Abstract
C57BL/6J and 129 substrains of mice are known to differ in their basal levels of anxiety and behavioral response to drugs of abuse. We have previously shown strain differences in heroin-induced conditioned place preference (CPP) between C57BL/6J (C57) and 129P3/J (129) mice, and in the regional expression of several receptor and peptide mRNAs. In this study, we examined the contribution of the GABAergic system in the cortex, nucleus accumbens (NAc), caudate putamen (CPu) and the region containing the substantia nigra and ventral tegmental area (SN/VTA) to heroin reward by measuring mRNA levels of 7 of the most commonly expressed GABA-A receptor subunits, and both GABA-B receptor subunits, in these same mice following saline (control) or heroin administration in a CPP design. Using real-time PCR, we studied the effects of strain and heroin administration on GABA-A α1, α2, α3, β2, and γ2 subunits, which typically constitute synaptic GABA-A receptors, GABA-A α4 and δ subunits, which typically constitute extrasynaptic GABA-A receptors, and GABA-B R1 and R2 subunits. In saline-treated animals, we found an experiment-wise significant strain difference in GABA-Aα2 mRNA expression in the SN/VTA. Point-wise significant strain differences were also observed in GABA-Aα2, GABA-Aα3, and GABA-Aα4 mRNA expression in the NAc, as well as GABA-BR2 mRNA expression in the NAc and CPu, and GABA-BR1 mRNA expression in the cortex. For all differences, 129 mice had higher mRNA expression compared to C57 animals, with the exception of GABA-BR1 mRNA in the cortex where we observed lower levels in 129 mice. Therefore, it may be possible that known behavioral differences between these two strains are, in part, due to differences in their GABAergic systems. While we did not find heroin dose-related changes in mRNA expression levels in C57 mice, we did observe dose-related differences in 129 mice. These results may relate to our earlier behavioral finding that 129 mice are hyporesponsive to the rewarding effects of heroin.
Keywords: Strain differences, GABA receptor mRNA, Brain region mRNA expression, Heroin
1. Introduction
Gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain, plays a role in various complex neurobiological processes such as anxiety, reward, and learning and memory, by binding to ionotropic GABA-A receptors and metabotropic GABA-B receptors. GABA-A receptors are typically thought to produce both phasic and tonic inhibition (Churchill et al., 1992), while GABA-B receptors are thought to produce slow, prolonged inhibitory signals (Jones et al., 1998).
GABA-A receptors are hetero-pentameric ligand-gated ion channels, and are assembled from 5 of 19 subunits, each encoded by its own gene. Each receptor consists of two α subunits, two β subunits, and an alternative subunit (usually γ). Eleven heteropentameric receptors have been identified in vivo, the most prevalent of which consists of the α1β2γ2 subunits (Benke et al., 1991; Olsen and Sieghart, 2008; Somogyi et al., 1996). The location of these receptors, either synaptically or extrasynaptically, determines the type of conductance they mediate. When activated, synaptic GABA-A receptors are briefly exposed to low millimolar concentrations of endogenous GABA, and subsequently mediate phasic inhibition. However, extrasynaptic GABA-A receptors experience a “spillover effect” from synapses such that GABA exists at a thousand-fold lower concentration but is maintained consistently over time (Caraiscos et al., 2004; Mortensen and Smart, 2006; Mtchedlishvili and Kapur, 2006; Stell and Mody, 2002). This results in the continual activation of extrasynaptic GABA-A receptors, and subsequently tonic inhibition (Farrant and Nusser, 2005). No GABA-A receptor subunit has yet been found to have an exclusively synaptic location, however the α4, α6 and δ subunits are predominantly if not exclusively found extra-synaptically (Farrant and Nusser, 2005).
GABA-B receptors are G-protein coupled receptors that regulate potassium and calcium channels. There are two subunits of the GABA-B receptor, R1 and R2, both of which are required to produce a fully functional GABA-B receptor (Jones et al., 1998). When expressed alone in mammalian cells, the GABA-BR1 receptor subunit exhibits relatively low affinity for GABA in comparison to the endogenous GABA-B receptor. However, when the GABA-BR2 subunit forms a heterodimer with the GABA-BR1 subunit, it results in a fully functional GABAB receptor at the cell surface with high affinity for GABA (White et al., 1998). The GABA-BR1 subunit contains the agonist-binding site, while the GABA-BR2 subunit is necessary for G-protein coupling and activation (Couve et al., 1998).
Depressant drugs, such as barbiturates and benzodiaze-pines, potentiate the inhibitory effect of GABA via interactions with the GABA-A receptor. Benzodiazepines exert their effects by binding to the interface between the α and γ subunits, while barbiturates bind to the β subunit. GABA-A receptor activation by benzodiazepines has been found to be both anxiolytic and sedative, an effect which was originally believed to depend on receptor subunit composition (Low et al., 2000; McKernan et al., 2000; Morris et al., 2006; Rowlett et al., 2005; Rudolph et al., 1999; Rudolph and Mohler, 2004). However, more recent research has shown that many drugs with preclinical anxiolytic profiles fail to exhibit anxiolysis in a clinical setting (Skolnick, 2012). Therefore, it has been concluded that drugs with high efficacy at α2-containing GABA-A receptors do not necessarily lead to anxiolysis, and drugs with high efficacy at α1-containing receptors do not necessarily lead to sedation (Skolnick, 2012). Despite these recent findings, it is still believed that GABA receptors play a prominent role in anxiety-related behaviors (Lydiard, 2003; Nemeroff, 2003; Nutt and Malizia, 2001), and perhaps this role is not subunit specific but rather depends on a combination of various subtypes of GABA-A and GABA-B receptors in brain regions crucial to anxiety.
Mice of the C57BL/6J (C57) strain and various 129 substrains (both commonly used in the generation of knockout animals) have been found to differ in their basal levels of anxiety and responsiveness to diazepam. C57 mice show relatively high levels of basal locomotor activity and lower levels of anxiety-like behaviors compared to most other strains studied. Many substrains of 129 mice, on the other hand, have been found to be less active than C57 mice, and all substrains of 129 mice show more anxiety-like behavior than C57 mice (e.g. (Lad et al., 2010)). Even after accounting for differences in locomotor activity between the two strains, 129 mice display higher levels of anxiety-like behavior relative to C57 mice (Rodgers et al., 2002). These strain-related behavioral differences may be related to differential expression of GABAergic mRNAs. Studies in humans with anxiety disorders have shown reductions in binding to GABA-A receptors in cortical, striatal and limbic brain areas (reviewed in (Nikolaus et al., 2009)).
Opiates, both endogenous and exogenous, inhibit GABAergic activity, and therefore disinhibit dopaminergic (DA) projection neurons. Morphine, the biologically active metabolite of heroin (Inturrisi et al., 1983), binds to the mu opioid receptor (MOP-r) on GABAergic neurons in the VTA, thus disinhibiting DAergic projection neurons (Johnson and North, 1992b), resulting in the release of DA in the mesocorticolimbic and nigrostriatal projection fields such as the NAc and frontal cortex (Di Chiara and Imperato, 1988). Both GABA-A and GABA-B receptors, located on DAergic neurons as well as non-DAergic neurons within the mesocorticolimbic DAergic system, have been suggested to play a role in the positive reinforcing effects of heroin (e.g. (Xi and Stein, 1999; Yoon et al., 2009)).
One way to study individual differences in vulnerability to develop addictive diseases is to examine inbred strains of mice that are known to differ in their response to drugs of abuse. Mice of the C57BL/6 J strain and of various 129 substrains differ in their response to drugs of abuse. 129 substrains of mice consistently express less stereotypic behavior following cocaine administration than do C57 mice (Kuzmin and Johansson, 2000; Kuzmin et al., 2000; Miner, 1997; Schlussman et al., 1998, 2003). Mice of 129 substrains also consistently appear less responsive to the locomotor effects of cocaine (Kuzmin and Johansson, 2000; Kuzmin et al., 2000; Miner, 1997; Schlussman et al., 1998, 2003). We have reported significant strain-related differences in the locomotor stimulating and rewarding properties of heroin administration in the same mice described in the study presented here (Schlussman et al., 2008). While heroin produced dose-dependent increases in the locomotor activity of both strains of mice, C57 mice developed CPP to relatively low doses of heroin, while 129 mice developed preference only to higher doses (Schlussman et al., 2008). This suggests that 129 mice are less sensitive to the rewarding effects of heroin than are C57 mice.
We have previously reported significant strain differences and heroin related effects on the expression levels of mRNAs of the somatostinergic system (Schlussman et al., 2010), the opioidergic system (Schlussman et al., 2011), and the DAergic system (Schlussman et al., 2011) in the same mice used in the present report. Here we extend our studies by examining the relative expression of GABA receptor subunit mRNAs in various brain regions of these C57BL/6J and 129P3/J mice.
2. Results
2.1. Point-wise significant strain differences in GABA-A receptor subunits in saline-treated animals
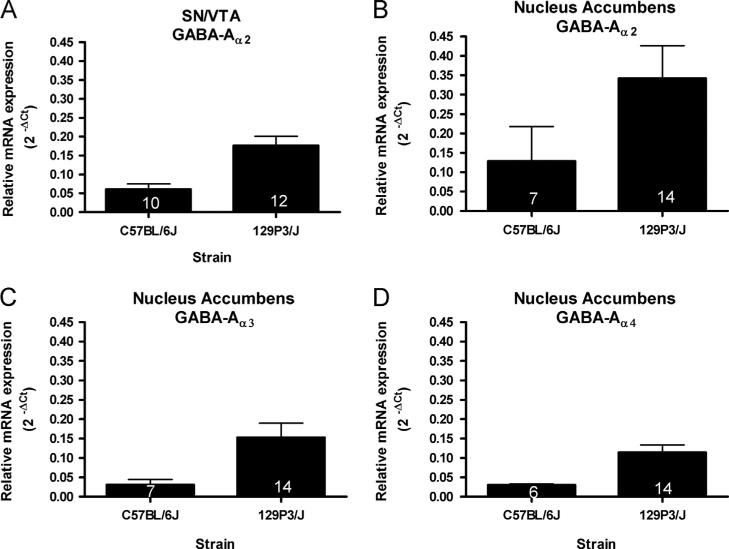
Expression of GABA-A receptor subunit mRNAs varied between strains in a region-specific manner (Fig. 1). In the SN/VTA, a significant strain-related difference was observed in the expression of GABA-Aα2 mRNA (Fig. 1A), such that 129 mice had higher levels of GABA-Aα2 mRNA than did C57 mice (t(20)=3.836, p<0.001). In the NAc, expression levels of GABA-Aα2 (Fig. 1B), GABA-Aα3 (Fig. 1C), and GABA-Aα4 mRNA (Fig. 1D) were significantly higher in the NAc of 129 compared to C57 mice (U=12.00, p<0.005; t(19)=2.273, po0.05; and t(18)=2.780, po0.05 respectively). No significant strain-related differences were found in GABA-A receptor subunit mRNA expression levels in the cortex or CPu.
Fig. 1.
Region-specific strain differences in the levels of GABA-A receptor subunits mRNA. (A) GABA-Aα2 mRNA expression in the SN/VTA was higher in 129 mice than in C57 animals (t(20)=3.836, p<0.001). B) GABA-Aα2 mRNA levels were higher in the NAc of 129 compared to C57 mice (U=12.00, p<0.005). C) Levels of GABA-Aα3 mRNA were higher in the NAc of 129 compared to C57 mice (t(19)=2.273, p<0.05). D) GABA-Aα4 mRNA expression in the SN/VTA was higher in 129 mice than in C57 animals (t(18)=2.780, p<0.05). The number of animals in each group is indicated within each bar.
2.2. Point-wise significant strain differences in GABA-B receptor subunits in saline-treated animals
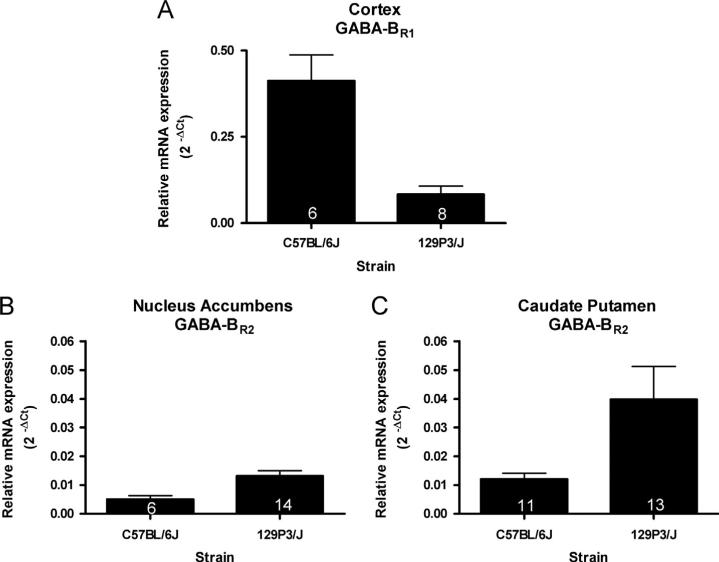
Strain differences in the levels of GABA-B receptor subunit mRNAs were also observed regionally. In the cortex, GABABR1 mRNA levels were significantly lower in 129 mice compared to C57 mice (t(6)=4.168, p<0.01; Fig. 2A). However, in the NAc (Fig. 2B) and CPu (Fig. 2C), levels of GABA-BR2 mRNA were significantly higher in 129 compared to C57 mice (t(18)=2.753, p<0.05; U=24, p<0.005 respectively).
Fig. 2.
Region-specific strain differences in the levels of GABA-B receptor subunits mRNA. (A) GABA-BR1 mRNA levels in the cortex were significantly lower in 129 mice compared to C57 mice (t(6)=4.168, p<0.01). (B) Levels of GABA-BR2 mRNA in the NAc were significantly higher in 129 compared to C57 mice (t(18)=2.753, p<0.05). and (C) Levels of GABA-BR2 mRNA in the CPu were significantly higher in 129 compared to C57 mice (U=24, p<0.005). The number of animals in each group is indicated within each bar.
2.3. Experiment-wise significant strain differences in saline-treated animals using the Bonferroni correction
To control for multiple comparisons, the Bonferroni correction was applied to all point-wise significant findings in GABA-A and GABA-B receptor subunit mRNA expression levels. In the SN/ VTA, the observed difference in GABA-Aα2 mRNA expression levels was experiment-wise significant (Bonferroni corrected p<0.05); however, all other strain-related differences in subunit mRNA expression levels were not. It should be noted that the Bonferroni correction is very conservative. The level of expected false positive significant differences between strains, with 36 independent comparisons (9 independent subunit mRNAs in 4 separate brain regions), at a level of p<0.05, is 1.8, based on the Poisson distribution. In analyzing GABA-A and GABA-B receptor subunit mRNAs, we found 7 strain-related differences at this level of confidence, highly suggestive that many of these differences are in fact real, and not resulting from random variance. The presence of a substantially greater number of results with p valueso0.05 than would be expected based on the Poisson distribution is a compelling argument to not reject these differences as insignificant.
2.4. Heroin-induced effects on GABA receptor subunit mRNA levels
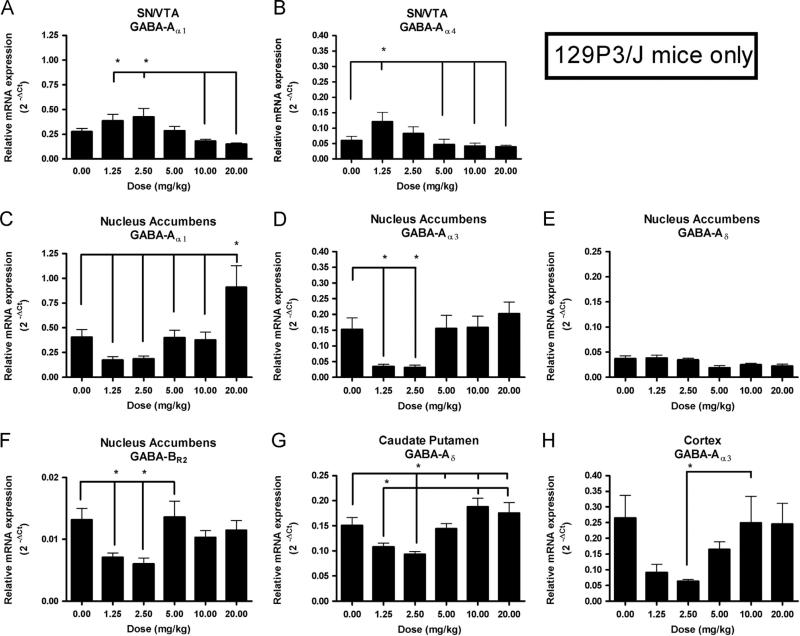
In the present study, heroin-induced effects were not observed in subunit mRNA expression levels in the brains of C57 mice. However, multiple region-specific heroin-induced effects were observed in mRNA expression levels in 129 mice. In the SN/VTA of 129 mice, a significant main effect of Dose was observed in GABA-Aα1 and GABA-Aα4 mRNA expression levels (F(5,51)=5.73, p<0.0005, Fig. 3A; F(5,51)=3.20, p<0.05, Fig. 3B respectively). Newman–Keuls post hoc tests showed that, in regards to GABA-Aα1, 129 mice receiving the two lowest doses of heroin (1.25 mg/kg and 2.5 mg/kg) had significantly higher levels of expression than did those receiving the two highest doses of heroin (10 mg/kg and 20 mg/kg; p<0.05). In addition, expression levels of GABA-Aα4 mRNA in 129 mice receiving the lowest dose of heroin were significantly higher than were those of mice receiving saline, or the three highest doses (5 mg/kg, 10 mg/kg and 20 mg/kg; p<0.05).
Fig. 3.
Region- and gene- specific effects of heroin on levels of GABA receptor subunit mRNAs in 129P3/J mice. In the SN/VTA, there were significant main effects of Dose on expression levels of (A) GABA-Aα1 mRNA (F(5,51) =5.73, p<0.0005), and (B) GABA-Aα4 mRNA (F(5,51) =3.20, p<0.05). In the NAc, there were significant main effects of Dose on expression levels of (C) GABA-Aα1 mRNA (F(5,52) =8.194, p<0.00001), (D) GABA-Aα3 mRNA (F(5,60) =5.72, p<0.0005), (E) GABA-Aδ mRNA (F(5,60) =2.94, p<0.05), and (F) GABA-BR2 mRNA (F(5,58) =4.499, p<0.005). In the CPu, there was a significant main effect of Dose on expression levels of (G) GABA-Aδ mRNA (F(5,45) =7.13, p<0.0001), and in the cortex there was a significant main effect of Dose on expression levels of (H) GABA-Aα3 mRNA (F(5,58) =3.55, p<0.01). Lines marked with asterisks are used to illustrate significant differences, according to the Newman–Keuls post hoc test.
In the NAc of 129 mice, a significant main effect of Dose was observed in the expression levels of GABA-Aα1 (Fig. 3C), GABA-Aα3 (Fig. 3D), GABA-Aδ (Fig. 3E) and GABA-BR2 (Fig. 3F) mRNA (F(5,52)=8.194, p<0.00001; F(5,60)=5.72, p<0.0005; F(5,60)=2.94, p<0.05; F(5,58)=4.499, p<0.005 respectively). Newman–Kuels post hoc tests showed that 129 mice receiving the two lowest doses of heroin expressed significantly lower expression levels of GABAAα3 and GABA-BR2 compared to saline controls, and that 129 mice receiving the highest dose of heroin expressed significantly higher expression levels of GABA-Aα1 compared to those receiving saline and all other heroin doses (p<0.05).
Lastly, in the CPu and the cortex, a significant main effect of Dose was observed in the expression levels of GABA-Aδ (Fig. 3G) and GABA-Aα3 (Fig. 3H) mRNA (F(5,45)=7.13, p<0.0001; F(5,58)=3.55, p<0.01), respectively. Newman–Keuls post hoc tests showed that, in the CPu, expression levels of GABA-Aδ were significantly lower in 129 mice receiving the two lowest doses of heroin compared to 129 mice receiving the two highest doses (p<0.05), and that mice receiving the second-lowest dose of heroin (2.5 mg/kg) also had significantly lower expression levels than did mice receiving saline and 5 mg/kg (p<0.05). In the cortex, Newman-Keuls post hoc tests showed that expression levels of GABA-Aα3 mRNA in mice receiving the second-lowest dose of heroin were significantly lower compared to mice receiving the second-highest dose (p<0.05).
In order to control for multiple testing, the Bonferroni correction was also applied to all point-wise significant heroin-related differences in mRNA expression levels, and 4 out of 8 differences were found to be experiment-wise significant. In the SN/VTA of 129 mice, the main effect of Dose in GABA-Aα1 mRNA expression levels was observed to be experiment-wise significant (Bonferroni corrected p<0.01). In the NAc of 129 mice, the main effects of Dose in GABA-Aα1 and GABA-Aα3 mRNA expression levels were also observed to be experiment-wise significant (Bonferroni corrected p<0.001 and p<0.01, respectively). Lastly, in the CPu of 129 mice, the main effect of Dose in GABA-Aδ mRNA expression levels was found to be experiment-wise significant (Bonferroni corrected p<0.005).
3. Discussion
In the present report, we examined the relative mRNA expression levels of several important GABA-A and GABA-B receptor subunits, in C57 and 129 mice. We observed regional strain differences in the relative expression levels of GABA-A and GABA-B receptor subunits. For GABA-A receptor subunits, we report an experiment-wise significant finding that compared to C57 animals, 129 mice had higher levels of GABA-Aα2 mRNA in the SN/VTA. We also report point-wise significant differences in the NAc, in which 129 mice had higher levels of GABA-Aα2, GABA-Aα3, and GABA-Aα4 mRNA than did their C57 counterparts. For GABA-B receptor subunits, we report the point-wise significant findings that compared to C57 animals, 129 mice had lower levels of GABA-BR1 mRNA in the cortex and higher levels of GABA-BR2 mRNA in both the NAc and CPu. Furthermore, while we did not observe any heroin dose-related differences in C57 mice, we did observe eight region-specific dose-related differences in GABA-A and GABA-B receptor subunits in 129 mice.
GABA, the major inhibitory neurotransmitter in the CNS, is crucial to diverse neurobiological processes such as anxiety, reward, and learning and memory. The GABAergic system, specifically in forebrain areas, is believed to mediate, at least in part, the expression of anxiety disorders (Lydiard, 2003; Nemeroff, 2003; Nutt and Malizia, 2001), although the exact neurobiological mechanisms behind anxiety are not yet fully understood (for review, see Kalueff and Nutt, (2007)). Gene expression studies have found a down-regulation of both GABA-A and GABA-B genes in the cortex (Kroes et al., 2006; Wang et al., 2003), amygdala (Mei et al., 2005), and hypothalamus (Verkuyl et al., 2004) of highly anxious rats. One study found that infusions of the GABA-A receptor agonist, muscimol, into the prefrontal cortex reduced anxiety-like behaviors in rats (Shah et al., 2004). Additionally, mice lacking GABA-B receptors have been found to display heightened anxiety-related behaviors (Mombereau et al., 2004, 2005). Behaviorally, all substrains of 129 mice display more anxiety-related behaviors than do C57 mice even after accounting for basal differences in locomotor activity (Rodgers et al., 2002). We and others have reported that 129 mice are consistently less responsive to the effects of drugs of abuse than are C57 mice, a difference which has been hypothesized to be mediated by differential expression of anxiety-like behavior rather than by differences in the reward system (e.g. (Dockstader and van der Kooy, 2001)). Consequently, we would expect to find decreased mRNA expression levels of GABA-A and/or GABA-B receptor subunits in the cortex of 129 compared to C57 mice. While we did not see significant differences in GABA-A mRNA expression levels in the cortex, we did observe significantly lower GABA-BR1 mRNA expression levels in 129 compared to C57 mice in this region. Interestingly, the cortex is the only region in which we observed lower mRNA expression levels in 129 compared to C57 mice. This finding is consistent with the hypothesis that lower levels of GABA-B receptors in the cortex might, in part, mediate heightened expression of anxiety-like behaviors and decreased demonstration of opiate reward.
We have previously reported significant differences in the expression of CPP to heroin in the same C57 and 129 mice utilized in this study. While both strains showed significant CPP to heroin, the C57 mice showed this conditioned effect only to the two lowest doses of heroin tested, while the 129 mice expressed this behavior only to the three highest doses used (Schlussman et al., 2008). This and other data (e.g. (Szumlinski et al., 2005)) suggests that compared to C57 mice, 129 animals are hyporesponsive to the rewarding effects of heroin. It has been shown previously that the development of CPP following morphine administration is largely a DA-dependent process (Shippenberg et al., 1992; Stinus et al., 1992), and therefore it is possible that this hyporesponsiveness is not only due to a difference in anxiety phenotypes, but also to a difference in heroin-induced release of mesolimbic DA, which may be driven by the strain-dependent differences in GABA function.
There is evidence to suggest that GABA-A and GABA-B receptors in the VTA are involved in DA transmission in the NAc and VTA, and consequently heroin self-administration and reward. While ventral tegmental GABA-B receptor activation has been shown to be centrally involved in the inhibition of DA release into the NAc (Westerink et al., 1996; Xi and Stein, 1998), as well as the attenuation of heroin self-administration behaviors (Xi and Stein, 1999) and morphine-induced CPP (Tsuji et al., 1996), we have not observed any strain-related differences in GABA-B receptor subunit mRNA expression in the SN/VTA. However, our finding that GABA-Aα2 mRNA levels were higher in 129 compared to C57 mice suggests that GABA-A receptors in this region may partially mediate the hyporesponsiveness of 129 mice to the rewarding effects of heroin. GABA-A receptors in the VTA reside on both DAergic projection neurons (Johnson and North, 1992a; Xi and Stein, 1998) as well as GABAergic interneurons (O'Brien and White, 1987; Steffensen et al., 1998), and the effect of GABA-A receptor activation on accumbal DA release has been shown to be directly inhibitory and indirectly disinhibitory, respectively (Churchill et al., 1992; Johnson and North, 1992a; Kalivas et al., 1990; Klitenick et al., 1992; O'Brien and White, 1987; Sugita et al., 1992). Interestingly, it has been found that GABA-A receptor agonists, such as muscimol, dose-dependently activate GABA-A receptors on both DAergic projection neurons and GABAergic interneurons (Doherty and Gratton, 2007; Grace and Bunney, 1979; Kalivas et al., 1990; Klitenick et al., 1992; Waszczak and Walters, 1980; Xi and Stein, 1998). Muscimol has also been shown to both decrease (Guan and McBride, 1989; Westerink et al., 1996) and increase (Xi and Stein, 1998) DA levels in the NAc, effects which have both been reversed by microinjections of the GABA-A receptor antagonist, bicuculline, into the VTA(Westerink et al., 1996; Xi and Stein, 1998). Since VTA GABA-A receptors appear to be primarily located on non-DA neurons (Churchill et al., 1992), it has been suggested that these GABA-A receptors are activated preferentially, and it is at high doses of muscimol that GABA-A receptors on DAergic projection neurons are also activated (Doherty and Gratton, 2007). Additionally, it has been found that iontophoretically applied GABA was substantially more potent in binding to GABA-A receptors on non-DA neurons (Grace and Bunney, 1979). Therefore, in order to fully understand the effect of differential GABA-Aα2 mRNA expression between C57 and 129 mice, the relative localization of α2-containing GABA-A receptors to either DAergic or GABAergic neurons in the VTA would need to be distinguished in a matter not possible using the methods of the current investigation. Furthermore, studying the development of heroin-induced CPP in 129 α2-knockout mice would help determine whether the heightened α2 subunit mRNA expression we observed in fact contributes to a differential expression of heroin reward.
Important to the effect of GABA-A receptor activation in the VTA on subsequent DA release is the distinction between synaptic and extra-synaptic GABA-A receptors. Muscimol has been found to act preferentially at high-affinity extrasynaptic GABA-A receptors typically containing the α4 and δ subunits (Korpi et al., 2002; Mihalek et al., 1999; Mortensen et al., 2010; Quirk et al., 1995); however, it also acts as a prototypic GABAA receptor agonist (Chandra et al., 2010). α4- and δ-knockout mice have been found to possess fewer binding sites in forebrain areas for muscimol, as well as a reduced behavioral sensitivity to muscimol administration (Chandra et al., 2010), suggesting that extrasynaptic GABA-A receptors in the fore-brain contribute to the behavioral effects of muscimol. In the VTA, muscimol administration has been shown to be rewarding as measured by CPP (Laviolette and van der Kooy, 2001, 2004) and to differentially affect morphine-induced CPP depending on the dose injected (Sahraei et al., 2005). However, gaboxadol—a GABA-A receptor agonist which acts predominantly on extrasynaptic receptors but does not act as a prototypic GABA-A receptor agonist (Belelli et al., 2005; Chandra et al., 2010; Jia et al., 2005)— has not been found to be rewarding. One study demonstrated that while gabaxadol induced glutamatergic synaptic plasticity on DAergic neurons in the VTA, an effect which is common in response to various drugs of abuse, intravenous self-administration of gaboxadol failed to be rewarding in mice or baboons (Vashchinkina et al., 2012). Therefore, it is possible that the rewarding effects of muscimol in the VTA are primarily due to the binding of synaptic, as opposed to extrasynaptic, GABA-A receptors. Our results indicate that despite robust strain-related behavioral differences to heroin administration between 129 and C57 mice, there were no differences in either GABA-Aα4 or GABA-Aδ mRNA expression levels in the SN/VTA. This observation is consistent with the suggestion that extrasynaptic GABA-A receptors in the VTA may not be crucial to the mediation of drug reward. A study investigating the effect of intra-ventral tegmental injection of gaboxadol on morphine or heroin-induced CPP would be a worthwhile test of this hypothesis.
In addition to the VTA, GABA receptors in the NAc may also be involved in DA transmission and subsequently heroin self-administration and reward. It has been suggested previously that decreased GABA release might mediate drug-induced elevations of accumbal DA levels (Ferraro et al., 1996; Tanganelli et al., 1994) via an intra-accumbal mechanism. Furthermore, it is possible that this effect occurs by activation of GABA-A, rather than GABA-B receptors. While we observed a significant difference between 129 and C57 mice in expression levels of GABA-BR2 mRNA in the NAc, several studies have suggested that GABA-B receptors in the NAc may not in fact be crucial to opiate reward. Despite the finding that accumbal infusions of the GABA-B receptor antagonist 2-OH saclofen causes an increase in extracellular DA in the NAc (Saigusa et al., 2008), microinjections of baclofen directly into the NAc have consistently failed to affect morphine and heroin self-administration behavior in rats (Xi and Stein, 1999; Yoon et al., 2009). On the other hand, not only have accumbal injections of the GABA-A receptor antagonist bicuculline been found to significantly increase extracellular DA levels in the NAc (Yan, 1999), but microinjections of muscimol into the NAc have also been found to decrease morphine self-administration(Yoon et al., 2009). It was therefore suggested that this effect of muscimol in the NAc was mediated by the tonic inhibition of DA release by GABA-A receptors on DA terminals within the NAc. However, similar to the VTA, the relative localization of GABA-A receptors to either DAergic nerve endings or GABAergic interneurons in the NAc is not yet fully understood. One group hypothesized that since both muscimol and bicuculline administered into the NAc increase accumbal DA efflux, muscimol may act at GABA-A receptors on GABAergic interneurons, while bicuculline may act at GABA-A receptors on DAergic nerve endings (Aono et al., 2008). In the present study, we found higher expression levels of GABA-Aα2, GABA-Aα3, and GABA-Aα4 mRNA, as well as higher expression levels of GABA-BR2 mRNA in the NAc of 129 mice. Taking into account the hyporesponsiveness of 129 mice to heroin administration, our results would suggest that the GABA-A receptors crucial to the counter-modulation of opiate reward may reside primarily on DAergic nerve terminals in the NAc. Furthermore, since we observed differences in mRNA expression levels of GABA-A subunits associated with extrasynaptic receptors (α4) as well as subunits associated with synaptic receptors (α2 and α3) in the NAc, it is possible that both types of GABA-A receptors in the NAc contribute to the hyporesponsiveness of 129 mice to heroin self-administration. Although GABA-B receptors also localize on DAergic nerve terminals in the NAc, and GABA has been found to exhibit an inhibitory tone on accumbal DA levels by acting at both GABA-A and GABA-B receptors, only GABA-A receptor agonists have been found to attenuate opiate reward (Yoon et al., 2009). It has previously been suggested that the two types of GABA receptors are activated by different sources (and therefore “pools”) of GABA in the NAc (Aono et al., 2008; Saigusa et al., 2008, 2012), and therefore it is possible that this difference also affects the role of accumbal GABA-A and GABAB receptors in the countermodulation of opiate reward.
Lastly, in the present study we observed eight region-specific heroin dose-related differences in GABA-A receptor subunit mRNA expression levels in 129 mice. Interestingly, we did not observe any heroin dose-related differences in C57 mice. This would suggest that not only do 129 mice appear to have a distinct organization of GABA receptors in the mesolimbic DA pathway compared to C57 mice, but also that the mRNA expression levels of these receptors are affected differently by heroin administration. Furthermore, heroin-related dose effects in 129 mice were observed to follow two distinct patterns. In the SN/VTA, low doses of heroin administration were generally associated with increased mRNA expression levels relative to saline and/or higher doses of heroin. Alternatively, in all other regions, low doses of heroin administration were generally associated with decreased mRNA expression levels relative to saline and/or higher doses. Therefore, it appears that heroin administration alters GABA receptor subunit mRNA expression levels in 129 mice differently in the SN/VTA compared to the DAergic terminal fields such as the NAc, CPu and cortex.
In summary, we report various region-specific strain differences in the expression of key components of the GABAergic system between 129 and C57 mice. These differences may be related to known strain differences in the response to drugs of abuse and expression of anxiety-like behaviors.
4. Experimental procedure
4.1. Animals
Fifty-six C57BL/6J and 72 age-matched 129P3/J male mice (6 weeks old on arrival; Jackson Laboratory, Bar Harbor, ME) were utilized in this study. Mice were individually housed in an environmentally controlled room dedicated to this study and animals were allowed two weeks to acclimate prior to the start of the experiments. Mice of each strain were randomly assigned to one of six groups, based on dose of heroin administered (0, 1.25, 2.5, 5, 10, or 20 mg/kg). Three animals died during the course of these studies (two 129P3/J and one C57BL/6J) and were not included in analyses. This study was approved by the Rockefeller University Institutional Animal Care and Use Committee and included provisions to minimize pain and discomfort. Heroin (3,6-diacetyl-morphine HCl) was obtained from NIH-NIDA.
Mice whose tissue was studied here were from the abovementioned study of heroin-induced CPP, and has been reported elsewhere (Schlussman et al., 2008). All animals in non-saline groups received i.p. injections of heroin or saline on alternate days for a total of eight days, half receiving heroin and half saline on the first day. Animals in the 0 mg/kg group received isotonic saline [0.9% NaCl] on all days (for details see (Schlussman et al., 2008)). Animals were sacrificed immediately following the testing session (24.5 h following the last conditioning session), and brains were rapidly removed. The cortex (an area containing the cingulate cortex area 1, the primary and secondary motor cortices and the prelimbic cortex) (Franklin and Paxinos, 1997), nucleus accumbens (NAc), caudate putamen (CPu) and a region containing both the substantia nigra and the ventral tegmental area (SN/VTA) were dissected and homogenized in guanidium thiocyanate as previously described (Branch et al., 1992). RNA from the CPu and the cortex was isolated using RNAqueous (Ambion [ABI], Austin, TX). RNA from the NAc and SN/VTA was isolated using an acid phenolic extraction(Chomczynski and Sacchi, 1987). Following RNA isolation, all samples were treated with DNase (Turbo DNA-free™, Ambion [ABI], Austin, TX). The quantity and quality of RNA in each extract was determined with the Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA).
4.2. cDNA synthesis
cDNA was synthesized from each sample using the Super Script™ III first strand synthesis kit (Invitrogen, Carlsbad, CA). Five hundred ng of RNA from the CPu and the cortex was used for reverse transcription, while the entire NAc and SN/VTA were utilized for generation of cDNAs. cDNAs were diluted 1:10 for real time PCR (RT-PCR) analysis.
4.3. RT-PCR analysis
RT-PCR analysis of the relative mRNA expression levels of GABA-Aα1, GABA-Aα2, GABA-Aα3, GABA-Aα4, GABA-Aβ2, GABA-Aγ2, GABA-Aδ, GABA-BR1 and GABA-BR2 in all four regions was conducted using commercially available primers and master mix (RT2qPCR™ primer assays and RT2 Real Time™ SYBRs Green PCR Master Mix; Qiagen, Valencia, CA) in an ABI Prism 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA). Water controls for each primer set were included in every assay. Any sample with a cycle threshold (Ct) greater than that of the water control, or a Ct≥5, was not included in data analysis. All data was normalized to Gapdh and reported as 2−ΔCt where ΔCt is the cycle threshold of the mRNA of interest minus the cycle threshold of Gapdh.
4.4. Statistics
Strain effects were analyzed by t-test or Mann–Whitney U tests, as appropriate, in saline control animals. Heroin Dose effects were analyzed within each strain by one-way ANOVA. Any sample that was42.5 standard deviations from the strain mean was considered an outlier and dropped from the analysis. To control for multiple comparisons (9 independent subunit mRNAs in 4 separate brain regions), the Bonferroni correction was applied to all Strain and Dose effects in GABA receptor subunit mRNA expression levels.
Acknowledgments
The authors thank Dr. Brian Reed for his critical reading of the manuscript and helpful comments. 3, 6 diacetyl-morphine HCl was generously provided by NIH-NIDA, Division of Drug Supply and Analytical Services. This work was supported by grants from NIH-NIDA (DA05130), the Arcadia Charitable Trust and the Carson Family Charitable Trust to MJK.
REFERENCES
- Aono Y, Saigusa T, Mizoguchi N, Iwakami T, Takada K, Gionhaku N, Oi Y, Ueda K, Koshikawa N, Cools AR. Role of GABAA receptors in the endomorphin-1-, but not endomorphin-2-, induced dopamine efflux in the nucleus accumbens of freely moving rats. Eur. J. Pharmacol. 2008;580:87–94. doi: 10.1016/j.ejphar.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J. Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke D, Mertens S, Trzeciak A, Gillessen D, Mohler H. GABAA receptors display association of gamma 2-subunit with alpha 1- and beta 2/3-subunits. J. Biol. Chem. 1991;266:4478–4483. [PubMed] [Google Scholar]
- Branch AD, Unterwald EM, Lee SE, Kreek MJ. Quantitation of preproenkephalin mRNA levels in brain regions from male Fischer rats following chronic cocaine treatment using a recently developed solution hybridization assay. Brain Res. Mol. Brain Res. 1992;14:231–238. doi: 10.1016/0169-328x(92)90178-e. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. USA. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Halonen LM, Linden AM, Procaccini C, Hellsten K, Homanics GE, Korpi ER. Prototypic GABA(A) receptor agonist muscimol acts preferentially through forebrain high-affinity binding sites. Neuropsychopharmacology. 2010;35:999–1007. doi: 10.1038/npp.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Churchill L, Dilts RP, Kalivas PW. Autoradiographic localization of gamma-aminobutyric acidA receptors within the ventral tegmental area. Neurochem. Res. 1992;17:101–106. doi: 10.1007/BF00966870. [DOI] [PubMed] [Google Scholar]
- Couve A, Filippov AK, Connolly CN, Bettler B, Brown DA, Moss SJ. Intracellular retention of recombinant GABAB receptors. J. Biol. Chem. 1998;273:26361–26367. doi: 10.1074/jbc.273.41.26361. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockstader CL, van der Kooy D. Mouse strain differences in opiate reward learning are explained by differences in anxiety, not reward or learning. J. Neurosci. 2001;21:9077–9081. doi: 10.1523/JNEUROSCI.21-22-09077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M, Gratton A. Differential involvement of ventral tegmental GABA(A) and GABA(B) receptors in the regulation of the nucleus accumbens dopamine response to stress. Brain Res. 2007;1150:62–68. doi: 10.1016/j.brainres.2007.02.081. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tanganelli S, O'Connor WT, Antonelli T, Rambert F, Fuxe K. The vigilance promoting drug modafinil decreases GABA release in the medial preoptic area and in the posterior hypothalamus of the awake rat: possible involvement of the serotonergic 5-HT3 receptor. Neurosci. Lett. 1996;220:5–8. doi: 10.1016/s0304-3940(96)13212-2. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. l. Academic Press, Inc.; New York: 1997. [Google Scholar]
- Grace AA, Bunney BS. Paradoxical GABA excitation of nigral dopaminergic cells: indirect mediation through reticulata inhibitory neurons. Eur. J. Pharmacol. 1979;59:211–218. doi: 10.1016/0014-2999(79)90283-8. [DOI] [PubMed] [Google Scholar]
- Guan XM, McBride WJ. Serotonin microinfusion into the ventral tegmental area increases accumbens dopamine release. Brain Res. Bull. 1989;23:541–547. doi: 10.1016/0361-9230(89)90198-6. [DOI] [PubMed] [Google Scholar]
- Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ. Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci. 1983;33(Suppl 1):773–776. doi: 10.1016/0024-3205(83)90616-1. [DOI] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J. Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J. Physiol. 1992a;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 1992b;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Eberhardt H. Modulation of A10 dopamine neurons by gamma-aminobutyric acid agonists. J. Pharmacol. Exp. Ther. 1990;253:858–866. [PubMed] [Google Scholar]
- Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depression Anxiety. 2007;24:495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, DeWitte P, Kalivas PW. Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. J. Neurosci. 1992;12:2623–2632. doi: 10.1523/JNEUROSCI.12-07-02623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Mihalek RM, Sinkkonen ST, Hauer B, Hevers W, Homanics GE, Sieghart W, Luddens H. Altered receptor subtypes in the forebrain of GABA(A) receptor delta subunit-deficient mice: recruitment of gamma 2 subunits. Neuroscience. 2002;109:733–743. doi: 10.1016/s0306-4522(01)00527-9. [DOI] [PubMed] [Google Scholar]
- Kroes RA, Panksepp J, Burgdorf J, Otto NJ, Moskal JR. Modeling depression: social dominance-submission gene expression patterns in rat neocortex. Neuroscience. 2006;137:37–49. doi: 10.1016/j.neuroscience.2005.08.076. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B. Reinforcing and neurochemical effects of cocaine: differences among C57, DBA, and 129 mice. Pharmacol. Biochem. Behav. 2000;65:399–406. doi: 10.1016/s0091-3057(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B, Fredholm BB, Ogren SO. Genetic evidence that cocaine and caffeine stimulate locomotion in mice via different mechanisms. Life Sci. 2000;66:PL113–PL118. doi: 10.1016/s0024-3205(99)00647-5. [DOI] [PubMed] [Google Scholar]
- Lad HV, Liu L, Paya-Cano JL, Parsons MJ, Kember R, Fernandes C, Schalkwyk LC. Behavioral battery testing: Evaluation and behavioral outcomes in 8 inbred mouse strains. Physiol. Behav. 2010;99:301–316. doi: 10.1016/j.physbeh.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. GABA(A) receptors in the ventral tegmental area control bidirectional reward signalling between dopaminergic and non-dopaminergic neural motivational systems. Eur. J. Neurosci. 2001;13:1009–1015. doi: 10.1046/j.1460-9568.2001.01458.x. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. GABAA receptors signal bidirectional reward transmission from the ventral tegmental area to the tegmental pedunculopontine nucleus as a function of opiate state. Eur. J. Neurosci. 2004;20:2179–2187. doi: 10.1111/j.1460-9568.2004.03665.x. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Lydiard RB. The role of GABA in anxiety disorders. J. Clin. Psychiatry. 2003;64(Suppl 3):21–27. [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat. Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Mei B, Li C, Dong S, Jiang CH, Wang H, Hu Y. Distinct gene expression profiles in hippocampus and amygdala after fear conditioning. Brain Res. Bull. 2005;67:1–12. doi: 10.1016/j.brainresbull.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc. Natl. Acad. Sci. USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner LL. Cocaine reward and locomotor activity in C57BL/6J and 129/SvJ inbred mice and their F1 cross. Pharmacol. Biochem. Behav. 1997;58:25–30. doi: 10.1016/s0091-3057(96)00465-0. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacol.: Official Publ. Am. Coll. Neuropsychopharmacol. 2004;29:1050–1062. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Gassmann M, Bettler B, van der Putten H, Cryan JF. Altered anxiety and depression-related behaviour in mice lacking GABAB(2) receptor subunits. Neuroreport. 2005;16:307–310. doi: 10.1097/00001756-200502280-00021. [DOI] [PubMed] [Google Scholar]
- Morris HV, Dawson GR, Reynolds DS, Atack JR, Stephens DN. Both alpha2 and alpha3 GABAA receptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emotional response paradigm. Eur. J. Neurosci. 2006;23:2495–2504. doi: 10.1111/j.1460-9568.2006.04775.x. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic alphabeta subunit GABAA receptors on rat hippocampal pyramidal neurons. J. Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Ebert B, Wafford K, Smart TG. Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J. Physiol. 2010;588:1251–1268. doi: 10.1113/jphysiol.2009.182444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol. Pharmacol. 2006;69:564–575. doi: 10.1124/mol.105.016683. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacol. Bull. 2003;37:133–146. [PubMed] [Google Scholar]
- Nikolaus S, Antke C, Muller HW. In vivo imaging of synaptic function in the central nervous system: II. Mental and affective disorders. Behav. Brain Res. 2009;204:32–66. doi: 10.1016/j.bbr.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br. J. Psychiatry: J. Ment. Sci. 2001;179:390–396. doi: 10.1192/bjp.179.5.390. [DOI] [PubMed] [Google Scholar]
- O'Brien DP, White FJ. Inhibition of non-dopamine cells in the ventral tegmental area by benzodiazepines: relationship to A10 dopamine cell activity. Eur. J. Pharmacol. 1987;142:343–354. doi: 10.1016/0014-2999(87)90072-0. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk K, Whiting PJ, Ragan CI, McKernan RM. Characterisation of delta-subunit containing GABAA receptors from rat brain. Eur. J. Pharmacol. 1995;290:175–181. doi: 10.1016/0922-4106(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Boullier E, Chatzimichalaki P, Cooper GD, Shorten A. Contrasting phenotypes of C57BL/6JOlaHsd, 129S2/SvHsd and 129/SvEv mice in two exploration-based tests of anxiety-related behaviour. Physiol. Behav. 2002;77:301–310. doi: 10.1016/s0031-9384(02)00856-9. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc. Natl. Acad. Sci. USA. 2005;102:915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu. Rev. Pharmacol. Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Sahraei H, Amiri YA, Haeri-Rohani A, Sepehri H, Salimi SH, Pourmotabbed A, Ghoshooni H, Zahirodin A, Zardooz H. Different effects of GABAergic receptors located in the ventral tegmental area on the expression of morphine-induced conditioned place preference in rat. Eur. J. Pharmacol. 2005;524:95–101. doi: 10.1016/j.ejphar.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Saigusa T, Aono Y, Mizoguchi N, Iwakami T, Takada K, Oi Y, Ueda K, Koshikawa N, Cools AR. Role of GABA B receptors in the endomorphin-1-, but not endomorphin-2-, induced dopamine efflux in the nucleus accumbens of freely moving rats. Eur. J. Pharmacol. 2008;581:276–282. doi: 10.1016/j.ejphar.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Saigusa T, Aono Y, Sekino R, Uchida T, Takada K, Oi Y, Koshikawa N, Cools AR. In vivo neurochemical evidence that newly synthesised GABA activates GABA(B), but not GABA(A), receptors on dopaminergic nerve endings in the nucleus accumbens of freely moving rats. Neuropharmacology. 2012;62:907–913. doi: 10.1016/j.neuropharm.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Ho A, Zhou Y, Curtis AE, Kreek MJ. Effects of “binge” pattern cocaine on stereotypy and locomotor activity in C57BL/6J and 129/J mice. Pharmacol. Biochem. Behav. 1998;60:593–599. doi: 10.1016/s0091-3057(98)00047-1. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Kane S, Stewart CL, Ho A, Kreek MJ. Locomotion, stereotypy, and dopamine D1 receptors after chronic “binge” cocaine in C57BL/6J and 129/J mice. Pharmacol. Biochem. Behav. 2003;75:123–131. doi: 10.1016/s0091-3057(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Hsu NM, Allen JM, Ho A, Kreek MJ. Heroin-induced locomotor activity and conditioned place preference in C57BL/6J and 129P3/J mice. Neurosci. Lett. 2008;440:284–288. doi: 10.1016/j.neulet.2008.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlussman SD, Cassin J, Levran O, Zhang Y, Ho A, Kreek MJ. Relative expression of mRNA for the somatostatin receptors in the caudate putamen of C57BL/6J and 129P3/J mice: strain and heroin effects. Brain Res. 2010;1345:206–212. doi: 10.1016/j.brainres.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlussman SD, Cassin J, Zhang Y, Levran O, Ho A, Kreek MJ. Regional mRNA expression of the endogenous opioid and dopaminergic systems in brains of C57BL/6J and 129P3/J mice: strain and heroin effects. Pharmacol. Biochem. Behav. 2011;100:8–16. doi: 10.1016/j.pbb.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AA, Sjovold T, Treit D. Inactivation of the medial prefrontal cortex with the GABAA receptor agonist muscimol increases open-arm activity in the elevated plus-maze and attenuates shock-probe burying in rats. Brain Res. 2004;1028:112–115. doi: 10.1016/j.brainres.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A, Spanagel R, Bals-Kubik R, Stein C. Conditioning of opioid reinforcement: neuroanatomical and neurochemical substrates. Ann. N Y Acad. Sci. 1992;654:347–356. doi: 10.1111/j.1749-6632.1992.tb25980.x. [DOI] [PubMed] [Google Scholar]
- Skolnick P. Anxioselective anxiolytics: on a quest for the Holy Grail. Trends Pharmacol. Sci. 2012;33:611–620. doi: 10.1016/j.tips.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Fritschy JM, Benke D, Roberts JD, Sieghart W. The gamma 2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the alpha 1 and beta 2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology. 1996;35:1425–1444. doi: 10.1016/s0028-3908(96)00086-x. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J. Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J. Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinus L, Cador M, Le Moal M. Interaction between endogenous opioids and dopamine within the nucleus accumbens. Ann. N Y Acad. Sci. 1992;654:254–273. doi: 10.1111/j.1749-6632.1992.tb25972.x. [DOI] [PubMed] [Google Scholar]
- Sugita S, Johnson SW, North RA. Synaptic inputs to GABAA and GABAB receptors originate from discrete afferent neurons. Neurosci Lett. 1992;134:207–211. doi: 10.1016/0304-3940(92)90518-c. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Frys KA, Middaugh LD. Genetic variation in heroin-induced changes in behaviour: effects of B6 strain dose on conditioned reward and locomotor sensitization in 129-B6 hybrid mice. Genes Brain Behav. 2005;4:324–336. doi: 10.1111/j.1601-183X.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- Tanganelli S, O'Connor WT, Ferraro L, Bianchi C, Beani L, Ungerstedt U, Fuxe K. Facilitation of GABA release by neurotensin is associated with a reduction of dopamine release in rat nucleus accumbens. Neuroscience. 1994;60:649–657. doi: 10.1016/0306-4522(94)90493-6. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Nakagawa Y, Ishibashi Y, Yoshii T, Takashima T, Shimada M, Suzuki T. Activation of ventral tegmental GABAB receptors inhibits morphine-induced place preference in rats. Eur. J. Pharmacol. 1996;313:169–173. doi: 10.1016/0014-2999(96)00642-5. [DOI] [PubMed] [Google Scholar]
- Vashchinkina E, Panhelainen A, Vekovischeva OY, Aitta-aho T, Ebert B, Ator NA, Korpi ER. GABA site agonist gaboxadol induces addiction-predicting persistent changes in ventral tegmental area dopamine neurons but is not rewarding in mice or baboons. J. Neurosci. 2012;32:5310–5320. doi: 10.1523/JNEUROSCI.4697-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkuyl JM, Hemby SE, Joels M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur. J. Neurosci. 2004;20:1665–1673. doi: 10.1111/j.1460-9568.2004.03568.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhu YZ, Wong PT, Farook JM, Teo AL, Lee LK, Moochhala S. cDNA microarray analysis of gene expression in anxious PVG and SD rats after cat-freezing test. Exp. Brain Res. Exp. Hirnforsch. Exp. Cerebrale. 2003;149:413–421. doi: 10.1007/s00221-002-1369-1. [DOI] [PubMed] [Google Scholar]
- Waszczak BL, Walters JR. Intravenous GABA agonist administration stimulates firing of A10 dopaminergic neurons. Eur. J. Pharmacol. 1980;66:141–144. doi: 10.1016/0014-2999(80)90308-8. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Kwint HF, deVries JB. The pharmacology of mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J. Neurosci. 1996;16:2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Nucleus accumbens dopamine release modulation by mesolimbic GABAA receptors-an in vivo electrochemical study. Brain Res. 1998;798:156–165. doi: 10.1016/s0006-8993(98)00406-5. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Baclofen inhibits heroin self-administration behavior and mesolimbic dopamine release. J. Pharmacol. Exp. Ther. 1999;290:1369–1374. [PubMed] [Google Scholar]
- Yan Q. Focal bicuculline increases extracellular dopamine concentration in the nucleus accumbens of freely moving rats as measured by in vivo microdialysis. Eur. J. Pharmacol. 1999;385:7–13. doi: 10.1016/s0014-2999(99)00699-8. [DOI] [PubMed] [Google Scholar]
- Yoon SS, Kim JA, Lee BH, Choi KH, Shim I, Choi SH, Hwang M, Yang CH. Role for GABA agonists in the nucleus accumbens in regulating morphine self-administration. Neurosci. Lett. 2009;462:289–293. doi: 10.1016/j.neulet.2009.07.018. [DOI] [PubMed] [Google Scholar]