Abstract
Background
Opening of the mitochondrial permeability transition pore (MPTP) has been shown to contribute to myocardial ischemia/reperfusion injury. We sought to demonstrate that the myocardial protective effect of inhibiting MPTP opening with cyclosporine A (CsA) results in stabilization of mitochondrial morphology and is independent of CsA-induced calcineurin inhibition.
Methods
Thirty-seven rabbits were divided into three groups: control (n = 15), CsA (MPTP and calcineurin inhibitor, n = 12), or FK506 (calcineurin inhibitor, n = 10). Each group received a 1-hour infusion of either a saline vehicle, 25 mg/kg CsA or 1 mg/kg FK506. All animals underwent 30 minutes of regional ischemia and 3 hours of reperfusion. Myocardial infarct size was determined using Evans blue dye and triphenyltetrazolium chloride. In situ oligo ligation was used to assess apoptotic cell death. Transmission electron microscopy was used to quantitatively evaluate morphologic differences in the mitochondria between groups.
Results
Infarct size in the CsA group (39% ± 3%) was significantly reduced compared with the control group (60% ± 2%, p < 0.001) and FK506 group (55% ± 3%, p = 0.001). Apoptotic cell death was also attenuated in the CsA group (1.2% ± 0.5%) compared with the control group (4.3% ± 0.8%, p = 0.01) and FK506 group (4.1% ± 0.9%, p = 0.05). Transmission electron microscopy revealed a preservation of normal mitochondrial morphology and a reduction in the percentage of disrupted mitochondria in the CsA group (20% ± 7%) compared with the control group (53% ± 12%) and FK506 group (47% ± 9%).
Conclusions
Cyclosporine A–induced MPTP inhibition preserves mitochondrial morphology after myocardial ischemia/reperfusion and limits myocyte necrosis and apoptosis. These effects are independent of calcineurin inhibition.
A growing body of evidence over the past 2 decades has implicated the mitochondrial permeability transition pore (MPTP) as a critical determinant of myocardial cell death. It has been demonstrated that the MPTP remains closed during normal physiologic conditions and during ischemia, but opens upon reperfusion of the ischemic myocardium. When this pore opens, the mitochondria lose their ability to maintain a pH gradient, preserve membrane potential, and produce ATP. Instead they actively degrade ATP, which leads to a loss of ionic and metabolic homeostasis and ultimately cell death [1]. The opening of this nonspecific ion channel located in the inner membrane of the mitochondria has been linked to lethal myocardial reperfusion injury resulting in both necrotic and apoptotic cardiomyocyte death [1]. The discovery of the involvement of the MPTP in myocardial ischemia/reperfusion injury began with experiments using cyclosporine A (CsA), a widely used immunosuppressant drug. The addition of CsA to isolated cardiomyocytes subjected to anoxia and reoxygenation resulted in a reduction of the anoxic injury [2]. Subsequent experiments in a Langendorff perfused heart model demonstrated a reduction in infarct size with the addition of CsA before ischemia, during ischemia and directly at the onset of reperfusion [3–5]. Argaud and colleagues [6] built on these in vitro experiments by demonstrating the cardioprotective effects of CsA in an in vivo rabbit model of ischemia/reperfusion. These results suggested that MPTP inhibition with CsA was cardioprotective. Halestrap’s group [7, 8] confirmed these hypotheses by showing MPTP uptake of the radioactive tracer [3H]2-deoxygluocse (3H-DOG) at the onset of reperfusion, and inhibition of 3H-DOG uptake with the administration of CsA.
The interactions of CsA with cyclophilin proteins in the cytosol produce different downstream effects. The binding of CsA to cyclophilin-A results in inhibition of calcineurin; however, the binding of CsA to cyclophilin-D results in inhibition of MPTP opening. Calcineurin is a ubiquitous protein that plays a key role in calcium homeostasis, and has been described as having both proapoptotic and antiapoptotic properties. Although the cardioprotective attributes of CsA have been well described, it has not been clearly elucidated whether it is the inhibition of the MPTP or calcineurin that is the responsible mechanism.
The goals of this study were threefold. We sought to confirm the effects of CsA on MPTP inhibition in an in vivo model of myocardial ischemia/reperfusion injury. Additionally, we sought to provide conclusive data to support the hypothesis that the cardioprotective effects of CsA were due to MPTP inhibition, rather than calcineurin inhibition. Therefore, we compared the effects of CsA with FK506, another immunosuppressant drug that inhibits calcineurin but has no known effect on the MPTP. Finally, we sought to quantitatively assess the effect of MPTP inhibition on ischemia/reperfusion-induced changes in myocardial mitochondrial morphology.
Material and Methods
Animals were treated under experimental protocols approved by the University of Pennsylvania’s Institutional Animal Care and Use Committee and in compliance with the National Institutes of Health (Publication No. 85-23, revised 1996). Thirty-nine male New Zealand White rabbits (2.8 to 4.0 kg) were used in this study. Thirty-seven animals underwent the ischemia/reperfusion experimental protocol described below, and 2 additional animals were used solely for transmission electron microscopy analysis.
Surgical Protocol
Thirty-seven rabbits were sedated with intramuscular ketamine (50 mg/kg), glycopyrrolate (0.2 mg/kg), and buprenorphine (0.05 mg/kg). After intubation, rabbits were ventilated with a mechanical respirator (Hallowell EMC Model AWS; Hallowell, Pittsfield, Massachusetts) using room air enriched with 0.6 L/min oxygen. Catheters were introduced into a small auricular artery and vein, and into the right jugular vein for the continuous measurement of blood pressure and the administration of intravenous medications. Anesthesia was maintained with an intravenous infusion of ketamine (0.02 to 0.04 mg · kg−1 · min−1) and supplemental pentothal (2.5 to 5 mg/kg) as needed. Additionally, a pressure transducer (SPR-524; Millar Instruments, Houston, Texas) was introduced through the right carotid artery into the left ventricle. Heart rate, blood pressure, surface electrocardiogram, and rectal temperature were continuously monitored (Hewlett Packard 78534C; Palo Alto, California).
A left thoracotomy was performed, and a coronary snare was constructed by passing a suture around a large branch of the circumflex coronary artery at approximately 50% of the distance from base to apex of the heart, and threaded through a small piece of polyethylene tubing.
A hyper/hypothermia unit was used to maintain core temperature between 39.5° and 40.5°C in rabbits. Arterial blood gases were measured in all animals and pH was maintained between 7.35 and 7.45 throughout the protocol.
Experimental Protocol
Thirty-seven rabbits were divided into three separate groups: control group (n = 15), CsA (n = 12), and FK506 (n = 10). After instrumentation, baseline hemodynamic data were recorded. Next, all animals received a 1-hour, continuous 20-mL infusion of either a phosphate-buffered saline (PBS) vehicle (control), 25 mg/kg CsA, or 1 mg/kg FK506. The CsA and FK506 were both dissolved in a PBS vehicle.
Coronary snares were tightened to produce an ischemic region of the left ventricle. Ischemia was confirmed by a visible color change in the ischemic myocardial region, ST elevations on the electrocardiogram, and regional wall motion abnormalities on echocardiogram. At the end of the 30-minute ischemic period, coronary snares were loosened and the previously ischemic myocardium was reperfused for 3 hours. Hemodynamic measurements were recorded throughout the reperfusion period.
Analysis of Area at Risk and Infarct Size
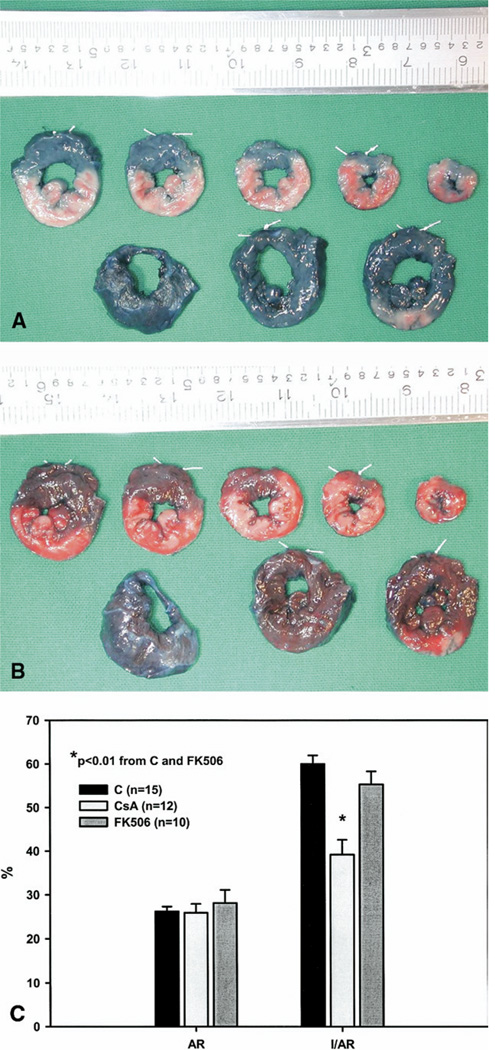
At the end of the protocol, the coronary snare was reapplied; vascular clamps were used to occlude the aorta, pulmonary artery and inferior vena cava; and the right atrium was incised. Evans Blue Dye (Sigma-Aldrich, St. Louis, MO), 5 mL of 1%, was injected into the left atrium to delineate the myocardial area at risk (AR). This was followed by a 20 mEq intra-atrial bolus of potassium chloride to arrest the heart. The heart was explanted, and the left ventricle (LV) was isolated and fixed in a 20% gelatin solution for 20 minutes. After fixation, the left ventricle was sectioned into eight 2- to 3-mm transverse slices (Fig 1A). The thickness of each slice was measured with a digital micrometer, and all slices were photographed. Infarct area was delineated by photographing and measuring the slices after 20 minutes of incubation in 2% triphenyltetrazolium chloride at 37° C (Fig 1B). All photographs were imported into an image analysis program (Image Pro Plus; MediaCybernetics, Silver Spring, Maryland), and computerized planimetry was performed. The AR is expressed as a percentage of the LV, and the infarct size is expressed as a percentage of the AR (I/AR).
Fig. 1.
Photographs of rabbit left ventricle sliced perpendicular to its long axis. (A) Evans blue dye staining to delineate the ischemic area at risk (AR). The AR is the unstained myocardium. (B) Triphenyltetrazolium chloride (TTC) staining to delineate viable myocardium (brick red) from nonviable myocardium (pale) in the AR. (C) Comparisons of area at risk (AR) and infarct size measured as a percentage of the area at risk (I/AR) in rabbits receiving either a phosphate- buffered saline vehicle (control [C], black bars; n = 15), cyclosporine A (CsA [light gray bars; n= 12]), or FK506 (dark gray bars; n = 10). *p < 0.001 from the control group.
Temperature and Hemodynamic Measurements
Arterial blood pressure, left ventricular pressure, heart rate, surface electrocardiogram, and rectal temperature were continuously monitored throughout the protocol in all animals. Hemodynamic, heart rate, and temperature measurements were recorded at baseline, after drug (PBS vehicle, CsA, or FK506) infusion, at 15 minutes of ischemia, immediately after the release of the coronary snares, and after 3 hours of reperfusion. The rate pressure product was calculated by multiplying the heart rate by the systolic blood pressure at all time points [9].
Tissue Preparation
The entire AR from each LV slice was excised. A 1- to 2-mm transmural specimen was removed from the AR, snap frozen in liquid nitrogen, and stored at −80°C. The remainder of the AR was fixed for 24 hours in 10% formalin and subsequently embedded in paraffin.
In Situ Oligo Ligation Assay
For the identification of apoptotic cells, an in situ oligo ligation (ISOL) assay (Intergen 7200; Intergen, Purchase, NY) with a high specificity for staining the specific DNA fragmentation characteristic of apoptosis was selected [10]. This assay utilizes T4 DNA ligase to bind synthetic biotinylated oligonucleotides to 3’-dT overhangs. Paraffin- embedded tissue was sectioned into 5-µm slices and deparaffinized by three changes of xylene, followed by three changes of absolute ethanol. Subsequently, endogenous peroxidase was quenched in 3% hydrogen peroxide in PBS. After washing the tissue sections, they were treated with 20 µg/mL proteinase K in PBS, washed again, and placed in an equilibration buffer. Next, a solution of T4 DNA ligase and oligonucleotides was applied to the slides and incubated overnight at 16° to 22°C. ApopTag detection of ligated oligonucleotides was accomplished by applying a streptavidin-peroxidase conjugate that was developed with diaminobenzidine. Finally, tissue sections were counterstained in hematoxylin.
Entire tissue sections were digitalized using a scanning microscope and analyzed using an image analysis software package (Image Pro Plus; MediaCybernetics, Silver Spring, MD). ISOL-positive and ISOL-negative nuclei were counted in the AR. Results are expressed as an apoptic index, which is defined as the percentage of ISOL positive cells per total number of cells in the entire AR.
Transmission Electron Microscopy
Myocardial punch biopsies were obtained from the AR from 2 rabbits from each of the control, CsA, and FK506 groups. Tissue was also obtained from 2 normal rabbits that were not subjected to the ischemia/reperfusion protocol. Biopsies were preserved in fixative (2.5% glutaraldehyde, 2.0% paraformaldehyde, 0.1 M sodium cacodylate [NaCaC]) for 24 hours at 4°C. After several washes in 0.1M NaCaC, samples were post-fixed with buffered 2% osmium tetroxide for 1 hour at 4°C. Subsequent washes in 0.1M NaCaC, water, and 2% aqueous uranyl acetate were used to destain samples. Tissue samples were dehydrated in serial washes of ethanol and propylene oxide, before a slow infiltration with EPON 812. Samples were cured at 70°C for 48 hours and cut, stained, and imaged on a Jeol-10-10 transmission electron microscope (Jeol, Akishima, Japan). Random images were captured from each sample for comparative analysis. To assess the degree of mitochondrial disruption, five random images of mitochondria at 12,000 magnification per rabbit were captured from each specimen. Morphologic differences in mitochondria were assessed in the nuclear cap, a region surrounding the cell nucleus. The total number of mitochondria and the number of disrupted mitochondria were counted and averaged. The mean percentage of disrupted mitochondria was calculated and reported for each group.
Statistics
Measurements are reported as means ± SEM. A one-way analysis of variance (ANOVA) with a post-hoc Tukey honestly significant difference (HSD) test was used for all comparisons between groups. A repeated measures ANOVA with a post-hoc Tukey HSD test was used for all comparisons within groups. All analyses were completed using SPSS version 11.0 (SPSS, Chicago, Illinois). Statistically significant differences were established at p les than 0.05.
Results
Risk Area and Infarct Size Measurements
The sizes of the ischemic myocardial AR were similar among the three groups of rabbits (26% ± 1% control versus 26% ± 1% CsA versus 28% ± 3% FK506, p > 0.05). Infarct size (I/AR) in the CsA group (39% ± 3%) was significantly reduced compared with both the control group (60% ± 2%, p < 0.001) and the FK506 group (55% ± 3%, p = 0.001). There was no significant difference in infarct sizes between the control and FK506 groups (p > 0.05, Fig 1C).
Temperature, Heart Rate, and Rate Pressure Product
Core temperatures were equivalent (p > 0.05) in all groups throughout the protocol. Baseline heart rates were similar in all groups. The infusion of CsA or FK506 resulted in a reduction in heart rate compared with the PBS vehicle alone (272 ± 6 beats per minute control versus 247 ± 5 beats per minute CsA [p < 0.01] versus 252 ± 6 bpm FK506 [p < 0.05]). Differences in heart rate between the control group and the other two groups persisted throughout ischemia and reperfusion. There were no significant differences in heart rate between the CsA and FK506 groups at any time point. Within the CsA and FK506 groups, rabbits had significantly lower heart rate at the onset and after 3 hours of reperfusion compared with their baseline values. There were no differences between groups in rate pressure product at any of the time points. Within the CsA and FK506 groups, rabbits had attenuated rate pressure product values during ischemia, at the onset, and after 3 hours of reperfusion compared with baseline values. Within the control group, there were no significant differences in heart rate or rate pressure product values throughout the protocol (Table 1).
Table 1.
Heart Rate (HR) and Rate Pressure Product (RPP) in Rabbits
| Baseline | Post-Drug | Ischemia | Reperfusion-0 | Reperfusion-165 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | RPP | HR | RPP | HR | RPP | HR | RPP | HR | RPP | |
| Control | 269 ± 7 | 19,188 ± 998 | 272 ± 6 | 19.856 ± 881 | 264 ± 5 | 18,173 ± 844 | 253 ± 6 | 17,373 ± 981 | 248 ± 4 | 16,255 ± 1,282 |
| CsA | 263 ± 5 | 18,403 ± 942 | 247 ± 5b,c | 17,129 ± 955 | 240 ± 9a,c | 16,392 ± 1233c | 235 ± 6a,c | 16,705 ± 1,309c | 213 ± 5b,c,d | 13,283 ± 1,013c,d |
| FK506 | 262 ± 9 | 20,148 ± 1331 | 252 ± 6a | 19,597 ± 1,018 | 238 ± 5b,c | 16,018 ± 991c | 233 ± 4a,c | 14,966 ± 549c | 216 ± 5b,c,d | 13,259 ± 466c,d |
Values are means ± SEM.
p < 0.05 between-group comparison with control group;
p < 0.01 between-group comparison with control group;
p < 0.05 within-group comparison with baseline value;
p < 0.05 within-group comparison with post-drug, ischemia, and reperfusion-0 values.
CsA = cyclosporine A; HR = heart rate (beats per minute); RPP = rate pressure product (beats per minute · mm Hg).
Apoptosis
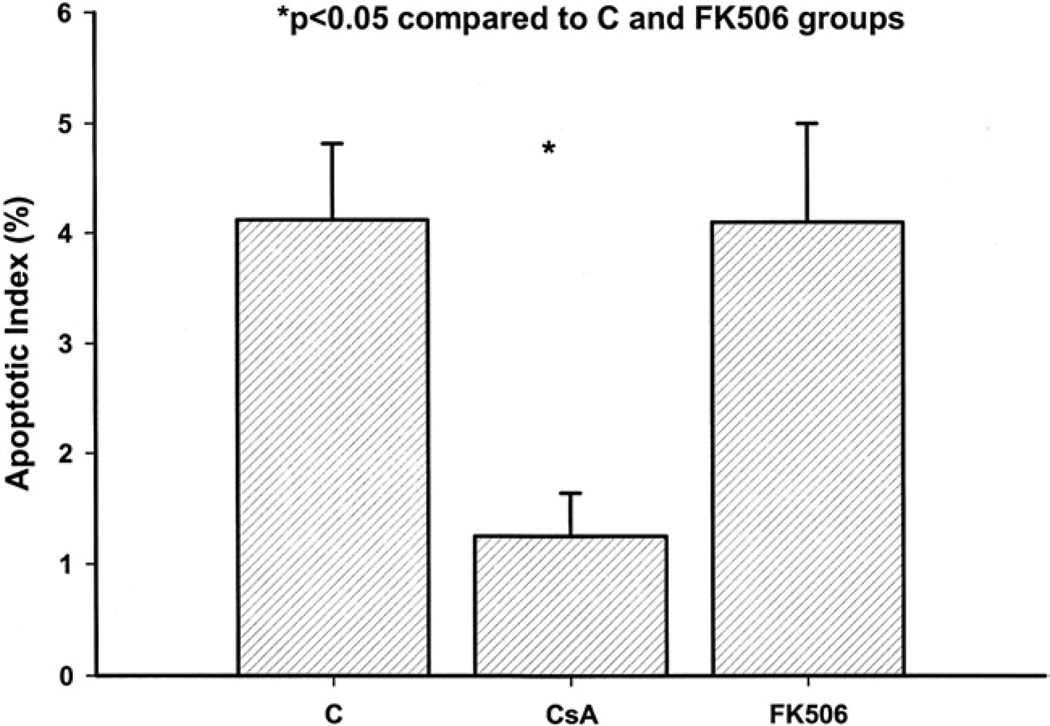
Apoptotic cell death was studied with the use of the ISOL technique, a highly specific test for the DNA cleavage fragments characteristic of apoptotic cell death [10]. The addition of CsA (1.2% ± 0.5%) to reperfusion therapy resulted in a significant reduction in the apoptotic cell index when compared with the control group (4.3% ± 0.8%, p = 0.01) and FK506 group (4.1% ± 0.9%, p = 0.05; Fig 2).
Fig. 2.
Comparisons of apoptotic index in rabbits receiving either a phosphate-buffered saline vehicle (control [C]), cyclosporine A (CsA), or FK506 and undergoing the ischemia/reperfusion protocol. *p < 0.05 from the control and FK506 groups.
Transmission Electron Microscopy
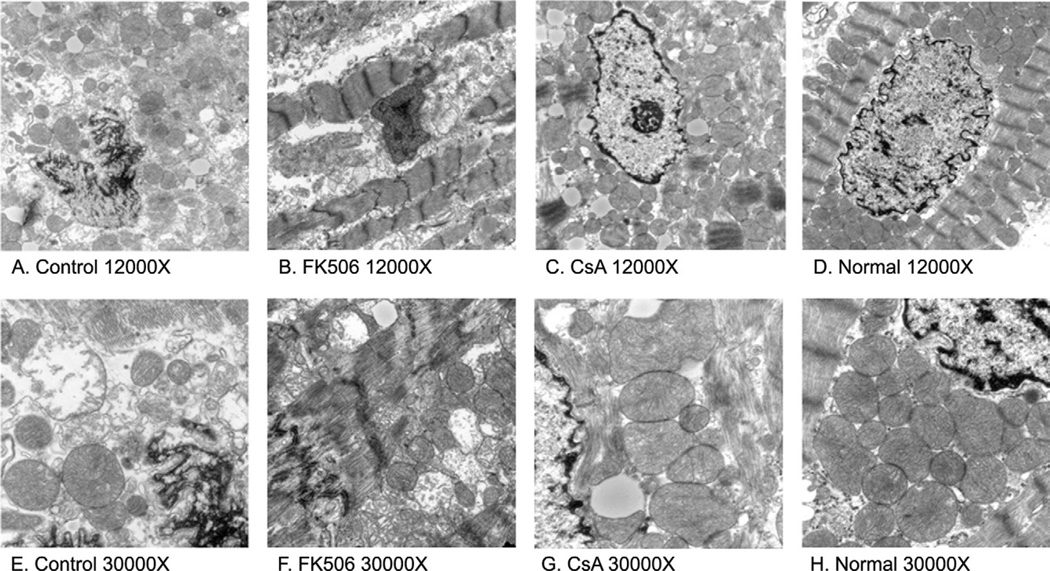
In the normal animals, the nuclear cap was densely populated with mitochondria that had well-defined membranes with tightly packed cristae (Fig 3D and H). The nuclear cap in the control group (Fig 3A and E) and FK506 group (Fig 3B and F) contained fewer mitochondria that were vacuolated, swollen, or possessed poorly defined or completely absent outer membranes with unraveling cristae. Compared with the normals, the nuclear cap in the CsA group (Fig 3C and G) was less densely populated with mitochondria, and contained a few mitochondria with dilated cristae. However, the majority of the mitochondria had well-defined outer membranes with normal-appearing cristae. Overall, the CsA group demonstrated a morphology that was more consistent with that of the normal animals than the nuclear cap morphology observed in the control and FK506 groups. Quantification of these structural differences revealed a significantly higher percentage of disrupted mitochondria in the control group (53% ± 12%) and FK506 group (47% ± 9%) compared with the CsA group (20% ± 7%, p = 0.01) or normal rabbits (1.7% ± 1%, p = 0.001).
Fig. 3.
Transmission electron micrographs of rabbit myocardium. Representative images at × 12,000 compare the differences among the mitochondria of the (A) control, (B) FK506-treated, (C) CsA-treated, and (D) normal rabbit groups. At ×30,000, mitochondrial differences are more evident. The mitochondria of the (G) CsA-treated and (H) normal group are tightly packed, possessing an intact mitochondrial membrane and well-organized cristae, unlike the (E) control group and (F) FK506-treated group that are characterized by swollen, loosely packed, vacuolated, and completely disrupted mitochondria.
Comment
The MPTP has been shown to be a critical determinant of cell death in many different models of ischemia/reperfusion [11]. In this study, we demonstrated that the cardioprotective effects of CsA are calcineurin-independent, and that CsA-mediated inhibition of the MPTP limits the pathologic changes in mitochondrial morphology that are associated with myocardial ischemia/ reperfusion injury.
The immunosuppressive effects of CsA and FK506 are due to their affinity for the cytosolic protein cyclophilin A. The binding of these drugs to cyclophilin A results in the inhibition of calcineurin, and the limitation of early T-cell activation at the DNA transcriptional level. Calcineurin is a calcium-calmodulin activated protein phosphatase, which is ubiquitous in all cells and is activated by the binding of calcium [12]. The role of calcineurin in cardiomyocyte ischemia/reperfusion injury and its effect on the apoptotic pathway have not been fully elucidated. It has been proposed that calcineurin plays a role in calcium-induced apoptosis in mammalian cells [13–15]. However, Lotem and colleagues [16] have demonstrated that calcineurin activation can activate opposing pathways that can either induce or suppress apoptosis.
Cyclosporine A also has an affinity for binding the mitochondrial cyclophilin D protein, which prevents opening of the MPTP [17]; FK506 does not bind cyclophilin D or affect the function of the MPTP [18].
In the current study, we demonstrated that the administration of CsA limited ischemia/reperfusion-induced myocyte necrosis and apoptosis, resulting in a 20% reduction in myocardial infarct size. To prove that the myocardial protective effects of CsA were due to its inhibition of MPTP and were independent of its effects on calcineurin, we administered FK506 to a separate group of rabbits. Calcineurin inhibition with FK506 had no significant impact on myocardial salvage, cardiomyocyte apoptosis, or mitochondrial morphology. These results provide conclusive evidence that calcineurin is not an important activator or inhibitor of the apoptotic pathway for cardiac myocytes after ischemia/reperfusion-induced injury.
Controlling for the potential confounding effects of CsA’s calcineurin inhibition properties was an important element of the experimental design. Calcineurin influences intracellular calcium handling, which could impact myocardial salvage after myocardial ischemia/reperfusion injury. Recently, it has been reported that both FK506 and CsA are effective in reducing injury in rabbit spinal cord ischemia/ reperfusion models. This suggests that in neural tissue the calcineurin-inhibiting property of CsA is the protective mechanism [19].
We also examined mitochondrial morphology from normal (no ischemia/reperfusion), control, CsA, and FK506 treated animals using transmission electron microscopy. Analysis of the nuclear cap regions from all groups suggests that CsA preserves mitochondrial structure compared with control and FK506 animals. Figure 3 demonstrates that that the number and morphology of mitochondria in CsA rabbits are more consistent with the normal animals than the control group. The nuclear cap in the control and FK506 groups is characterized by frequent vacuolated and disrupted mitochondria. Quantitative measurements of the nuclear cap region demonstrate a higher percentage of disrupted mitochondria in control animals (53% ± 12%) and FK506 animals (47% ± 9%) than in CsA-treated animals (20% ± 7%, p = 0.01).
The reduction in heart rate observed after the administration of both CsA and FK506 can only be explained as a drug effect, as it is present immediately after infusion and persisted throughout ischemia and reperfusion (Table 1). Bradycardia is a known adverse effect of FK506, but not CsA. Nevertheless, the reductions in heart rate were not significant enough to produce differences in rate pressure product, a sensitive indicator of myocardial oxygen consumption, among the three groups at any time point [9].
The results of this study add to a growing body of literature that identifies the MPTP as a prime therapeutic target for limiting myocardial ischemia/reperfusion injury [1, 4, 7, 8, 20]. The full understanding of the role of the MPTP is yet to be elucidated; however, it is believed to be involved in the cardioprotective effects of ischemic preconditioning and postconditioning [20–23]. Inhibition of MPTP opening in our study resulted in a reduction of both necrotic and apoptotic cardiomyocyte death and preservation of mitochondrial morphology. Preliminary evidence in isolated human atrial myocytes suggests that pharmacologic suppression of the MPTP may have cardioprotective effects [24]. The reduction in apoptosis demonstrated with MPTP inhibition could impact post-CABG myocardial stunning, an event that has been linked with myocyte apoptosis [25]. The myocardial salvage benefit demonstrated in these preclinical animal model studies are the basis for the current clinical trial evaluating intravenous administration of CsA before reperfusion therapy in patients undergoing percutaneous coronary interventions for acute myocardial infarction. [23].
Acknowledgments
This research was supported by Grants HL71137, HL63954 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland.
References
- 1.Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 2.Nazareth W, Yafei N, Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin. A. J Mol Cell Cardiol. 1991;23:1351–1354. doi: 10.1016/0022-2828(91)90181-k. [DOI] [PubMed] [Google Scholar]
- 3.Weinbrenner C, Liu GS, Downey JM, Cohen MV. Cyclosporine A limits myocardial infarct size even when administered after onset of ischemia. Cardiovasc Res. 1998;38:678–684. doi: 10.1016/s0008-6363(98)00064-9. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths EJ, Halestrap AP. Protection by cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 5.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 6.Argaud L, Gateau-Roesch O, Muntean D, et al. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol. 2005;38:367–374. doi: 10.1016/j.yjmcc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307:93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halestrap AP, Connern CP, Griffiths EJ, Kerr PM. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/ reperfusion injury. Mol Cell Biochem. 1997;174:167–172. [PubMed] [Google Scholar]
- 9.Wilkinson PL, Moyers JR, Ports T, Chatterjee K, Ullyott D, Hamilton WK. Rate-pressure product and myocardial oxygen consumption during surgery for coronary artery bypass. Circulation. 1979;60:170–173. doi: 10.1161/01.cir.60.2.170. [DOI] [PubMed] [Google Scholar]
- 10.Lesauskaite V, Epistolato MC, Ivanoviene L, Tanganelli P. Apoptosis of cardiomyocytes in explanted and transplanted hearts. Comparison of results from in situ TUNEL, ISEL, and ISOL reactions. Am J Clin Pathol. 2004;121:108–116. doi: 10.1309/7MNN-4E8T-FAKH-3XC9. [DOI] [PubMed] [Google Scholar]
- 11.Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie. 2002;84:153–166. doi: 10.1016/s0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- 12.Jayaraman T, Marks AR. Calcineurin is downstream of the inositol 1,4,5-trisphosphate receptor in the apoptotic and cell growth pathways. J Biol Chem. 2000;275:6417–6420. doi: 10.1074/jbc.275.9.6417. [DOI] [PubMed] [Google Scholar]
- 13.Shibasaki F, Kondo E, Akagi T, McKeon F. Suppression of signalling through transcription factor NF-AT by interactions between calcineurin and Bcl-2. Nature. 1997;386:728–731. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]
- 14.Shibasaki F, McKeon F. Calcineurin functions in Ca(2+)-activated cell death in mammalian cells. J Cell Biol. 1995;131:735–743. doi: 10.1083/jcb.131.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HG, Pathan N, Ethell IM, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 16.Lotem J, Kama R, Sachs L. Suppression or induction of apoptosis by opposing pathways downstream from calcium-activated calcineurin. Proc Natl Acad Sci USA. 1999;96:12016–12020. doi: 10.1073/pnas.96.21.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion— a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 18.Nathan M, Friehs I, Choi YH, et al. Cyclosporin A but not FK-506 protects against dopamine-induced apoptosis in the stunned heart. Ann Thorac Surg. 2005;79:1620–1626. doi: 10.1016/j.athoracsur.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 19.Tachibana T, Shiiya N, Kunihara T, et al. Immunophilin ligands FK506 and cyclosporine A improve neurologic outcome after transient spinal cord ischemia in rabbits. J Thorac Cardiovasc Surg. 2005;129:123–128. doi: 10.1016/j.jtcvs.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 20.Di Lisa F, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res. 2006;70:191–199. doi: 10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- 22.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 23.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 24.Shanmuganathan S, Hausenloy DJ, Duchen MR, Yellon DM. Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol. 2005;289:H237–H242. doi: 10.1152/ajpheart.01192.2004. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt JP, Schroder J, Schunkert H, Birnbaum DE, Aebert H. Role of apoptosis in myocardial stunning after open heart surgery. Ann Thorac Surg. 2002;73:1229–1235. doi: 10.1016/s0003-4975(02)03401-x. [DOI] [PubMed] [Google Scholar]