Abstract
Background
In 2010 the World Health Assembly called for action to improve the care and prevention of congenital disorders, noting that technical guidance would be required for this task, especially in low- and middle-income countries. Responding to this call, we have developed a freely available web-accessible Toolkit for assessing health needs for congenital disorders.
Methods
Materials for the Toolkit website (http://toolkit.phgfoundation.org) were prepared by an iterative process of writing, discussion and modification by the project team, with advice from external experts. A customized database was developed using epidemiological, demographic, socio-economic and health-services data from a range of validated sources. Document-processing and data integration software combines data from the database with a template to generate topic- and country-specific Calculator documents for quantitative analysis.
Results
The Toolkit guides users through selection of topics (including both clinical conditions and relevant health services), assembly and evaluation of qualitative and quantitative information, assessment of the potential effects of selected interventions, and planning and prioritization of actions to reduce the risk or prevalence of congenital disorders.
Conclusions
The Toolkit enables users without epidemiological or public health expertise to undertake health needs assessment as a prerequisite for strategic planning in relation to congenital disorders in their country or region.
Keywords: chromosomal disorders, congenital disorders, health services, population-based and preventative services
Background
Throughout the world, but particularly in low- and middle-income countries (LMICs), the burden of congenital disorders contributes substantially to death, disability and disadvantage for individuals, families, communities and countries.
The terms ‘congenital disorder’ or ‘birth defect’ refer to any abnormality affecting body structure or function that is present from birth. Congenital disorders include chromosomal and single-gene disorders, conditions with multifactorial inheritance (e.g. malformations of single or multiple organs or limbs) and disorders caused by maternal or environmental factors such as exposure to teratogens, maternal infections and diabetes.
A range of determinants influence the occurrence and severity of congenital disorders. Risk factors include advanced parental age, parental consanguinity, residence in or origin from places where malaria is (or was) common, micronutrient deficiencies (e.g. folate and iodine), and infection during pregnancy. Wider determinants of outcomes include the country's level of development; access to, and quality of health and other services and the influence of cultural, religious, ethical and legal factors.
The epidemiological transition in many LMICs has been characterized by reductions in infant and child morbidity and mortality, especially from infectious diseases.1 As the burden of disease from these conditions falls, an increasing proportion of the remaining morbidity and mortality in the population is related to congenital disorders.2,3 Every year, ∼5 million babies are born with a severe genetic disorder or congenital malformation, and nearly 3 million children under the age of 5 years die from one of these conditions.1,4 This burden is further highlighted by the recent Global Burden of Disease (GBD) 2010 study5 update which ranked congenital disorders as one of the leading causes of deaths and disability-adjusted life years (DALYs) for under-5s at the global level.
There is a widespread misconception that little can be done about congenital disorders. On the contrary, up to 70% of congenital disorders can be either prevented, or managed in such a way as to considerably reduce mortality and disability.4,6 However in LMICs, where more than 90% of affected births occur,1 preventive and therapeutic services are often still rudimentary.
Effective actions to reduce the burden of congenital disorders have been recognized as essential for achieving the Millennium Development Goal for child mortality.7 Significantly, the May 2010 World Health Assembly (WHA) passed a resolution calling on member states to ‘prevent birth defects wherever possible, to implement screening programmes, and to provide ongoing support and care to children with birth defects and their families’.8 The WHA resolution noted that ‘international technical guidance will be required to help ministries with organized assessment of requirements and costs and with support in choosing priorities’.
The task of developing programmes to tackle congenital disorders is challenging for many countries. Problems include the diverse range of conditions, the paucity of reliable epidemiological and health-services data, and lack of expertise and resources. Recognizing these difficulties, the Foundation for Genomics and Population Health (PHG Foundation) established the Born Healthy programme in 2009. The major component of this programme is a freely available web-accessible ‘Toolkit’: a step-wise guide based on the principles of health needs assessment (HNA) as a prerequisite for strategic planning in relation to congenital disorders.
HNA involves integration of three main sets of data: (i) demographic and epidemiological; (ii) effectiveness of interventions and (iii) assessment of current services, to develop an agreed set of priorities for action. In practice the planning process often stalls at the outset because of a perceived lack of data. The Toolkit addresses this problem by providing a framework within which users can begin to gather data relevant to their own country, and giving them access to country-specific demographic and epidemiological estimates where they do not have direct evidence from their own sources. These data are intended to provide a starting point and to help users to make the initial case that data need to be collected on the burden of these conditions.
We envisage that officers at Ministries of Health, public health professionals and geneticists will be the main users of the Toolkit, but others such as primary care providers, health promotion specialists, voluntary and patient organizations may also find it useful; the Toolkit is designed to be usable without specialist public health or clinical knowledge.
Methods
Development of the Toolkit
The Toolkit materials were prepared by a core team of epidemiologists, public health specialists and policy analysts from the PHG Foundation, with data and informatics support from the Centre for Health Informatics and Multiprofessional Education (CHIME) at University College London (UCL). Materials were developed by an iterative process of writing, discussion and modification by the project team, with advice from external experts.
Part of the Toolkit development process included an international workshop attended by ∼50 invited experts from 12 countries in Europe, the Americas, Africa and Asia. Participants included medical geneticists and other clinicians, public health specialists and policy-makers from governments and NGOs. The Toolkit materials were revised in the light of the workshop participants' suggestions.
The Toolkit website (http://toolkit.phgfoundation.org) was developed in compliance with core web standards. The aims were to minimize file sizes and to provide documents in familiar and/or free software formats to minimize both the cost to users and the IT skills needed.
Scope of the Toolkit
The Toolkit includes both ‘clinical topics’ and ‘service topics’. Clinical topics were chosen for inclusion as examples of congenital conditions that have a substantial impact on public health worldwide.
Clinical topics include the following:
Congenital heart disease
Congenital hypothyroidism and iodine deficiency disorder
Congenital rubella
Congenital syphilis
Down syndrome
Fetal alcohol spectrum disorder
Glucose-6-phosphate dehydrogenase deficiency
Neural tube defects (NTDs)
Orofacial clefts
Rhesus D haemolytic disease of the newborn
Sickle cell disease
Thalassaemias.
The principle underlying the choice of service topics was that the care and prevention of congenital disorders requires a continuum of care that covers women in reproductive age and during pregnancy and childbirth, the neonate and the developing child up to adulthood. Within this continuum, opportunities for interventions include carrier screening, family planning, preconception care and counselling, prenatal care and screening, obstetric services, neonatal care and screening, and health and social care throughout childhood and adult life.
Service topics include the following:
Health services (capacity and organization)
Newborn screening (including screening for inherited metabolic disorders)
Preconception care and screening
Prenatal care and screening
Teratogens (including teratogen information services).
The Toolkit is not intended to provide detailed clinical information on diagnosis or management, but rather to guide the process of assessing the availability and quality of existing services and interventions and identifying areas for action and improvement.
Toolkit data
The Toolkit provides indicative demographic, health service, socio-economic and epidemiological data drawn from a customized database (the PHGDB) developed by the PHG Foundation. These data are provided as a starting point, or to provide modelled estimates where no local data are available. Users are encouraged to use and evaluate their own data wherever possible.
Data in the PHGDB relate to specific countries and, in some cases, to world regions. The PHGDB groups countries into the same 21 geographical regions as the GBD 2010 Study.9 Demographic data for each country were compiled from a range of resources.10–14 Particular data, such as population data, were chosen to provide a coherent set and to allow quick comparisons between countries and so were not necessarily the most up-to-date data in every case.
The principal source for epidemiological data on congenital disorders was the Modell Global Database of Constitutional Congenital Disorders (MGDB), developed by Modell and colleagues at UCL CHIME1 with data updated in 2011 for submission to the GBD study. The MGDB provides country-level epidemiological estimates for severe congenital anomalies (those classified by ICD10 codes Q00–Q99: ‘congenital malformations, deformations and chromosomal abnormalities’). (Note that the MGDB does not include data for congenital disorders caused by environmental factors such as teratogen exposure or maternal infection.) The MGDB is constructed from data obtained by extensive literature searches and from registries of congenital anomalies, supplemented by data derived from mathematical modelling.1 Outcomes from the modelling process include, for each condition and country, the potential birth prevalence for the condition in the absence of any interventions and the estimated actual birth prevalence based on the estimated effects of selected preventive interventions. The incorporation of data on current and past mortality, and on the operation and coverage of health services and therapeutic interventions, allows estimation of the number of people living with the condition by age group, gender and degree of severity, and of age-specific condition-specific mortality. When country-level data from the GBD study9 and the Child Health Epidemiology Reference Group (CHERG, www.cherg.org) are publicly available, the Toolkit will include reference to these data.
Document-processing and data integration software, also developed for the Toolkit, combines relevant PHGDB data with a template to generate topic- and country-specific Calculator documents in multiple formats (Adobe PDF, Microsoft Excel and Open Document Spreadsheet Formats).
Results
Conducting the HNA
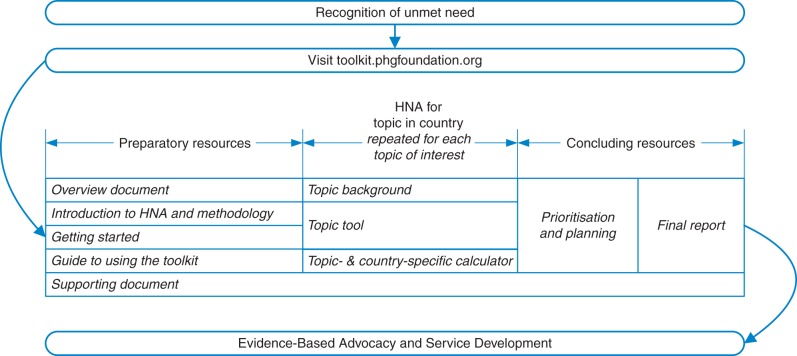
The procedure for a full HNA is outlined in Fig. 1 and described in detail in the Toolkit website (http://toolkit.phgfoundation.org). The user first completes the ‘Getting Started' document, which guides them through a structured approach to assembling the necessary expertise and resources, and choosing their HNA topic(s), then navigates from the home page to a World Health Organization (WHO) region, a country and then their chosen topic. A ‘Supporting Document' provides supplementary information on parental consanguinity; ethical, legal and social issues; patient engagement; health economics; prioritization; overview of methods used by the MGDB and a glossary.
Fig. 1.
Schematic representation of use of the Toolkit to carry out HNA, showing possible iteration over multiple topics
For each topic, the main substance of the HNA is contained within the HNA ‘Tool' supported by the ‘Background' document and the ‘Calculator'. The ‘Background' document provides a core knowledge base for a specific topic. The HNA ‘Tool' leads the user through the HNA process and the recording of qualitative and summary information, while the associated ‘Calculator' enables the user to record and compare quantitative data on epidemiological indicators and to calculate the potential effects of selected interventions on the risk or prevalence of congenital disorders. At present these interventions include folic acid fortification of food and targeted dietary folic acid supplementation, termination of pregnancy (where appropriate) and measures to reduce risks associated with advanced maternal age.
HNA for each clinical topic consists of seven sections:
Country profile
Epidemiology
Interventions
Needs assessment
Situation assessment
Initial prioritization
Summary report.
When HNA is carried out for more than one topic, the ‘Prioritization and Planning' document enables the results of separate HNAs to be brought together so that the user can prioritize action areas and devise an integrated plan of action. The ‘Final Report' enables users to consolidate the findings into a summary report for stakeholders.
Examples of using the Toolkit
An important strength of the Toolkit is its flexibility. Not all users may need, or have the resources, to carry out a full HNA for a topic. They can choose, for example, just to use the epidemiology section to collate data on the burden of disease or to concentrate on qualitative evaluation of their existing services. A full HNA can be completed at a later date if resources allow.
Here we provide a few examples in which features of the Toolkit have been used for specific purposes.
Developing a country-specific profile
At a recent WHO South East Asia Regional Office meeting, countries were asked to put together a brief country profile as a baseline for developing a strategy for tackling congenital disorders. Box 1 shows an example of a profile that could have been readily assembled from the Toolkit by an individual without epidemiological expertise. The data, drawn from the PHGDB, include both demographic and health-services data (sources and year of data shown in brackets), and epidemiological data (sourced from the MGDB) on specific types of congenital disorders. Together, this information, based on well-referenced and up-to-date sources, provides an immediate snapshot of the burden of congenital disorders in the country and the context for developing services for care and prevention.
Box 1. Profile for Bhutan.
Bhutan is a small country with a population of ∼634 000 (UN 2011, data for 2005). It is situated in the Himalayan region between India and China, and falls within the WHO South East Asia region. The population of Bhutan is largely rural, with the majority of the 12 000 annual births (UNICEF 2007) taking place at home. Approximately 88% of pregnant women have at least one prenatal visit (WHO, 2007) and 71.5% of births are attended by a skilled health professional (WHO, 2007). Infant mortality is 44 per 1000 births (UNICEF, 2011, data for 2010) and average life expectancy at birth is 63 years (WHO, 2009). There are relatively few doctors and nurses/midwives per capita [0.2 and 3.2 per 10 000 population, respectively (WHO, 2007)], suggesting that much of the health burden falls on the immediate and extended family.
Data from PHGDB estimate that 857 children are born with congenital disorders annually in Bhutan. These comprise 327 with congenital heart disease, 220 with NTDs, 111 with orofacial clefts, 109 with Down syndrome, 40 with rhesus D haemolytic disease of the newborn, 34 with G6PD deficiency and 16 with congenital hypothyroidism.
Regional comparisons
Table 1 compares epidemiological data from the Toolkit for the birth prevalence of beta-thalassaemia and overall haemoglobin disorders in India with data from a recent systematic review of the literature. The generally close correspondence confirms that the Toolkit offers a reliable way of estimating the magnitude of the burden of disease. Table 2, showing data from the PHGDB both for India and for the wider South Asian Region, illustrates how the Toolkit enables comparisons between countries and regions; such a comparative approach can help to highlight areas of particular need.
Table 1.
Epidemiological information on beta-thalassaemia and overall haemoglobin disorders in India
| India | PHGDBa | Sinha et al.20 |
|---|---|---|
| Beta-thalassaemia carrier frequency | 4.6% | 3–4%21 |
| No. of beta-thalassaemia carriers | 49.0 million | 35.1–46.8 million |
| Beta-thalassaemia births per year | 6000b | 10 000–15 00022–24 |
| Pathological haemoglobin disordersc births per year | 32 500b | 32 400 |
| Pathological haemoglobin disordersc birth rate | 1.23/1000 | 1.2/10001 |
aModell: parental consanguinity factored in (p2 + Fpq) + 2(pq − Fpq) + (q2 + Fpq) = 1, where p is the frequency of variable 1, q the frequency of variable 2 and F the population coefficient of parental consanguinity.25
bModell estimates are the most conservative that are permitted by the data available (i.e. believed to be minimum estimates).25
cPathological haemoglobin disorders are sickle cell disease, beta-thalassaemia, alpha zero thalassaemia and Hb H disease.
Table 2.
PHGDB data for haemoglobin disorders in India and the South Asia Region
| India | Sickle cell disease in India | Sickle cell disease in SAR | Beta-thalassaemia in India | Beta-thalassaemia in SAR |
|---|---|---|---|---|
| Live birth prevalence/1000 | 1.02 | 0.73 | 0.24 | 0.43 |
| No. of affected live births | 26 451 | 26 805 | 6076 | 15 618 |
| Infant mortality rate/1000 births | 0.23 | 0.17 | 0.01 | 0.03 |
| No. of infant deaths | 6104 | 6252 | 298 | 935 |
| Under-5 mortality rate/1000 births | 0.29 | 0.21 | 0.1 | 0.23 |
| No. of under-5 deaths | 7631 | 7815 | 2683 | 8289 |
SAR, South Asia Region consisting of India, Pakistan, Afghanistan, Bangladesh, Bhutan and Nepal.
Estimating the potential effects of interventions
The Toolkit can be used to evaluate the potential effects of selected new interventions or of changes (for example, coverage) in existing interventions such as folic acid fortification of foods, which is known to affect the birth prevalence of NTDs. For example, in a population with a 2/1000 birth prevalence of NTD and where the coverage of folic acid food fortification at 2 ppm is 80%, the Toolkit Calculator predicts that a change in both coverage (from 80 to 90%) and in concentration of folic acid in flour (from 2 to 2.5 ppm) could potentially reduce birth prevalence by 20% to 1.6/1000.
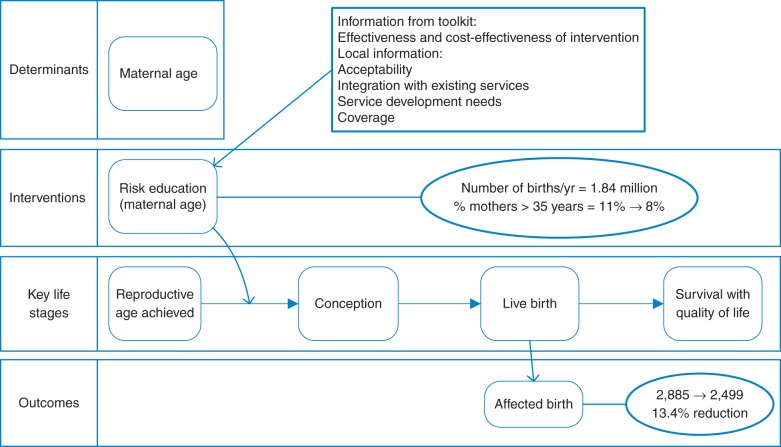
Figure 2, based on estimates for Egypt, shows that a reduction from 11 to 8% in the prevalence of motherhood over 35 years of age (for example through education and/or improved family planning services) in a country with 1.84 million births per year could lead, in the absence of any other interventions, to a 13.4% reduction in the birth prevalence of Down syndrome, corresponding to nearly 400 fewer affected births per year. The relative effect will be higher, the higher the initial proportion of births from mothers over 35 years old. Figure 2 also illustrates the Toolkit's approach of considering the effects of services and interventions targeting the risk or prevalence of congenital disorders at key life stages.
Fig. 2.
The potential impact of maternal age reduction alone on birth prevalence of Down syndrome
Discussion
Main findings of this study
Our HNA Toolkit is a flexible, user-friendly resource suitable for users without epidemiological or public health expertise—particularly those from LMICs—enabling them to put together a valid and compelling case with priorities for action to reduce the burden of congenital disease in their country or region.
What is already known on this topic
The need for action to tackle the burden of congenital disorders has been recognized by the WHA, as has the requirement for ‘international technical guidance … to help ministries with organized assessment of requirements and costs and with support in choosing priorities’ in relation to these conditions. The recent GBD 2010 study update further highlighted this need, ranking congenital disorders as a leading cause of under-5 death and DALYs at the global level.5 HNA is a well-established approach for strategic planning of health services and thus appropriate for application in the field of congenital disorders.
What this study adds
The Toolkit provides a way for countries to respond to the WHA's call for action. A recent WHO consultation on community genetics services in LMICs15 also recognized that epidemiological studies and needs assessment are an essential prerequisite to planning and improving services for the care and prevention of congenital disorders. The Toolkit complements the WHO's community genetics initiative by providing a practical, step-by-step approach for undertaking this initial task.
Needs assessment alone does not bring about improved health services but if conducted properly it can act as a powerful stimulus and catalyst. From the outset, the Toolkit user is reminded of the importance of involving—as well as the core team who will carry out the basic work of the HNA—a broad range of stakeholders including representation from national (and local) Ministries of Health or equivalent institutions; the public health services; clinical and laboratory genetics; maternal and child health services; medical and health educators; patients' representatives, such as charities supporting those with congenital disorders; expertise in ethical, legal and social issues; expertise in health economics; professional societies (e.g. genetics) and researchers. This inclusive approach ensures not only that the core team has access to a broad range of advice and expertise, but also that the full range of those with an interest in the problem—and in particular those who are in a position to champion change and drive it forward—know about the initiative and support it.
To date, the Toolkit has been used by health professionals, including medical geneticists, from three countries in South America: Brazil, Uruguay and Argentina. Each team chose a mix of clinical and service topics and worked through the full HNA process, resulting in some cases in the planning and initiation of new services or interventions [for example, in Brazil a higher prevalence at birth of NTDs has been observed due to the lower concentration of folic acid and planning is underway to address this situation (Lavinia Schuler-Faccini, personal communication)]. The experience of the Argentinian team, which has been published,16 was that the Toolkit was ‘action oriented’, providing a context for building a team of stakeholders who then had an interest in pushing forward the agenda identified by the HNA. The team found that the Toolkit materials were user friendly, flexible and offered a systematic approach to data collection and analysis that could be readily undertaken by those unfamiliar with the HNA process. An additional benefit was that parallel work using the Toolkit in other countries in the region enabled the establishment of a regional community of interest for those working on the public health problem of congenital disorders. The Argentinian team estimated that their full HNA, which assessed policies, programmes and interventions to reduce the burden of NTDs, required the equivalent of 3 months' full-time work by one person.
Limitations of this study
The countries that have used the Toolkit so far have been middle-income countries in which professional and technical resources are reasonably extensive, and basic health services are in place. Nevertheless, even in these countries poorly served regions or population groups were identified, highlighting the need for improvement. Although very poorly resourced countries may find using the Toolkit challenging, it has been designed to enable progress to be made from any starting point, and technical and financial requirements have been kept to a minimum. Recently, epidemiological elements of the Toolkit related to the prevalence of NTDs and associated risk factors have been used in India. Feedback from lower income countries about the utility of the Toolkit and the relevance of current and future topics would also be valuable to identify any barriers to uptake by this group of users.
A limitation of the research field and therefore also of the Toolkit is the scarcity of reliable local level epidemiological information for many countries.17–19 The mathematical modelling used to derive data for the Toolkit is particularly useful for countries with no or limited data.1 Because of these uncertainties in the resulting estimates, particularly for small populations, or where there are known high rates of congenital disorders caused by teratogen exposure or maternal infection, the use of local data, where available, is always encouraged. It is hoped that use of the Toolkit may act as a spur for the establishment of new registries and research studies to collect data that will strengthen both the HNA process and the research field.
The Toolkit is designed to allow for updating and development, including the production of new materials to suit needs of specific populations. It will be particularly important that the underlying data and evidence are kept up to date (for example, with reference to publicly available country-level GBD and CHERG data), a commitment that will require significant resources and leadership.
Funding
This work was supported primarily by the PHG Foundation, with additional financial support from the Mothercare Foundation and contributions from Costello Medical Consulting and Wellbeing of Women. C.G. was funded by an ESRC internship. Funding to pay the Open Access publication charges for this article was provided by the Foundation for Genomics and Population Health, a charitable company registered in England and Wales Company Number: 5823194, Charity Number: 1118664.
Acknowledgements
The PHG Foundation thanks all those who contributed to the development of the Toolkit. A full list of contributors can be found at http://toolkit.phgfoundation.org/index.htm.
References
- 1.Christianson AC, Howson CP, Modell B. New York: March of Dimes Birth Defects Foundation; 2006. The March of Dimes global report on birth defects: the hidden toll of dying and disabled children. [Google Scholar]
- 2.Institute of Medicine of the National Academies. Washington, DC: National Academy Press; 2003. Reducing birth defects: meeting the challenge in the developing world. [PubMed] [Google Scholar]
- 3.World Health Organisation. Services for the prevention and management of genetic disorders and birth defects in developing countries. http://whqlibdoc.who.int/hq/1999/WHO_HGN_GL_WAOPBD_99.1.pdf. 24 February 2012, date last accessed. [DOI] [PubMed]
- 4.Christianson A, Modell B. Medical genetics in developing countries. Annu Rev Genomics Hum Genet. 2004;5:219–65. doi: 10.1146/annurev.genom.5.061903.175935. doi:10.1146/annurev.genom.5.061903.175935. [DOI] [PubMed] [Google Scholar]
- 5.Global Burden of Disease Study 2010. Seattle, USA: Institute for Health Metrics and Evaluation (IHME); 2012. Global Burden of Disease Study 2010 (GBD2010). Results by cause 1990–2010. [Google Scholar]
- 6.Czeizel AE, Intody Z, Modell B. What proportion of congenital abnormalities can be prevented? BMJ. 1993;306(6876):499–503. doi: 10.1136/bmj.306.6876.499. doi:10.1136/bmj.306.6876.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howse JL, Howson CP, Katz M. Reducing the global toll of birth defects. Lancet. 2005;365(9474):1846–7. doi: 10.1016/S0140-6736(05)66611-1. doi:10.1016/S0140-6736(05)66611-1. [DOI] [PubMed] [Google Scholar]
- 8.World Health Assembly. Sixty-third world health assembly: birth defects. http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R17-en.pdf. 24 February 2012, date last accessed.
- 9.The Global Burden of Disease Study. The global burden of disease study operations manual. http://www.globalburden.org/gbdops.html. 24 February 2012, date last accessed.
- 10.Bittles A. The Consanguinity/Endogamy Resource. http://consang.net/index.php/Main_Page. 24 February 2012, date last accessed.
- 11.The United Nations. Demographic and social statistics website. http://unstats.un.org/unsd/demographic/default.htm. 24 February 2012, date last accessed.
- 12.UN Inter-agency Group on Child Mortality Estimation. Unicef Childinfo statistical tables: child survival and health. http://www.childinfo.org/mortality_tables.php. 24 February 2012, date last accessed.
- 13.United Nations Statistics Division. United Nations statistical databases. http://unstats.un.org/unsd/databases.htm. 24 February 2012, date last accessed.
- 14.World Health Organisation. World Health Organisation global health observatory data repository. http://apps.who.int/ghodata/ 24 February 2012, date last accessed.
- 15.World Health Organization. Community genetics services: report of a WHO Consultation on community genetics in low- and middle-income countries. 2010. http://whqlibdoc.who.int/publications/2011/9789241501149_eng.pdf. 28 February 2012, date last accessed.
- 16.Groisman B, Liascovich R, Barbero P, et al. The use of a Toolkit for health needs assessment on neural tube defects in Argentina. J Community Genet. 2013;4(1):77–86. doi: 10.1007/s12687-012-0120-2. doi:10.1007/s12687-012-0120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darlison MW, Modell B. Sickle-cell disorders: limits of descriptive epidemiology. Lancet. 2013;381(9861):98–9. doi: 10.1016/S0140-6736(12)61817-0. doi:10.1016/S0140-6736(12)61817-0. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–61. doi: 10.1016/S0140-6736(12)60560-1. doi:10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 19.Modell B, Berry RJ, Boyle CA, et al. Global regional and national causes of child mortality. Lancet. 2012;380(9853):1556–7. doi: 10.1016/S0140-6736(12)61878-9. doi:10.1016/S0140-6736(12)61878-9. [DOI] [PubMed] [Google Scholar]
- 20.Sinha S, Black ML, Agarwal S, et al. Profiling beta-thalassaemia mutations in India at state and regional levels: implications for genetic education, screening and counselling programmes. Hugo J. 2009;3(1–4):51–62. doi: 10.1007/s11568-010-9132-3. doi:10.1007/s11568-010-9132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organisation. Geneva: World Health Organisation (NLM Classification: WH 190); 2008. Joint WHO-TIF meeting on management of haemoglobin disorders (2nd: 2008: Nicosia, Cyprus) [Google Scholar]
- 22.Edison ES, Shaji RV, Devi SG, et al. Analysis of beta globin mutations in the Indian population: presence of rare and novel mutations and region-wise heterogeneity. Clin Genet. 2008;73(4):331–7. doi: 10.1111/j.1399-0004.2008.00973.x. doi:10.1111/j.1399-0004.2008.00973.x. [DOI] [PubMed] [Google Scholar]
- 23.Sheth JJ, Sheth FJ, Pandya P, et al. Beta-thalassemia mutations in western India. Indian J Pediatr. 2008;75(6):567–70. doi: 10.1007/s12098-008-0109-3. doi:10.1007/s12098-008-0109-3. [DOI] [PubMed] [Google Scholar]
- 24.Tamhankar PM, Agarwal S, Arya V, et al. Prevention of homozygous beta thalassemia by premarital screening and prenatal diagnosis in India. Prenat Diagn. 2009;29(1):83–8. doi: 10.1002/pd.2176. doi:10.1002/pd.2176. [DOI] [PubMed] [Google Scholar]
- 25.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86(6):480–7. doi: 10.2471/BLT.06.036673. doi:10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]