Abstract
Relatively little attention has been devoted to the risks from mercury in saltwater fish, that were caught by recreational fisherfolk. Although the US Food and Drug Administration has issued advisories based on mercury for four saltwater species or groups of fish, there are few data on how mercury levels vary by size, season, or location. This paper examines total mercury levels in muscle of bluefish (Pomatomus saltatrix) collected from coastal New Jersey, mainly by recreational fishermen. Of primary interest was whether there were differences in mercury levels as a function of location, weight and length of the fish, and season, and in what risk mercury posed to the food chain, including people. Selenium was also measured because of its reported protective effects against mercury. Mercury levels averaged 0.35±0.02 (mean and standard error) ppm, and selenium levels averaged 0.37±0.01ppm (N = 206). In this study, 41% of the fish had mercury levels above 0.3 ppm, 20% had levels above 0.5 ppm, and 4% had levels above 1 ppm. Size was highly correlated with mercury levels, but not with selenium. While selenium levels did not vary at all with season, mercury levels decreased significantly. This relationship was not due to differences in the size of fish, since the fish collected in the summer were the smallest, but had intermediate mercury levels. Mercury levels declined from early June until November, particularly for the smaller-sized fish. While there were significant locational differences in mercury levels (but not selenium), these differences could be a result of size. The levels of mercury in bluefish are not sufficiently high to cause problems for the bluefish themselves, based on known adverse health effects levels, but are high enough to cause potential adverse health effects in sensitive birds and mammals that eat them, and to provide a potential health risk to humans who consume them. Fish larger than 50cm fork length averaged levels above 0.3 ppm, suggesting that eating them should be avoided by pregnant women, children, and others who are at risk.
Keywords: Mercury, Fish, Bluefish, Atlantic Ocean, New Jersey, Consumption, Risk assessment, US FDA
1. Introduction
While fish are an important source of protein for many people, fishing provides recreational, cultural and aesthetic pleasures (Toth and Brown, 1997; Harris and Harper, 1998; Burger, 2000, 2002). High fishing rates occur in a wide range of cultures, including rural recreation and within urban areas (Burger et al., 1999, 2001a, b; Bienenfeld et al., 2003), among Native Americans (Harris and Harper, 1998, 2000; Burger, 1999; Burger et al. 2007a,b; Harper and Harris 2008), and in other regions of the world (Burger et al., 2003). Further, within aquatic ecosystems, fish play a key role in the food chain and community dynamics. Changes in fish populations brought about by either contaminants or overfishing can have effects on prey populations and their predators.
Fish are an excellent, low-fat source of protein that contributes to low blood cholesterol (Anderson and Wiener, 1995). Fish contain omega-3 (n-3) fatty acids that reduce cholesterol levels and the incidence of heart disease, stroke, and pre-term delivery (Daviglus et al., 2002; Patterson, 2002). Yet levels of PCBs and mercury in some fish are high enough to potentially cause effects on the fish themselves, on top-level predators, and on people (WHO 1989; NRC, 2000; Consumer Reports, 2003). In humans, levels of methylmercury are sufficiently high in some fish to cause adverse health effects if consumed too often or in large enough quantities (IOM, 1991, 2006; Grandjean et al., 1997; Gochfeld, 2003; Hightower and Moore, 2003; Hites et al., 2004), particularly in offspring (Amin-zaki et al., 1978; Crump et al., 1998; Steuerwald et al., 2000). Methylmercury can counteract the cardioprotective effects of fish consumption (Guallar et al., 2002; Rissanen et al., 2000; Salonen et al., 1995) and damage developing fetuses and young children (NRC, 2000), leading to significant behavioral deficits in children (JECFA, 2003).
The major source of methylmercury for fish is from mercury that has been methylated after atmospheric transport and precipitation or runoff, followed by food chain biomagnification (Montiero et al., 1996; Downs et al., 1998). Fish consumption is the only significant source of methylmercury exposure for the public (Rice et al., 2000). Communities that rely on fish intake for daily nutrient sustenance may be at risk from chronic, high exposure to methylmercury (Grandjean et al., 1997) as well as other persistent organic pollutants. Similarly, high-end fish consumers, whether recreational or subsistence, are at risk from mercury exposure (Hightower and Moore, 2003). The US Food and Drug Administration (US FDA, 2001, 2005) issued a series of consumption advisories based on methylmercury that suggested that pregnant women and women of childbearing age who may become pregnant should limit their fish consumption, should avoid eating four types of marine fish (shark, swordfish, king mackerel, tilefish), and should also limit their consumption of all other fish to just 12 ounces per week (US FDA, 2001, 2003).
Considerable attention has been devoted to mercury levels in a wide range of freshwater fish, where variations in pH can account for up to 70% of the variation in mercury levels (Haines et al., 1992, 1994; Watras et al., 1998), but less research has been devoted to marine ecosystems (Legrand et al., 2005). Similarly, much of the data dealing with the effects of fish size on mercury levels comes from freshwater fish. Yet for many coastal states, consumption of saltwater fish is an important potential source of mercury exposure that has been largely ignored until recently.
In this study the levels of total mercury and selenium in bluefish (Pomatomus satlatrix) from coastal New Jersey are examined as a function of location, size, and season Levels of selenium were analyzed because selenium is thought to be protective for mercury exposure, either by protecting against the harmful effects, or affecting absorption of mercury (Satoh et al., 1985). Fish are an important dietary item of the people living along coastal New Jersey, and people often freeze bluefish for consumption at all times of the year (Pottern et al., 1989; Burger, 2005; Gobeille et al., 2005). It is therefore important to understand how to reduce the risk from mercury. While newer techniques, such as fin clips (Rolfhus et al., 2008) offer nonlethal methods for monitoring mercury in fish, it remains important to actually test fish that people are catching and eating.
2. Methods
Bluefish were collected from several sites along the New Jersey shore (Fig. 1), mainly from recreational fisherfolk who were either fishing individually or were taking part in fishing tournaments. In many coastal regions there are a number of fishing tournaments that focus on bluefish. Fish from tournaments were either taken home for consumption by the families of the fishermen, or were donated to orphanages or other facilities. In addition, some bluefish were collected from the NJ Department of Environmental Protection trawls; these samples were collected to obtain fish smaller than the legal size limit allowed.
Fig. 1.
Map showing the locations of sampling for bluefish from New Jersey (2005–2008).
Fish were kept in coolers, covered with ice, brought to the Environmental and Occupational Health Sciences Institute (EOHSI) of Rutgers University where they were immediately frozen and subsequently dissected for analysis. At EOHSI, a 2 g (wet weight) sample of fish tissue was digested in ultrex ultrapure nitric acid in a microwave (MD 2000 CEM), using a digestion protocol of three stages of 10 min each under 50, 100 and 150 lb in.2 (3.5, 7, and 10.6 kg/cm2) at 80× power. Digested samples were subsequently diluted in 100 ml deionized water. All laboratory equipment and containers were washed in 10% HNO3 solution and deionized water rinse prior to each use (Burger et al., 2001a).
Mercury was analyzed by the cold vapor technique using the Perkin Elmer FIMS-100 mercury analyzer, with an instrument detection level of 0.2 ng/g, and a matrix level of quantification of 0.002 μg/g. Selenium was analyzed by graphite furnace atomic absorption, with Zeeman correction. All concentrations are expressed in parts per million (ppm = μg/g) on a wet weight basis. Many studies have shown that almost all of the mercury in fish tissue is methylmercury, and 90% is a reasonable approximation of this proportion (Duffy et al., 1999), which does vary somewhat among fish types and laboratories, but not by age of the fish (Lansens et al., 1991). However, Bloom (1991) reported that over 95% of mercury present in fish is methylmercury, and that lower levels may have been biased by analytical and homogeneity variability.
A DORM-2 Certified dogfish tissue was used as the calibration verification standard. Recoveries between 90% and 110% were accepted to validate the calibration. All specimens were run in batches that included blanks, a standard calibration curve, 2 spiked specimens, and one duplicate. The accepted recoveries for spikes ranged from 85% to 115%; no batches were outside of these limits. 10% of samples were digested twice and analyzed as blind replicates (with agreement within 15%). For further quality control on mercury, the laboratory periodically runs a random subset of samples in the Quebec Laboratory of Public Health; the correlation between the two laboratories is over 0.90 (p<0.0001, see Burger and Gochfeld, 2004).
Multiple regression procedures were used to determine if tissue, length, weight or age contributed to explaining the variations in amount of mercury in samples (PROC GLM, SAS, 1995). The procedure adds the variable that contributes the most to the R2, then adds the next variable that increases the R2 the most, continuing until all significant variables are added. Thus, variables that vary colinearly are entered only if they add independently to explaining the variation. Size and weight were used as continuous variables, although we present size data by class in the figures and tables. Kruskal–Wallis non-parametric one-way analysis of variance (generating a χ2 statistic) was used to examine differences among tissues and size measurements. Kendall correlations were used to examine relationships among metals and size variables. The level for significance was designated as p<0.05.
3. Results
For the 206 fish examined in this study, mercury levels averaged 0.35±0.02 ppm, and selenium levels averaged 0.37±0.01 ppm. In this study, 41% of the fish had mercury levels above 0.3 ppm, 20% had levels above 0.5 ppm, and 4% had levels above 1 ppm. The model explaining the most variation in mercury levels (F = 49.1, p<0.0001) in bluefish accounted for 64% of the differences by weight (F = 14.3, p<0.0002) and season (F = 9.0, p<0.0002). No model was significant in explaining variations in selenium levels in bluefish, and there was no significant variations as a function of catch location, fish size or season (see tables below).
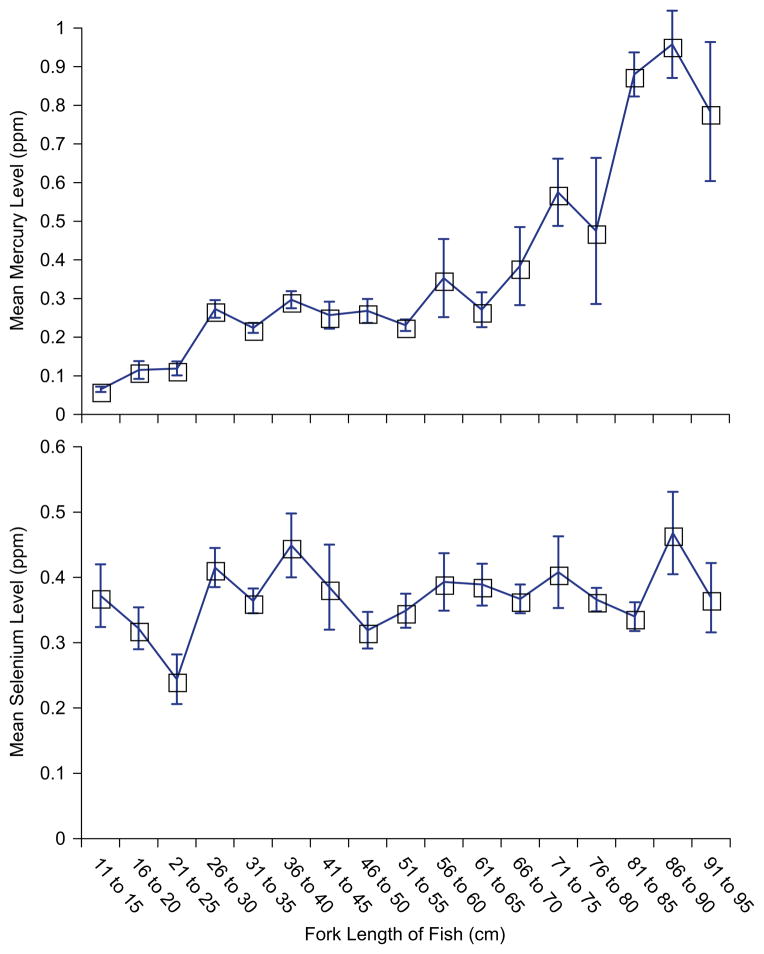
Size (length and weight) was highly correlated with mercury levels in bluefish, but not with selenium (Table 1). As expected, length and weight were also highly correlated. By inspection, there seemed to be two plateaus in mercury levels in bluefish (Fig. 2). Fish less than 25cm in fork length had lower levels than those between 26 and 50 cm, but thereafter levels increased quickly (Fig. 2). As might be expected, variation in mercury levels increased with fish size. Selenium levels remained relatively constant with size of the fish.
Table 1.
Correlation of size and contaminant levels in bluefish caught in New Jersey (2005–2008).
| All bluefish, N = 206 | Mercury | Selenium | Length | Weight |
|---|---|---|---|---|
| Mercury | ********* | 0.14 (0.003) | 0.49 (<0.0001) | 0.51 (<0.0001) |
| Selenium | ********* | ********* | 0.03 (NS) | 0.04 (NS) |
| Length | ********* | ********* | ********* | 0.90 (<0.0001) |
| Weight | ********* | ********* | ********* | ********* |
Given are Kendall tau correlations (p values are in parentheses).
Fig. 2.
Relationship of length (fork length), selenium levels, and mercury levels (ppm, wet weight) for bluefish caught in New Jersey (2005–2008).
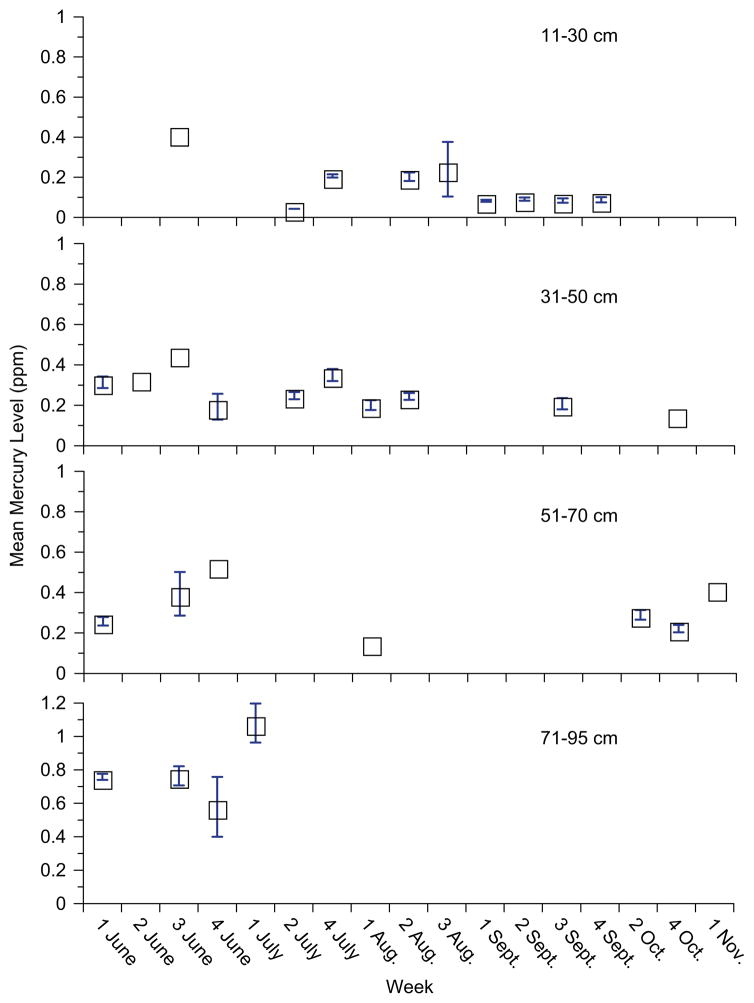
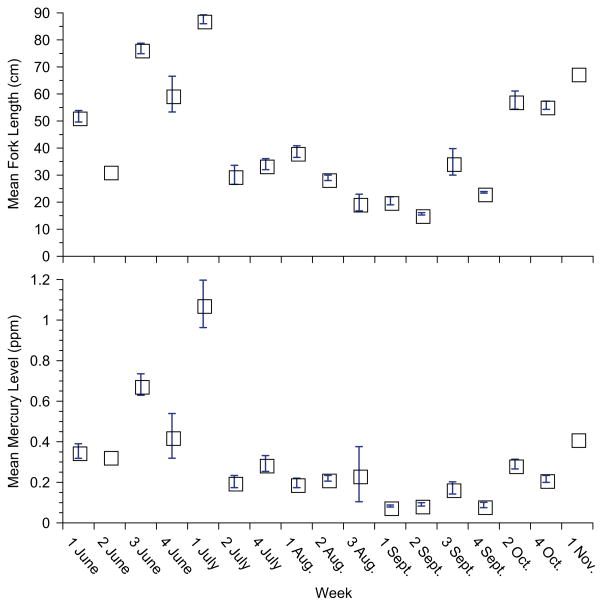
While selenium levels did not vary at all with season, mercury levels decreased significantly from June until November (Table 2; bluefish are not fished in the other months). This relationship is not due only to differences in the size of fish, since the fish collected in the summer were the smallest, but had intermediate mercury levels. Examining the data by size categories indicates that for smaller fish that were available throughout the sampling period, mercury levels declined seasonally: For 11–30cm fork length, r = −0.2 (p<0.04) and for fork length 31–50, r = −0.2 (p<0.009) (Fig. 3). Intermediate-sized fish (51–70cm fork length) were available only in the spring and fall, and large fish (over 71cm fork length) were only available (to fishermen) in the spring (Fig. 4). This is a result of the larger bluefish moving offshore after the spring (see Section 4).
Table 2.
Mean mercury and selenium levels by season.
| Year (n)
|
Total by season | Selenium (ppm) Mean±SE |
Mercury (ppm) Mean±SE |
Total length (cm) Mean±SE |
Weight (g) Mean±SE |
||||
|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | ||||||
| Spring | 53 | 8 | 9 | 70 | 0.37±0.01 0.35 |
0.56±0.04 0.45 (A) |
67.2±2.09 64.7 (A) |
2932±205 2281 (A) |
|
| Summer | 36 | 51 | 18 | 105 | 0.37±0.01 0.34 |
0.26±0.02 0.19 (B) |
33.9±1.66 30.6 (C) |
834±183 249 (C) |
|
| Autumn | 3 | 15 | 9 | 4 | 31 | 0.37±0.03 0.34 |
0.21±0.02 0.19 (B) |
47.6±2.73 44.5 (B) |
1811±230 1068 (B) |
| χ2 (p) | 0.27 (NS) | 51.2 (<0.0001) | 91.8 (<0.0001) | 67.2(<0.0001) | |||||
Spring fish were caught 6–18 June, summer fish were caught 23 June–10 September, and autumn fish were caught 17 September–5 November. Given are arithmetic means±SE (geometric means below) with Kruskal–Wallis χ values and p values. Duncan values are given in parenthesis.
Fig. 3.
Mean mercury levels (ppm, wet weight) as a function of season for four size classes of bluefish collected in New Jersey (2005–2008).
Fig. 4.
Mean fork length and mercury levels as a function of season for bluefish caught in New Jersey (2005–2008).
Although there were significant locational differences in mercury (but not selenium) levels, when this factor is examined alone, these differences could be a result of size (Table 3). Further study is required to examine locational differences since the ratio of mercury to fork length was higher (0.8) for fish caught in central New Jersey than in the other locations (0.6).
Table 3.
Mean mercury and selenium levels (ppm, wet weight) by location.
| Species | N | Selenium (ppm) Mean±SE |
Mercury (ppm) Mean±SE |
Total length (cm) Mean±SE |
Weight (g) Mean±SE |
|---|---|---|---|---|---|
| Bluefish | |||||
| North | 68 | 0.35±0.02 0.33 |
0.32±0.03 0.26 (B) |
52.3±1.81 50 (A) |
1918±194 1353 (A) |
| Central | 79 | 0.38±0.01 0.36 |
0.49±0.04 0.38 (A) |
57.6±2.73 52 (A) |
2522±235 1422 (A) |
| South | 59 | 0.36±0.02 0.33 |
0.19±0.02 0.15 (C) |
28.1±1.90 25 (B) |
441±185 98 (B) |
| χ2 (p) | 1.7 (NS) | 40.6 (<0.0001) | 69.2 (<0.0001) | 58.8 (<0.0001) | |
North includes fish from Jersey City, Raritan Bay, and Sandy Hook. Central includes fish caught between Point Pleasant and Atlantic City. South includes all locations south of Atlantic City. Given are arithmetic means±SE (geometric means below) with Kruskal–Wallis χ2 values and p values. Duncan values are given in parenthesis. Note: 89% of the bluefish from the South were caught in trawls or seines, probably accounting for the smaller size.
Additionally, there were significant yearly differences when the data are combined within a year (regardless of size or collection location (Table 4). However, when mercury levels are considered as a function of size class (Table 5), yearly differences are not significant.
Table 4.
Mean mercury and selenium levels (ppm, wet weight) by year.
| Species | N | Selenium (ppm) Mean±SE |
Mercury (ppm) Mean±SE |
Total length (cm) Mean±SE |
Weight (g) Mean±SE |
|---|---|---|---|---|---|
| Bluefish | |||||
| 2005 | 56 | 0.35±0.01 0.33 (B) |
0.60±0.05 0.47 (A) |
70.0±2.36 67.3 (A) |
3256±223 2609 (A) |
| 2006 | 59 | 0.36±0.02 0.32 (B) |
0.34±0.04 0.26 (B) |
50.2±2.43 46.9 (B) |
2013±277 1116 (B) |
| 2007 | 60 | 0.41±0.02 0.39 (A) |
0.20±0.01 0.17 (C) |
29.9±1.48 27.8 (C) |
569±151 198 (C) |
| 2008 | 31 | 0.33±0.02 0.31 (B) |
0.24±0.03 0.19 (B,C) |
35.1±2.83 31.3 (C) |
702±151 298 (C) |
| χ2 (p) | 10.1 (0.02) | 48.8 (<0.0001) | 99.6 (<0.0001) | 74.1 (<0.0001) | |
Given are arithmetic means±SE (geometric means below) with Kruskal–Wallis χ2 values and p values. Duncan values are given in parenthesis.
Table 5.
Mean mercury levels (ppm, wet weight) by year.
| Species | N | 11–30cm Mean±SE |
31–50cm Mean±SE |
51–70cm Mean±SE |
71–95cm Mean±SE |
χ2 (p) |
|---|---|---|---|---|---|---|
| Bluefish | ||||||
| 2005 | 56 | 0.42±a 0.42 (B) |
0.29±0.03 0.28 (B) |
0.38±0.10 0.28 (B) |
0.76±0.06 0.65 (A) |
18.1 (0.0004) |
| 2006 | 59 | 0.13±0.03 0.11 (B |
0.24±0.02 0.22 (B) |
0.25±0.03 0.23 (B) |
0.81±0.10 0.68 (A) |
22.6 (<0.0001) |
| 2007 | 60 | 0.17±0.02 0.13 (A) |
0.23±0.02 0.22 (A) |
0.24±0.03 0.24 (A) |
6.5 (0.04) | |
| 2008 | 31 | 0.18±0.04 0.12 (A) |
0.29±0.04 0.26 (A) |
0.28±0.02 0.28 (A) |
5.5 (0.06) | |
| χ2 (p) | 3.1 (NS) | 3.7 (NS) | 3.1 (NS) | 0.0007 (NS) | ||
Given are arithmetic means±SE (geometric means below) with Kruskal–Wallis χ2 values and p values. Duncan values (given in parenthesis) compare fish sizes, not years.
Standard error not given because n = 1.
4. Discussion
4.1. Mercury levels and fish size
In general, mercury levels increase with the size and age of the fish (Lange et al., 1994; Bidone et al., 1997; Burger et al., 2001a; Pinho et al., 2002; Green and Knutzen, 2003), however, this is not always the case (Stafford and Haines, 2001). At low mercury levels, the size relationships may not hold (see Park and Curtis, 1997). Further, Trudel and Rasmussen (1997) found that elimination rate is negatively correlated with size, suggesting another reason for larger fish to have significantly higher mercury levels. Most of the studies that have examined the relationship between size and mercury levels have concentrated on freshwater fish, and few have dealt with large marine predatory fish. However, Storelli et al. (2002) reported that size and mercury levels were highly correlated for swordfish (Xiphias gladius) and bluefin tuna (Thunnus thynnus) from the Mediterranean Sea. Although yellowfin tuna (Thunnus albacares) showed a positive relationship between mercury and size (length and weight), albacore tuna (Thunnus alalunga) did not (Fiji, 2006). Luten et al. (1987) found a positive correlation between size and mercury content in Atlantic cod. Alexander et al. (1973) reported a correlation between weight and mercury concentrations for bluefish and striped bass off Montauk Point on Long Island. Usually the age of individual fish is unknown, and size is used as a surrogate for age (Boening, 2000). However, Braune (1987) found that in known-aged herring (Clupea harengus harengus), mercury level was more strongly correlated with age than with weight or length. In a study of Pacific cod (Gadus macrocephalus), Burger and Gochfeld (2007c) found that age was the variable that first entered the regression model explaining variation in mercury levels. In the present study, mercury levels varied significantly by size (age information was not available), and mercury levels showed a greater increase in bluefish over 50cm in fork length. These data clearly indicated that mercury exposure to humans and other top-level predators can be reduced by eating smaller fish. It also indicates, however, that scavengers that eat large fish could be at risk (see below).
There was no correlation between size and selenium levels in bluefish, and Pakkala et al. (1972) also did not find any correlation between size and selenium levels in striped bass (Morone saxatilis).
4.2. Seasonal, yearly and locational differences
There were significant seasonal differences in mercury levels in bluefish, both when examined overall for all fish caught, but also within size classes. For fish less than 50cm fork length, mercury levels decreased with season in the fish collected in this study from New Jersey. With larger fish the differences were less clear, partly because of the inability to catch bluefish of larger sizes during all seasons (refer to Fig. 3). The largest bluefish were only available in the late spring, and intermediate fish were not available (“catchable”) in mid-summer. This reflects their migration patterns. Most North American bluefish are migratory, spending their summers in the north, and their winters around Florida and the Gulf Stream (Pottern et al., 1989; Adams et al., 2003). The larger fish move into the bays and estuaries in late spring (early summer), spawn in the bays and estuaries, and move offshore, while schools of intermediate-sized fish and smaller fish remain inshore in bays, estuaries and marsh creeks (Able and Fahay, 1998; Neuman et al., 2004).
While there were significant locational differences in mercury levels in the bluefish collected in New Jersey, these may be mainly a result of differences in size and season. Similarly, methylmercury levels in 46 bluefish from Long Island Sound averaged 0.14±0.06ppm (Hammerschmidt and Fitzgerald, 2006), while those from northern New Jersey averaged 0.32±0.03ppm (this study). While the levels in the northern New Jersey fish are clearly higher than those reported from Long Island Sound, the differences may partly be related to size of the fish. To tease apart these differences requires a study to obtain the same size fish at every location and time in the season, a protocol that would require extensive capture by trawl, rather than by recreational fishermen.
Although there were yearly variations in mercury levels when all fish are considered together, these differences disappeared when fish were considered by size class. This clearly indicates that contaminants data in bluefish, and perhaps other fish, should always be examined as a function of size class, and not just all sizes combined.
4.3. Risk to the food chain
Methylmercury in fish pose a risk both to the fish themselves, and to their predators. In fish, dietary uptake probably accounts for more that 90% of the total uptake (Wiener et al., 2003), and the assimilation efficiency dietary uptake of methylmercury is 65–80% (Wiener and Spry, 1996). Methylmercury is a neurotoxin in fish, and at toxic levels can cause lack of coordination, diminished appetite, inability to feed, diminished responsiveness, lowered swimming activity, starvation, and mortality (Wiener et al., 2003). In the fish themselves, muscle levels of 5–20ppm are associated with toxicity (Wiener et al., 2003). Differences in sensitivity relate to species of fish, bioavailability, and bioaccumulation rate (Niimi and Kissoon, 1994). The mean mercury level of 0.35ppm in bluefish in this study was well below these levels, indicating that the fish themselves are not at risk from mercury.
Much of the methylmercury in fish accumulates in skeletal muscle, which is protective for the fish itself because the mercury exposure to the central nervous system is reduced (Wiener and Spry, 1996). However, mercury concentrations in muscle are available to predators, including humans, that eat fish. And since the mercury then accumulates in larger and larger fish, mercury magnifies as it moves up the food chain (to humans or other top-level predators). Mercury toxicity is also affected by temperature, salinity, dissolved oxygen and water hardness (Boening, 2000). The critical effects levels for consumption by piscivorous mammals are 0.1 ppm, and for birds are 0.02ppm (Yeardley et al., 1998), although seabirds are generally less sensitive (Furness, 1996). The mean mercury levels in the bluefish from New Jersey (0.35+0.02ppm in muscle) are clearly higher than the levels known to pose a problem for sensitive birds or mammals that scavenge them along the shore, or for sensitive marine mammals.
Finally, the protective effects of selenium on mercury toxicity have been known for over a quarter of a century (Ganther et al., 1972; Kim et al., 1977; Ringdal and Julshamn, 1985). Levels of mercury and selenium are correlated in some studies (Eisler, 1985; Kuehl and Haebler, 1995; Wagemann et al., 1996), and this was also the case for the NJ bluefish, although the correlation was low. It should be noted, however, that as mercury levels rise, selenium does not, and the ratio of mercury to selenium becomes higher in the larger fish, which could potentially increase the risk of toxicity from methylmercury.
4.4. Risk to humans
The USFDA action level for methylmercury in fish is 1.0 μg/g (ppm w/w), but this is a regulatory action level, rather than a risk level (US FDA, 2001). Originally the FDA had set 0.5ppm as the action level, comparable to many other nations (reviewed in Burger and Gochfeld, 2004). In contrast, the critical value for human consumption used by the US EPA is 0.2ppm (Rothschild and Duffy, 2002). The United Kingdom and the European Union have established criteria for certain metals in fish (e.g. the level for mercury is 0.5ppm in edible fish, with up to 1ppm allowed for certain exempt fish species, all of which are predatory). China has set standards for methylmercury in canned fish (ppm wet weight) of 0.5ppm (except 1ppm is allowed in shark, sailfish, tuna, pike and other high-mercury fish). In 1982, the European Commission set an Environmental Quality Standard for mercury; the mean concentration in mercury of a representative sample of fish shall not exceed 0.3ppm (wet weight). The US EPA (2001) promulgated 0.3ppm as an ambient freshwater quality standard in 2001. Muscle in the bluefish in this study averaged about 0.35 ppm, with 41% of the bluefish having levels above 0.3 ppm. The data from this study indicate that it would be wise to avoid eating bluefish above 50cm fork length (ca. 20 in.), which is a number that could be clearly presented to the public.
Risk assessments for fish consumption generally examine chronic exposure, and not a single meal. However, Ginsberg and Toal (2000) have suggested that there may be risk during pregnancy for even a single-meal exposure, particularly for fish with levels of over 2.0 ppm. The risk from a pulsed exposure should also be examined, particularly its impact on a developing fetus at a critical developmental period. In the present study, 4% of fillets of bluefish were above 1 ppm, clearly a risk to consumers. The percent of fillets that were above 0.5ppm are also reported because of the need to know the percent of times an exposure in a single meal may approach the tolerable daily intake (Berti et al., 1998). Providing information on risk from single-meal exposures, especially for pregnant women, is a risk communication challenge that should be considered by the FDA. Egeland and Middaugh (1997) have called attention to the countervailing nutritional importance of fish, which increases the importance of identifying suitable local fish with low contaminant levels, especially during pregnancy. It is a matter of risk balance (Gochfeld and Burger, 2005; IOM, 2006).
4.5. Risk management
Bluefish are an important sport and commercial fish in New Jersey and along the northeastern United States (Burger, 2005), and bluefish are available in over 80% of the supermarkets and fish markets in New Jersey (Burger et al., 2004, 2005). In the lower Hudson River area (NY/NJ harbor estuary), 64% of the anglers eat bluefish (Gobeille et al., 2005). It is thus of some interest to understand how mercury levels vary in bluefish by size, location and season to provide useful information to consumers, many of whom eat bluefish regularly. Providing people who fish and consume self-caught fish with information on mercury levels is thus of public health importance. In this paper data are provided on mercury levels as a function of fish size, location where caught, and season because people are not consistent in the sizes of fish they eat or where they caught them. Catching fish for tournaments is a big sport in New Jersey and elsewhere along the east coast, which results in people bringing back the largest fish they catch. Since people often bring back more fish to eat than “the big one”, a public health campaign aimed at providing information on the effect of size might be effective. This is complicated by the fact that bluefish often travel in schools of the same age or size, and fisherfolk who come upon such a school might well catch only large or medium-sized fish. The desire to continue catching (and keeping) large fish needs to be overcome to reduce consumption of large-sized bluefish. Thus, it would be possible for a pregnant woman to have several meals in a row from large-sized bluefish at the high end of mercury exposure. It should also be mentioned that there is an advantage to bluefish populations of leaving the larger fish to ensure that they breed; thus catching (or keeping for consumption) only the smaller fish both reduces the risk of mercury toxicity to people, but aids in population viability of bluefish.
However, there is also additional room for public health risk management since the data in this paper show clear differences as a function of season, even within the same size fish. Bluefish had higher levels of mercury in the spring than in the fall. These data thus suggest that smaller bluefish should be specifically targeted for consumption in the spring, and that smaller fish caught in the fall could wisely be frozen for later consumption, or both. While many of the fishermen and women were aware that contaminant levels were often higher in larger fish, no one was aware that mercury levels might vary by season, even in the same-sized fish.
In conclusion, fish consumption is a matter of risk balancing (Egeland and Middaugh, 1997; Egeland et al., 1998; Ponce et al., 2000; Gochfeld and Burger, 2005). There are clearly both benefits and risks from fish consumption, and the public should be provided with as much information as possible to allow them to maximize the positive health benefits, while minimizing the risks from contaminants (IOM, 2006). The availability of information on how mercury varies in fish (size, season, fishing location) can be valuable, especially for pregnant women and others at high risk, in reducing their mercury exposure while still consuming fish. To be effective, development of risk communication tools should involve scientists, health professionals, regulators, and the general public (Jardine et al., 2003; Knuth et al., 2003; Burger et al., 2005). But such communications must be informed by data on how mercury varies within each species of fish.
Acknowledgments
This research was partly supported by the Jersey Coast Angler’s Association (JCAA), the Jersey Coast Shark Anglers Association (JCSA), NIEHS Center Grant (P30ESO05022), and the Consortium for Risk Evaluation with Stakeholder Participation (Department of Energy, no. DE-FC01-06EW07053), Wildlife Trust, and EOHSI. This research was conducted under a Rutgers University protocol, and fish samples were obtained from recreational anglers and NJ DEP trawls. I particularly thank M. Gochfeld and T. Fote for advice and help throughout the study, C. Jeitner and M. Donio for field and laboratory assistance, and the many anglers in New Jersey who allowed us to collect samples from their fish, or who collected the samples for us. The views and conclusions expressed in this paper are solely those of the author, and do not reflect the funding agencies.
References
- Able KW, Fahay MP. The First Year in the Life of Estuarine Fishes in the Middle Atlantic Bight. Rutgers University Press; New Brunswick, NJ: 1998. [Google Scholar]
- Adams DH, McMichael RH, Henderson GE. Florida Marine Research Institute Technical Reports. Florida Fish and Wildlife Commission; 2003. Mercury levels in marine estuarine fishes of Florida 1989–2001. [Google Scholar]
- Amin-zaki L, Najeed MS, Clarkson TW, Greenwood MR. Methylmercury poisoning in Iraqi children: clinical observations over two years. Br Med J. 1978;1:163–616. doi: 10.1136/bmj.1.6113.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PD, Wiener JB. Eating fish. In: Graham JD, Wiener JB, editors. Risk Versus Risk: Tradeoffs in Protecting Health and the Environment. Harvard University Press; Cambridge, MA: 1995. [Google Scholar]
- Alexander JE, Foehrenbach, Fisher S, Sullivan D. Mercury in striped bass and bluefish. N Y Fish Game J. 1973;20:147–151. [Google Scholar]
- Berti PR, Receveur O, Chan HM, Kuhnlein HV. Dietary exposure to chemical contaminants from traditional food among adult Dene/Metis in the Western Northwest Territories, Canada. Environ Res. 1998;76:131–142. doi: 10.1006/enrs.1997.3797. [DOI] [PubMed] [Google Scholar]
- Bidone ED, Castilhos ZC, Santos TJS, Souza TMC, Lacerda LD. Fish contamination and human exposure to mercury in Tartarugalzinho River, Northern Amazon, Brazil: a screening approach. Water Air Soil Pollut. 1997;97:9–15. [Google Scholar]
- Bienenfeld LS, Golden AL, Garland EJ. Consumption of fish from polluted waters by WIC participants in East Harlem. J Urban Health. 2003;80:349–358. doi: 10.1093/jurban/jtg036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom NS. On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci. 1991;49:1010–1017. [Google Scholar]
- Boening DW. Ecological effects, transport, and fate of mercury: a general review. Chemosphere. 2000;40:1335–1351. doi: 10.1016/s0045-6535(99)00283-0. [DOI] [PubMed] [Google Scholar]
- Braune BM. Mercury accumulation in relation to size and age of Atlantic Herring (Clupea harengus harengus) from the Southwestern Bay of Fundy, Canada. Arch Environ Contam Toxicol. 1987;16:311–320. doi: 10.1007/BF01054948. [DOI] [PubMed] [Google Scholar]
- Burger J. American Indians, hunting and fishing rates, risk and the Idaho National Engineering and Environmental Laboratory. Environ Res. 1999;80:317–329. doi: 10.1006/enrs.1998.3923. [DOI] [PubMed] [Google Scholar]
- Burger J. Consumption advisories and compliance: the fishing public and the deamplification of risk. J Environ Plan Manage. 2000;43:471–488. [Google Scholar]
- Burger J. Consumption patterns and why people fish. Environ Res. 2002;90:125–135. doi: 10.1006/enrs.2002.4391. [DOI] [PubMed] [Google Scholar]
- Burger J. Fishing, fish consumption, and knowledge about advisories in college students and others in central New Jersey. Environ Res. 2005;98:268–275. doi: 10.1016/j.envres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Mercury in canned tuna: white versus light and temporal variation. Environ Res. 2004;96:239–249. doi: 10.1016/j.envres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Burger J, Pflugh KK, Lurig L, von Hagen LA, von Hagen SA. Fishing in urban New Jersey: ethnicity affects information sources, perception, and compliance. Risk Anal. 1999;19:217–229. doi: 10.1023/a:1006921610468. [DOI] [PubMed] [Google Scholar]
- Burger J, Gaines KF, Gochfeld M. Ethnic differences in risk from mercury among Savannah River fishermen. Risk Anal. 2001a;21:533–544. doi: 10.1111/0272-4332.213130. [DOI] [PubMed] [Google Scholar]
- Burger J, Gaines KF, Boring CS, Stephens WL, Jr, Snodgrass J, Gochfeld M. Mercury and selenium in fish from the Savannah River: species, trophic level, and locational differences. Environ Res. 2001b;87:108–118. doi: 10.1006/enrs.2001.4294. [DOI] [PubMed] [Google Scholar]
- Burger J, Fleischer J, Gochfeld M. Fish, shellfish, and meat meals of the public in Singapore. Environ Res. 2003;93:254–261. doi: 10.1016/s0013-9351(03)00015-x. [DOI] [PubMed] [Google Scholar]
- Burger J, Stern AH, Dixon C, Jeitner C, Shukla S, Burke S, Gochfeld M. Fish availability in supermarkets and fish markets in New Jersey. Sci Total Environ. 2004;333:89–97. doi: 10.1016/j.scitotenv.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Burger J, Stern AH, Gochfeld M. Mercury in commercial fish: optimizing individual choices to reduce risk. Environ Health Perspect. 2005;113:266–271. doi: 10.1289/ehp.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, Burke S, Stamm T, Snigaroff R, Snigaroff E, Patrick D, Weston J. Mercury levels and potential risk from subsistence foods from the Aleutians. Sci Total Environ. 2007a;384:93–105. doi: 10.1016/j.scitotenv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, Burke S, Stamm T. Metal levels in flathead sole (Hippoglossoides elassodon) and great sculpin (Myoxocephalus polyacanthocephalus) from Adak Island, Alaska: potential risk to predators and fishermen. Environ Res. 2007b;103:62–69. doi: 10.1016/j.envres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Risk to consumers from mercury in Pacific cod (Gadus macrocephalus) from the Aleutians: fish age and size effects. Environ Res. 2007c;105:276–284. doi: 10.1016/j.envres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Consumer Reports. American’s fish: fair or foul? Consumers Union; New York: 2003. [accessed 1 April 2004]. Available: < http://www.consumerreports.org/special/consumerInteret/Reports/0102fis0.html>. [Google Scholar]
- Crump KS, Kjellstrom T, Shipp AM, Silvers A, Stewart A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: benchmark analysis of a New Zealand cohort. Risk Anal. 1998;18:701–713. doi: 10.1023/b:rian.0000005917.52151.e6. [DOI] [PubMed] [Google Scholar]
- Daviglus M, Sheeshka J, Murkin E. Health benefits from eating fish. Comments Toxicol. 2002;8:345–374. [Google Scholar]
- Downs SG, Macleod CL, Lester JM. Mercury in precipitation and its relation to bioaccumulation in fish: a literature review. Water Air Soil Pollut. 1998;108:149–187. [Google Scholar]
- Duffy LK, Scofield E, Rodgers T, Patton M, Bowyer RT. Comparative baseline levels of mercury, HSP 70 and HSP 60 in subsistence fish from the Y-K Delta region of Alaska. Comp Biochem Physiol. 1999;124C:181–186. doi: 10.1016/s0742-8413(99)00055-9. [DOI] [PubMed] [Google Scholar]
- Egeland GM, Feyk LA, Middaugh JP. The Use of Traditional Foods in a Healthy Diet in Alaska. Alaska Division of Public Health; Juneau, AL: 1998. [Google Scholar]
- Egeland GM, Middaugh JP. Balancing fish consumption benefits with mercury exposure. Science. 1997;278:904–1906. doi: 10.1126/science.278.5345.1904. [DOI] [PubMed] [Google Scholar]
- Eisler R. US Fish and Wildlife Service Report. 1.10 Vol. 85. Washington, DC: 1985. Selenium hazards to fish, wildlife and invertebrates: a synoptic review. [Google Scholar]
- Environmental protection agency (EPA) Freshwater criterion for fish. 2001 < http://www.epa.gov/fedrgstr/EPA-WATER/2001/January/Day-08/w217.htm>.
- Fiji. [Accessed 10 February 2006];Mercury levels in Fijian seafood. 2006 < http://www.wpro.who.int/NR/rdonlyres/437775AO-671F-41AE-A068>.
- Furness RW. Cadmium in birds. In: Beyer WN, Heinz WN, Redmon-Norwood AW, editors. Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations. Lewis Publ; Boca Raton, FL: 1996. pp. 389–404. [Google Scholar]
- Ganther HE, Goudie C, Sunde ML, Kopecky MJ, Wagner R, Sang-Hwang OH, Hoekstra WG. Selenium relation to decreased toxicity of methylmercury added to diets containing tuna. Science. 1972;72:1122–1124. doi: 10.1126/science.175.4026.1122. [DOI] [PubMed] [Google Scholar]
- Ginsberg GL, Toal BF. Development of a single-meal fish consumption advisory for methylmercury. Risk Anal. 2000;20:41–47. doi: 10.1111/0272-4332.00004. [DOI] [PubMed] [Google Scholar]
- Gobeille AK, Morland KV, Bopp RF, Godbold JH, Landrigan PJ. Body burdens of mercury in lower Hudson River area anglers. Environ Res. 2005;101:205–212. doi: 10.1016/j.envres.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Gochfeld M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol Environ Saf. 2003;56:174–179. doi: 10.1016/s0147-6513(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Gochfeld M, Burger J. Good fish/bad fish: a composite benefit–risk by dose curve. Neurotoxicology. 2005;26:511–520. doi: 10.1016/j.neuro.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorenson N, Jorgensen PJ. Cognitive deficit in 7-year old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:418–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Green NW, Knutzen J. Organohalogens and metals in marine fish and mussels and some relationships to biological variables at reference localities in Norway. Mar Pollut Bull. 2003;46:362–377. doi: 10.1016/S0025-326X(02)00515-5. [DOI] [PubMed] [Google Scholar]
- Guallar E, Sanz-Gallardo MI, van’t Veer P, Bode P, Aro A, Gomez-Aracena J. Heavy metals and myocardial infarction study group: mercury, fish oils, and the risk of myocardial infarction. New Engl J Med. 2002;347:1747–1754. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- Haines TA, Komov V, Jagoe CH. Lake acidity and mercury content of fish in Darwin National Reserve, Russia. Environ Pollut. 1992;78:107–112. doi: 10.1016/0269-7491(92)90017-5. [DOI] [PubMed] [Google Scholar]
- Haines TA, Komov VT, Jagoe CH. Mercury concentration in perch (Perca fluviatilis) as influenced by lacustrine physical and chemical factors in two regions of Russia. In: Watras CJ, Huckabee JW, editors. Mercury Pollution: Integration and Synthesis. Lewis Publ; Boca Raton, FL: 1994. pp. 397–407. [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF. Bioaccumulation and trophic transfer of methylmercury in Long Island sound. Archiv Environ Contam Toxicol. 2006;51:416–424. doi: 10.1007/s00244-005-0265-7. [DOI] [PubMed] [Google Scholar]
- Harper BL, Harris SG. A possible approach for setting a mercury risk-based action level based on tribal fish ingestion rates. Environ Res. 2008;107:60–68. doi: 10.1016/j.envres.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Harris SG, Harper BL. Native American exposure scenarios and a tribal risk model. Risk Anal. 1998;17:789–795. doi: 10.1111/j.1539-6924.1997.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Harris SG, Harper BL. Using eco-cultural dependency webs in risk assessment and characterization of risks to tribal health and cultures. Environ Sci Pollut Res. 2000;2:91–100. [Google Scholar]
- Hightower JM, Moore D. Mercury levels in high-end consumers of fish. Environ Health Perspec. 2003;111:604–608. doi: 10.1289/ehp.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA, Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. Global assessment of organic contaminants in farmed salmon. Science. 2004;303:226–229. doi: 10.1126/science.1091447. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Seafood Safety. National Academy Press; Washington, DC: 1991. [Google Scholar]
- Institute of Medicine (IOM) Seafood Choices: Balancing Benefits and Risks. National Academy Press; Washington, DC: 2006. [Google Scholar]
- Jardine CG, Hrudey SE, Shortreed JH, Craig L, Krewski D, Furgal C, McColl S. Risk management frameworks for human health and environmental risks. J Toxicol Environ Health B. 2003b;6:569–641. doi: 10.1080/10937400390208608. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) [Accessed March 2005];2003 < www.who.int/pcs/jecfa/jecra-htm>.
- Kim JG, Birks E, Heisinger JF. Protective action of selenium against mercury in northern creek chubs. Bull Environ Contam Toxicol. 1977;17:132–136. doi: 10.1007/BF01685539. [DOI] [PubMed] [Google Scholar]
- Knuth B, Connelly NA, Sheeshka J, Patterson J. Weighing health benefits and health risk information when consuming sport-caught fish. Risk Anal. 2003;23:1185–1197. doi: 10.1111/j.0272-4332.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- Kuehl DW, Haebler R. Organochlorine, organobromine, metal, and selenium residues in bottlenose dolphin (Tursiope truncatus) collected during an unusual mortality event in the Gulf of Mexico. Arch Environ Contam Toxicol. 1995;28:494–499. doi: 10.1007/BF00211632. [DOI] [PubMed] [Google Scholar]
- Lange TR, Royals HE, Connor LL. Mercury accumulation in largemouth bass (Micropterus salmoides) in a Florida Lake. Arch Environ Contam Toxicol. 1994;27:466–471. doi: 10.1007/BF00214837. [DOI] [PubMed] [Google Scholar]
- Lansens P, Leermakers M, Vaeyens W. Determination of methylmercury in fish by headspace-gas chromatography with microwave-induced-plasma detection. Water Air Soil Pollut. 1991;56:103–115. [Google Scholar]
- Legrand M, Arp P, Ritchie C, Chan HM. Mercury exposure in two coastal communities of the Bay of Funday, Canada. Environ Res. 2005;98:14–21. doi: 10.1016/j.envres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Luten JB, Bouquet W, Riekwel-Booy G, Rauchbaar AB, Scholte MWM. Mercury in flounder, Platichtys flesus, Cod, Gadus morhua, and Perch, Perca fluviatilis, in relation to their length and environment. Bull Environ Contam Toxicol. 1987;38:318–323. doi: 10.1007/BF01606681. [DOI] [PubMed] [Google Scholar]
- Montiero LR, Costa V, Furness RW, Santos RS. Mercury concentrations in prey fish indicate enhanced bioaccumulation in mesopelagic environments. Mar Ecol Prog Ser. 1996;141:21–25. [Google Scholar]
- National Research Council (NRC) Toxicological Effects of Methylmercury. National Academy Press; Washington, DC: 2000. [Google Scholar]
- Neuman MJ, Ruess G, Able KW. Species composition and food habits of dominant fish predators in salt marshes of an urbanized estuary, the Hackensack Meadowlands, New Jersey. Urban Habitats. 2004;2:3–22. [Google Scholar]
- Niimi AJ, Kissoon GP. Evaluation of the critical body burden concept based on inorganic and organic mercury toxicity to rainbow trout (Oncorhynchus mykiss) Arch Environ Contam Toxicol. 1994;26:169–178. doi: 10.1007/BF00224801. [DOI] [PubMed] [Google Scholar]
- Park JG, Curtis LR. Mercury distribution in sediments and bioaccumulation by fish in two Oregon reservoirs: point-source and nonpoint source impacted systems. Arch Environ Contam Toxicol. 1997;33:423–429. doi: 10.1007/s002449900272. [DOI] [PubMed] [Google Scholar]
- Patterson J. Introduction—comparative dietary risk: balance the risks and benefits of fish consumption. Comments Toxicol. 2002;8:337–344. [Google Scholar]
- Pakkala IS, Gutenmann WH, Lisk DJ, Durdick GE, Harris EJ. A survey of the selenium content of fish from 49 New York state waters. Pest Monit J. 1972;6:107–114. [PubMed] [Google Scholar]
- Pinho AP, Guimaraes JRD, Marins AS, Costa PAS, Olavo G, Valentin J. Environ. Res. 2002;89:250–258. doi: 10.1006/enrs.2002.4365. [DOI] [PubMed] [Google Scholar]
- Ponce RA, Bartell SM, Wong EY, LaFlamme D, Carrington C, Lee RC, Patrick DI, Faustman EM, Bolger M. Use of quality-adjusted life year weights with dose–response models for public health decisions: a case study of the risks and benefits of fish consumption. Risk Anal. 2000;20:529–542. doi: 10.1111/0272-4332.204050. [DOI] [PubMed] [Google Scholar]
- Pottern GB, Huish MT, Kerby JH. Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (mid-Atlantic): bluefish. US Fish Wildlife Service Biol Rep. 1989;82 (11.94):1–21. [Google Scholar]
- Rice G, Swartout J, Mahaffey K, Schoeny R. Derivation of US EPS’s oral reference dose (RfD) for methylmercury. Drug Chem Toxicol. 2000;23:41–54. doi: 10.1081/dct-100100101. [DOI] [PubMed] [Google Scholar]
- Ringdal O, Julshamn K. Effect of selenite on the uptake of methylmercury in cod (Gadus morhua) Bull Environ Contam Toxicol. 1985;35:335–344. doi: 10.1007/BF01636519. [DOI] [PubMed] [Google Scholar]
- Rissanen T, Voutilainen S, Nyyssonen K, Lakka TA, Salonen JT. Fish oil-derived fatty acids, docosahexaenoic acid and docosaphentaenoic acid, and the risk of acute coronary events: the Kuopio ischaemic heart disease risk factor study. Circulation. 2000;102:2677–2679. doi: 10.1161/01.cir.102.22.2677. [DOI] [PubMed] [Google Scholar]
- Rolfhus K, Sandheinrich MR, Wiener JG, Bailey SW, Thorenson KR, Hammerschmidt CR. Analysis of fin clips as a nonlethal method for monitoring mercury in fish. Environ Sci Technol. 2008;42:871–877. doi: 10.1021/es071427+. [DOI] [PubMed] [Google Scholar]
- Rothschild RN, Duffy LK. Preliminary study on total mercury in the common prepared subsistence foods of a rural Alaskan village. Alaska Med. 2002;44:89–103. [PubMed] [Google Scholar]
- Salonen JT, Seppanen K, Nyyssonen K, Korpela H, Kauhanen J, Kantola M, Tuomilehto J, Esterbauer H, Tatzber F, Salonen R. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation. 1995;91:645–655. doi: 10.1161/01.cir.91.3.645. [DOI] [PubMed] [Google Scholar]
- Satoh H, Yasuda N, Shimai S. Development of reflexes in neonatal mice prenatally exposed to methylmercury and selenite. Toxicol Lett. 1985;25:199–203. doi: 10.1016/0378-4274(85)90082-7. [DOI] [PubMed] [Google Scholar]
- Stafford CP, Haines TA. Mercury contamination and growth rate in two piscivore populations. Environ Toxicol Chem. 2001;20:2099–2101. [PubMed] [Google Scholar]
- Statistical Analysis System (SAS) SAS Users’ Guide. Statistical Institute Inc; Cary, NC: 1995. [Google Scholar]
- Storelli MM, Stuffler RG, Marcotrigiano GO. Total and methylmercury residues in tuna-fish from the Mediterranean Sea. Food Additiv Contam. 2002;19:715–720. doi: 10.1080/02652030210153569. [DOI] [PubMed] [Google Scholar]
- Steuerwald U, Weihe P, Jorgansen PJ, Bjerve K, Brock J, Heinzow B, Budtz-Jorgensen E, Grandjean P. Maternal seafood diet, methylmercury exposure, and neonatal neurological function. J Pediatr. 2000;136:599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- Toth JF, Jr, Brown RB. Racial and gender meanings of why people participate in recreational fishing. Leisure Sci. 1997;19:129–146. [Google Scholar]
- Trudel M, Rasmussen JB. Modeling the elimination of mercury by fish. Environ Sci Technol. 1997;31:1716–1722. [Google Scholar]
- US Food and Drug Administration (US FDA) FDA consumer advisory. US Food and Drug Administration; Washington, DC: 2001. [accessed 1 December 2001]. Available: < http://www.fda.gov/bbs/topics/ANSWERS/2000/advisory.html>. [Google Scholar]
- US Food and Drug Administration (US FDA) FDA consumer advisory. US Food and Drug Administration; Washington, DC: 2003. [accessed 1 January 2004]. Available: < http://www.fda.gov/bbs/topics/ANSWERS/2000/advisory.html>. [Google Scholar]
- US Food and Drug Administration (US FDA) Mercury Levels in Commercial Fish and Shellfish. US Food and Drug Administration; Washington, DC: 2005. [accessed 1 January 2005]. Available < http://www.fda.gov/bbs/topics/ANSWERS/2000/advisory.html>. [Google Scholar]
- Wagemann R, Innes S, Richard PR. Overview and regional and temporal differences of heavy metals of whales and ringed seals in the Canadian Arctic. Sci Total Environ. 1996;186:41–66. doi: 10.1016/0048-9697(96)05085-1. [DOI] [PubMed] [Google Scholar]
- Watras CJ, Back RC, Halvorsen S, Hudson RJM, Morrison KA, Wente SP. Bioaccumulation of mercury in pelagic freshwater food webs. Sci Total Environ. 1998;219:183–208. doi: 10.1016/s0048-9697(98)00228-9. [DOI] [PubMed] [Google Scholar]
- Wiener JG, Spry DJ. Toxicological significance of mercury in freshwater fish. In: Beyer WN, Heins GH, Redmon-Norwood AW, editors. Environmental Contaminants in Wildlife. Lewis Publ; Boca Raton, FL: 1996. pp. 297–339. [Google Scholar]
- Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer M. Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr, editors. Handbook of Ecotoxicology. Lewis Publ; Boca Ration, FL: 2003. pp. 409–463. [Google Scholar]
- World Health Organization (WHO) Mercury—Environmental Aspects. WHO; Geneva, Switzerland: 1989. [Google Scholar]
- Yeardley RB, Jr, Lazorchak JM, Paulsen SG. Elemental fish tissue concentration in northeastern US Lakes: evaluation of an approach to regional assessment. Environ Toxicol Chem. 1998;17:1875–1884. [Google Scholar]