Abstract
R-Ras is a member of the Ras superfamily of small GTPases, which are regulators of various cellular processes including adhesion, survival, proliferation, trafficking and cytokine production. R-Ras is expressed by immune cells and has been shown to modulate DC function in vitro and has been associated with liver autoimmunity. We used Rras-deficient mice to study the mechanism whereby R-Ras contributes to autoimmunity using experimental autoimmune encephalomyelitis (EAE), a mouse model of the CNS autoimmune disease multiple sclerosis (MS). We found that a lack of R-Ras in peripheral immune cells resulted in attenuated EAE disease. Further investigation revealed that during EAE, absence of R-Ras promoted the formation of MHC IIlo DC concomitant with a significant increase in proliferation of natural T regulatory cells (nTreg) resulting in an increase in their cell numbers in the periphery. Our study suggests a novel role for R-Ras in promoting autoimmunity through negative regulation of nTreg numbers by inhibiting the development of MHCIIlo DC with tolerogenic potential.
Introduction
The Ras superfamily is composed of small GTPases that act as molecular switches to activate downstream signaling pathways regulating numerous cellular responses including survival, proliferation, trafficking, retention, and cytokine production (1-5). In the off state GTPases are bound to guanosine-diphosphate (GDP) and they become activated by guanine-nucleotide-exchange-factors (GEF), which exchange guanosine-triphosphate (GTP) for GDP facilitating a change in conformation and the ability to interact with downstream effectors (2). Ras family proteins are also indispensable for proper functioning of the immune system including regulating dendritic cell (DC) migration and adhesion (6). They have also been implicated in autoimmunity (3, 7). Involvement of altered Ras signaling in autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis has recently been shown (3, 8, 9). One member of the Ras family, R-Ras, is associated with cholangitis an autoimmune disease of the liver, with high expression correlating with increased incidence (7). R-Ras is expressed by immune cells including T cells, B cells and DC (10) and has been shown to enhance the phagocytic activity of macrophages (11). Recently, we identified a unique role for R-Ras in the maturation and function of DC in vitro. Rras-deficiency resulted in impaired immunologic synapse formation between DC and T cells and led to reduced T cell proliferation in response to allogenic stimulation in vitro (10).
DC are an important immune sentinel and are essential for innate immune responses and adaptive immunity via antigen presentation and co-stimulation. Although DC are essential for conferring protection against pathogens, they can also initiate self-reactive immune responses leading to autoimmunity. In contrast, DC play important roles in controlling autoimmune responses by maintaining tolerance (12-14). DC endowed with negative immune-regulatory functions are termed tolerogenic DC and have been reported to express lower surface expression of co-stimulatory and major histocompatibility complex class II (MHCII) molecules (15-18). Tolerogenic DC are thought to inhibit T cell responses at least in part by maintaining T regulatory cell (Treg) homeostasis (17, 19-23). Natural (n) Treg develop in the thymus and express the transcription factor Foxp3 (24, 25). Treg can also be induced in the periphery (iTreg) in the presence of TGF-β and IL-2 (26, 27). Treg are essential for the maintenance of tolerance to self-tissues and in their absence both humans and mice quickly acquire autoimmune syndromes (24, 28-30).
Experimental autoimmune encephalomyelitis (EAE) is a well-established animal model for the central nervous system (CNS) autoimmune disease multiple sclerosis (MS). Both MS and EAE are thought to be mediated by CD4 T cells and are characterized by inflammatory lesions, demyelination and neuronal damage within the CNS (31, 32). A role for DC in both the induction and control of EAE has been demonstrated (21). In addition, Treg have been found to be a critical inhibitor of both EAE onset and disease progression (33-37).
In this study, we investigated the mechanisms whereby R-Ras regulated T cell immune responses in vivo during EAE. We found that mice deficient in R-Ras exhibited attenuated EAE, which was dependent upon R-Ras expression in peripheral immune cells, but not the CNS. The reduced EAE severity was accompanied by a significant reduction in the absolute number of immune cells in the CNS including CD4+ T cells producing IFN-γ or IL-17. The reduced disease was not due to altered T cell priming, but was associated with a significant increase in the number of both MHCIIlo DC and Tregs in the draining lymph node and spleen. Treg from Rras−/− mice also exhibited a higher rate of proliferation as compared to WT. In addition, using both in vitro and in vivo assays, we demonstrated that MHCIIlo DC from rRas−/−, as compared to WT mice, significantly induced greater Treg expansion. These data suggest that R-Ras expression negatively regulates the formation of DC with a MHCIIlo tolerogenic phenotype thereby promoting adaptive immune responses by limiting Treg expansion.
Materials and Methods
Mice
The generation of Rras-deficient mice (Rras−/−) on the C57BL/6 background has been described previously (10). Rras+/+ mice were generated from littermates (WT) and Rras−/− mice were housed and bred in the Translational Biomedical Research Center of the Medical College of Wisconsin. B6.SJL-Ptprca Pep3b/BoyJ (WT C57BL/6 CD45.1+) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6.Cg-FoxP3tm2Tch/J mice (Foxp3EGFP) were provided by Dr. Calvin Williams (Medical College of Wisconsin) (38). All animal procedures were approved by the Institutional Animal Care and Use Committee.
Peptide and antibodies
Myelin oligodendrocyte glycoprotein 35-55 (MOG35-55) peptide (MEVGWYRSPFSRVVHLYRNGK) was generated by the Protein Core laboratory of the Blood Research Institute, BloodCenter of Wisconsin. The 2.4G2 hybridoma was obtained from the American Tissue Culture Collection. Anti-mouse CD4-APC-eFluor 780, CD25-Alexa Fluor 700, CD11b-PE, CD11b-biotin, IL-17-Alexa Fluor 647, CD11c-Biotin, B220-Biotin, CD8-Biotin, CD11b-Biotin, Foxp3-PE and streptavidin-PE Cy5.5 were purchased from eBioscience (San Diego, CA). Anti-mouse B220-PE-Texas Red, IFN-γ-PE, anti-active caspase 3-FITC and anti-human Ki67-FITC were purchased from BD Biosciences (San Diego, CA). Anti-mouse Ly-6C-APC and Ly-6G-APC-Cy7 were purchased from Biolegend (San Diego, CA). Anti-mouse/rat Neuropilin-1-APC was obtained from R&D Systems (Minneapolis, MN). Monoclonal antibodies SMI-32 (anti-nonphosphorylated neurofilament-H) and SMI-99 (anti-myelin basic protein (MBP)) were purchased from Covance (Emeryville, CA). Strepavidin Alexa 405 and goat anti-mouse Alexa 546 (H + L) were purchased from Life Sciences Advanced Technologies (St. Petersburg, FL). Anti-Biotin microbeads were purchased from MiltenyiBiotec (Auburn, CA).
EAE induction
Mice were subcutaneously immunized with 200 μg MOG35-55 peptide emulsified in a equal volume of complete Freund’s adjuvant (CFA) (Chondrex, Redmond, WA) containing 4 mg/ml mycobacterium followed by two i.p. injections of 200 ng pertussis toxin (List Biological Laboratories, Campbell, CA) 48 h apart. Clinical symptoms of EAE were scored daily as follows: 0, no disease; 1, limp tail; 1.5, hind limb ataxia; 2, hind limb paresis; 2.5, partial hind limb paralysis; 3, total hind limb paralysis; 4, hind and fore limb paralysis; and 5, death.
Cell isolation and flow cytometry
CNS infiltrating mononuclear cells were obtained as described previously (34) and single cell suspensions were prepared from the spleen and the draining brachial lymph node (BLN). Cells were stained with fluorochrome-conjugated antibodies and were acquired on a LSRII flow cytometer (BD Biosciences, San Diego, CA). Data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR). Intracellular Foxp3, Ki67, and active caspase 3 staining was performed using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience, San Diego, CA) as per manufacturer’s recommended protocol. For the detection of intracellular cytokines, mononuclear cells isolated from the CNS were stimulated in vitro with PMA (50 ng/ml) and ionomycin (500 ng/ml) (Sigma-Aldrich, St. Louis, MO) for 4 h in the presence of BD GolgiStop™ (BD Biosciences, San Diego, CA). Intracellular IFN-γ and IL-17 staining was performed using the Cytofix/Cytoperm Kit (BD Biosciences, San Diego, CA).
Histological analysis
Anesthetized mice were sequentially perfused with PBS and fixative and brain and spinal cords were collected, fixed and frozen as previously described (39). Spinal cord sections (10 μm) were stained with anti-mouse CD11b-biotin and anti-mouse MBP or with SMI-32 followed by staining with streptavidin Alexa 405 and goat anti-mouse Alexa 546. Laser scanning confocal microscopy was performed using the Olympus Fluoview FV1000 MPE Multiphoton Laser Scanning microscope as described (40).
Bone marrow (BM) chimeras
For the generation of BM chimeras, 5 × 106 donor BM cells from WT or Rras−/− mice were transplanted into lethally irradiated (two sequential doses of 575 rad each, 6 h apart, totaling 1150 rad) WT or Rras−/− recipient mice. To generate mixed BM chimeric mice, WT (CD45.1+) and Rras−/− (CD45.2+) donor BM cells were mixed in a 1:1 ratio and a total of 5 × 106 cells were transplanted into lethally irradiated (1150 rad) WT (CD45.2+) mice, and allowed to reconstitute for 8-10 weeks before induction of EAE.
T cell recall response
On day 7 after EAE induction, CD4+ cells from the BLN of WT and Rras−/− mice were sorted by negative selection using magnetic columns and labeled with 3 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Invitrogen). 1 × 105 labeled cells were cultured in the presence of 1 × 105 irradiated (3000 rad) T cell-depleted syngeneic splenocytes and varying concentrations (0.1- 50 μg/ml) of MOG35-55 peptide. The percentage of proliferating CD4+ cells was determined by CFSE dye dilution by flow cytometry.
In vitro coculture of dendritic cells and Treg
Splenic MHCIIlo and MHCIIhi CD11c+ cells were sorted from WT and Rras−/− mice on day 17 after EAE induction. CD4+EGFP+ Treg from Foxp3EGFP mice were sorted and labeled with 3 μM Cell Proliferation Dye eFluor® 670 (eBioscience, San Diego, CA). Treg (0.5 × 105) were cocultured with sorted DC (1 × 105) in the presence of anti-CD3 (2 μg/ml). Post 96 h culture, the cells were stained with CD4 and proliferation of CD4+EGFP+ cells was determined by dye dilution using flow cytometry.
In vivo maintenance and proliferation of nTreg
Splenic EGFP+ Tregs were sorted from WT Foxp3EGFP mice and labeled with 3 μM Cell Proliferation Dye eFluor® 670 (eBioscience, San Diego, CA). Tregs (0.2 × 106) were i.v. transferred into WT and Rras−/− mice on day 11 after EAE induction. Seven days later, the absolute numbers and proliferation level of the transferred CD4+EGFP+ Treg in the spleen was determined.
Statistical analysis
Data were analyzed using GraphPad prism software (GraphPad Software, Inc., La Jolla, CA) and were presented as mean ± SEM. Statistical significance was determined using the nonparametric Mann-Whitney test. p-values <0.05 were considered significant.
Results
Rras−/− mice exhibit an attenuated EAE disease course
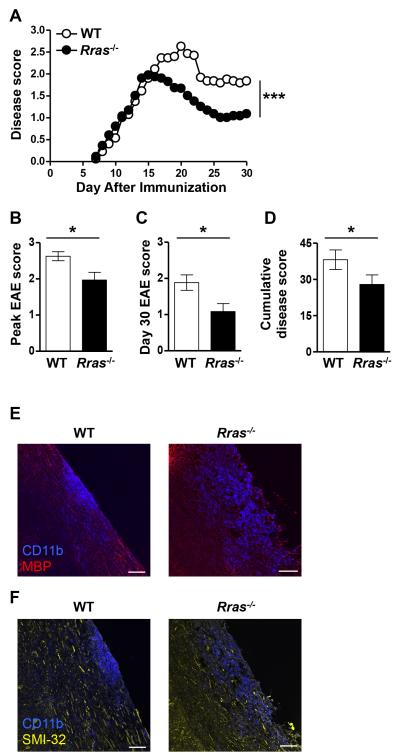
To determine whether a deficiency in R-Ras would alter T cell effector functions associated with autoimmunity in vivo, we induced EAE in WT and Rras−/− mice by immunization with MOG35-55 peptide. In this model of EAE, disease onset is dependent upon T cell priming in the LN and their subsequent migration into the CNS. WT and Rras−/− mice exhibited a similar disease onset which progressed comparably until day 15 at which time the WT mice continued to progress while disease in the Rras−/− mice peaked followed by a quick recovery (Fig. 1A). The EAE disease score in WT mice peaked on day 20, which was significantly higher than the disease score of the Rras−/− mice, which peaked on day 15 (Fig. 1B). WT mice exhibited a more severe disease course as compared to Rras−/− mice as indicated by their significantly higher disease score on day 30 (Fig. 1C) and cumulative disease score (Fig. 1D). To determine whether Rras-deficiency alters lesion formation, we generated frozen sections and localized inflammatory infiltrates by staining for CD11b, which identifies macrophages and microglial cells. We found that both WT and Rras−/− mice exhibited lesion formation accompanied with demyelination (Fig. 1E). Because R-ras could potentially alter neuronal function (41, 42), we also examined neuronal damage in areas of infiltration (CD11b+) (Fig. 1F). As shown in Fig. 1F, neuronal damage was present in both groups of mice as detected by staining with the SMI-32 antibody, which recognizes nonphosphorylated neurofilament-H and is considered a marker of neuronal damage (39, 43-45). These cumulative data demonstrate that R-Ras plays a role in promoting immune responses that lead to autoimmunity.
Figure 1. Rras−/− mice exhibit attenuated EAE disease severity.
A-F, EAE was induced in WT or Rras−/− mice at 6-8 wk of age by s.c. immunization with MOG35-55 peptide. A, Clinical signs of EAE were evaluated daily starting on day seven and the data shown are the daily average of 15 WT and 15 Rras−/− mice. B, The mean ± SE peak disease score is shown for WT mice on day 20 and day 15 for Rras−/− mice. C, The mean ± SE disease score at the end of the experiment on day 30 is shown. D, The cumulative disease score was calculated by adding the daily scores of each mouse from day 7-30 and shown as the mean ± SE of all the mice in each group. E-F, longitudinal frozen sections from the spinal cord of mice 17 days after EAE induction were generated from WT and Rras−/− mice. The sections were stained with anti-CD11b (blue) (E,F), anti-MBP (red) (E) and with the SMI-32 antibody (yellow) (F) and analyzed by immunofluorescence. Overlaid images of CD11b and MBP (E) or SMI-32 (F) are shown. Data shown are representative of two mice in each group. *p<0.05, ***p<0.001. Scale bar: 80 μm.
Attenuated EAE disease in Rras-deficient mice is associated with a reduction in immune cell infiltrates and IL-17- and IFN-γ-producing T cells in the CNS
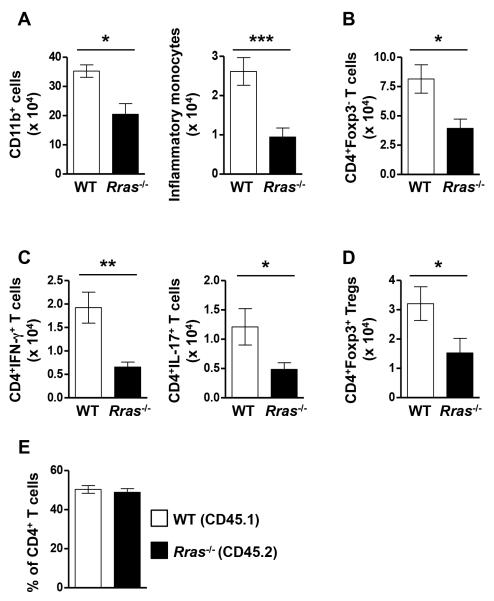
In the MOG model of EAE, myeloid and CD4+ T cells from the periphery migrate into the CNS and the pathogenic CD4 cells produce the pro-inflammatory cytokines IL-17 and IFN-γ leading to inflammation resulting in disease pathology (34, 46, 47). Therefore, the reduced EAE disease course in Rras−/− mice could be due to decreased immune cell infiltration and impaired pro-inflammatory cytokine production by T cells in the CNS. To test this possibility, we quantitated absolute cell numbers of CNS infiltrating myeloid cells (CD11b+) and CD4 cells during EAE by flow cytometry (Fig. 2). We chose day 17 post-immunization because this is the timepoint at which the WT and Rras−/− EAE curves diverged (Fig. 1A). In the CNS of Rras−/− mice, the absolute number of CD11b+ cells and inflammatory monocytes (CD11b+Ly6ChiCCR2+), thought to contribute to EAE pathology (46), were significantly reduced (Fig. 2A). Furthermore, the total number of pathogenic T cells (CD4+Foxp3−) (Fig. 2B) and those producing IFN-γ and IL-17 (Fig. 2C) were also significantly reduced in the CNS of Rras−/− mice compared to WT. In addition, the absolute number of Tregs (CD4+Foxp3+) in the CNS were also significantly reduced (Fig 2D).
Figure 2. The absolute number of CD11b+ cells, CD4+ T cells and those producing IFN-γ or IL-17 are reduced in the CNS of Rras−/− mice during EAE.
A-E, EAE was induced in WT and Rras−/− mice at 6-8 wk of age by immunization with MOG35-55 peptide. A-D, CNS mononuclear cells were isolated 17 days after EAE induction and the absolute number of total myeloid cells (CD11b+) (A), inflammatory monocytes (CD11b+Ly6C+CCR2+) (A), effector CD4 T cells (CD4+Foxp3−) (B), IFN-γ and IL-17 producing CD4+ cells (C) and Treg (CD4+Foxp3+) (D) were determined by flow cytometry. Pooled data from three (A-C) or four (D) independent experiments with nine (A,B), 8-9 (C) or 12 (D) total mice in each group are shown. E, lethally irradiated WT mice were transplanted with an equal mix of CD45.1+ WT and CD45.2+ Rras−/− BM cells to generate mixed BM chimera mice. Ten weeks after BM reconstitution, EAE was induced by immunization with MOG35-55 peptide. At the peak of EAE disease, the percentages of WT (CD45.1+) and Rras−/− (CD45.2+) cells amongst CNS infiltrating effector T cells (CD4+Foxp3−) was determined. Pooled data from 2 independent experiments with eight mice in each group are shown. *p<0.05, **p<0.01, ***p<0.001.
One explanation for the reduced number of CD4 cells in the CNS of Rras−/− mice could be due to decreased proliferation. Using Ki67, a marker expressed by cycling cells, we found no difference in proliferation between the CNS infiltrating CD4 cells from WT and Rras−/− mice at day 17 of EAE (Fig. S1A). We also determined that the reduced numbers of CNS-infiltrating CD4 cells in Rras-deficient mice was not due to enhanced apoptosis as measured by the expression of active caspase 3 (Fig. S1B). Another possibility could be an intrinsic migration defect of Rras-deficient CD4 T cells, since a role for R-Ras in cellular migration has been shown (4, 48). To address this question, we generated mixed BM chimera mice by transplanting an equal mix of WT (CD45.1) and Rras−/− (CD45.2) BM into lethally irradiated recipient mice. In the resultant chimera mice, the peripheral immune cells were an equal mix of WT and Rras−/− cells (Fig. S2). In these mixed BM chimera mice, we observed comparable numbers of WT and Rras−/− CD4 cells in the CNS at the peak of EAE disease (Fig. 2E), ruling out any intrinsic defect of Rras-deficient CD4 cells in migrating to the CNS during EAE. These data demonstrate that Rras-deficiency limits the number of peripheral immune cells in the CNS during EAE without affecting their proliferation, survival or migration into the CNS and suggests that R-Ras contributes to the regulation of pathogenic CD4 cells during EAE.
Rras-deficiency in peripheral immune cells results in reduced EAE severity
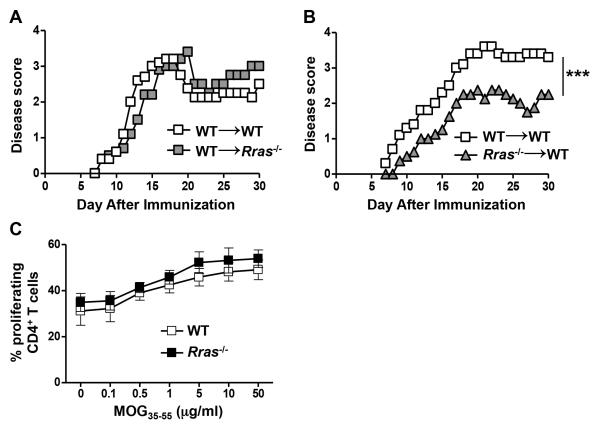
In addition to peripheral immune cells, R-Ras is expressed in the CNS by microglial cells, astrocytes and neurons, in which it is involved in axon formation and guidance (10, 41, 42, 49). Thus to determine whether R-Ras regulated EAE severity was mediated by radioresistant CNS resident cell populations, we generated BM chimera mice by transplanting WT BM mice into lethally irradiated Rras−/− mice (WT→Rras−/−) with WT→WT serving as the controls. In these chimeras, we found that the EAE disease curves were essentially identical (Fig. 3A). We then performed the reciprocal experiment to determine if R-Ras regulation was mediated by radiosensitive hematopoietic cells and found that when BM from Rras−/− mice was transplanted into WT mice (Rras−/−→WT), EAE was less severe as compared to controls with a similar disease profile as the global Rras−/− mouse (Fig. 3B versus Fig. 1A). These data suggest that R-Ras expression in the radiosensitive peripheral immune system, but not the radioresistant CNS, is required to drive pathogenic immune responses.
Figure 3. Rras-deficiency in the periphery, but not in the CNS, results in attenuated EAE severity.
A,B, chimera mice in which either the CNS and peripheral radioresistant cells (WT→Rras−/−) or the peripheral immune cells (Rras−/−→WT) were deficient in R-Ras were generated by transplanting lethally irradiated Rras−/− (A) or WT (B) recipient mice with WT (A) or Rras−/− (B) BM cells. Control chimeras were generated by transplanting WT BM into WT mice (WT→WT) (A,B). Ten weeks after BM reconstitution, EAE was induced by immunization with MOG35-55 peptide and EAE disease was scored daily starting on day 7. Representative data of two independent experiments with five mice in each group (A) or data from one experiment with six mice in the WT→WT and four mice in the Rras−/−→WT group are shown (B). C, EAE was induced in WT or Rras−/− mice with MOG35-55 peptide and seven days later purified CD4+ cells from the draining BLN were stimulated in vitro with varying concentrations of MOG35-55 peptide and their proliferation was determined by CFSE dye dilution by flow cytometry. Dead cells were excluded using DAPI. Percentages of proliferating CD4+CD11b−DAPI− cells at different concentrations of MOG35-55 peptide are shown. Pooled data from three independent experiments with six mice in each group are shown. ***p<0.001.
We have previously shown that R-Ras is required for efficient synapse formation between DC and T cells in vitro (10), thus it is possible that the absence of R-Ras in peripheral immune cells could result in ineffective priming of encephalitogenic T cells resulting in reduced disease severity. To address whether there was a CD4 T cell priming defect in Rras−/− mice, we performed a recall response using CD4 T cells purified from the draining LN at day seven post-immunization. However, we found no apparent defect in CD4 T cell priming in Rras−/− mice as determined by their similar ability to proliferate in a dose-dependent manner in an in vitro recall response compared to WT (Fig. 3C).
Increased numbers of MHCIIlo DC are present in the periphery of Rras-deficient mice during EAE
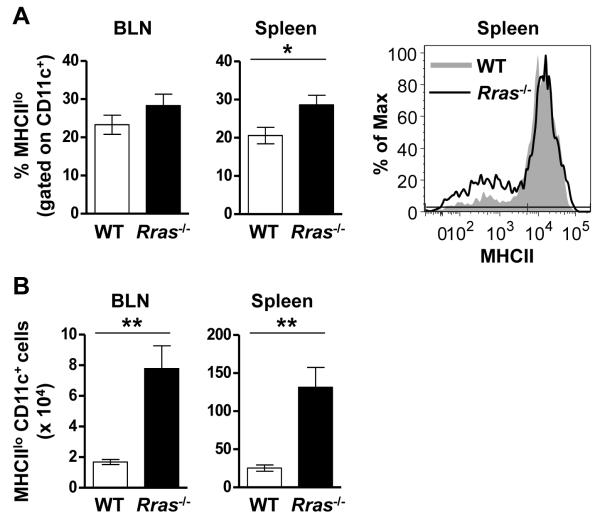
The similar T cell recall response between WT and Rras−/− mice indicates that R-Ras does not influence cognate interactions between naïve T cells and DC in the draining LN. However, R-Ras could regulate the maturation and differentiation of regulatory DC populations. To test this possibility, we phenotyped BLN and splenic CD11c+ DC for the MHCIIlo marker reported to be expressed by tolerogenic DC (15-17). We choose day 17 after EAE induction because at this timepoint EAE in the WT mice was still progressing while Rras−/− mice were undergoing recovery (Fig. 1A). When we compared the percentage of MHCIIloCD11c+ DC in the draining LN there was no difference between WT and Rras−/− mice (Fig. 4A). In contrast, a significant increase in the percentage of MHCIIlo DC was observed in the spleen of Rras−/− mice (Fig. 4A). When we quantitated the absolute number of MHCIIlo DC they were significantly increased in both the BLN and the spleen in Rras−/− mice (Fig. 4B). These data suggest that R-ras promotes an ongoing immune response by inhibiting the generation of MHCIIlo DC in the draining LN and spleen.
Figure 4. Increased numbers of MHCIIlo DC are present in the BLN and spleen of Rras−/− mice during EAE.
A,B, EAE was induced as for Fig. 1 and the presence of MHCIIlo DC was evaluated 17 days later in WT and Rras−/− mice. A, The percentage of CD11c-gated MHCIIlo cells in the BLN (left panel) and spleen (middle panel) is shown. One representative experiment showing MHCII expression levels in the spleen is shown (right panel). B, The absolute number of CD11c-gated MHCIIlo cells in the BLN (left panel) and spleen (middle panel) is shown. Data shown are the mean ± SE of two independent experiments with six mice in each group. *p<0.05, **p<0.01.
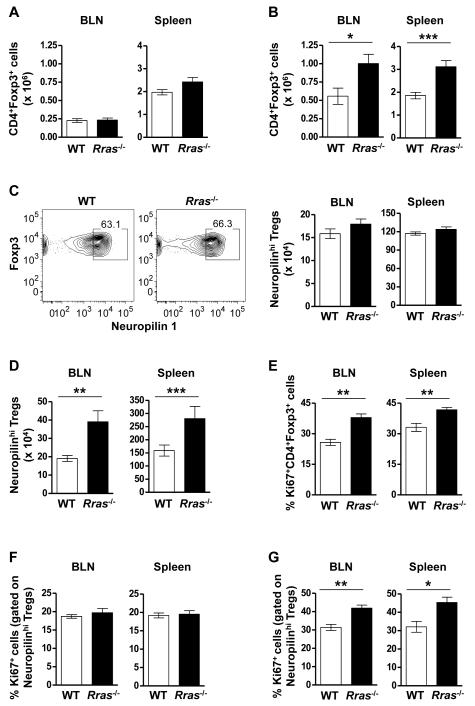
Enhanced proliferation of nTreg results in their increased numbers in the LN and spleen of Rras−/− mice during EAE
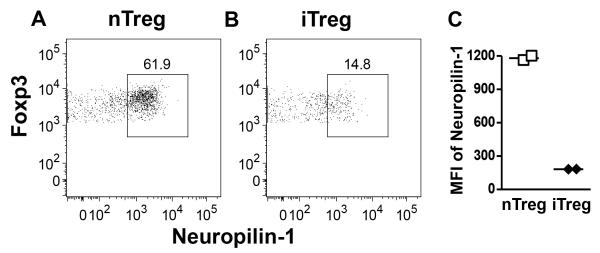
Because MHCIIlo DC are thought to be tolerogenic via the maintenance of Tregs (20), we quantitated the absolute number of CD4+Foxp3+ Treg in the BLN and the spleen at the same EAE timepoint. We first confirmed that Rras-deficiency did not alter the steady state levels of Treg in naïve mice in the BLN and spleen (Fig. 5A). We also determined that Treg from WT and Rras−/− mice were similarly suppressive in vitro (Fig. S3). When we quantitated Treg numbers during EAE, we observed a significant increase in the absolute number of Treg in the BLN and the spleen of Rras−/− mice (Fig. 5B). We next asked whether the increase in Treg was due to the generation of iTregs. To address this question, we differentiated nTreg and iTreg by Neuropilin-1 expression, which is highly expressed on nTregs in the thymus, but at a lower level on in vitro induced Treg (Fig. S4) (50, 51). In naïve mice, we found no difference in the percentage or absolute number of Foxp3+ Treg expressing high levels of Neuropilin-1 between WT and Rras−/− mice (Fig. 5C). In contrast during EAE, there was a significant increase the absolute number of Neuropilinhi nTregs in both the BLN and the spleen of Rras−/− mice (Fig. 5D). These data suggest that the increase in the number of Tregs in Rras−/− mice during EAE is not due to the generation of iTreg and likely due to the expansion of nTreg. To test this possibility, we determined the proliferation rate of Treg on day 17 of EAE and found that their proliferation was significantly increased in both the BLN and spleen of Rras−/− mice (Fig. 5E). Because this increase in proliferation could be due to abnormal homeostasis of nTreg, we determined that the steady state level of nTreg proliferation was not altered in naive Rras−/− mice in the BLN or the spleen as compared to WT mice (Fig. 5F). In contrast during EAE, Neuropilinhi nTregs in Rras−/− mice were undergoing a significantly higher rate of proliferation in both the BLN and spleen (Fig. 5G).
Figure 5. Enhanced proliferation of leads to increased numbers of nTreg in the BLN and spleen of Rras−/− mice during EAE.
A,B, The absolute number of CD4+Foxp3+ cells in the BLN (left panel) and spleen (right panel) of WT and Rras−/− mice when naïve (A) or 17 days after EAE induction (as for Fig. 1) (B) is shown. Data shown are the mean ± SE of pooled data from three to four independent experiments with 8-12 mice in each group. C,D, The percentage (C, left panel) and absolute number (C, right panel and D) of CD4+Foxp3+ cells expressing high levels of Neuropilin-1 (Neuropilinhi) was determined in the spleen (C, left and right panel and D) and the BLN (C, right panel and D) in WT and Rras−/− naïve mice (C) and 17 days after EAE induction (D). Data shown are the mean ± SE of pooled data from two to three independent experiments with 6-10 mice in each group. E,G) The proliferation of CD4+Foxp3+ (E) and CD4+Foxp3+ Neuropilinhi (F,G) cells was determined by Ki67 expression in WT and Rras−/− naïve mice (F) and 17 days after EAE induction (E,G). Data shown are the mean ± SE of pooled data from two independent experiments with 6-7 mice in each group. *p<0.05, **p<0.01, ***p<0.001.
MHCIIlo DC induce the proliferation of natural Tregs and promote enhanced maintenance of Tregs in vivo
To determine whether MHCIIlo DC were efficient inducers of Treg proliferation, we FACS purified MHCIIlo and MHCIIhi DC from the spleens of WT and Rras−/− mice on day 17 after EAE induction and co-cultured them with FACS purified WT EGFP+ Tregs. When we measured Treg proliferation, we found that MHCIIlo DC from both WT and Rras−/− mice induced higher levels of Treg proliferation as compared to MHCIIhi DC (Fig. 6A). In addition, both MHCIIlo and MHCIIhi DC from Rras−/− mice induced higher levels of Treg proliferation than the WT controls (Fig. 6A). Next, to determine whether DC-Treg interactions occur in vivo, we adoptively transferred sorted EGFP+ Tregs into WT and Rras−/− mice on day 11 after EAE induction and seven days later measured Treg recovery and proliferation. As compared to WT, 3-fold more splenic EGFP+ Tregs were recovered from Rras−/− mice (Fig. 6B). In addition, significantly more Rras−/− Treg had undergone proliferation as compared to WT (Fig. 6C). These data suggest that the increase in Treg numbers in Rras−/− mice during EAE is mediated by MHCIIlo DC via increased proliferation of nTreg increasing their numbers and cumulative suppressive capacity resulting in attenuated EAE.
Figure 6. Rras−/− MHCIIlo DC promote increased maintenance and proliferation of natural Tregs.
A, Splenic Treg (CD4+EGFP+) were FACS purified and labeled with cell proliferation dye, and cocultured with FACS purified splenic CD11c+MHCIIlo or CD11c+MHCIIhi cells from WT or Rras−/− mice at day 17 after EAE induction in the presence of soluble anti-CD3. Four days post culture, proliferation of CD4+EGFP+ Treg was determined by flow cytometry and the percentage of cells that had undergone proliferation is shown. Representative data from two independent experiments are shown. B, C, Sorted and labeled splenic Treg (CD4+EGFP+) were i.v. transferred into WT or Rras−/− mice on day 11 after EAE induction. Seven days later, the absolute number (mean ± SE) (B) and proliferation (C) of the transferred EGFP+ Tregs in the spleen of the recipient mice was determined by flow cytometry. Pooled data (B) from two independent experiments with six mice in each group or representative (C (left panel)) and pooled (C (right panel)) data from one of two experiments with three mice in each group are shown. *p<0.05, **p<0.01.
Discussion
In this study, we extended our in vitro findings that Rras-deficient DC have a maturation defect and a reduced capacity to interact with and induce the proliferation of T cells (10) to an in vivo disease model that is dependent upon T cell:DC interactions. Using EAE, we found that mice deficient in R-Ras exhibited an attenuated disease course that was associated with reduced disease parameters. Interestingly, the reduced disease severity was not due to a deficiency in peripheral T cell priming. Upon further investigation, we found that Rras−/− mice with EAE harbored increased numbers of both MHCIIlo DC and nTreg in the draining LN and spleen. The increase in nTreg in Rras−/− mice was shown to be due to their higher rate of proliferation. Collectively, these data demonstrate a novel role for R-Ras in promoting adaptive immune responses by constraining the maturation/function of MHCIIlo DC with tolerogenic potential thereby limiting the expansion of Treg.
To further study the role of R-Ras in DC function, we chose the EAE model because we have previously shown that the adoptive transfer of self-antigen loaded DC was sufficient to drive the priming of antigen-specific T cells leading to EAE (52). In addition, using in vitro assays, we previously demonstrated that Rras−/− DC had an impaired ability to induce the proliferation of allogeneic T cells and formed fewer cognate interactions with antigen-specific T cells (10). Based on these findings, we predicted that the attenuated EAE disease severity in Rras−/− mice (Fig. 1A) would be due to a deficiency in the priming of MOG-specific encephalitogenic T cells. To our surprise, we found no defect in the priming of Rras−/− CD4+ T cells (Fig. 3C). The essentially identical proliferation rates of WT and Rras−/− T cells to increasing doses of MOG35-55 peptide indicates that R-Ras does not regulate T cell proliferation. This finding is important because R-Ras as been implicated in inducing the proliferation of several nonhematopoietic cell types (11, 53).
Although DC are generally thought to play a role in the priming of encephalitogenic T cells, they were shown to be dispensable for the induction of EAE in a study whereby DC were conditionally depleted prior to immunization with MOG peptide (21, 54). Thus it is remains unclear whether R-Ras plays a role in DC priming of naïve T cells in EAE. In addition to T cell priming in the draining LN, encephalitogenic T cells must reencounter antigen within the CNS for the initiation of EAE, a process that is likely mediated by DC (55). Thus the reduction in EAE disease severity in R-Ras mice could be due to inefficient presentation of self-antigen by DC resulting in reduced activation of MOG-specific encephalitogenic T cells thereby attenuating their proliferation and effector function within the CNS. Consistent with that possibility, there were reduced numbers of myeloid cells and IFN-γ- and Th17-producing CD4 T cells in the CNS of Rras−/− mice with EAE (Fig. 2). However, when we measured proliferation and apoptosis rates in WT versus Rras−/− T cells within the CNS, we observed no difference (Fig. S1). From these cumulative data, we concluded that R-Ras deficiency does not alter DC-mediated T cell activation in vivo during EAE. However, we cannot rule out a role for R-Ras in DC antigen presentation in other disease models.
Other than immune cells, R-Ras is also expressed in neurons and plays an important role in neuronal growth cone collapse and formation of neuronal networks (41, 42). Since neuronal damage is a prominent feature of EAE (39, 56-58), R-Ras could be playing a direct role in neurons facilitating their demise. Although hematopoietic in origin, microglial cells are resident to the CNS and while their exact functions in EAE pathogenesis are not well understood they are thought to phagocytize degenerated myelin and play a critical role in CNS inflammation (59-61). Previously, it has been reported that blockade of Ras resulted in the inhibition of phagocytosis by microglia; however, R-Ras was not specifically studied (62). A specific role for R-Ras in phagocytosis was demonstrated by the microinjection of R-Ras into a macrophage cell line leading to the activation of the αMβ2 integrin and increased phagocytosis of RBC (11). Thus to determine whether CNS R-Ras was responsible for the attenuated EAE observed, we utilized a BM chimera approach whereby Rras-deficiency was restricted to the CNS and peripheral radioresistant cells. In these chimeras no difference in the onset or severity of EAE was observed (Fig. 3A). These data indicate that R-Ras plays little or no role within the CNS in the inflammatory processes leading to CNS tissue damage in EAE, including neuronal dysfunction.
Contrary to the above result, localization of the R-Ras deficiency to peripheral immune cells, using the same BM chimera approach, resulted in a recapitulation of the attenuated disease observed in the global Rras-deficient mice (Fig. 3B). This finding indicates that R-Ras impacts EAE severity outside of the CNS. A number of studies have demonstrated a role for R-Ras in cell adhesion and migration, largely through the modulation of integrin activation (5, 48, 63, 64). Since T cells require the integrin α4β1 to migrate into the CNS (65, 66), the decreased numbers of Rras−/− encephalitogenic T cells in the CNS during EAE (Fig. 2B,C) could reflect a reduction in their ability to migrate into the CNS. To test whether Rras-deficient T cells exhibit a migration defect during EAE, we generated mixed BM chimera whereby WT mice were transplanted with an equal mix of WT: Rras−/− BM cells. During EAE, we found comparable numbers of WT and Rras−/− CD4 T cells in the CNS (Fig. 2E). These data suggest that although R-Ras plays an important role in cellular migration, it is dispensable for the trafficking of CD4 T cells to the CNS during EAE. Thus R-Ras inhibitors could be used to attenuate CNS autoimmunity without affecting the migration of cells required for immune surveillance in the CNS and thus avoid the development of JC virus-mediated fatal progressive multifocal leukoencephalopathy as observed with integrin blocking therapy in MS (67, 68).
Since we didn’t observe a defect in T cell priming, proliferation or migration of Rras−/− T cells, we reasoned that the attenuated EAE was due to peripheral tolerance mechanisms. Given the important role of DC in peripheral tolerance mechanisms that restrain autoimmunity (17, 21), we phenotyped DC cell populations in the draining LN and the spleen of mice following EAE induction. Specifically, we found that in Rras−/− mice there was a significant increase in the absolute number of DC (CD11c+) expressing low levels of MHC class II (Fig. 4B). This phenotype is consistent with a tolerogenic phenotype (15-17). A primary mechanism whereby tolerogenic DC contribute to peripheral tolerance is by facilitating the homeostasis of peripheral Treg (17, 19-23). When we quantitated Treg numbers in the BLN and the spleen of unmanipulated WT and Rras−/− mice we found no difference (Fig. 5A), indicating that R-Ras is not required for Treg homeostasis in the steady state. In contrast, Treg cell numbers were significantly increased in both the spleen and BLN of Rras−/− mice with EAE (Fig. 5B), which is consistent with a similar increase in MHCIIlo DC (Fig. 4B). Furthermore, MHCIIlo DC from Rras−/− EAE mice induced increased proliferation of Tregs in vitro (Fig. 6A), and 3-fold more EGFP+ transferred Tregs, displaying significantly increased cell cycle progression, were recovered from Rras−/− mice compared to WT (Fig. 6B). In addition to Treg homeostasis, DC are also potent inducers of peripheral iTreg (69, 70). Recently, the expression of Neuropilin-1 has been used to distinguish between nTreg and iTreg, with nTreg expressing high and iTreg low levels of Neuropilin-1 (Fig. S4) (50, 51). When we characterized Neuropilin-1 expression on Treg during EAE, the number of Neuropilinhi nTreg were significantly increased in Rras−/− mice (Fig. 5D). Consistent with a role for DC in Treg expansion, there was a significant increase in the percentage of nTreg undergoing proliferation in Rras-deficient mice (Fig. 5G). Recently, it was shown that the stability and function of Treg is maintained by Neuropilin-1-semaphorin-4A interactions (71). In the nervous system, semaphoring-4A represses R-Ras leading to growth cone collapse (41, 42). Thus Neuropilin-1-semaphorin-4A signaling may require the removal of R-Ras in order to promote survival. Our data demonstrating that Tregs lacking R-Ras undergo greater proliferation during EAE is consistent with R-Ras exerting a negative effect on Treg. In other words, when R-Ras is absent in Treg the brake preventing Neuropilin-1 signaling via interaction with semaphorin-4 on DC is absent and the cells undergo greater levels of proliferation.
Our cumulative data suggest that R-Ras plays a role in promoting adaptive immune responses by curtailing the generation of MHCIIlo DC thereby limiting Treg expansion. Specifically, we found the R-Ras regulates the expansion of nTreg and not the generation of iTreg. Since R-Ras was globally knocked out in this study, we cannot rule out the possibility of a DC-independent expansion of nTreg during EAE. We recently demonstrated that the increased disease severity in B cell-deficient mice is due to an essential role for B cells in the homeostatic expansion of Treg (34). Thus it is highly possible that R-Ras also modulates B cell regulatory activity.
Although it is not known how R-Ras alters the generation of MHCIIlo DC, one possibility is DC stimulation through toll-like receptor (TLR) ligands present in CFA used for EAE induction. Indeed, we previously found a defect in the maturation of Rras−/− DC in vitro in response to LPS, a TLR4 ligand (10). During EAE, in the absence of R-Ras, TLR signaling might lead to the generation of immature MHCIIlo DC that display a tolerogenic phenotype leading to attenuated disease severity suggesting that R-Ras enhances TLR signaling and DC maturation. How R-Ras signaling could promote DC maturation is unknown. Indeed very little is known regarding R-Ras signaling pathways, which unlike Ras does not include the activation of ERK, JNK or p38/Mpks MAP kinases (11, 72, 73), all considered to be pro-inflammatory signaling molecules important for DC function. However, R-Ras does bind phosphatidylinositol 3-kinase α (73), which regulates a number of cellular processes. In addition to regulating DC maturation, it is also possible that an Rras-deficiency has a direct affect on Treg expansion and function. We have largely ruled out this possibility by showing that Treg numbers are not reduced in Rras−/− mice (Fig. 5A) and that Rras-deficient Treg isolated from the spleens of mice with are equally suppressive to WT in vitro (Fig. S3).
Given that Ras and R-Ras, at least in part, utilize distinct signaling pathways it should be possible to generate a specific inhibitor for R-Ras to facilitate the generation of MHCIIlo DC and the expansion of Treg for the treatment of autoimmunity. Along this line, the pharmacological Ras inhibitor Farnesythiosalicylic acid (FTS) has been shown to attenuate experimental autoimmune diseases (74-79), including EAE (80, 81). However, the role of individual Ras family members was not elucidated. Ras proteins undergo prenylation, either farnesylation or geranylgeranylation, for anchoring to inner membranes and FTS functions by inhibiting the prenylation of Ras disrupting membrane localization thereby enhancing their degradation (82, 83). Although FTS blocks farnesylation and inhibits several members of the Ras family including H-Ras, N-Ras and K-Ras, it does not affect R-Ras activity, which unlike other Ras family proteins undergoes geranylgeranylation to anchor to membrane proteins (84). This differential anchoring mechanism provides a target for the development of specific R-Ras inhibitors.
In summary, we identified a novel role for R-Ras in promoting the adaptive immune response leading to EAE. Of clinical relevance is our finding that lack of R-Ras resulted in increased Treg proliferation. This finding has implications for the development of Treg therapies aimed at enhancing Treg numbers for combating autoimmunity and the induction of immune tolerance during transplantation. Furthermore, these studies identify a role of R-Ras in the generation of MHCIIlo DC, which suggests that R-Ras could be specifically targeted on DC to either to block its function or enhance its activity enhancing DC-based treatments strategies for the treatment of autoimmune diseases and cancer.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Xiaocai Yan and Shelley Morris for assistance with the mice and technical help, Dr. Calvin Williams for providing the Foxp3EGFP mice and purified TGF-β, and Dr. Subramaniam Malarkannan for providing anti-mouse CD28.
References
- 1.Czyzyk J, Chen HC, Bottomly K, Flavell RA. p21 Ras/impedes mitogenic signal propagation regulates cytokine production and migration in CD4 T cells. J. Biol. Chem. 2008;283:23004–23015. doi: 10.1074/jbc.M804084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson DS, Chen YH. Ras family of small GTPases in immunity and inflammation. Curr. Opin. Pharmacol. 2012;12:458–463. doi: 10.1016/j.coph.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reedquist KA, Tak PP. Signal transduction pathways in chronic inflammatory autoimmune disease: small GTPases. Open Rheumatol. J. 2012;6:259–272. doi: 10.2174/1874312901206010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang X, Cancelas JA, Li L, Guo F, Liu W, Johnson JF, Ficker A, Daria D, Geiger H, Ratner N, Zheng Y. R-Ras and Rac GTPase cross-talk regulates hematopoietic progenitor cell migration, homing, and mobilization. J. Biol. Chem. 2011;286:24068–24078. doi: 10.1074/jbc.M111.226951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Vuori K, Wang H, Reed JC, Ruoslahti E. Integrin activation by R-ras. Cell. 1996;85:61–69. doi: 10.1016/s0092-8674(00)81082-x. [DOI] [PubMed] [Google Scholar]
- 6.Katagiri K, Ohnishi N, Kabashima K, Iyoda T, Takeda N, Shinkai Y, Inaba K, Kinashi T. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat. Immunol. 2004;5:1045–1051. doi: 10.1038/ni1111. [DOI] [PubMed] [Google Scholar]
- 7.Zhao R, Li H, Shen C, Zheng S. RRAS: A key regulator and an important prognostic biomarker in biliary atresia. World J. Gastroenterol. 2011;17:796–803. doi: 10.3748/wjg.v17.i6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King PD. Lupus-like autoimmunity caused by defects in T-cell signal transduction. Curr. Opin. Investig. Drugs. 2004;5:517–523. [PubMed] [Google Scholar]
- 9.Yasuda S, Stevens RL, Terada T, Takeda M, Hashimoto T, Fukae J, Horita T, Kataoka H, Atsumi T, Koike T. Defective expression of Ras guanyl nucleotide-releasing protein 1 in a subset of patients with systemic lupus erythematosus. J. Immunol. 2007;179:4890–4900. doi: 10.4049/jimmunol.179.7.4890. [DOI] [PubMed] [Google Scholar]
- 10.Singh G, Hashimoto D, Yan X, Helft J, Park PJ, Ma G, Qiao RF, Kennedy CR, Chen SH, Merad M, Chan AM. R-Ras is required for murine dendritic cell maturation and CD4+ T-cell priming. Blood. 2012;119:1693–1701. doi: 10.1182/blood-2011-05-357319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Self AJ, Caron E, Paterson HF, Hall A. Analysis of R-Ras signalling pathways. J. Cell. Sci. 2001;114:1357–1366. doi: 10.1242/jcs.114.7.1357. [DOI] [PubMed] [Google Scholar]
- 12.Amodio G, Gregori S. Dendritic cells a double-edge sword in autoimmune responses. Front. Immunol. 2012;3:233. doi: 10.3389/fimmu.2012.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immuno.l. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 15.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J. Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 16.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 17.Hubert P, Jacobs N, Caberg JH, Boniver J, Delvenne P. The cross-talk between dendritic and regulatory T cells: good or evil? J. Leukoc. Biol. 2007;82:781–794. doi: 10.1189/jlb.1106694. [DOI] [PubMed] [Google Scholar]
- 18.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Ad.v Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat. Rev. Immunol. 2013;13:566–577. doi: 10.1038/nri3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J. Exp. Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-β-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 25.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 26.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J. Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 28.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 29.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 30.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 31.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 32.Croxford AL, Kurschus FC, Waisman A. Mouse models for multiple sclerosis: historical facts and future implications. Biochim. Biophys. Acta. 2011;1812:177–183. doi: 10.1016/j.bbadis.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Gultner S, Kuhlmann T, Hesse A, Weber JP, Riemer C, Baier M, Hutloff A. Reduced Treg frequency in LFA-1-deficient mice allows enhanced T effector differentiation and pathology in EAE. Eur. J. Immunol. 2010;40:3403–3412. doi: 10.1002/eji.201040576. [DOI] [PubMed] [Google Scholar]
- 34.Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J. Immunol. 2012;188:3188–3198. doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B Cells (B10 Cells) and Regulatory T Cells Have Independent Roles in Controlling Experimental Autoimmune Encephalomyelitis Initiation and Late-Phase Immunopathogenesis. J. Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGeachy MJ, Stephens LA, Anderton SM. Natural Recovery and Protection from Autoimmune Encephalomyelitis: Contribution of CD4+CD25+ Regulatory Cells within the Central Nervous System. J. Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 37.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 38.Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J. Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 39.Shriver LP, Dittel BN. T-cell-mediated disruption of the neuronal microtubule network: correlation with early reversible axonal dysfunction in acute experimental autoimmune encephalomyelitis. Am. J. Pathol. 2006;169:999–1011. doi: 10.2353/ajpath.2006.050791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller NM, Shriver LP, Bodiga VL, Ray A, Basu S, Ahuja R, Jana A, Pahan K, Dittel BN. Lymphocytes with cytotoxic activity induce rapid microtubule axonal destabilization independently and before signs of neuronal death. ASN NEURO. 2013;5:e00105. doi: 10.1042/AN20120087. doi:10.1042/AN20120087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- 42.Iwasawa N, Negishi M, Oinuma I. R-Ras controls axon branching through afadin in cortical neurons. Mol. Biol. Cell. 2012;23:2793–2804. doi: 10.1091/mbc.E12-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl. J. Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 44.Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126:515–530. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- 45.Petzold A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J. Neurol. Sci. 2005;233:183–198. doi: 10.1016/j.jns.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 46.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat. Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wozniak MA, Kwong L, Chodniewicz D, Klemke RL, Keely PJ. R-Ras controls membrane protrusion and cell migration through the spatial regulation of Rac and Rho. Mol. Biol. Cel.l. 2005;16:84–96. doi: 10.1091/mbc.E04-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhai J, Strom AL, Kilty R, Venkatakrishnan P, White J, Everson WV, Smart EJ, Zhu H. Proteomic characterization of lipid raft proteins in amyotrophic lateral sclerosis mouse spinal cord. FEBS J. 2009;276:3308–3323. doi: 10.1111/j.1742-4658.2009.07057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, Yang Y, Floess S, Huehn J, Oh S, Li MO, Niec RE, Rudensky AY, Dustin ML, Littman DR, Lafaille JJ. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 2012;209:1723–1742. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, Bluestone JA. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 2012;209:1713–1722. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dittel BN, Visintin I, Merchant RM, Janeway CA., Jr Presentation of the self antigen myelin basic protein by dendritic cells leads to experimental autoimmune encephalomyelitis. J. Immunol. 1999;163:32–39. [PubMed] [Google Scholar]
- 53.Yu Y, Feig LA. Involvement of R-Ras and Ral GTPases in estrogen-independent proliferation of breast cancer cells. Oncogene. 2002;21:7557–7568. doi: 10.1038/sj.onc.1205961. [DOI] [PubMed] [Google Scholar]
- 54.Isaksson M, Lundgren BA, Ahlgren KM, Kampe O, Lobell A. Conditional DC depletion does not affect priming of encephalitogenic Th cells in EAE. Eur. J. Immunol. 2012;42:2555–2563. doi: 10.1002/eji.201142239. [DOI] [PubMed] [Google Scholar]
- 55.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 56.Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, Linington C, Schmidbauer M, Lassmann H. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 2000;157:267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogt J, Paul F, Aktas O, Muller-Wielsch K, Dorr J, Dorr S, Bharathi BS, Glumm R, Schmitz C, Steinbusch H, Raine CS, Tsokos M, Nitsch R, Zipp F. Lower motor neuron loss in multiple sclerosis and experimental autoimmune encephalomyelitis. Ann. Neurol. 2009;66:310–322. doi: 10.1002/ana.21719. [DOI] [PubMed] [Google Scholar]
- 58.Siffrin V, Radbruch H, Glumm R, Niesner R, Paterka M, Herz J, Leuenberger T, Lehmann SM, Luenstedt S, Rinnenthal JL, Laube G, Luche H, Lehnardt S, Fehling HJ, Griesbeck O, Zipp F. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity. 2010;33:424–436. doi: 10.1016/j.immuni.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 59.Rinner WA, Bauer J, Schmidts M, Lassmann H, Hickey WF. Resident microglia and hematogenous macrophages as phagocytes in adoptively transferred experimental autoimmune encephalomyelitis: an investigation using rat radiation bone marrow chimeras. Glia. 1995;14:257–266. doi: 10.1002/glia.440140403. [DOI] [PubMed] [Google Scholar]
- 60.Sierra A, Abiega O, Shahraz A, Neumann H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front. Cell. Neurosci. 2013;7:6. doi: 10.3389/fncel.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neurosci. Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 62.Rotshenker S, Reichert F, Gitik M, Haklai R, Elad-Sfadia G, Kloog Y. Galectin-3/MAC-2, Ras and PI3K activate complement receptor-3 and scavenger receptor-AI/II mediated myelin phagocytosis in microglia. Glia. 2008;56:1607–1613. doi: 10.1002/glia.20713. [DOI] [PubMed] [Google Scholar]
- 63.Holly SP, Larson MK, Parise LV. The unique N-terminus of R-ras is required for Rac activation and precise regulation of cell migration. Mol. Biol. Cell. 2005;16:2458–2469. doi: 10.1091/mbc.E03-12-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sethi T, Ginsberg MH, Downward J, Hughes PE. The small GTP-binding protein R-Ras can influence integrin activation by antagonizing a Ras/Raf-initiated integrin suppression pathway. Mol. Biol. Cell. 1999;10:1799–1809. doi: 10.1091/mbc.10.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 66.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J. Exp. Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N. Engl. J. Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 68.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 69.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 70.Sela U, Olds P, Park A, Schlesinger SJ, Steinman RM. Dendritic cells induce antigen-specific regulatory T cells that prevent graft versus host disease and persist in mice. J. Exp. Med. 2011;208:2489–2496. doi: 10.1084/jem.20110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, Vignali DA. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osada M, Tolkacheva T, Li W, Chan TO, Tsichlis PN, Saez R, Kimmelman AC, Chan AM. Differential roles of Akt, Rac, and Ral in R-Ras-mediated cellular transformation, adhesion, and survival. Mol. Cell. Bio.l. 1999;19:6333–6344. doi: 10.1128/mcb.19.9.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marte BM, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. R-Ras can activate the phosphoinositide 3-kinase but not the MAP kinase arm of the Ras effector pathways. Cur. Biol. 1997;7:63–70. doi: 10.1016/s0960-9822(06)00028-5. [DOI] [PubMed] [Google Scholar]
- 74.Aizman E, Mor A, George J, Kloog Y. Ras inhibition attenuates pancreatic cell death and experimental type 1 diabetes: possible role of regulatory T cells. Eur. J. Pharmacol. 2010;643:139–144. doi: 10.1016/j.ejphar.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 75.Kafri M, Kloog Y, Korczyn AD, Ferdman-Aronovich R, Drory V, Katzav A, Wirguin I, Chapman J. Inhibition of Ras attenuates the course of experimental autoimmune neuritis. J. Neuroimmunol. 2005;168:46–55. doi: 10.1016/j.jneuroim.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 76.Katzav A, Kloog Y, Korczyn AD, Molina V, Blank M, Shoenfeld Y, Chapman J. Inhibition of ras by farnesylthiosalicylate significantly reduces the levels of autoantibodies in two animal models of the antiphospholipid syndrome. Immunobiol. 2003;207:47–50. doi: 10.1078/0171-2985-00208. [DOI] [PubMed] [Google Scholar]
- 77.Katzav A, Kloog Y, Korczyn AD, Niv H, Karussis DM, Wang N, Rabinowitz R, Blank M, Shoenfeld Y, Chapman J. Treatment of MRL/lpr mice, a genetic autoimmune model, with the Ras inhibitor, farnesylthiosalicylate (FTS) Clin. Exp. Immuno.l. 2001;126:570–577. doi: 10.1046/j.1365-2249.2001.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mor A, Kloog Y, Keren G, George J. Ras inhibition increases the frequency and function of regulatory T cells and attenuates type-1 diabetes in non-obese diabetic mice. Eur. J. Pharmacol. 2009;616:301–305. doi: 10.1016/j.ejphar.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 79.Oron T, Elad-Sfadia G, Haklai R, Aizman E, Brazowski E, Kloog Y, Reif S. Prevention of induced colitis in mice by the ras antagonist farnesylthiosalicylic acid. Dig. Dis. Sci. 2012;57:320–326. doi: 10.1007/s10620-011-1880-y. [DOI] [PubMed] [Google Scholar]
- 80.Aizman E, Mor A, Chapman J, Assaf Y, Kloog Y. The combined treatment of Copaxone and Salirasib attenuates experimental autoimmune encephalomyelitis (EAE) in mice. J. Neuroimmunol. 2010;229:192–203. doi: 10.1016/j.jneuroim.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 81.Karussis D, Abramsky O, Grigoriadis N, Chapman J, Mizrachi-Koll R, Niv H, Kloog Y. The Ras-pathway inhibitor, S-trans-trans-farnesylthiosalicylic acid, suppresses experimental allergic encephalomyelitis. J. Neuroimmunol. 2001;120:1–9. doi: 10.1016/s0165-5728(01)00385-x. [DOI] [PubMed] [Google Scholar]
- 82.Aharonson Z, Gana-Weisz M, Varsano T, Haklai R, Marciano D, Kloog Y. Stringent structural requirements for anti-Ras activity of S-prenyl analogues. Biochim. Biophys. Acta. 1998;1406:40–50. doi: 10.1016/s0925-4439(97)00077-x. [DOI] [PubMed] [Google Scholar]
- 83.Haklai R, Weisz MG, Elad G, Paz A, Marciano D, Egozi Y, Ben-Baruch G, Kloog Y. Dislodgment and accelerated degradation of Ras. Biochem. 1998;37:1306–1314. doi: 10.1021/bi972032d. [DOI] [PubMed] [Google Scholar]
- 84.Wurtzel JG, Kumar P, Goldfinger LE. Palmitoylation regulates vesicular trafficking of R-Ras to membrane ruffles and effects on ruffling and cell spreading. Small GTPases. 2012;3:139–153. doi: 10.4161/sgtp.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.