang W, Spiden S, et al. A SALL4 mutant mouse model useful for studying the role of SALL4 in early embryonic development and organogenesis. Genesis. 2007;45:51–58. doi: 10.1002/dvg.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohlhase J, Schubert L, Liebers M, et al. Mutations at the SALL4 locus on chromosome 20 result in a range of clinically overlapping phenotypes, including Okihiro syndrome, Holt-Oram syndrome, acro-renal-ocular syndrome, and patients previously reported to represent thalidomide embryopathy. J Med Genet. 2003;40:473–478. doi: 10.1136/jmg.40.7.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohlhase J. SALL4-Related Disorders. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. GeneReviews™ [Internet] University of Washington, Seattle; Seattle (WA): Aug 16, 2004. pp. 1993–2013. [updated 2008 Mar 12] [Google Scholar]
- 8.Ma Y, Cui W, Yang J, et al. SALL4, a novel oncogene, is constitutively expressed in human acute myeloid leukemia (AML) and induces AML in transgenic mice. Blood. 2006;108:2726–2735. doi: 10.1182/blood-2006-02-001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohlhase J, Heinrich M, Liebers M, et al. Cloning and expression analysis of SALL4, the murine homologue of the gene mutated in Okihiro syndrome. Cytogenet Genome Res. 2002;98:274–277. doi: 10.1159/000071048. [DOI] [PubMed] [Google Scholar]
- 10.Cao D, Guo S, Allan RW, et al. SALL4 is a novel sensitive and specific marker of ovarian primitive germ cell tumors and is particularly useful in distinguishing yolk sac tumor from clear cell carcinoma. Am J Surg Pathol. 2009;33:894–904. doi: 10.1097/PAS.0b013e318198177d. [DOI] [PubMed] [Google Scholar]
- 11.Cao D, Li J, Guo CC, Allan RW, et al. SALL4 is a novel diagnostic marker for testicular germ cell tumors. Am J Surg Pathol. 2009;33:1065–1077. doi: 10.1097/PAS.0b013e3181a13eef. [DOI] [PubMed] [Google Scholar]
- 12.Liu A, Cheng L, Du J, et al. Diagnostic utility of novel stem cell markers SALL4, OCT4, NANOG, SOX2, UTF1, and TCL1 in primary mediastinal germ cell tumors. Am J Surg Pathol. 2010;34:697–706. doi: 10.1097/PAS.0b013e3181db84aa. [DOI] [PubMed] [Google Scholar]
- 13.Wang F, Liu A, Peng Y, et al. Diagnostic utility of SALL4 in extragonadal yolk sac tumors: an immunohistochemical study of 59 cases with comparison to placental-like alkaline phosphatase, alpha-fetoprotein, and glypican-3. Am J Surg Pathol. 2009;33:1529–1539. doi: 10.1097/PAS.0b013e3181ad25d5. [DOI] [PubMed] [Google Scholar]
- 14.Cao D, Humphrey PA, Allan RW. SALL4 is a novel sensitive and specific marker for metastatic germ cell tumors, with particular utility in detection of metastatic yolk sac tumors. Cancer. 2009;115:2640–2651. doi: 10.1002/cncr.24308. [DOI] [PubMed] [Google Scholar]
- 15.Ushiku T, Shinozaki A, Shibahara J, et al. SALL4 represents fetal gut differentiation of gastric cancer, and is diagnostically useful in distinguishing hepatoid gastric carcinoma from hepatocellular carcinoma. Am J Surg Pathol. 2010;34:533–40. doi: 10.1097/PAS.0b013e3181d1dcdd. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi D, Kuribayashi K, Tanaka M, et al. Overexpression of SALL4 in lung cancer and its importance in cell proliferation. Oncol Rep. 2011;26:965–970. doi: 10.3892/or.2011.1374. [DOI] [PubMed] [Google Scholar]
- 17.Forghanifard MM, Moghbeli M, Raeisossadati R, et al. Role of SALL4 in the progression and metastasis of colorectal cancer. J Biomed Sci. Jan 30. 2013;20:6. doi: 10.1186/1423-0127-20-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miettinen M. A simple method for generating multitissue blocks without special equipment. Appl Immunohistochem Mol Morphol. 2012;20:410–412. doi: 10.1097/PAI.0b013e318245c82f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyoda D, Taguchi A, Chiga M, et al. SALL4 is transiently expressed in the caudal Wolffian duct and the ureteric Bud, but dispensable for kidney development. PloS One. 2013;8:e68508. doi: 10.1371/journal.pone.0068508. Print 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korkava H, Wicha MS. HER2 and breast cancer stem cells: more than meets the eye. Cancer Res. 2013;73:3489–3493. doi: 10.1158/0008-5472.CAN-13-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi D, Kuribayshi K, Tanaka M, et al. SALL4 is essential for cancer cell proliferation and is overexpressed at early clinical stages in breast cancer. Int J Oncol. 2011;38:933–939. doi: 10.3892/ijo.2011.929. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda H, Sato Y, Yoneda N, et al. α-Fetoprotein-producing gastric carcinoma and combined hepatocellular and cholangiocarcinoma show similar morphology but different histogenesis with respect to SALL4 expression. Hum Pathol. 2012;43:1955–1963. doi: 10.1016/j.humpath.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Roibon N, Katz B, Chaux A, et al. Immunohistochemical expression of SALL4 in hepatocellular carcinoma, a potential pitfall in the differential diagnosis of yolk sac tumors. Hum Pathol. 2013;44:1293–1299. doi: 10.1016/j.humpath.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Deisch J, Raisanen J, Rakheja D. Immunoexpression of SALL4 in Wilms tumors and developing kidney. Pathol Oncol Res. 2011;17:639–644. doi: 10.1007/s12253-011-9364-0. [DOI] [PubMed] [Google Scholar]
- 25.Deisch J, Raisanen J, Rakheja D. Immunohistochemical expression of embryonic stem cell markers in malignant rhabdoid tumors. Pediatr Dev Pathol. 2011;14:353–359. doi: 10.2350/10-09-0902-OA.1. [DOI] [PubMed] [Google Scholar]
- 26.Venneti S, Le P, Martinez D, et al. Malignant rhabdoid tumors express stem cell factors, which relate to the expression of EZH2 and Id proteins. Am J Surg Pathol. 2011;35:1463–1472. doi: 10.1097/PAS.0b013e318224d2cd. [DOI] [PubMed] [Google Scholar]
- 27.Esheba GE, Pate LL, Longacre TA. Ocofetal protein glypican-3 distinguishes yolk sac tumor from clear cell carcinoma of the ovary. Am J Surg Pathol. 2008;32:600–607. doi: 10.1097/PAS.0b013e31815a565a. [DOI] [PubMed] [Google Scholar]
- 28.Maeda D, Ota S, Takazawa Y, et al. Glypican-3 expression in clear cell adenocarcinoma of the ovary. Mod Pathol. 2009;22:824–832. doi: 10.1038/modpathol.2009.40. [DOI] [PubMed] [Google Scholar]
- 29.Cui W, Kong NR, Ma Y, Amin HM, Lai R, Chai L. Differential expression of the novel oncogene, SALL4, in lymphoma, plasma cell myeloma, and acute lymphoblastic leukemia. Mod Pathol. 2006;19:1585–1592. doi: 10.1038/modpathol.3800694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.