Abstract
Neurons that express high levels of polysialylated neural cell adhesion molecule (PSANCAM) in adult spinal substantia gelatinosa also express the μ-opioid receptor. While PSA removal from NCAM by spinal intrathecal injection of endoneuraminidase-N (endo-N) did not detectably change opioid receptor expression, morphine-induced analgesia was significantly increased. This analgesic strengthening was detected as early as 15 minutes after endo-N treatment and persisted for at least 7 days. In addition, the tolerance that develops with chronic morphine treatment was overcome in the absence of PSA. Interestingly, the same effects on analgesia and tolerance were also produced by selective deletion of the NCAM-180 isoform.
Keywords: Opiate, Polysialic acid, NCAM, Antinociceptive, Tolerance, Pain
1. Introduction
The polysialylated neural cell adhesion molecule (PSA-NCAM) is abundantly expressed in the developing brain, and although it is lost from most adult brain areas, it is retained within distinct regions that exhibit morphological or physiological plasticity (Bonfanti et al., 1992; El Maarouf, 2005; Rutishauser, 2008). One example of adult PSA-NCAM expression is the spinal substantia gelatinosa. Previously, we showed that enzymatic removal of PSA in this region revealed that this polymer helps to moderate neuropathic hyperalgesia (El Maarouf et al., 2005). PSA is a long linear homopolymer of -2,8-linked sialic acid typically attached to the neural cell adhesion molecule (NCAM). It attenuates cell interactions mediated by NCAM as well as by other adhesion receptors such as cadherins and integrins (Fujimoto et al., 2001). PSA removal can impact contact-dependent signaling, e.g., NCAM activation of p59Fyn kinase and MAPK (Petridis et al., 2004; Seidenfaden et al., 2003). PSA modulation has also been shown to affect the function of soluble molecules such as brain-derived neurotrophic factor (BDNF) (Burgess and Aubert, 2006; Vutskits et al., 2001), NMDA (Hammond et al., 2006) and AMPA signaling (Vaithianathan et al., 2004).
The spinal substantia gelatinosa expresses the μ-opioid receptor (Abbadie et al., 2000) that transduces the analgesic effect of opiates such as morphine (Law et al., 2000). Opiates are commonly used in the control of pain, including high doses in patients with refractory pain (Pasternak, 2007; Plante and VanItallie, 2010). Unfortunately, chronic treatment is often associated with development of tolerance to the opiates, and dose escalation to manage pain is often limited by side effects (Pasternak, 2007; Ruan, 2007).
In the present study, we found that PSA and μ-opioid receptor are expressed by the same neurons, and that either the enzymatic removal of PSA or genetic deletion of the NCAM-180 isoform result in a surprisingly substantial strengthening of morphine-induced analgesia as well as a delay in the development of tolerance.
2. Results
2.1. Neurons of the dorsal spinal cord co-express PSA and μ-opioid receptor
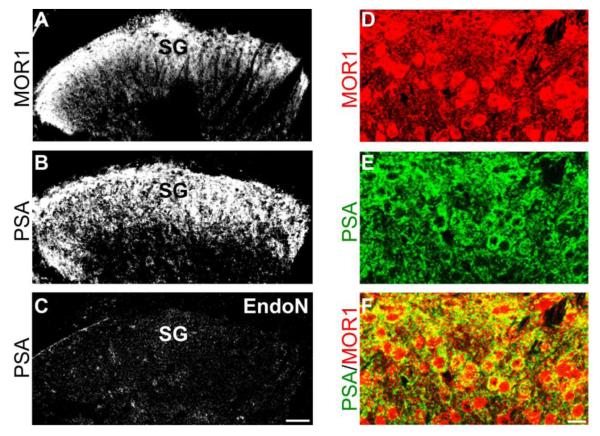
Lumbar spinal cross-sections from untreated male mice were immunostained for PSA and the μ-opioid receptor. High levels of PSA expression occur in the substantia gelatinosa of superficial dorsal horn where the μ-opioid receptor is also expressed (Fig. 1A,B). PSA is clearly present on cell surfaces (Fig. 1E), and the PSA-expressing neurons also express the μ-opioid receptor both intracellularly and on their surface membrane (Fig. 1D-F).
Figure 1. PSA and μ-opioid receptor are co-expressed in neurons of spinal substantia gelatinosa.
A: Image of superficial layers of the dorsal horn in a mouse lumbar spinal cross-section. Immunostaining with MOR1 antibody shows that the μ-opioid receptor is expressed in the substantia gelatinosa (SG). B: A similar cross-section showing that PSA is also expressed the substantia gelatinosa (SG:). C: substantia gelatinosa PSA-staining disappears completely after in vivo intrathecal injection of endo-N (Scale bar: 50 μm). At higher magnification, the μ-opioid receptor expression (red) appears to occur on the cell surface and in the cytoplasm (D), while PSA (green) is expressed mostly on cell surfaces (E). Double labeling reveals that PSA-expressing neurons also co-express μ-opioid receptor (yellow) (F) (Scale bar: 20 μm).
2.2. Removal of PSA does not detectably alter expression of μ-opioid receptor
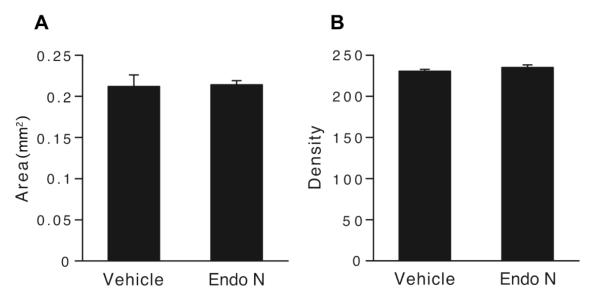
The anatomical co-localization of PSA and the μ-opioid receptor raised the possibility of a structural or functional interaction. Intrathecal endo-N administration was used to assess possible effects of PSA removal on expression of the μ-opioid receptor. Tissue immunostaining showed that endo-N completely removed PSA from lumbar spinal cord (Fig. 1C), whereas expression of the μ-opioid receptor in the substantia gelatinosa appeared unchanged 24 hours later. Quantitative analyses confirmed that PSA removal had no detectable effect on μ-opioid receptor distribution; both the density and area of immunostaining remained unaffected (Fig. 2A,B).
Figure 2. Expression of μ-opioid receptor is not detectably changed after PSA removal.
A: Graph showing the surface area (mm2) of μ-opioid receptor immunostaining in the substantia gelatinosa as measured by NIH-Image program in spinal cross-sections 1 day after vehicle or endo-N-treatment (3 animals each, 3 sections per animal). As shown in B, the density of staining was also assessed in these sections. Both parameters remain unchanged.
2.3. PSA removal induces a rapid and persistent increase in morphine-induced analgesia
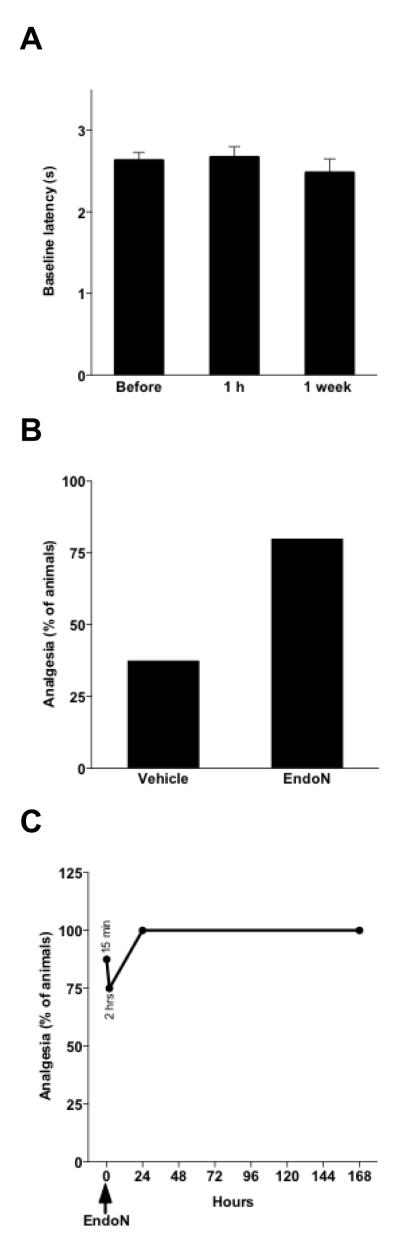
Mean tail-flick baseline latencies remained unchanged 1 week after endo-N treatment (Fig. 3A). While subcutaneous administration of morphine (5 mg/kg) to vehicle-treated animals was effective in producing a 37.5% analgesic effect in the tail-flick test, with endo-N pretreatment (1hr 30 min before morphine injection), the same dose of morphine produced a considerably higher analgesic effect (p < 0.001; Fig 3B). This analgesic amplification was observed as early as 15 minutes after endo-N treatment (p < 0.001; morphine was injected 30 min and endo-N 15 min before testing); the effect reached 100% when the animals were tested one day after endo-N injection and persisted at this level for at least 7 days (p < 0.001; Fig. 3C).
Figure 3. PSA removal rapidly and persistently enhances morphine-induced analgesia.
A: Graph showing tail-flick latencies both before endo-N intrathecal injection and 1 hour or 1 week after. PSA removal from the spinal cord does not change tail-flick latencies (n = 10; error bars: SEM). B: When animals receive a subcutaneous morphine (5 mg/Kg) injection 1h 30 min after endo-N (n = 10) or vehicle (n = 10) intrathecal administration, tail-flick-assessed analgesia 30 min later is substantially much higher (p < 0.001) in the absence of PSA. The analgesic effect was determined quantally as the percentage of animals displaying a doubling or more of individual baseline latencies. C: This boost in analgesia occurs as early as 15 min after endo-N administration (p < 0.001; morphine was injected 15 min before; n = 8), and persists for at least 1 week (n = 20 at 2, 24 and 168 hrs) (first 2 points from left represent levels at 15 min and 2 hours after endo-N administration).
2.4. PSA removal helps to overcome morphine tolerance
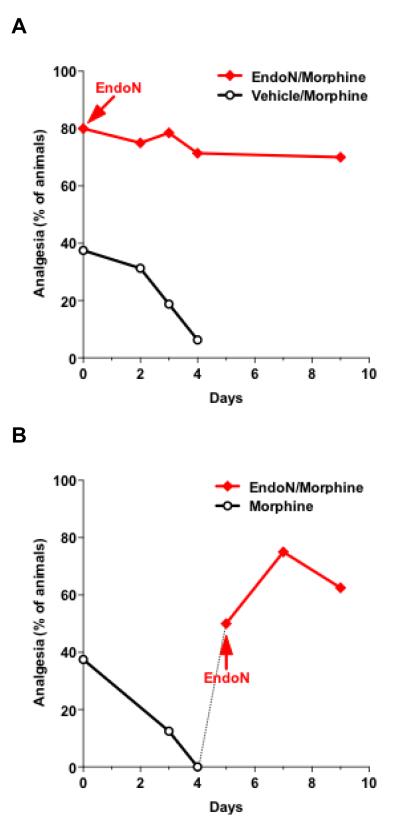
The effect of PSA removal on analgesia was re-examined with chronic morphine administration (5 mg/kg). As expected, in vehicle-treated mice, tolerance developed after only 4 daily administrations of morphine (p < 0.001), whereas in the absence of PSA (Endo-N-treated), the levels of morphine-induced analgesia remained constantly elevated for at least 9 days of morphine treatment (Fig. 4A; p = 0.14). It is noteworthy that after 4 daily morphine injections, PSA immunostaining (n = 3) remained similar to levels in non-morphine treated samples shown in figure 1B,C.
Figure 4. Morphine tolerance is overcome by PSA removal.
A. Graph showing analgesic levels following repetitive daily morphine treatment (5 mg/kg) in the presence and absence of PSA. Analgesic levels drastically decreased in the vehicle-treated group (black line with circles) by day 4 (p < 0.001) of daily injections of morphine (n = 16), while they remained significantly higher in the absence of PSA for at least 9 days (p = 0.14) of morphine treatment (red line with squares; n = 28). B. After development of tolerance in the vehicle-treated group (black line with circles), endo-N injection (on day 5, arrow, n = 8) 30 min before morphine (5 mg/kg) considerably increased (p < 0.001) analgesic levels (red line with squares), which became afterwards comparable to values in the group treated with endo-N on day 0 (p = 0.29).
Interestingly, after development of tolerance in vehicle-treated mice, intrathecal injection of endo-N (on day 5; n = 8) rapidly and sustainably reversed that tolerance (Fig. 4B; p < 0.001), despite the continuation of daily morphine injections. Values in the following days became comparable to those obtained with endo-N injection at the beginning of morphine regimen (p = 0.29). Together, these observations reveal that the enhancement of morphine analgesia by PSA removal helps both to prevent and overcome the effect of chronic morphine.
2.5. PSA removal effects are reproduced by selective deletion of the NCAM-180
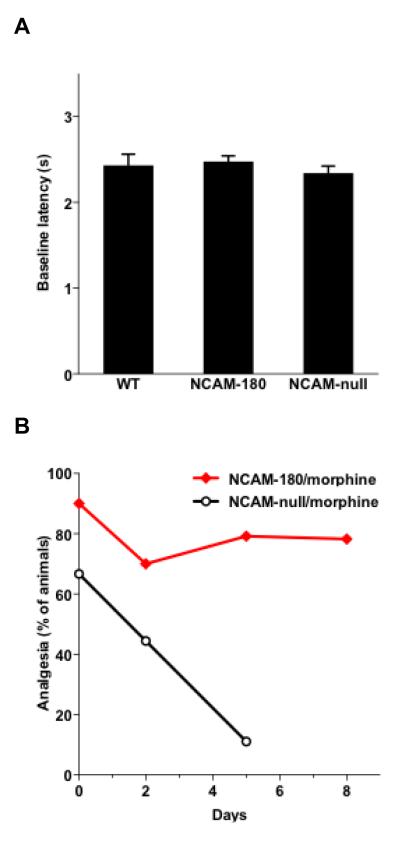
There are 3 major NCAM isoforms, NCAM-120, 140, and 180, which differ with respect to their cytoplasmic domain (lipid-linked, small domain, large domain respectively), but all share the same extracellular domains and can be polysialylated. Mice lacking all isoforms (NCAM-null) have been generated. While NCAM-120 or 140 specific mutants are not available, the long cytoplasmic tail of NCAM-180 can be selectively targeted, and it has been possible to generate mice that specifically lack NCAM-180, but still retain the other isoforms. As with removal of PSA, selective genetic deletion of the NCAM-180 variant did not affect baseline tail-flick latencies, ranging between 2 and 3 seconds (Fig. 5A).
Figure 5. Selective deletion of NCAM-180 isoform reproduces the effects of PSA removal by endo-N.
There are 3 major NCAM isoforms, NCAM-120, 140, and 180. A. Selective deletion of the NCAM-180 variant or total genetic ablation of all NCAM isoforms does not detectably change baseline thermal sensitivity of the tail in untreated animals. Tail-flick latencies were the same in the presence (WT) or absence of NCAM (NCAM-null), as well as when only the 180 isoform was deleted. B. Analgesic levels following repetitive daily morphine treatment (5 mg/kg) were measured in NCAM-180 mutants and in NCAM-null animals (lacking all isoforms). Mutation of NCAM-180 (red line with squares; n = 20) produced effects similar to PSA removal by endo-N. There was a high morphine analgesic effect at day 0, which was maintained throughout the study. The NCAM-null mutation had a milder effect on morphine analgesia (day 0), with occurrence of morphine tolerance (p < 0.001; black line with discs; n = 9).
Interestingly, with selective deletion of the NCAM-180 isoform, the levels of morphine-induced analgesia were shifted to values similar to those obtained after PSA removal by endo-N. That is, analgesia was high (p < 0.001) after a single morphine (5 mg/kg) injection (Fig. 5B; day 0), and tolerance was not observed after 9 consecutive daily injections of the same dose (Fig. 5B; n = 20). Values obtained with NCAM-180 mutation and endo-N, at the end of the chronic morphine treatment, were comparable (p = 0.26). The effect of null mutation of NCAM, which removes all isoforms was also tested. While the baseline was not affected (Fig. 5A), the change in morphine analgesia (5 mg/kg) was milder (Fig. 5B; day 0). The effect on tolerance produced by endo-N or NCAM-180 deletion was not observed in the null animals, with the same morphine regimen (Fig. 5B; n = 9). The loss in analgesic effect by day 5 was statistically significant (p < 0.001).
3. Discussion
The present findings show that PSA removal enhances morphine-induced analgesia and interferes with tolerance to this drug. There is some precedent to an effect of PSA removal on surface receptors, in that its cleavage increases BDNF biological activity in embryonic septal neurons (Burgess and Aubert, 2006) and the in vivo action of the AMPA antagonist NBQX (Potschka et al., 2008). As the endo-N effect on morphine-induced analgesia was fast (less than 15 min) and immunostaining showed no noticeable changes in receptor abundance, the observed effects of PSA removal did not likely result from more receptor expression, but probably from a potentiation of receptor function.
It is interesting that in addition to the heightened morphine analgesia produced by PSA removal, no tolerance to this drug (daily 5 mg/Kg) was observed during the entire study period. This is probably due to a much delayed manifestation of tolerance rather than its complete abolition, as repetitive daily injection of a smaller dose of morphine (2.5 mg/Kg) revealed a delayed tolerance onset (Data not shown).
The observation that selective deletion of the NCAM-180 variant and removal of PSA by endo-N have the same effects (Fig. 6) suggests that the PSA that modulates the opioid system is carried by the NCAM-180 isoform. The result that deletion of all NCAM isoforms (NCAM-null) did not reproduce the endo-N effects attests of a role for increased NCAM function following PSA removal. However levels of morphine analgesia in NCAM-null animals are not identical to those in control wildtypes. This is not easily interpreted, but PSA could modulate other molecule(s) in addition to NCAM (Fujimoto et al., 2001; Rutishauser, 2008). Other studies have implicated another cell adhesion molecules in the opioid system. The opioid-binding cell adhesion molecule (OBCAM), which is also expressed in the spinal substantia gelatinosa, influences the activity of the opioid receptor (Govitrapong et al., 1993; Hachisuka et al., 1999; Loh and Smith, 1990). It would be interesting to examine if OBCAM function is also sensitive to PSA removal.
Figure 6. Possible mode of action of PSA and NCAM on opioid receptor.
A possible interpretation of the present findings is that in wildtype animals, the chains of polysialic acid that modulate morphine-induced analgesia are attached to the NCAM-180 variant. PSA occupies large hydrated volumes (grey halo on the cell surface), which hinders the function of all NCAM isoforms. Upon removal of PSA by endo-N (Wildtype + endo-N) or by genetic deletion of PSA-NCAM-180 (NCAM-180-/-), the NCAM protein becomes able to interact (red double arrow) either directly or indirectly with the opioid receptor, thereby strengthening the morphine analgesia. Although this effect does not require the cytoplasmic domain unique to NCAM-180, it is not clear if is specific to one isoform or can be produced by any NCAM variant in the absence of PSA. Tolerance develops in the absence of all NCAM isoforms (NCAM-null), which confirms that the observed effect depends on NCAM.
Development of tolerance is under complex control, involving factors at the receptor level as well as the cellular and neuronal network levels (Chen and Sommer, 2009; Pasternak, 2007). Different mechanisms could potentially be involved in the NCAM effects. For example, receptor internalization contributes to tolerance, and NCAM has been shown to play a role in vesicular trafficking (Hata et al., 2007; Polo-Parada et al., 2004) including exocytosis (Chernyshova et al., 2011). Therefore the possibility exists that NCAM facilitates replenishment of surface receptors. A role for NMDA receptor activation of nitric oxide synthase (NOS) in morphine tolerance is also well established (Kolesnikov et al., 1993; Pasternak, 2007), and NCAM has the ability to change the distribution of NMDA receptors through interaction with spectrin (Sytnyk et al., 2006). However, it is not known if such redistribution would reduce NOS activation and block tolerance. Chronic morphine activates PLC-gamma (Wolf et al., 2007) as well as MAPK, which have been implicated in morphine tolerance (Chen and Sommer, 2009). NCAM also has the capacity to activate PLC-gamma (Kolkova et al., 2000) and MAPK (Petridis et al., 2004; Seidenfaden et al., 2003); however an increase in NCAM interactions should promote tolerance not the opposite. An alternative hypothesis is binding of PLC-gamma to NCAM (Buttner et al., 2005) that would sequestrate it away from the opioid signaling pathway.
It is also interesting that while spinal PSA levels did not visibly change with chronic morphine, an increase in NCAM expression has been described (Suzuki et al., 2006). PSA expression is elevated in the hippocampus of human heroin addicts (Weber et al., 2006), which might suggest that adjustments in PSA levels could provide a means to control excessive activation of the opioid system in this region.
While the mechanisms remain unclear, the magnitude of the effects obtained by PSA and/or NCAM perturbation raise the possibility of new therapeutic approaches to pain. Reducing PSA expression by enzymatic or biochemical means could help achieve a desired analgesic level with a smaller amount of drug, and at the same time reduce the risk of morphine tolerance. However, we have found in other studies that such treatments could also exacerbate neuropathic pain (El Maarouf et al., 2005). Further understanding of the interactions that are modified following PSA removal and that change morphine analgesia will be required in order to indicate the nature of potential drug targets for pain treatment.
4. Experimental Procedure
Adult CD-1/CRL locR/6 male mice (25-30 g) were purchased from Charles River Laboratories. Male NCAM-null mutants (lacking all NCAM isoforms) (Cremer et al., 1994), NCAM-180 mutants (lacking the NCAM-180 isoform only) (Tomasiewicz et al., 1993) and control wildtype littermates were bred in our facility. All animals were housed in a 12:12-h light–dark cycle temperature-controlled room with free access to food and water. Animal care and experimentation were carried out in accordance with institutional and NIH guidelines. Drugs were obtained from the Research Technology Branch of the National Institute on Drug Abuse (Rockville, MD).
4.1. Endo-N injection
Preparation of endo-N was carried out as described previously (El Maarouf and Rutishauser, 2003). This enzyme specifically cleaves -2,8-linked sialic acid chains with minimum chain length of 8, sparing other sialic acid-containing moieties (Hallenbeck et al., 1987; Vimr et al., 1984). This selectivity for PSA and the rapid diffusion in neural tissues make it suitable for in vivo application (El Maarouf et al., 2005; El Maarouf and Rutishauser, 2003; Glass et al., 2000). Under light halothane anesthesia, animals received a 1 μl intrathecal injection of endo-N (200 U/μl), which is effective in completely removing PSA from the spinal cord (El Maarouf et al., 2005). Injection of a similar volume of vehicle solution was used as control.
4.2. Assessment of morphine analgesia in radiant heat tail-flick test
The radiant heat tail-flick assay was used to assess the drug antinociceptive effect (Kolesnikov et al., 2003). The apparatus uses a light beam to stimulate the tail. A photoelectric cell-controlled timer gives the latency when the tail moves (i.e., flicks). The animals were tested twice at baseline and at the indicated times, and the two readings’ mean was used. Baseline tail-flick latencies typically ranged from 2 to 3 s. Analgesia was considered quantally as the doubling or more of individual baseline latencies; it was determined as the percentage of animals displaying the analgesic response. The animals were always tested 30 min after subcutaneous morphine administration (5 mg/Kg). For tolerance studies, morphine was injected daily (5 mg/Kg, subcutaneously) and animals tested in the tail-flick assay when indicated. Fisher’s exact test was used for statistical comparison of quantal data.
4.3. Immunostainings
Under deep anesthesia, the animals were transcardially perfused with phosphate buffer saline (7.4 pH; 25 ml) followed by a solution of 4% paraformaldehyde (in 0.1 M, 7.4 pH phosphate buffer; 25 ml). After dissection, the lumbar spinal cords were postfixed in the same fixative overnight at 4°C, then 50 μm transverse sections were prepared using a vibratome.
The 5A5, a mouse monoclonal antibody specific for PSA (El Maarouf et al., 2005; El Maarouf and Rutishauser, 2003), and the MOR-1, a guinea pig monoclonal antibody specific for the μ-opioid receptor (Abbadie et al., 2000) were applied to floating sections at 1:2000 and 1:1000 dilutions, respectively. Appropriate fluorescent secondary antibodies were used to visualize immunostaining using a Zeiss 510 LSM confocal microscope. The NIH image analysis software was used for morphometric quantifications, and a one-way ANOVA was used for comparison.
Highlights.
- In the dorsal spinal cord, PSA is co-expressed with the μ-opioid receptor in the same neurons.
- The enzymatic removal of PSA induced a rapid and persistent increase in morphine analgesia.
- Morphine tolerance was not observed following PSA removal.
- After development of tolerance, PSA removal restored morphine analgesia.
- Selective deletion of the NCAM-180 isoform reproduced the effects of enzymatic removal of PSA.
- The effects of PSA removal on morphine analgesia and tolerance are produced by an increase in NCAM function.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Pan YX, Pasternak GW. Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J. Comp. Neurol. 2000;419:244–256. doi: 10.1002/(sici)1096-9861(20000403)419:2<244::aid-cne8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Olive S, Poulain DA, Theodosis DT. Mapping of the distribution of polysialylated neural cell adhesion molecule throughout the central nervous system of the adult rat: an immunohistochemical study. Neuroscience. 1992;49:419–436. doi: 10.1016/0306-4522(92)90107-d. [DOI] [PubMed] [Google Scholar]
- Burgess A, Aubert I. Polysialic acid limits choline acetyltransferase activity induced by brain-derived neurotrophic factor. J. Neurochem. 2006;99:797–806. doi: 10.1111/j.1471-4159.2006.04110.x. [DOI] [PubMed] [Google Scholar]
- Buttner B, Kannicht C, Reutter W, Horstkorte R. Novel cytosolic binding partners of the neural cell adhesion molecule: mapping the binding domains of PLC gamma, LANP, TOAD-64, syndapin, PP1, and PP2A. Biochemistry. 2005;44:6938–6947. doi: 10.1021/bi050066c. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sommer C. The role of mitogen-activated protein kinase (MAPK) in morphine tolerance and dependence. Mol. Neurobiol. 2009;40:101–107. doi: 10.1007/s12035-009-8074-z. [DOI] [PubMed] [Google Scholar]
- Chernyshova Y, Leshchyns’ka I, Hsu SC, Schachner M, Sytnyk V. The neural cell adhesion molecule promotes FGFR-dependent phosphorylation and membrane targeting of the exocyst complex to induce exocytosis in growth cones. J. Neurosci. 2011;31:3522–3535. doi: 10.1523/JNEUROSCI.3109-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- El Maarouf A, Rutishauser U. Polysialic Acid in Adult Brain Plasticity. In: Fukuda M, Rutishauser U, Schnaar RL, editors. Neuroglycobiology. Oxford University Press; London: 2005. pp. 39–57. [Google Scholar]
- El Maarouf A, Kolesnikov Y, Pasternak G, Rutishauser U. Polysialic acid-induced plasticity reduces neuropathic insult to the central nervous system. Proc. Natl. Acad. Sci. USA. 2005;102:11516–11520. doi: 10.1073/pnas.0504718102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Maarouf A, Rutishauser U. Removal of polysialic acid induces aberrant pathways, synaptic vesicle distribution, and terminal arborization of retinotectal axons. J. Comp. Neurol. 2003;460:203–211. doi: 10.1002/cne.10635. [DOI] [PubMed] [Google Scholar]
- Fujimoto I, Bruses JL, Rutishauser U. Regulation of cell adhesion by polysialic acid. Effects on cadherin, immunoglobulin cell adhesion molecule, and integrin function and independence from neural cell adhesion molecule binding or signaling activity. J. Biol. Chem. 2001;276:31745–31751. doi: 10.1074/jbc.M104525200. [DOI] [PubMed] [Google Scholar]
- Glass JD, Shen H, Fedorkova L, Chen L, Tomasiewicz H, Watanabe M. Polysialylated neural cell adhesion molecule modulates photic signaling in the mouse suprachiasmatic nucleus. Neurosci. Lett. 2000;280:207–210. doi: 10.1016/s0304-3940(00)00786-2. [DOI] [PubMed] [Google Scholar]
- Govitrapong P, Zhang X, Loh HH, Lee NM. Transfection of NG108-15 cells with antisense opioid-binding cell adhesion molecule cDNA alters opioid receptor-G-protein interaction. J. Biol. Chem. 1993;268:18280–18285. [PubMed] [Google Scholar]
- Hachisuka A, Nakajima O, Yamazaki T, Sawada J. Localization of opioid-binding cell adhesion molecule (OBCAM) in adult rat brain. Brain Res. 1999;842:482–486. doi: 10.1016/s0006-8993(99)01831-4. [DOI] [PubMed] [Google Scholar]
- Hallenbeck PC, Vimr ER, Yu F, Bassler B, Troy FA. Purification and properties of a bacteriophage-induced endo-N-acetylneuraminidase specific for poly-alpha-2,8-sialosyl carbohydrate units. J. Biol. Chem. 1987;262:3553–3561. [PubMed] [Google Scholar]
- Hammond MS, Sims C, Parameshwaran K, Suppiramaniam V, Schachner M, Dityatev A. Neural cell adhesion molecule-associated polysialic acid inhibits NR2B-containing N-methyl-D-aspartate receptors and prevents glutamate-induced cell death. J. Biol. Chem. 2006;281:34859–34869. doi: 10.1074/jbc.M602568200. [DOI] [PubMed] [Google Scholar]
- Hata K, Polo-Parada L, Landmesser LT. Selective targeting of different neural cell adhesion molecule isoforms during motoneuron myotube synapse formation in culture and the switch from an immature to mature form of synaptic vesicle cycling. J. Neurosci. 2007;27:14481–14493. doi: 10.1523/JNEUROSCI.3847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov YA, Cristea M, Pasternak GW. Analgesic synergy between topical morphine and butamben in mice. Anesth. Analg. 2003;97:1103–1107. doi: 10.1213/01.ANE.0000081060.63296.A8. [DOI] [PubMed] [Google Scholar]
- Kolesnikov YA, Pick CG, Ciszewska G, Pasternak GW. Blockade of tolerance to morphine but not to kappa opioids by a nitric oxide synthase inhibitor. Proc. Natl. Acad. Sci. USA. 1993;90:5162–5166. doi: 10.1073/pnas.90.11.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolkova K, Novitskaya V, Pedersen N, Berezin V, Bock E. Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein kinase C and the Ras-mitogen-activated protein kinase pathway. J. Neurosci. 2000;20:2238–2246. doi: 10.1523/JNEUROSCI.20-06-02238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu. Rev. Pharmacol. Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- Loh HH, Smith AP. Molecular characterization of opioid receptors. Annu. Rev. Pharmacol. Toxicol. 1990;30:123–147. doi: 10.1146/annurev.pa.30.040190.001011. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. When it comes to opiates, just say NO. J. Clin. Invest. 2007;117:3185–3187. doi: 10.1172/JCI34035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petridis AK, El-Maarouf A, Rutishauser U. Polysialic acid regulates cell contact-dependent neuronal differentiation of progenitor cells from the subventricular zone. Dev. Dyn. 2004;230:675–684. doi: 10.1002/dvdy.20094. [DOI] [PubMed] [Google Scholar]
- Plante GE, VanItallie TB. Opioids for cancer pain: the challenge of optimizing treatment. Metabolism. 2010;59(Suppl 1):S47–52. doi: 10.1016/j.metabol.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Polo-Parada L, Bose CM, Plattner F, Landmesser LT. Distinct roles of different neural cell adhesion molecule (NCAM) isoforms in synaptic maturation revealed by analysis of NCAM 180 kDa isoform-deficient mice. J. Neurosci. 2004;24:1852–1864. doi: 10.1523/JNEUROSCI.4406-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potschka H, Pekcec A, Weinhold B, Gerardy-Schahn R. Deficiency of neural cell adhesion molecule or its polysialylation modulates pharmacological effects of the AMPA receptor antagonist NBQX. Neuroscience. 2008;152:1093–1098. doi: 10.1016/j.neuroscience.2007.09.027. [DOI] [PubMed] [Google Scholar]
- Ruan X. Drug-related side effects of long-term intrathecal morphine therapy. Pain Physician. 2007;10:357–366. [PubMed] [Google Scholar]
- Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 2008;9:26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- Seidenfaden R, Krauter A, Schertzinger F, Gerardy-Schahn R, Hildebrandt H. Polysialic acid directs tumor cell growth by controlling heterophilic neural cell adhesion molecule interactions. Mol. Cell Biol. 2003;23:5908–5918. doi: 10.1128/MCB.23.16.5908-5918.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Narita M, Niikura K, Suzuki T. Chronic morphine treatment increases the expression of the neural cell adhesion molecule in the dorsal horn of the mouse spinal cord. Neurosci. Lett. 2006;399:202–205. doi: 10.1016/j.neulet.2006.01.066. [DOI] [PubMed] [Google Scholar]
- Sytnyk V, Leshchyns’ka I, Nikonenko AG, Schachner M. NCAM promotes assembly and activity-dependent remodeling of the postsynaptic signaling complex. J. Cell Biol. 2006;174:1071–1085. doi: 10.1083/jcb.200604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasiewicz H, Ono K, Yee D, Thompson C, Goridis C, Rutishauser U, Magnuson T. Genetic deletion of a neural cell adhesion molecule variant (NCAM-180) produces distinct defects in the central nervous system. Neuron. 1993;11:1163–1174. doi: 10.1016/0896-6273(93)90228-j. [DOI] [PubMed] [Google Scholar]
- Vaithianathan T, Matthias K, Bahr B, Schachner M, Suppiramaniam V, Dityatev A, Steinhauser C. Neural cell adhesion molecule-associated polysialic acid potentiates alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor currents. J. Biol. Chem. 2004;279:47975–47984. doi: 10.1074/jbc.M407138200. [DOI] [PubMed] [Google Scholar]
- Vimr ER, McCoy RD, Vollger HF, Wilkison NC, Troy FA. Use of prokaryotic-derived probes to identify poly(sialic acid) in neonatal neuronal membranes. Proc. Natl. Acad. Sci. USA. 1984;81:1971–1975. doi: 10.1073/pnas.81.7.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vutskits L, Djebbara-Hannas Z, Zhang H, Paccaud JP, Durbec P, Rougon G, Muller D, Kiss JZ. PSA-NCAM modulates BDNF-dependent survival and differentiation of cortical neurons. Eur. J. Neurosci. 2001;13:1391–1402. doi: 10.1046/j.0953-816x.2001.01516.x. [DOI] [PubMed] [Google Scholar]
- Weber M, Modemann S, Schipper P, Trauer H, Franke H, Illes P, Geiger KD, Hengstler JG, Kleemann WJ. Increased polysialic acid neural cell adhesion molecule expression in human hippocampus of heroin addicts. Neuroscience. 2006;138:1215–1223. doi: 10.1016/j.neuroscience.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Wolf DH, Nestler EJ, Russell DS. Regulation of neuronal PLCgamma by chronic morphine. Brain Res. 2007;1156:9–20. doi: 10.1016/j.brainres.2007.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]