Abstract
Individual differences in impulsivity occur at a cognitive and/or behavioural level and are associated with differing life outcomes. However there is a lack of empirical evidence to support the long-term stability of these characteristics in non-human animals. This study reports on the stability of convergent measures of impulsivity in domestic dogs assessed more than six years apart. Measures were (1) owner assessment by means of a questionnaire, the validated ‘Dog Impulsivity Assessment Scale’ (DIAS) and (2) dogs’ performance in a delayed reward choice test. Dogs had 15 minutes free access to two food dispensers, one dispensing a piece of food immediately, the other dispensing three pieces after a delay, which increased by one second every other time the dogs sampled it. Maximum delay reached in this task reflects decision making, or cognitive impulsivity, whereas the rate of extra presses on the delayed reward device during the delay can be considered as a measure of motor or behavioural impulsivity. DIAS scores were strongly and significantly correlated across years. The maximum delay reached in the behaviour test was also highly stable, whereas paw pressing rate was uncorrelated between the years. These results demonstrate that cognitive but not motor impulsivity is highly consistent over time in dogs.
Keywords: dogs Canis familiaris, impulsivity, delayed reward choice, personality, test-retest reliability, stability
Introduction
Many authors report two major facets of impulsivity, the ability to delay gratification (cognitive impulsivity) and the ability to, inhibit prepotent responses (motor impulsivity), Dougherty et al. 2003, Arce and Santisteban 2006). A common test of these abilities in humans and non-human animals is the delayed reward task in which subjects are given a choice between an immediate lower value reward and a higher value reward after a delay. Humans may be asked questions such as “Would you rather have (X amount) now or (Y amount) in (a given delay period)?”, with hypothetical or real rewards such as money, food, etc. (Odum 2011). Nonhuman animals may be given operant choices via lever presses or key pecks between an immediate lower value reinforcer (e.g. one piece of food) and a delayed higher value reinforcer (e.g. three pieces of food, Wright et al. 2012). Individuals’ delay choice can be considered as a measure of cognitive impulsivity, while the ability to refrain from responding during the delay can be considered as a measure of motor impulsivity. Across species, individuals tend to discount the value of rewards that are delayed, as reflected by a decreasing preference for an increasingly delayed reward. Nonetheless, this phenomenon, known as delay discounting, exhibits substantial individual differences (Kalenscher et al. 2006; Jimura et al. 2013), which are associated with a variety of life outcomes. High individual levels of impulsivity (fast discounting) are related to attention deficit hyperactivity disorder, substance abuse, pathological gambling, obesity and aggressive behaviour in humans (Cherek et al. 1997, Winstanley et al. 2006, Reynolds 2006, Odum 2011), as well as to aggressiveness in non-human animals (rats: van den Bergh et al. 2006; golden hamsters: Cervantes and Delville 2009) and to owner-reported ‘behaviour problems’ in domestic dogs (Wright et al. 2011).
In their pioneering work, Mischel and colleagues (1988) measured how long preschool children were able to resist taking an immediate reward (e.g. one marshmallow) to obtain a higher value reward later (e.g. two marshmallows). They found that ability to wait in preschoolers was predictive of attentiveness, academic and social competence, and ability to deal with frustration and stress as adolescents (Mischel et al. 1988). Moreover, individuals who were less able to delay gratification in preschool performed more poorly than those more able to delay in a go/no-go task 40 years later (Casey et al. 2011). However these studies remain rare examples, with behavioural tests of temporal stability of impulsivity in humans typically spanning only a few weeks. In behavioural tests, good long-term test-retest reliability has only been reported in relation to monetary rewards (Audrain-McGovern et al. 2009, Kirby 2009, Anokhin et al. 2011).
Models of impulsivity in animals similarly lack a robust demonstration of test-retest reliability over long-term time frames, despite suggestions that impulsivity is a stable characteristic persisting across experiments (Zaichenko and Merzhanova 2011). Although there has been increased interest in ‘animal personality’, defined as “individual differences in behavior that are consistent across time and contexts” (Stamps and Groothuis 2010), in recent years (Réale et al. 2007), the temporal consistency of traits is often neglected, especially over long time intervals. For instance, with one exception (van den Berg et al. 2006 on consistency of aggressive behaviour in dogs), intervals for assessing repeatability of behaviour in dogs have not exceeded 1.5 years (reviewed in Fratkin et al. 2013).
Here, we aimed to assess the stability of two measures of impulsivity in domestic dogs over a six-year period, one using a validated psychometric tool based on owners’ reports (the ‘Dog Impulsivity Assessment Scale’, DIAS), the other assessing performance in a delayed reward choice test. These measures have previously been shown to be related, demonstrating convergent validity (Wright et al. 2011, 2012).
Methods
This study is a follow-up of the work of Wright et al. (2011, 2012), conducted in 2006, with a repetition of two methods for assessing impulsivity on the same dogs. Thirteen dogs of mostly medium-sized breeds (Belgian Shepherd (Tervuren), two Border Collies, Cocker Spaniel, German Shepherd Dog, German Spitz, Labradoodle, Miniature Poodle, Spanish Water Dog, and four cross-breeds) that had participated in the original study were re-tested in 2013. The subjects’ age ranged from 7.5 to 11.5 years (mean 9 years).
Dog owners completed the Dog Impulsivity Assessment Scale (DIAS), a validated 18-item questionnaire (Wright et al. 2011). The questionnaire yields an overall questionnaire score (OQS) and values for three main factors, labelled ‘Behavioural Regulation’ (F1 – reflecting items relating to excitability and behavioural control), ‘Aggression and Response to Novelty’ (F2), and ‘Responsiveness’ (F3 – reflecting items relating to focus and ease to train). The DIAS has previously demonstrated convergent validity with both a delayed reward choice test and physiological markers of serotonergic and dopaminergic functioning (Wright et al. 2012). Dogs were tested in a delayed reward choice task as in 2006 (described in detail in Wright et al. 2012). After pre-training on a ‘neutral’ device to make sure that the dogs remembered the action of pressing the panel, they were given 10 forced choice trials on each of the two test devices. Pressing of the immediate reward device resulted in immediate delivery of a single piece of dry food, while pressing of the delayed reward device marked the start point of a three-second delay, after which three pieces of food were delivered. Following the forced choice trials, dogs were given continuous free access to the two devices for a 15 minute period as described in Wright et al. 2012. Starting at three seconds, the delay on the delayed reward device was increased by one second every other time the dogs chose the large reward (consistent with the method used by Wright et al. 2012). When a dog switched to the small immediate device before the delay was over, this cancelled the choice of the large delayed reward and resulted in the dispensing of one piece of food from the small immediate device. When the dogs subsequently selected the delayed reward device again, the time delay continued to increase from the delay that had been reached on the previous press of that panel (i.e. the delay was not reset by the alternative choice of the small immediate device). During testing, the dogs’ owners sat in a chair at the back of the test room, filled in the DIAS and did not interact with the dogs.
The maximum delay reached (MaxD) in the 15 minute session and number of extra presses (i.e. presses from first press to delivery of reward) on the delayed reward device during the waiting period were considered to be measures of cognitive and motor impulsivity respectively, since delayed choices reflect the ability to weigh the consequences of immediate and future events and consequently delay gratification (cognitive impulsivity), while rate of paw pressing is a form of response inhibition (motor impulsivity) (Arce & Santisteban 2006). Most dogs show an initial preference for the large delayed device, and although selecting the large delayed device does not lead to gain maximization at longer delays, maximum delay (MaxD) in this test has been found to be inversely correlated with dogs’ impulsivity, as assessed in the DIAS (Wright et al. 2012): The overall questionnaire score and ‘Behavioural Regulation’ factor of the DIAS were significantly negatively correlated with MaxD and significantly positively correlated with the rate of extra presses (Wright et al. 2012). Intraclass correlation coefficients (two-way random, absolute agreement, single measures. Lessells 1987) were calculated to assess repeatability of dogs’ performance in the behaviour test and questionnaire scores. Potential differences were analysed with Wilcoxon Signed Rank tests. To correct for multiple comparisons, False Discovery Rate control (FDR) was applied. Original p-values are indicated in the text. They remained significant after FDR correction unless indicated otherwise. Statistics were computed in PASW Statistics 21.0 software (SPSS Inc. 2012) and figures were produced in Statistica 6.1 (Statsoft Inc. 1984-2004).
Results
13 owners completed the DIAS scale on their dogs, but of these, one had a dog that never selected the delayed reward device in 2006 and two had dogs that did not in 2013. These subjects were therefore excluded from the analysis of the behaviour test since they did not comply with the operational requirement of sampling both devices available in the choice test, leaving 10 subjects in the behaviour test dataset.
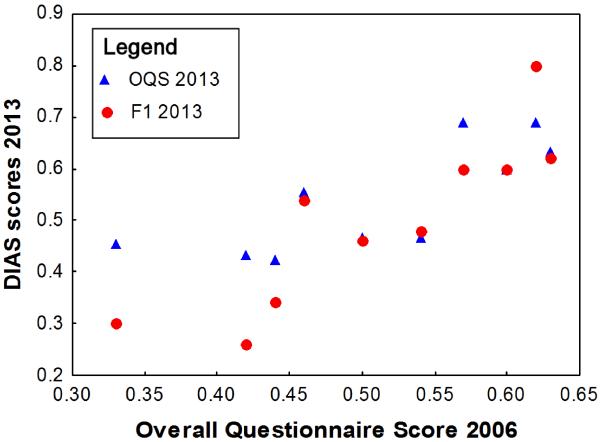
DIAS scores remained highly stable over time: Overall Questionnaire Score (ICC=0.76, n=13, p=0.002), Behavioural Regulation ICC=0.90, p<0.001; Figure 1) and Aggression and Response to Novelty (ICC=0.70, p=0.009) were highly correlated between 2006 and 2013. Responsiveness was the only factor that differed significantly between the years (Wilcoxon Z=2.47, p=0.01), showing a decrease over time. Although Responsiveness tended to correlate positively between the years (ICC=0.46, p=0.033), this was not significant after FDR correction.
Figure 1. Overall Questionnaire Score (OQS) 2013 and Behavioural Regulation Score (F1) in 2013 plotted against OQS in 2006.
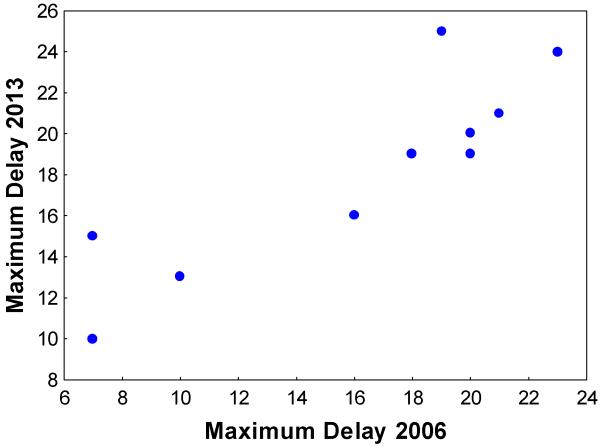
In 2013, the dogs reached maximum delays ranging from 10 - 25 s (median 19 s) in the delayed reward choice test, compared to 7 - 23 s (mean 18 s) in 2006. This slight increase (Wilcoxon, Z=2.02, p=0.04) was not significant following FDR correction. Individuals’ delay choices, reflected by MaxD, were significantly correlated between the years 2006 and 2013 (Intraclass Correlation Coefficient ICC= 0.80, n=10. p<0.001; Figure 2). In contrast, paw pressing rate was uncorrelated across the years (ICC=0.23, p=0.228). There was no statistically significant difference in the median rate of paw pressing in 2006 (median 0.51 presses per second) and 2013 (median 0.89 presses per second, Wilcoxon Z=1.17, p=0.24).
Figure 2. Maximum delay in 2013 plotted against maximum delay in 2006.
Discussion
Both the owners’ assessments of their dogs’ impulsivity according to the DIAS and the dogs’ delay choices in the behaviour test indicate that impulsivity remains highly stable in domestic dogs over at least six years (or half a dog’s lifetime). To our knowledge, this is longer than has been investigated in any previous study using a delayed reward paradigm in either humans or non-human animals.
The general construct of impulsivity, as assessed by DIAS, and cognitive impulsivity (MaxD), but not motor impulsivity (frequency of paw pressing), were found to be stable across time. Previous studies support the suggestion that motor and cognitive impulsivity may be independent (Dougherty et al. 2003; van den Bergh et al. 2006), and the results of the current study suggest that motor impulsivity may be more susceptible to changes over time than cognitive impulsivity. Furthermore, in pigeons, the rate of ineffective key pecks made during reinforcer delays in a delayed reward choice task was affected by food deprivation – unlike delay choice (Logue et al. 1985). These results suggest that motor impulsivity may also be more subject to influence from environmental factors (it is worth noting that, in both 2006 and 2013, owners were asked not to feed their dogs prior to the test, by way of control). It is furthermore possible that paw pressing rate simply reflects general motor activity.
However, delay choice and DIAS scores appear to be more accurate measures of trait impulsivity, having high temporal stability. These measures show a significant correlation with each other, demonstrating cross-situational consistency (Wright et al. 2012). Thus, cognitive but not motor impulsivity appears to be a stable individual characteristic in domestic dogs and can be regarded as a personality trait, being stable across time as well as across contexts (Stamps & Groothuis 2010). This highlights an important distinction between measures which simply allow differentiation between individuals (individual differences) and those relating to behavioural style with a biological basis (true personality traits, sensu Mills 2010). Individual differences do not need to be temporally stable, nor necessarily biologically meaningful; they simple need to allow the reliable discrimination between populations at a given time. The correlation coefficients obtained here are considerably higher than temporal correlations reported for other personality traits in adult domestic dogs, ranging from 0.47 to 0.49 (Fratkin et al. 2013), and this may reflect the grounding of the trait in a clear neurobiological basis (Wright et al. 2012).
More research is needed to determine at what age impulsivity can be considered a stable trait in domestic dogs and whether this is dependent on breed and/ or size of the dog. Furthermore, future studies should address the question to what extent individuals’ impulsivity can be modified through targeted training. It has been suggested for humans that interventions which increase tolerance to delay of reinforcement in one domain could provide beneficial reductions in impulsive behaviours in other domains that may not be as amenable to direct intervention (Odum 2011), and these results indicate that the domestic dog may be a useful experimental model for testing this question.
Acknowledgements
This research was financed by an Exchange Visit Grant awarded to SR by CompCog, an European Science Fund (ESF) Research Networking Programme. SR is furthermore funded by the FWF (Fonds zur Förderung der wissenschaftlichen Forschung) Project P21418 and the DK CogCom Programme (FWF Doctoral Programmes W1234). Our thanks go to Raquel Matos for help with experiments, Tom Pike, Claudia Wascher and an anonymous reviewer for constructive comments on the manuscript and to the dog owners and our canine participants for taking part in this study.
This research was approved by the University of Lincoln’s ethics committee and complies with British animal welfare legislation.
Footnotes
The authors declare that they have no conflict of interest.
Contributor Information
Stefanie Riemer, Messerli Research Institute, University of Veterinary Medicine Vienna, Veterinärplatz 1, 1210 Vienna, Austria; Department of Cognitive Biology, University of Vienna, Althanstr. 14, 1090 Vienna, Austria; Animal Behaviour, Cognition and Welfare Group, School of Life Sciences, University of Lincoln, Riseholme Park, Lincoln, LN2 2LG, UK.
Daniel Mills, Animal Behaviour, Cognition and Welfare Group, School of Life Sciences, University of Lincoln, Riseholme Park, Lincoln, LN2 2LG, UK.
Hannah Wright, Animal Behaviour, Cognition and Welfare Group, School of Life Sciences, University of Lincoln, Riseholme Park, Lincoln, LN2 2LG, UK, hwright@lincoln.ac.uk.
References
- Anokhin AP, Golosheykin S, Grant JD, Heath AC. Heritability of delay discounting in adolescence: A longitudinal twin study. Behav. Genet. 2011;41:175–184. doi: 10.1007/s10519-010-9384-7. doi: 10.1007/s10519-010-9384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce E, Santisteban C. Impulsivity: a review. Psicothema. 2006;18:213–220. [PubMed] [Google Scholar]
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, Jonides J, Berman MG, Wilson NL, Teslovich T, Glover G, Zavas V, Mischel W, Shoda Y. Behavioral and neural correlates of delay of gratification 40 years later. PNAS. 2011;108:14998–15003. doi: 10.1073/pnas.1108561108. doi: 10.1073/pnas.1108561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend. 2009;103(3):99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes MC, Delville Y. Serotonin 5-HT1A and 5-HT3 receptors in an impulsive-aggressive phenotype. Behav Neurosci. 2009;123:589–98. doi: 10.1037/a0015333. doi: 10.1037/a0015333. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Moeller FG, Dougherty DM, Rhoades H. Studies of Violent and Nonviolent Male Parolees: II. Laboratory and Psychometric Measurements of Impulsivity. Biol. Psychiatry. 1997;41:523–529. doi: 10.1016/s0006-3223(96)00426-x. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Harper RA, Marsh DM, Moeller FG, Mathias CW, Swann AC. Behavioral impulsivity paradigms: a comparison in hospitalized adolescents with disruptive behavior disorders. J. Child. Psychol. Psychiatry. 2003;44:1145–1157. doi: 10.1111/1469-7610.00197. doi: 10.1111/1469-7610.00197. [DOI] [PubMed] [Google Scholar]
- Fratkin L, Sinn DL, Patall EA, Gosling SD. Personality consistency in dogs: A meta-analysis. PLoS ONE. 2013;8:e54907. doi: 10.1371/journal.pone.0054907. doi:10.1371/journal.pone.0054907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Chushak MS, Braver TS. Impulsivity and Self-Control during Intertemporal Decision Making Linked to the Neural Dynamics of Reward Value Representation. J. Neurosci. 2013;33:344–357. doi: 10.1523/JNEUROSCI.0919-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalenscher T, Ohmann T, Güntürkün O. The neuroscience of impulsive and self-controlled decisions. Int. J. Psychophysiol. 2006;62:203–211. doi: 10.1016/j.ijpsycho.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Kirby KN. One-year temporal stability of delay-discount rates. Psychon. Bull. Rev. 2009;16:457–462. doi: 10.3758/PBR.16.3.457. [DOI] [PubMed] [Google Scholar]
- Lessells CM, Boag PT. Unrepeatable repeatabilities: a common mistake. Auk. 1987;104:116–121. [Google Scholar]
- Logue AW, Peña-Correal TE. The effect of food deprivation on self control. Behav. Process. 1985;10:355–368. doi: 10.1016/0376-6357(85)90036-1. doi: 10.1016/0376-63578590036-1. [DOI] [PubMed] [Google Scholar]
- Mills DS. Personality. In: Mills DS, editor. Encyclopedia of Applied Animal Behaviour. CABI; Wallingford: 2010. pp. 463–465. [Google Scholar]
- Mischel W, Shoda Y, Peake EK. The nature of adolescent competencies predicted by preschool delay of gratification. J. Pers. Soc. Psychol. 1988;54:687–696. doi: 10.1037//0022-3514.54.4.687. doi: 10.1037/0012-1649.26.6.978. [DOI] [PubMed] [Google Scholar]
- Odum AL. Delay discounting: Trait variable? Behav. Process. 2011;87:1–9. doi: 10.1016/j.beproc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament within ecology and evolution. Biol. Rev. 2007;82:291–318. doi: 10.1111/j.1469-185X.2007.00010.x. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav. Pharmacol. 2006;17:651–67. doi: 10.1097/FBP.0b013e3280115f99. doi:10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Stamps J, Groothuis TG. The development of animal personality: relevance, concepts and perspectives. Biol. Rev. 2010;85:301–325. doi: 10.1111/j.1469-185X.2009.00103.x. doi: 10.1111/j.1469-185X.2009.00103.x. [DOI] [PubMed] [Google Scholar]
- Van den Berg L, Schilder MBH, de Vries H, Leegwater PAG, van Oost BA. Phenotyping of aggressive behavior in Golden retriever dogs with a questionnaire. Behav. Genet. 2006;36:882–902. doi: 10.1007/s10519-006-9089-0. [DOI] [PubMed] [Google Scholar]
- Van den Bergh F, Spronk M, Ferreira L, Bloemarts E, Groenink L, Olivier B, Oosting R. Relationship of delay aversion and response inhibition to extinction learning, aggression, and sexual behaviour. Behav. Brain Res. 2006;175:75–81. doi: 10.1016/j.bbr.2006.08.003. doi:10.1016/j.bbr.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin. Psychol. Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. doi; 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright HF, Mills DS, Pollux PMJ. Development and validation of a psychometric tool for assessing impulsivity in the domestic dog Canis familiaris. Int. J. Comp. Psychol. 2011;24:210–225. doi: 10.1016/j.physbeh.2011.09.019. [Google Scholar]
- Wright HF, Mills DS, Pollux PMJ. Behavioural and Physiological correlates of Impulsivity in the Domestic Dog Canis familiaris. Physiol. Behav. 2012;105:676–682. doi: 10.1016/j.physbeh.2011.09.019. doi: 10.1016/j.physbeh.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Zaichenko MI, Merzhanova GKh. Studies of Impulsivity in Rats in Conditions of Choice between Food Reinforcements of Different Value. Neurosci. Behav. Physiol. 2011;41:445–451. [Google Scholar]