Abstract
Semantic knowledge (e.g., long-established knowledge about objects, facts, and word meanings) is known to be severely impaired by damage to the anterolateral temporal lobe. For example, patients with semantic dementia have prominent atrophy in anterolateral temporal cortex and also have significant damage within the medial aspect of the temporal lobe. However, there is uncertainty about the contribution of medial temporal lobe damage, including perirhinal cortex damage, to impaired semantic knowledge. Drawing largely on published material from multiple sources, we compared the performance of severely amnesic patients with large medial temporal lobe lesions and patients with semantic dementia on nine tests of semantic knowledge and two tests of new learning ability. On the tests of semantic knowledge, the amnesic patients performed markedly better than the patients with semantic dementia. By contrast, on the tests of new learning, the patients with semantic dementia performed markedly better than the amnesic patients. We conclude that medial temporal lobe damage impairs the formation of declarative memory, and that semantic knowledge is impaired to the extent that damage extends laterally in the temporal lobe. Reports that the extent of atrophy in perirhinal cortex correlated with the severity of impaired semantic knowledge may be understood by supposing that the extent of damage in many temporal lobe areas is intercorrelated in this progressive disease, and that the extent of atrophy in perirhinal cortex is a proxy for the overall severity of dementia.
Bilateral damage to the medial temporal lobe causes severe and lasting impairment in declarative memory (1). The important structures are thought to be the hippocampal region (the CA fields, the dentate gyrus, and the subicular complex) and the adjacent perirhinal, entorhinal, and parahippocampal cortices that make up much of the parahippocampal gyrus. In patients with damage limited largely to the hippocampal region (2-5), the memory impairment occurs against a background of intact intellectual function, intact remote memory for facts and events, and intact semantic knowledge.
The situation is less clear in the case of patients with severe memory impairment and larger lesions. Thus, it is of interest that, unlike patients with limited hippocampal lesions, two of the best-studied and most severely impaired patients (H.M. and E.P.) have some degree of impairment on tests of remotely acquired semantic knowledge (e.g., tests that assess long-established knowledge about the identity and function of common objects) (5). These two patients have large medial temporal lobe lesions as well as some damage to anterolateral temporal cortex, lateral to the medial temporal lobe (6, 7). The findings from a recent study of four patients, including H.M. and E.P., suggested that impaired semantic knowledge is related to the extent of damage to anterolateral temporal cortex, not to damage within the medial temporal lobe (5).
Additional important information about semantic knowledge comes from the study of patients with semantic dementia (SD), also known as the temporal variant of fronto-temporal dementia (8-10). These patients have progressive atrophy, prominently involving the anterolateral temporal lobes, and they have severe loss of conceptual knowledge about objects, facts, and word meanings. Several studies have documented quantitatively the sites of pathological change. In one report there was significant cortical atrophy, particularly in the left hemisphere, which involved the temporal pole, the fusiform gyrus, the inferior and middle temporal gyri, the amygdaloid complex, and ventromedial frontal cortex (11). It was also noted that anterior perirhinal cortex was likely affected in the region of the temporal pole.
Subsequent work also identified atrophy in anterolateral temporal cortex (fusiform gyrus, middle and inferior temporal gyri), but also identified marked atrophy in entorhinal cortex, anterior hippocampus, and in the total volume of the parahippocampal gyrus (12). Similar findings were reported in a larger volumetric study, which correlated impaired semantic memory to atrophy of the fusiform gyrus, temporal pole, and inferolateral temporal cortex (13). Yet it was noted again that the region of perirhinal cortex and parahippocampal gyrus were severely atrophied. Lastly, it was recently reported that the extent of perirhinal atrophy was correlated with the severity of impaired semantic knowledge (14). These findings have raised the suggestion that damage to structures within the medial temporal lobe might be an important contributor to SD (14).
To better understand the importance of perirhinal cortex and other medial temporal lobe structures for semantic knowledge, we have compared two kinds of patients: (i) memory-impaired patients with large medial temporal lobe lesions, including complete lesions of perirhinal cortex and variable damage to anterolateral temporal cortex; and (ii) patients with SD who have prominent damage to anterolateral temporal cortex, as well as atrophy in the anterior medial temporal lobe, including perirhinal cortex. Drawing largely on published reports in which the two kinds of patients have taken the same tests, we have assessed the patients' semantic knowledge and their capacity for new learning.
Participants
We assessed the performance of two patient groups on the same tests of semantic knowledge and anterograde memory. We compared severely amnesic patients with large medial temporal lobe lesions and variable additional damage to the anterolateral temporal lobe (MTL group) and patients with SD. Much of the data for the MTL group has been published (5). The data for SD come from a series of related publications that describe the pattern of sparing and loss in SD (15-18).
MTL Group. The MTL group (Fig. 1) consisted of three patients, all male, who have severe amnesia as a result of herpes simplex encephalitis (E.P., born 1922, 12 years of education, onset of amnesia in 1992; G.P., born in 1946, 16 years of education, onset of amnesia in 1987; and G.T., born in 1936, 12 years of education, onset of amnesia in 1990). New MRI measurements of the patients and three controls for each patient have been carried out to describe the lesions of the patients more thoroughly. The new measurements are similar to what had been previously reported (5, 7). Estimates of damage were based on quantitative analysis, following published procedures for segmenting the temporal lobe (19, 20).
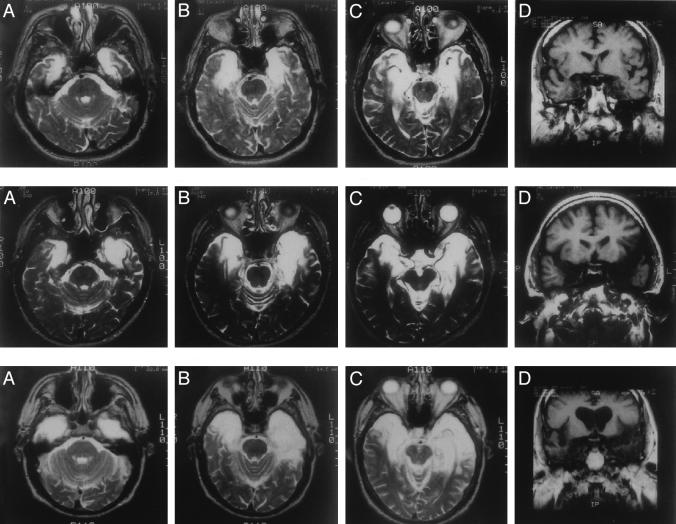
Fig. 1.
Magnetic resonance images showing the extent of bilateral temporal damage in patients E.P. (Top), G.P. (Middle), and G.T. (Bottom). A-C throughout are T-2 weighed axial images through the temporal lobe. The images are continuous 5-mm sections (with 2.5-mm gaps) and are arranged from ventral (A) to dorsal (C). Damaged tissue is indicated by bright signal. D throughout are coronal T-1 weighted images at the level of the amygdala. Damaged tissue is indicated by dark signal. See text for descriptions of the lesions.
E.P.'s lesion extends 7 cm caudally from the temporal pole bilaterally and includes all of the amygdala and all of the hippocampal region (dentate gyrus, cell fields of the hippocampus proper, and subicular complex except for a small tag of abnormally appearing vestigial tissue that comprises ≈10% of hippocampal volume). In addition, the damage includes all of entorhinal cortex, all of perirhinal cortex, and much of parahippocampal cortex (≈74% on the left and 79% on the right). The lesion also extends laterally to include the rostral portion of the fusiform gyrus (≈47% on the left and 71% on the right). Lateral temporal cortex (inferior, middle, and superior temporal gyri) has a normal volume (within 5% of controls). Lastly, the insula is reduced in size (≈40% on the left and 38% on the right).
Like E.P., G.P.'s lesion is primarily medial temporal, but his lesion extends further laterally. The damage extends through the anterior 7 cm of the left temporal lobe and the anterior 6 cm of the right temporal lobe. The damage includes bilaterally all of the amygdala, all of the hippocampal region, all of the entorhinal and perirhinal cortices, and much of parahippocampal cortex (≈87% on the left and 57% on the right). The lateral damage is most severe in the anterior 1 cm of the temporal lobe, and extends caudally into the fusiform gyrus (≈26% on the left and 43% on the right) and into lateral temporal cortex, the volume of which is reduced by ≈11% on the left and 20% on the right. Lastly, the insula is reduced in volume by ≈78% on the left and 44% on the right.
G.T.'s lesion includes the medial temporal lobe but also extends laterally to involve the anterior temporal lobes bilaterally. The damage involves the anterior 7 cm of the left temporal lobe, the anterior 5 cm of the right temporal lobe, and includes all of the amygdala, all of the hippocampal region, all of the entorhinal and perirhinal cortices, and much of parahippocampal cortex (≈85% on the left and 59% on the right). The fusiform gyrus is reduced in volume by ≈39% on the left and 55% on the right, and the volume of lateral temporal cortex is reduced by ≈52% on the left and 37% on the right. Lastly, the insula is reduced in volume by ≈68% on the left and 31% on the right.
CON-2 and CON-3. Eight healthy males served as controls (CON-2) for the MTL group (mean age = 74 years, mean education = 12.4 years). Only four of these controls took the Object Decision test. The control data (CON-3) for the two tests of declarative memory are from Squire and Shimamura (21).
SD, CON-1, CON-4, CON-5, and CON-6. The SD patients were reported in a series of related publications that characterize this condition in considerable detail. The data for the nine patients (6 female) who participated in tests 1-7 (see below) and in the Pyramids and Palm Trees Test were reported by Hodges et al. (15). These patients averaged 60 years of age (range = 56-72) and 11 years of education. CON-1 for these 7 tests refers to 9 controls (6 female, mean age = 61 years, education = 11 years), also from Hodges et al. (15). The data for the 11 patients (7 male) who took the Object Decision test were reported by Hovius et al. (16). The patients averaged 63.3 years of age (range = 52-78 years) and 11.6 years of education, and their 21 controls (CON-4) averaged 68.5 years of age and 11.0 years of education. The data for the 11 SD patients (7 male) who took the recognition memory test were reported by Lee et al. (17). The patients averaged 61.6 years of age and 12.1 years of education, and their 24 controls (CON-5; 6 male) averaged 69.7 years of age and 10.7 years of education. The 12 patients (7 female) who took the diagram test were reported by Simons et al. (18). The patients averaged 61.1 years of age, and their 10 controls (CON-6; 3 female) averaged 64.6 years of age.
Materials and Procedures
Seven of the tests are from the Semantic Test Battery, as originally introduced by Hodges and colleagues (9, 22) and subsequently amended (15, 23, 24). All seven tests were based on the same line drawings (25) of 24 animals and 24 objects (or their names). Each of the 48 items could further be assigned to one of 8 categories: 6 domestic land animals, 6 foreign land animals, 6 water creatures, and 6 birds; 6 electrical household items, 6 nonelectrical household items, 6 vehicles, and 6 musical instruments. Unless stated otherwise, there was no time limit for the tests.
Pointing to Picture (Cue: Name). Participants were given the name of an item as a cue and were asked to identify the appropriate picture from among eight pictures of the same category.
Naming (Cue: Picture). Participants were shown a picture of an item as a cue and asked to name it.
Naming (Cue: Description). Participants were given a verbal description of an item as a cue and asked to name it.
Semantic Features. Participants were asked eight yes/no questions about each of 24 items, 4 questions about an item's physical features and 4 questions about an item's associative (nonphysical) features, e.g., Is a toaster round? Does a zebra live in Africa?
Category Fluency (Living). Participants were asked to name in 1 min as many examples as they could from each of four categories of living things (Animals, Birds, Water Creatures, and Breeds of Dogs).
Category Fluency (Nonliving). Participants were asked to name in 1 min as many examples as they could from each of four categories of nonliving things (Household Items, Vehicles, Musical Instruments, and Types of Boat).
Subordinate Category Sorting. Participants sorted the 12 land animals and the 12 household items three different times into narrow categories (e.g., sort the land animals into foreign/domestic animals, fierce/nonfierce animals, and animals larger/smaller than a German shepherd dog). The original test involved three sorting tasks. Here we evaluated data for only the third, most difficult sorting task (maximum score = 72).
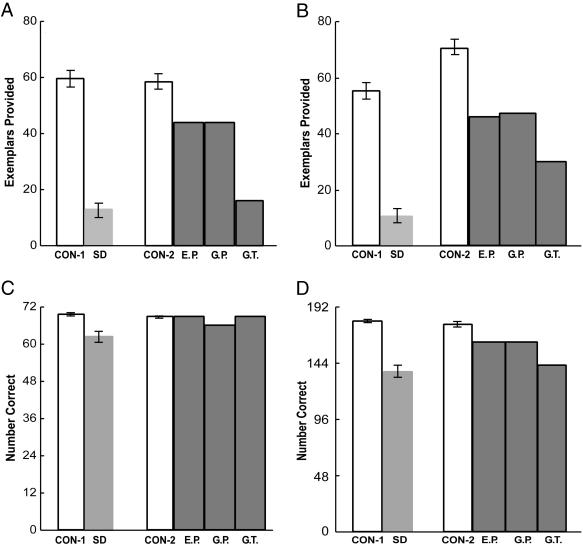
Two additional tests were included because they have been used previously to examine semantic knowledge in patients with SD or Alzheimer's disease (e.g., 26, 15). In the Object/Nonobject Discrimination Task (27, 28), participants saw line drawings of real objects and chimeric nonobjects (created by cutting and pasting parts of real objects) and were asked to indicate whether the object was real or not. The data reported for patients with SD and their controls (16) are from a test consisting of 32 real and 32 chimeric objects. The data for amnesic patients and their controls are from a similar test consisting of 30 items of each type. In the Pyramids and Palm Trees Test (29), participants were shown 52 cards, each containing a target picture and two test pictures. Patients were asked to indicate which one of the test pictures “goes with” the target picture. For example, a saddle was presented above drawings of a horse and a goat, and the participant was asked: Which one of these pictures at the bottom goes with the picture at the top?
Two tests were included to assess declarative memory (recall and recognition). For the Recognition Memory Test (30), participants studied a list of 50 words (or 50 faces) and then immediately took a two-alternative forced-choice recognition test. For the Rey-Osterrieth figure (31), participants were asked to copy a complex diagram and to reproduce it from memory after a delay. For the patients with SD and their controls, the delay was 45 min; for the amnesic patients and their controls, the delay was 12 min.
Results
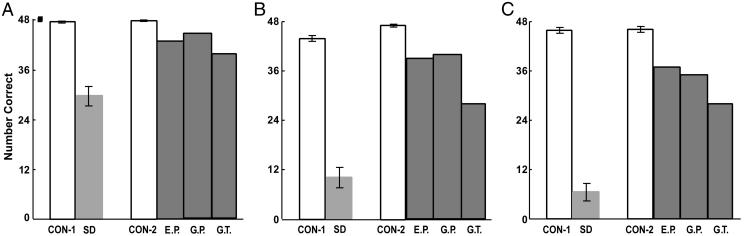
Figs. 2, 3, 4 show performance on the nine tests of semantic knowledge. The patients with SD were severely impaired relative to their controls (CON-1) on all nine tests (P < 0.05). Further, the amnesic patients with large medial temporal lobe lesions (E.P., G.P., and G.T.) were impaired relative to their control group (CON-2) on six of the nine tests (P < 0.05; all but Category Sorting, Object Decision, and Pyramids and Palm Trees). Importantly, on each of the nine tests, all three amnesic patients performed better than the mean score obtained by the patients with SD. Indeed, for 14 of 27 possible comparisons (9 tests × 3 patients), the three amnesic patients scored above the 95% confidence interval for the mean SD score.
Fig. 2.
The same 48 items were used for three different tests. (A) Participants were given the name of an item and were asked to identify the appropriate pictures from among eight pictures of the same category. (B) Participants were shown a picture of an item and were asked to name it. (C) Participants were given a verbal description of an item and asked to name it. CON-1 (n = 9) and SD (n = 9), patients with SD and their controls [data from ref. 15; in ref. 15, the naming to description (C) used 24 items rather than 48, and the means and SEMs for the CON-1 and SD groups have therefore been doubled]. CON-2 (n = 8), control group for E.P., G.P., and G.T., who are patients with large medial temporal lobe lesions and variable additional damage to the anterolateral temporal lobe (data from ref. 5). Brackets show SEM.
Fig. 3.
Participants were asked to name in 1 min as many examples as possible from each of four categories of living (A) and nonliving (B) things. (C) Participants were asked to sort 12 land animals and 12 household items into three subordinate categories each. (D) Participants were asked eight yes/no questions about semantic features (e.g., size, shape, habitat, usage) of 48 living and nonliving things. For control and patient groups, see Fig. 2. Brackets show SEM.
Fig. 4.
(A) Performance on the Pyramids and Palm Trees Test (maximum score = 52). For control and patient groups, see Fig. 2. (B) Participants were asked to judge whether line drawings depicted real or chimeric objects. Patients with SD (n = 11) and their control group (CON-4, n = 21) saw 32 real and 32 chimeric objects (data from ref. 16). Amnesic patients and their control group (CON-2, n = 4) saw 30 objects of each type (data from ref. 5). Brackets show SEM.
Of the three amnesic patients, G.T. was the most severely impaired (also see ref. 5). He obtained the lowest score of the MTL group on 6 of the 9 tests (and was tied for lowest in 2 others). Yet even G.T. performed better than the average SD patient on all nine tests, and he scored above their 95% confidence interval on four of the tests.
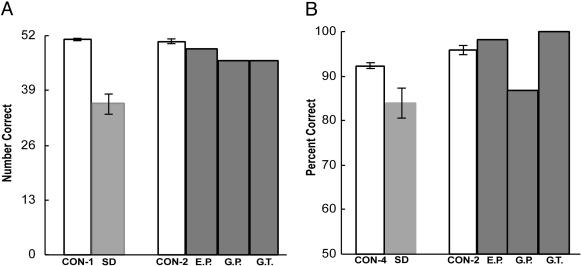
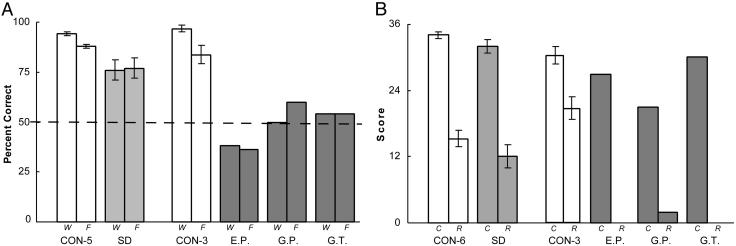
For the tests of recall and recognition, the results were the opposite. On the Words test of recognition memory (Fig. 5A), the patients with SD were impaired relative to their controls (P < 0.01), but nonetheless performed significantly above chance (P < 0.01). On the Faces test, the patients performed numerically below their controls but the difference did not reach significance. In contrast, the amnesic patients performed at chance and much more poorly than the SD patients. On the diagram test (Fig. 5B), the patients with SD were able to reproduce the figure from memory nearly as well as their control group (P > 0.10). In contrast, the three amnesic patients, despite being tested after only a 12-min delay (rather than after 45 min like the SD group) could not reproduce the diagram at all. G.P. obtained a score of 2, and E.P. and G.T. had no recollection of having seen the diagram earlier. The difference in copy scores for the amnesic patients and their controls, in comparison to the copy scores for the SD group and their controls, is most likely related to differences in how strictly the diagrams were scored.
Fig. 5.
(A) Recognition memory performance for 50 words (W) and 50 faces (F) for patients with SD (n = 11) and their control group (CON-5, n = 18, data from ref. 17; CON-3, n = 8, control data from ref. 21). The dashed line indicates chance performance. (B) Performance for the copy (C) and delayed recall (R) of the Rey-Osterreith figure for patients with SD (n = 12) and their control group (CON-6, n = 10, data for 45-min delayed recall from ref. 18; CON-3, n = 8, data for 12-min delayed recall from ref. 21. The recall score for E.P. and G.T. was zero. Brackets show SEM.
Discussion
Drawing largely on previously published data, we have compared the performance of severely amnesic patients with large medial temporal lobe lesions and patients with SD on nine tests of semantic knowledge and two tests of new learning ability. On the tests of semantic knowledge, the amnesic patients performed markedly better than the patients with SD. By contrast, on the tests of new learning, the patients with SD performed markedly better than the amnesic patients.
SD is a progressive disease with variable pathology, and the scores of some patients may differ from the scores of the patients summarized here. For example, in one study (32), patients with predominantly right temporal lobe atrophy performed noticeably worse on the Recognition Memory for Faces Test (<60% correct) than the patients with SD whose scores appear in Fig. 5A. Those patients also had significant medial temporal lobe damage, and the extent of atrophy in the right hippocampus and right parahippocampal gyrus correlated more highly with performance on the Recognition Memory for Faces Test than did other temporal areas measured. Importantly, those patients, like others with the diagnosis of SD, were also impaired on tests of semantic knowledge (e.g., they obtained a score of 36.3 on the Pyramids and Palm Trees Test; compare to Fig. 4A). Thus, although some patients with SD do exhibit impaired new learning capacity (because the pathology extends medially into the hippocampus and parahippocampal gyrus), these patients also exhibit severely impaired semantic knowledge. In that respect they differ strikingly from patients with damage that is primarily medial temporal, and we are unaware of reports that patients with SD have a pattern of impairment like that described here for medial temporal lobe amnesia.
The important point is that the amnesic patients with primarily medial temporal lobe damage exhibited only limited impairments on the tests of semantic knowledge. Among the three amnesic patients, patient G.T. had the most severe impairment on the tests of semantic knowledge and also had the most damage to anterolateral temporal cortex (although he still performed numerically better than the mean SD score on all nine tests). Further, patients E.P., G.P., and G.T. all have complete damage to the perirhinal and entorhinal cortices; yet G.T. performed worse than the other two patients on most of the tests of semantic knowledge. These findings appear to rule out a crucial contribution of the perirhinal and entorhinal cortices to deficits in semantic knowledge. This conclusion is also supported by the finding in SD that impaired semantic knowledge correlated with reduced volume of structures in lateral temporal cortex but not with reduced volume of the parahippocampal gyrus (13).
What then can be made of the observation that in patients with SD the extent of atrophy in perirhinal cortex was correlated with the severity of impaired semantic knowledge (14)? One likely possibility is that in a progressive condition like SD, which involves widespread pathology in the temporal lobes, the extent of damage in many temporal lobe areas will correlate with each other and with the severity of the condition. In this sense, the extent of atrophy in perirhinal cortex may serve as a proxy for the overall severity of dementia. Separate measurements of perirhinal cortex and other areas (for example, fusiform gyrus, anterolateral temporal pole, inferior and middle temporal gyri, and amygdala) at different disease stages could provide a test of this idea.
It is also worth emphasizing that all of the amnesic patients known to us who have significant medial temporal lobe damage that extends beyond the hippocampal region into the parahippocampal gyrus also have at least some damage to more lateral temporal cortex, even the extensively studied patient H.M. (5, 6). Accordingly, it is not possible to definitively exclude the possibility that some part of the limited impairment in semantic knowledge exhibited by the amnesic patients studied here might be caused by damage within the parahippocampal gyrus. Still, it is striking that the amnesic patients studied here have complete loss of entorhinal and perirhinal cortex; yet their semantic knowledge is much better than that of the patients with SD, and it is essentially intact on two of the tests of semantic knowledge (sorting for all three patients and object decision for E.P. and G.T.). It is also notable that patients with SD are much better at new learning than amnesic patients. These observations rule out the idea that damage to medial temporal lobe structures, including perirhinal cortex, make any large contribution to SD. Thus, it seems reasonable to conclude that medial temporal lobe damage impairs the formation of declarative memory and that semantic knowledge is impaired to the extent that more lateral damage occurs in the temporal lobe.
Acknowledgments
We thank Jennifer Frascino and Leah Swalley for assistance. This work was supported by the Medical Research Service of the Department of Veterans Affairs (National Institute of Mental Health Grant 24600) and the Metropolitan Life Foundation.
Abbreviations: SD, semantic dementia; MTL group, patients with large medial temporal lobe lesions and variable additional damage to the anterolateral temporal lobe.
References
- 1.Squire, L. R., Stark, C. E. L. & Clark, R. E. (2004) Annu. Rev. Neurosci., in press. [DOI] [PubMed]
- 2.Manns, J. R., Hopkins, R. O. & Squire, L. R. (2003) Neuron 37, 127-133. [DOI] [PubMed] [Google Scholar]
- 3.Bayley, P. J., Hopkins, R. O. & Squire, L. R. (2003) Neuron 37, 135-144. [DOI] [PubMed] [Google Scholar]
- 4.Schmolck, H., Stefanacci, L. & Squire, L. R. (2000) Hippocampus 10, 759-770. [DOI] [PubMed] [Google Scholar]
- 5.Schmolck, H., Kensinger, A., Corkin, S. & Squire, L. R. (2002) Hippocampus 12, 520-533. [DOI] [PubMed] [Google Scholar]
- 6.Corkin, S., Amaral, D. G., Gonzalez, R. G., Johnson, K. A. & Hyman, B. T. (1997) J. Neurosci. 17, 3964-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stefanacci, L., Buffalo, E. A., Schmolck, H. & Squire, L. R. (2000) J. Neurosci. 20, 7024-7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snowden, J. S., Goulding, P. J. & Neary, D. (1989) Behav. Neurol. 2, 167-182. [Google Scholar]
- 9.Hodges, J. R., Salmon, D. P. & Butters, N. (1992) Neuropsychologia 30, 301-314. [DOI] [PubMed] [Google Scholar]
- 10.Hodges, J. R. & Graham, K. S. (2001) Philos. Trans. R. Soc. London B 356, 1423-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mummery, C. J., Patterson, K., Price, C. J., Ashburner, J., Frackowiak, R. S. & Hodges, J. R. (2000) Ann. Neurol. 47, 36-45. [PubMed] [Google Scholar]
- 12.Chan, D., Fox, N. C., Scahill, R. I., Crum, W. R., Whitwell, J. L., Leschziner, G., Rossor, A. M., Stevens, J. M., Cipolotti, L. & Rossor, M. N. (2001) Ann. Neurol. 49, 433-442. [PubMed] [Google Scholar]
- 13.Galton, C. J., Patterson, K., Graham, K., Lambon-Ralph, M. A., Williams, G., Antoun, N., Sahakian, B. J. & Hodges, J. R. (2001) Neurology 57, 216-225. [DOI] [PubMed] [Google Scholar]
- 14.Davies, R. R., Xuereb, J. H. & Hodges, J. R. (2002) Neuropathol. Appl. Neurobiol. 28, 167-168. [Google Scholar]
- 15.Hodges, J. R., Patterson, K., Ward, R., Garrard, P., Bak, T., Perry, R. & Gregory, C. (1999) Neuropsychology 13, 31-40. [DOI] [PubMed] [Google Scholar]
- 16.Hovius, M., Kellenbach, M. L., Graham, K. S., Hodges, J. R. & Patterson, K. (2003) Neuropsychology 17, 100-107. [PubMed] [Google Scholar]
- 17.Lee, A. C., Rahman, S., Hodges, J. R., Sahakian, B. J. & Graham, K. S. (2003) Eur. J. Neurosci. 18, 1660-1670. [DOI] [PubMed] [Google Scholar]
- 18.Simons, J. S., Graham, K. S. & Hodges, J. R. (2002) J. Mem. Lang. 47, 197-213. [Google Scholar]
- 19.Insausti, R., Juottonen, K., Soininen, H., Insausti, A. M., Partanen, K., Vainio, P., Laakso, M. P. & Pitkanen, A. (1998) Am. J. Neuroradiol. 19, 659-671. [PMC free article] [PubMed] [Google Scholar]
- 20.Amaral D. G. & Insausti, R. (1990) in The Human Nervous System, ed. Paxinos, G. (Academic, San Diego), pp. 711-755.
- 21.Squire, L. R. & Shimamura, A. P. (1986) Behav. Neurosci. 100, 866-877. [DOI] [PubMed] [Google Scholar]
- 22.Hodges, J. R., Patterson, K., Oxbury, S. & Funnell, E. (1992) Brain 115, 1783-1806. [DOI] [PubMed] [Google Scholar]
- 23.Garrard, P., Perry, R. & Hodges, J. R. (1997) J. Neurol. Neurosurg. Psychiatry 62, 431-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodges, J. R., Patterson, K., Graham, N. & Dawson, K. (1996) Brain Lang. 54, 302-325. [DOI] [PubMed] [Google Scholar]
- 25.Snodgrass, J. G. & Vanderwart, M. (1980) J. Exp. Psychol. Hum. Learn. 6, 174-215. [DOI] [PubMed] [Google Scholar]
- 26.Breedin, S. D., Saffran, E. M. & Coslett, H. B. (1994) Cogn. Neuropsychol. 11, 617-660. [Google Scholar]
- 27.Kroll, J. F. & Potter, M. C. (1984) J. Verbal Learn. Verbal Behav. 23, 39-66. [Google Scholar]
- 28.Riddoch, M. J. & Humphreys, G. W. (1987) Cogn. Neurospsychol. 4, 131-185. [Google Scholar]
- 29.Howard, D. & Patterson, K. (1992) Pyramids and Palm Trees: A Test of Semantic Access from Pictures and Words (Thames Valley Test Company, Bury St. Edmunds).
- 30.Warrington, E. K. (1984) Recognition Memory Test (NFER-Nelson, Windsor, U.K.).
- 31.Osterrieth, P. A. (1944) Arch. Psychol. 30, 206-356. [Google Scholar]
- 32.Simons, J. S., Graham, K. S., Galton, C. J., Patterson, K. & Hodges, J. R. (2001) Neuropsychology 15, 101-114. [PubMed] [Google Scholar]